Abstract
Exosomes offer distinct advantages that uniquely position them as highly effective drug carriers. Comprised of cellular membranes with multiple adhesive proteins on their surface, exosomes are known to specialize in cell–cell communications and provide an exclusive approach for the delivery of various therapeutic agents to target cells. In addition, exosomes can be amended through their parental cells to express a targeting moiety on their surface, or supplemented with desired biological activity. Development and validation of exosome-based drug delivery systems are the focus of this review. Different techniques of exosome isolation, characterization, drug loading, and applications in experimental disease models and clinic are discussed. Exosome-based drug formulations may be applied to a wide variety of disorders such as cancer, various infectious, cardiovascular, and neuro-degenerative disorders. Overall, exosomes combine benefits of both synthetic nanocarriers and cell-mediated drug delivery systems while avoiding their limitations.
Keywords: Drug delivery, Exosomes, Extracellular vesicles, Nanotechnology
1. Introduction
In the midst of many exciting developments in drug delivery technologies, nanotechnology holds great promise for many new advances in targeted and controlled-release drug delivery platforms. Various drug nanoformulations have been developed to improve the therapeutic effect of drugs. Unfortunately, opsonization of drug-loaded synthetic nanoparticles in the bloodstream results in two distinct issues with drug nanoformulations: toxicity and rapid clearance by the mononuclear phagocyte system (MPS) [1]. To address these issues, coating the drug-loaded nanocarriers with a PEG corona has been introduced as a method for perpetuating stealth and decreasing clearance by the MPS. However, although PEGylation decreases clearance by the MPS, it also reduces interaction of the nanoformulation with target and barrier cells, thus decreasing the drug biodistribution in disease tissues [2–4]. In addition, the development of an immune response to the PEG corona significantly increases the clearance of PEGylated drug nanocarriers [5–7]. For example, PEGylated liposomes were reported to lose their long-circulating property in the second week following systemic administration in mice [7]. This may become a major problem in chronic disease conditions, which require prolonged drug treatment. In fact, it was reported that 22%–25% healthy blood donors already have preexisting PEG antibodies due to previous exposure to PEG in cosmetics, food, etc. [8,9].
In this respect, exosomes, nanosized vesicles secreted by a variety of cells, represent an important tool for both diagnostic and therapeutic purposes. Exosomes have the exceptional ability to interact with recipient cells (Fig. 1). Comprised of cellular membranes, extracellular vesicles such as exosomes can attach to target cells by a range of surface adhesion proteins and vector ligands (tetraspanins, integrins, CD11b and CD18 receptors), and deliver their payload to target cells [10,11]. Several studies indicate that extracellular vesicles, such as exosomes, have a specific cell tropism, according to their characteristics and origin, which can be used to target them to disease tissues and/or organs [12]. Exosomes can carry cell-type-specific proteins found in the membrane of the parent cell, such as myelin proteins in exosomes derived from oligodendrocytes, with the unique property of homing selectivity [13].
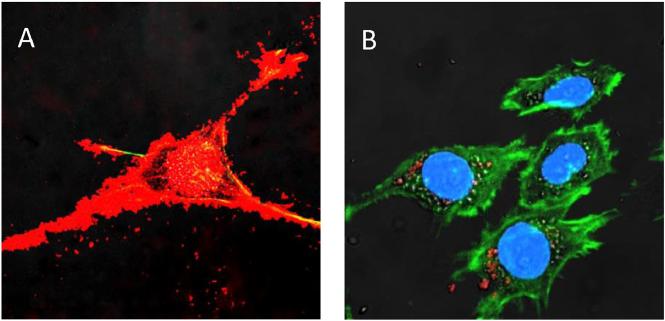
Fig. 1.
A profound accumulation of exosomes (A, red) compared to polymer-based nanoparticles (B, red) in target PC12 neuronal cells stained for actin microfilaments (green) and nuclei (blue). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Furthermore, collected from patients' tissues or blood (for example, bone marrow, monocytes or macrophages), allogenic exosomes may have an immune privileged status, which allows for decreased drug clearance compared to PEGylated nanoformulations. Thus, exosomes may function as an “invisibility cloak” for incorporated therapeutic agents, diminishing clearance by the MPS, and concurrently increasing drug transport to target tissues. Notably, of equal importance is using exosomes that were secreted from cells primed with an antigen immune response (dendritic cells or T cells) for vaccination. In fact, exosomes may comprise advantages of both synthetic nanocarriers and cell-mediated drug delivery, avoiding the rapid clearance and toxicity associated with synthetic vehicles, as well as the complexity in utilizing cell-mediated drug delivery systems in the clinic. Hence, an increasing number of investigations exploit this natural mechanism, using exosomes for the delivery of low molecular-weight therapeutics, nucleic acids, and proteins.
2. Biogenesis, isolation, and characterization of exosomes
Biogenesis, characterization, and functions of exosomes are exciting new fields of research that have triggered significant interest over the past three decades. Exosomes are 40–100 nm sized extracellular membrane-derived vesicles actively secreted by most cell types, in particular, cells of the immune system such as dendritic cells [10], macrophages [14], B cells [15], and T cells [16]; as well as mesenchymal stem cells [17], endothelial [18] and epithelial cells [19]. Exosomes and other types of microvesicles are also secreted by a variety of cancer cells [20].
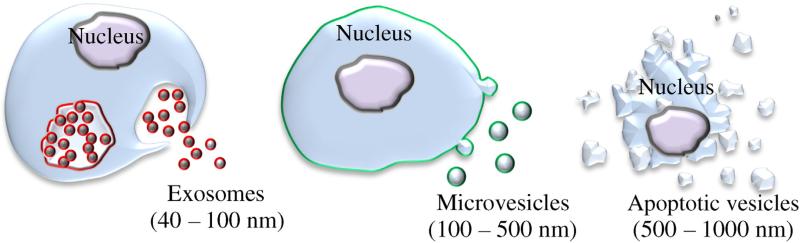
The unique properties of exosomes can be attributed to their biogenesis; the classical view of exosome biogenesis holds that they are initially produced by invagination of the endosomal membrane to create multivesicular bodies (MVB) (Fig. 2). In contrast, exosomes' close relative, microvesicles, are greater in size (100–500 nm) and bud directly from the plasma membrane. Consequently, exosomes and microvesicles are currently believed to have endosomal (red) and plasma (green) membrane origin, respectively (Fig. 2). Larger vesicles (500–1000 nm) are considered to be apoptotic bodies (Fig. 2). Many investigations, especially in the field of drug delivery, utilize both exosomes and microvesicles, defining them as extracellular vesicles, because a complete separation and purification of each type of vesicles is extremely laborious and difficult, if not impossible [21]. As research in the field of exosomes and extracellular vesicles continues, the nomenclature continues to be defined and refined. It should be noted that the absolute separation and definition of various extracellular vesicles (including exosomes) based on their size or biogenesis has yet to be established beyond doubt, and “there is currently no consensus on markers that distinguish the origin of these vesicles once they have left the cell [22].” In addition, exosomes themselves compose a fairly heterogeneous population in terms of their biochemical composition, the source (different cell lines or patient samples) often dictates exosome phenotype [23]; this has critical implications for the use of exosomes in the clinic. The reader would do well to keep these facts in mind when researching exosomes, as the field continues to grow and evolve. This review will refer to either exosomes or extracellular vesicles as is appropriate.
Fig. 2.
Schematic representation of different types of extracellular vesicles.
Exosomes can be characterized by the size, protein and lipid content. Different techniques were developed for the characterization of exosomes. Among them are flow cytometry, western blotting, nanoparticle tracking analysis (NTA), dynamic light scattering (DLS), mass spectrometry (MS) and several microscopy techniques [24]. The International Society for Extracellular Vesicles (ISEV) published a position paper in 2014, in which the characterization of exosomes is recommended by the presence of exosome-associated surface markers, as well as the absence of proteins not associated with exosomes [25]. Exosomal surface markers include TSG101, Alix, flotillin 1, tetraspanins (CD9, CD63, CD81), integrins, and cell adhesion molecules (CAM) [25]. Exosomes are highly enriched in cholesterol, sphingomyelin, and hexosylceramides at the expense of phosphatidylcholine and phosphatidylethanolamine [26]. The fatty acids in exosomes are mostly saturated or monounsaturated. Together with the high concentration of cholesterol, this may account for lateral segregation of these lipids into exosomes during their formation at MVB. Exosomes can be isolated from conditioned cell culture media or bodily fluids by differential centrifugation, filtration paired with centrifugation, immunoaffinity or size exclusion chromatography, or polymer-based precipitation, as well as microfluidic technologies utilizing principles from the aforementioned methods. Each method has its advantages and disadvantages, requires different methods of pre-processing of samples, and produces exosome preparations of varying purity and quality. The user may choose a method for exosome isolation based on the intended downstream use. It is beyond the scope of this review article to provide a comprehensive and detailed review on methods of sample preparation and exosome isolation, instead, we will briefly describe methods currently used to isolate exosomes and refer the reader to other reviews in the literature such as K.W. Witwer et al. [27].
2.1. Differential ultracentrifugation and density gradient centrifugation
This method is considered the “gold standard” for isolating exosomes [10]. It involves applying a centrifugal force to a solution containing exosomes, e.g. conditioned cell culture media or biological fluids. First, a low speed centrifugation step (400× g) is performed in order to remove cells and large cell debris. The supernatant is then subjected to 10,000–20,000× g to remove large debris and intact organ-elles. Finally, the supernatant is again subjected to high speed centrifugation (100,000–150,000× g) to pellet exosomes. It is worth noting that the type, quantity, and quality of exosomes isolated by this method is sensitive to the g force, rotor type, angle of rotor sedimentation, radius of centrifugal force, pelleting efficiency, and solution viscosity. One issue with differential ultracentrifugation is that it sediments exosomes as well as other vesicles, proteins, and/or protein-RNA aggregates. By including a sucrose density gradient, contaminants with densities different than exosomes may be separated from exosomes, allowing for recovery of a theoretically more pure fraction. Gradient centrifugation requires extensive (62–90 h) centrifugation time [28], but provides a more uncontaminated exosome isolate than ultracentrifugation alone. While differential centrifugation has the potential for high exosome yields, this method is subject to operator-dependent variability [29].
2.2. Immunoaffinity chromatography
Immunoaffinity chromatography is a process in which antibodies, covalently attached to beads, filters, or other matrices, bind to specific surface proteins or antigens on the target particle and non-target particles remain unbound. The unbound fraction is discarded, and the desired bound fraction may be collected by washing the stationary phase, typically with a low pH buffer. For the isolation of exosomes, antibodies to exosomal surface markers such as TSG101 or tetraspanins are used [10]. Because this method of exosomes isolation depends on antibody recognition of exosomal proteins, only a subset of all extracellular vesicles (those expressing the antibody-recognized protein) can be captured, resulting in a low yield, but the resulting exosomal isolate is much more pure than exosome isolates prepared by other methods which isolate exosomes based on their physical properties (size, density) [22].
2.3. Size exclusion chromatography
Size exclusion chromatography (SEC) is a method wherein a solution consisting of a heterogeneous population of differently sized components is separated based on their size. A column containing heteroporous beads is used in SEC; components (such as various vesicles and contaminants in a solution containing exosomes) with a smaller hydrodynamic radius are able to pass through the many small pores, akin to a maze, resulting in a longer time to elute. Components with a larger hydrodynamic radius (such as exosomes) are unable to penetrate through as many pores, and thus elute earlier from the column. In this manner, exosomes may be separated from other vesicles and contaminants of different sizes. The advantages of SEC are that it preserves the integrity and biological activity of exosomes and other molecules being separated; because SEC is typically performed using gravity flow, vesicle structure and integrity remains intact [30]. (It should be noted that the use of force to filter exosomes may result in the deformation and breaking-up of larger vesicles, which may potentially skew results [22]). Furthermore, SEC has excellent reproducibility and sensitivity. However, because SEC uses gravity flow, it requires a long run time which limits its scalability for high-throughput applications. In addition, SEC is often used in combination with ultracentrifugation or other techniques in order to concentrate the final exosome preparation [22].
2.4. Polymer precipitation
Polymer precipitation has been used to isolate viruses and other macromolecules for more than 50 years, typically by use of a solution containing polyethylene glycol (PEG). The most commonly used commercial polymer precipitation-based product for exosome isolation is ExoQuick-TC™ from System Biosciences. Typically, to isolate exosomes, a precipitation solution consisting of PEG with a molecular weight of 8,000 Da is used. This precipitation solution is combined with biofluid containing exosomes and is incubated overnight at 4 °C. The mixture is then centrifuged at low speed to form a pellet containing exosomes. The product is relatively easy to use and does not require specialized equipment or a lengthy run time. However, it has been shown that this method co-precipitates non-vesicular contaminants such as lipo-proteins, as well as polymer material [31]. These issues may be addressed by pre- and post-isolation steps. Pre-isolation typically involves the removal of subcellular particles such as lipoproteins through centrifugation. Post-isolation involves removal of the polymer, typically by using a Sephadex G-25 column [28].
2.5. Microfluidic technologies
Although microfluidic-based techniques for the isolation of exosomes are at an early-stage of development, they hold great promise for use in the clinic as they typically require smaller volumes of starting material and provide highly pure exosome preparations with minimal processing time [32]. Microfluidic technologies for isolating exosomes are typically used for diagnostic purposes due to their low yield and high sensitivity [32]. There are three main techniques for isolating exosomes or microvesicles using microfluidics: (A) immunoaffinity, (B) sieving, and (C) trapping exosomes on porous structures [32]. The immunoaffinity-based microfluidic approach to isolating exosomes is similar to the immunoaffinity chromatography technique mentioned above, and operates on similar principles (through the use of antibodies to exosomal surface proteins which are covalently bound to the chip in order to separate exosomes from contaminants) but at a much lower scale. Multiple groups [33–36] have developed chip-based immunoaf-finity microfluidic approaches to isolating exosomes and microvesicles, allowing for quantitative and high-throughput analysis of exosome contents. Davies et al. developed a method based on sieving extracellular vesicles through a porous membrane with a specific size [37]. In this approach, extracellular vesicles are collected by sieving whole blood through a membrane, with filtration driven by either pressure or electrophoresis. The applied electric field assists in separating exosomes from contaminants: proteins are less affected by the electric field due to their lower negative charge as compared to phospholipidic vesicles [38]. Finally, Wang et al. [39] developed a method of trapping exosomes or “exosome-like” vesicles in a porous ciliated silicon microstructure which selectively traps particles 40–100 nm in size. Wang et al. were able to show the selective trapping of lipid vesicles 83 nm and 120 nm in size, but failed to validate their technique with clinical samples, and no analysis of exosomal protein or RNA was performed.
Of note, the rapid production, isolation, purification, and standardization of exosomes in sufficient quantities is one of the main drawbacks for their use in clinic. Most of the developed techniques for the production of clinical grade (cGMP) exosomes are highly labor-intensive and time-consuming, although recently pioneered microfluidic technologies may be able to mitigate these drawbacks. Additional challenges include the reproducibility and consistency of the product lots. Also, exosome yields are typically low and require a large amount of starting material. Therefore, novel approaches are needed for the mass-production of exosome-based drug formulations.
3. Natural functions of exosomes and their intrinsic biological activity
Exosomes play a significant and diverse role in intercellular communication that is an essential process for the development and function of multicellular organisms. These extracellular vesicles were initially thought to be a mechanism for removing unneeded membrane proteins from reticulocytes. Recent studies have shown that they are specialized in long-distance intercellular communications [40,41] facilitating transfer of proteins [42,43], and functional mRNAs and microRNAs for subsequent protein expression in target cells [44,45]. This mechanism of secretion, signaling and communication is a highly efficient, robust, and economic manner of exchanging information between cells. Thus, exosomes themselves exert unique biological activity, even without any loaded drug that may be used for therapeutic purposes.
3.1. Immune regulation by exosomes
Tumor cells are poorly immunogenic and this has hampered the development of effective cancer immunotherapy. By transporting ligands and receptors, exosomes can trigger an anti-tumor response by presenting tumor antigens to immune cells. Initially, tumor-derived exosomes that carry antigens have been suggested as a source of specific stimulus for the immune response against tumors [46]. These exosomes were shown to induce anti-tumor responses more efficiently than irradiated tumor cells, apoptotic bodies, or tumor cell lysates. For example, mouse B lymphoma cells were reported to release exosomes that carry a number of heat shock proteins (HSP) that can induce significant anti-tumor immune responses in T cells [47].
Later, it was demonstrated that tumor-derived exosomes can also possess immunosuppressive properties [48], promote oncogenesis, metastasis [49,50], and drug resistance development [51–53]. Therefore, the attention was turned to the exosomes released by activated antigen presenting cells (APCs), such as dendritic cells (DCs), macrophages, T lymphocytes, and B cells. The presence of MHC class I and class II, as well as T cell co-stimulatory molecules, on the surface of these exosomes is an important mechanism of antigen presentation [54]. Furthermore, the immune response cells primed with antigens can package cellular components from cancer cells in exosomes that then promote immune responses [55–61]. In particular, exosomes secreted by DCs that were primed with acid-eluted tumor peptides were reported to eradicate established tumors in mice [55]. According to another study, DCs-secreted exosomes incubated with human breast adenocarcinoma cells (SK-BR-3) were able to induce tumor-sensitized T cells to secrete high levels of Interferon-γ (IFN-γ) [56]. Qazi et al. [57] reported a significant anti-cancer activity of exosomes secreted by DCs that were exposed to chicken egg ovalbumin (OVA). These exosomes elicited specific transgenic T cell proliferation in vitro. Interestingly, two different methods of OVA loading into exosomes were compared: OVA peptide that was directly loaded into exosomes (Pep-Exo), or exosomes released from OVA-pulsed DCs (OVA-Exo). Pep-Exo formulation was more efficient in specific transgenic T cell proliferation in vitro. However, only exosomes released from OVA-pulsed DCs were efficient in vivo, highlighting the importance of formulation strategies in some cases [57]. Noteworthy, DCs-derived exosomes may also exert undesirable effects, such as triggering the anti-donor T cell response that causes allograft rejection [62].
Along with the improving immune responses, exosomes released from T cells were shown to destroy tumor stroma, and prevent tumor invasion and metastases. In addition, cross-talk between T lymphocytes and endothelial cells through extracellular vesicles was reported [58]. Thus, T cell-derived extracellular vesicles were shown to modulate endothelial cell responses to vascular endothelial growth factor (VEGF) and alter tube formation and gene expression in target endothelial cells. Mechanistic studies revealed that overexpression of thrombospondin-1 and its receptor CD47 on extracellular vesicles derived from T cells allowed targeted and facilitated internalization of these extracellular vesicles into endothelial cells. CD47 transferred to the tumor vasculature by extracellular vesicles modulated tumor angiogenesis and inhibited pro-angiogenic signaling in endothelial cells [58]. Noteworthy, the induction of immune responses may be mediated not only by the bioactive lipids and proteins present in exosomes, but also by exosome- and extracellular vesicle-associated RNAs [59]. Contained inside exosomes, microRNAs (miRNAs) play a key role in mediating biological functions due to their prominent role in gene regulation. Thus, Aucher et al. [60] reported that human macrophages can transfer miRNAs to hepato-carcinoma cells (HCCs) and functionally inhibit proliferation of these cancerous cells. The transport of these miRNA was associated with extracellular vesicles.
Regarding infectious diseases, a successful immunization against diphtheria and Leishmania infections was achieved by use of DC-derived exosomes that were exposed to diphtheria toxin [63] or Leish-mania major [64] antigens, respectively. Furthermore, exosomes found in human breast milk can boost the immune response and alter the T cell balance toward a regulatory phenotype [65,66]. This mechanism may be crucial for the development of the infants' immune system. Thus, exosomes are potent immune regulators, and may be utilized for the design of vaccine adjuvants and therapeutic intervention strategies to modulate immune responses.
3.2. Protective and regenerative effects of exosomes
Exosomes (as well as other types of extracellular vesicles) play a vital role in regulating a broad range of physiological and pathological cellular processes [67] that may be utilized for therapeutic purposes. Mesenchymal stem cells (MSCs) derived from bone marrow, adipose tissue, cord blood, and other origins have recently received much attention as potential therapeutic agents with regenerative properties [68–75]. It was reported that MSCs-derived exosomes produced significant cardio-protective paracrine effects against myocardial ischemia/reperfusion injury in pig and mouse models [68,69]. These exosomes were also beneficial in pulmonary hypertension (HP). HP is a kind of malignant pulmonary vascular disease characterized by an increase in pulmonary artery pressure, which may lead to heart failure and even death. MSCs-derived exosomes directly suppressed early pulmonary inflammation and vascular remodeling [70] through the suppression of hyper-proliferative pathways, including signal transducer and activator of transcription 3 (STAT3)-mediated signaling.
Exosomes secreted from cardiosphere-derived cells (CDCs) were shown to produce a range of diverse cardio-protective effects, including anti-inflammatory, anti-oxidative, anti-apoptotic, anti-fibrotic, and cardiomyogenic effects [75,76]. CDCs-released exosomes stimulated angiogenesis, promoted cardiomyocyte proliferation, and decreased programmed cell death in vitro. Furthermore, the regenerative capacity of these exosomes was demonstrated in a model of chronic myocardial infarction (MI) in rats [77]. These diverse effects were attributed to the ability of exosomes to reduce collagen deposition and exert anti-fibrotic efficacy via paracrine mechanisms [78]. CDCs-released exosomes improved cardiac function, imparted structural benefits, and increased viable mass after MI. The observed therapeutic effects were associated with normalized oxygen consumption, induced ATP production, and preserved mitochondrial integrity.
Exosomes derived from endothelial cells were suggested to be a promising strategy to combat atherosclerosis [79]. Atherosclerosis, the underlying cause of myocardial infarction and stroke, occurs predominantly in predisposed spots in the large arteries. Systemic administration of exosomes released from human umbilical vein endothelial cells (HUVECs) reduced atherosclerotic lesions in mice fed a high-fat diet. It is known that shear stress and its central transcriptional regulator KLF2 elicit properties to the endothelium by regulating the expression of atheroprotective genes. Exosomes secreted by shear-stress-stimulated HUVECs were enriched in multiple miRNAs, most prominently miR-143 and miR-145. HUVECs-derived exosomes transported these miRNAs to smooth muscle cells (SMCs) which resulted in controlled target gene expression and reduction of atherosclerotic lesion formation in the mouse aorta [79].
MSCs-derived exosomes were shown to have neuroprotective effects in stroke. The release, as well as the content, of the MSC-generated exosomes can be modified by environmental conditions. Thus, stroke induces changes in the miRNA profile of these exosomes [80,81], especially in miRNAs that actively participate in the recovery process after stroke [82]. MSCs-derived exosomes transferred their therapeutic factors to recipient cells, altered gene expression, and thereby promoted neurite growth in rat primary neurons [72]. Furthermore, a hepatic regeneration was shown by use of MSC-derived exosomes in drug-induced liver injury models [83]. The higher survival rate was associated with up-regulation of the priming-phase genes during liver regeneration, which subsequently led to higher expression of proliferation proteins (PCNA and cyclin D1) in the exosome-treated group. Therapeutic effects of exosomes derived from human adipose tissue-derived MSCs were also reported for the treatment of Alzheimer disease (AD) [71]. It was demonstrated that these exosomes carry enzymatically active neprilysin (NEP), the most important enzyme that degrades β-amyloid (Aβ) peptide plugs in the brain. MSCs-derived exosomes decreased both intracellular and extracellular Aβ levels in a neuroblastoma cell line N2A in vitro.
Finally, multivesicular bodies have been also identified in plants, and leaderless secreted proteins can be released in vesicles, as described recently [84,85]. Ju et al. showed protective effects of exosome-like nano-particles isolated from crushed grapes [86]. In particular, the oral administration of exosome-like nanoparticles from grapes to mice led to the significant proliferation of the intestinal epithelium. These exosomes are being tested for their effects on oral mucositis and related pain after radio- and chemotherapeutic treatment of head and neck cancers in an ongoing clinical trial (NCT01668849).
4. Using exosomal carriers for therapeutics
4.1. Drug loading into exosomes
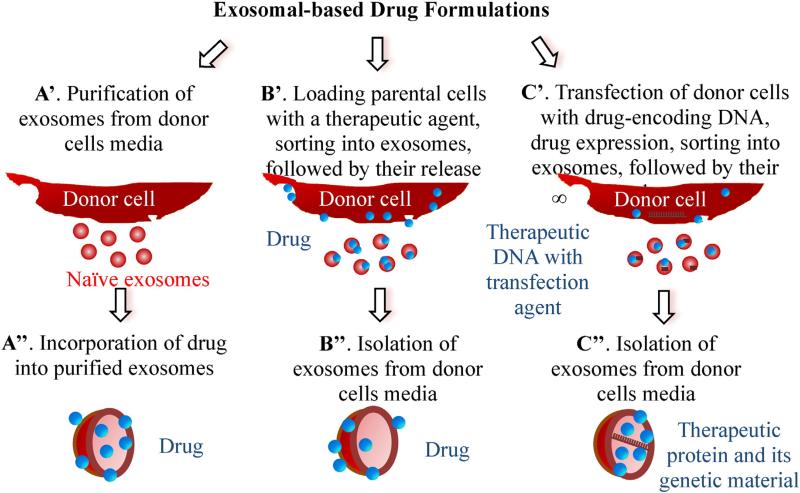
Several distinct approaches can be utilized for the loading of exosomal carriers with therapeutic cargo (Fig. 3): (A) loading naïve exosomes isolated from parental cells ex vitro; (B) loading parental cells with a drug, which is then released in exosomes; and finally, (C) transfecting/infecting parental cells with DNA encoding therapeutically active compounds which are then released in exosomes. Each approach has its advantages and limitations, and may be dictated by the type of therapeutic cargo, site of the disease, and conditions suitable for a specific type of exosome-encapsulated cargo.
Fig. 3.
Different approached for drug loading into exosomes.
Regarding ex vitro loading of naïve exosomes (Fig. 3, path A), different methods for drug incorporation were suggested. In most cases, lipophilic small molecules were passively loaded into exosomes during co-incubation with exosomes or exosome-like vesicles [87–93]. Thus, low molecular antioxidant, curcumin [89,90], anticancer agents, Doxorubicin (Dox) [92,93] and Paclitaxel (PTX) [94], and a model drug Rhoda-mine 123 [94] were loaded into exosomes or exosome-like vesicles by incubation at room temperature (RT). The drug loading was determined by HPLC and varied from 7.2% for PTX to 11.7% for Dox. It is worth noting that exosomes are a very distinct type of nanocarrier that, by their nature, already carries numerous proteins and nucleic acids. This may explain the relatively low loading capacity achieved with these carriers.
Extracellular vesicles, including exosomes, naturally deliver mRNA, miRNA, various noncoding RNA, mitochondrial DNA, and genomic DNA [11,95]. Therefore, they were suggested as carriers for nucleic acids transfer. Similar to the incorporation of genetic material into living cells, electroporation of purified exosomes was proposed for loading of exogenous RNA [13,96–99]. Alvarez-Erviti et al. pioneered this method, electroporating siRNA into DC-derived exosomes [13]. The same method was used to load exosomes with miRNA to epidermal growth factor receptor (EGFR)-expressing breast cancer cells [100]. About 3000 miRNA molecules were loaded per exosome. It should be taken into consideration that electroporation of extracellular vesicles with siRNA may be accompanied by extensive siRNA aggregate formation, which may cause overestimation of the amount of siRNA actually loaded into exosomes [101]. The authors of this report suggested that electroporation is far less efficient than previously reported, and highlighted the necessity for alternative methods to prepare siRNA-loaded exosomes. Exosomes are known to carry a negative surface charge, hence precluding electrostatic siRNA complexation. Pre-complexation of siRNA via cationic liposomes followed by the fusion with isolated exosomes has been suggested for their loading with siRNA by Wahlgren et al. [102]. Furthermore, elevated temperature (37 °C) may be used for improved siRNA loading into exosomes or other types of extracellular vesicles [103].
Exosomes are also known to be nature's way of delivering different proteins [104]. We suggested harnessing this mechanism for the delivery of a potent antioxidant, catalase, in exosomes [105]. Catalase is a large protein (MW 240 kDa) that represents a challenge for incorporation into exosomes. Therefore, different loading procedures (incubation at RT, freeze/thaw cycles, sonication, extrusion, and permeabilization with saponin) were examined. The extensive reformation and reshaping of exosomes upon sonication and extrusion procedures enabled catalase diffusion across the relatively tight and highly structured lipid bilayers, and resulted in the high loading efficiency of exosomal carriers (20%–26% loading capacity). Furthermore, treatment with saponin, an efficient permeabilization agent, also increased catalase loading into exosomes [106]. Notably, aside from proteins, these methods for loading into exosomes can be applied to other therapeutic and imaging agents, in particular, gold nanoparticles [105]. Regarding the quantity, standardization and uniformity of exosomal drug formulations, this approach seems to be the most appropriate, as it allows obtaining large lots of exosomes combined from several isolations, and then loading them with the therapeutic cargo.
As a second approach, parental cells can be loaded with exogenous compounds, which then are released into the conditioned media inside exosomes (Fig. 3, path B). Thus, MSCs-secreted exosomes were loaded with PTX by incubating the parental cells with the drug [107]. It was reported that the murine SR4987 cells that were used as MSCs model produced a significant amount of PTX-loaded exosomes as demonstrated by HPLC [107]. Unfortunately, the authors were not able to determine the amount of PTX associated with MSCs-secreted exosomes; therefore the antitumor activity of this exosome-based formulation referred to the concentration of proteins of the conditioned media bound to PTX. A similar result was reported for HepG2 cells that were incubated with different anticancer agents: PTX, Etoposide, Carboplatin, Irinotecan, Epirubicin, and Mitoxantrone [108]. Exosomes released from drug-treated HepG2 cells demonstrated strong anti-proliferative activity on the human pancreatic cell line CFPAC-1 and induced immunogenicity and HSPs-specific NK cell responses [108]. In another study, the breakdown of parental cells (monocytes/macrophages) loaded with anticancer agents, Dox, Gentamicin, 5-Fluorouracil, or Carboplatin with subsequent isolation of exosome-like nanoparticles was also suggested [91]. An interesting method to pack hydrophobic photosensitizers into membrane vesicles was developed by professor Ji-Ho Park and his team in South Korea [109]. The researchers treated parental cells with synthetic membrane fusogenic liposomes loaded with hydrophobic therapeutics. The drug-loaded liposomes were efficiently incorporated into the membrane of membrane vesicles in the parental cells and were consequently secreted from the cells.
We developed a new approach of loading parental cells (monocytes/macrophages) with catalase followed by isolation of drug-loaded exosomes from conditioned media (Fig. 3, path B) [105,110]. To preserve the therapeutic protein against degradation in host cells and increase loading capacity, catalase was incorporated into a polymer-based nanocontainer before the loading. Importantly, the formulation design of this polymer-based nanocontainer was different from the commonly held approach, where a drug nanoformulation is prepared for systemic administration. Protective nanoparticles are typically size-restricted to avoid entrapment in MPS, focusing on small size nanoparticles with a PEG corona (to perpetuate a stealth effect). In contrast, the optimal nanoformulation for loading into parental cells had a relatively large size (c.a. 200 nm) that resulted in improved accumulation in parental cells, and drug reshuffling into exosomes. The cross-linking of polymer-based nanoparticles with an excess of a non-biodegradable linker ensured low cytotoxicity of the nanoformulation and efficient catalase protection in the parental cells [105,111].
Finally, isolation of drug-loaded exosomes secreted from genetically-modified parental cells has been suggested as a third way of manufacturing exosome-based formulations (Fig. 3, path C) [21,87, 110,112]. In this elegant approach, chicken egg ovalbumin, OVA, was loaded into membrane vesicles when parental cells were transfected with OVAC1C2 fusion complementary DNA (cDNA) consisting of the cargo-encoding gene, OVA, and the gene encoding a protein known to localize to membrane vesicles, C1C2 [112]. We developed a new drug delivery system for different therapeutic proteins, where macrophages were transfected with plasmid DNA (pDNA) encoding therapeutic proteins, catalase [113], or glial cell-line derived neurotropic factor (GDNF) [110] to treat neurodegenerative disorders. Another interesting approach for the incorporation of adeno-associated virus (AAV) capsids into extracellular vesicles to diminish their immunogenicity and improve gene delivery was suggested by Maguire et al. [114]. It was reported that during production, a fraction of released AAV vectors were associated with exosomes, termed vexosomes (vector-exosomes), which outperformed conventionally purified AAV vectors in transduction efficiency in vitro.
4.2. Therapeutic effects of drug-loaded exosomes
Since exosomal carriers can provide advantages of both cell-based drug delivery and nanotechnology, interest in using exosomes for therapeutic approaches has exploded in recent years. Similar to viruses, these remarkable carriers are capable of traveling from one cell to another, easily passing their contents across the cell membrane due to their unique characteristics, and delivering their cargo in a biologically active form. Noteworthy, exosomes possess an intrinsic ability to cross biological barriers, including the most difficult to penetrate: the blood brain barrier (BBB).
Exosomes have been exploited as drug delivery vehicles for low molecular-weight drugs in several investigations [89–94,107,109]. In one of the first reports, exosomes loaded with an anti-inflammatory small molecule compound, curcumin, were shown to protect mice from lipopolysaccharides-induced brain inflammation [89,90]. The incorporation of curcumin in exosomes improved its solubility, increased circulation time, preserved drug therapeutic activity, and improved brain delivery. In another study, exosomes or exosome-like vesicles loaded with different chemotherapeutics, Dox or PTX, were shown to traffic to tumor tissues and reduce tumor growth in mice without the adverse effects observed with the equipotent free drug [91–93]. Notably, the therapeutic effects of Dox-loaded exosomemimetic nanovesicles were greater than the commercially available Dox-loaded liposomes, Doxil; the liposomal formulation was inefficient in reducing tumor growth in this model [91]. Pascucci et al. observed that PTX-treated MSCs mediated strong anti-tumorigenic effects because of their capacity to take up the drug and later release it in extracellular vesicles [107]. In this study, PTX-treated extracellular vesicles induced a dose-dependent inhibition of human pancreatic adenocarcinoma (CFPAC-1) cell proliferation, and 50% inhibition of tumor growth in vivo. Next, membrane vesicles loaded with hydrophobic photosensitizers exhibited superior phototherapeutic effects compared to the polymer-based synthetic nanoparticles [109]. Thus, membrane vesicles fused more effectively with the plasma membrane of cancer cells than polymer-based synthetic nanoparticles, and enabled co-delivery of hydrophobic and hydrophilic compounds into the cellular membrane and cytosol, respectively, largely bypassing the endosome/lysosome pathway. This strategy allowed hydrophobic photosensitizers to significantly penetrate both spheroids and in vivo tumors, thereby enhancing the therapeutic efficacy. Finally, exosomes derived from brain endothelial cell line, bEND.3, were loaded with anticancer drugs and used for systemic delivery across the BBB to treat gliomas [94]. This study tested the hypothesis that bEND.3-derived exosomes can be utilized for the treatment of brain cancer in a xenotransplanted zebrafish model of brain cancer. Exosome-delivered Dox and PTX significantly decreased the fluorescent intensity of xenotransplanted cancer cells and tumor growth marker.
Another therapeutic avenue involves the use of exosomes to deliver exogenous siRNA [13,96–99,102,115–117]. Wahlgren et al. reported the efficient silencing of the target MAPK gene in monocytes and lymphocytes using peripheral blood exosomes with incorporated exogenous siRNAs [102]. In another investigation, Shtam et al. introduced two different exogenous siRNAs against RAD51 and RAD52 into exosomes derived from HeLa cells [115]. The exosome-delivered siRNA against RAD51 was functional and caused massive reproductive cell death of recipient cancer cells. The effect of exosome-siRNA gene silencing has also been validated in [116,117]. As an example, extracellular vesicles were used to transport siRNA targeted to miR-150, an oncomir, due to its promotional effect on VEGF [117]. It was demonstrated that the neutralization of miR-150 down-regulated VEGF levels in mice and attenuated angiogenesis.
The genetic modification of donor cells may be also used for targeting exosomes to the disease site. As an example, targeting of exosomes to the brain was achieved by engineering the parental DCs to express lysosomal-associated membrane protein 2 (Lamp2b), fused to the neuron-specific peptide derived from rabies virus glycoprotein (RVG) [13]. Systemically administered RVG-targeted exosomes delivered glyceraldehyde 3-phosphate dehydrogenase (GAPDH) siRNA specifically to neurons, microglia, oligodendrocytes in the brain, resulting in specific gene knockdown. The therapeutic potential of exosome-mediated siRNA delivery was demonstrated by the strong mRNA (60%) and protein (62%) knockdown of BACE1, a therapeutic target in Alzheimer's disease, in wild-type mice [13].
Exosomes released from macrophages genetically-modified to express antioxidant, catalase, and glial cells-derived neurotrophic factor (GDNF) were suggested for the treatment of Parkinson's disease (PD) [110,113]. Mechanistic studies revealed that exosomes secreted from genetically-modified parental cells contained the encoded therapeutic protein, as well as its genetic material (DNA and mRNA), and NF-κb, a transcription factor involved in the encoded gene expression [113]. Drug-loaded exosomes were able to efficiently transfer their contents to contiguous neurons resulting in de novo protein synthesis in target cells. The transfected brain tissues showed month-long expression of the encoded protein and prolonged attenuation of neuroinflammation (over 40 days) in mice with neuroinflammation [113]. Overall, these reports indicate that exosomes may function as exceptional gene delivery vectors that are safe, efficient, organ/cell-specific, and nonimmunogenic. Nevertheless, significant efforts are required to develop these therapies for clinical use.
5. Using exosomal drug formulations in the clinic
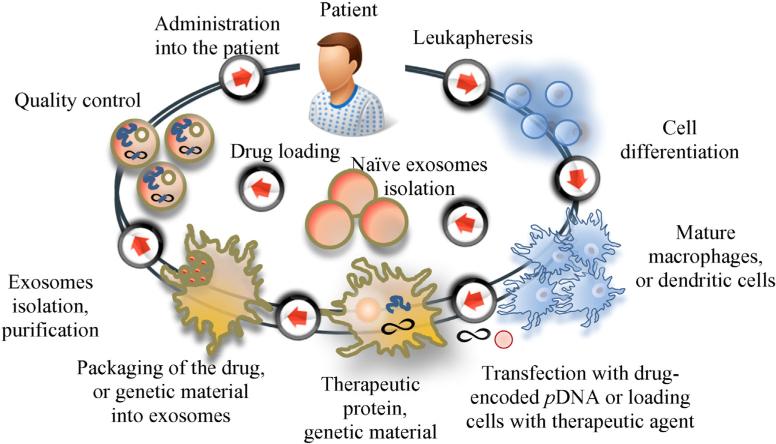
In clinical settings, several approaches may be applied to introduce exosomal-based drug delivery systems. First, leukocytes harvested from peripheral blood by apheresis may be propagated and cultured, differentiated to specific cell types if necessary, and then exosomal carriers can be loaded with a therapeutic agent and re-administered back into the patient (Fig. 4). One of the major challenges in developing this approach is whether the production of exosomes could be scalable or reproducible [118]. Indeed, the exosome yield per cell will impact the final production cost as well as clinical applications. In this respect, the choice of parental cells is critical. For example, MSCs are known to produce large amounts of exosomes, suggesting that these cells may be efficient for exosome production in a clinically applicable scale [119]. Next, extended culturing of donor cells may considerably increase exosomal production. For example, culturing DCs for extended time period [29], or at low pH [120] increased the release of exosomes 5–10 fold. In another study, the breakdown of parental cells (monocytes/macrophages) loaded with anticancer agents, and isolation of exosome-like nanoparticles allowed a 100-fold higher production yield of the drug carriers [91]. Finally, specifically designed bioreactors that resemble bioreactors for tissue engineering [121] can be utilized for exosome production scale-up. Notably, exosomes can be concentrated, lyophilized, and reconstituted in aqueous solutions, as was reported in [105].
Fig. 4.
The flow of the production and delivery of exosomal drug formulations to the patient.
As an alternative approach, MSCs may be harvested from bone marrow, propagated in culture to obtain specific cell types, or even sub-types, and then exosomes may be loaded with a therapeutic agent. Although this approach would require a more invasive procedure, a significant amount of, as well as storage of, well-characterized exosomal carriers would be possible [122]. Furthermore, large scale production of therapeutically efficacious exosomes can be achieved through the immortalization of donor cells; for example, MSCs can be transfected by lentivirus carrying a MYC gene as was reported in [123]. MYC is a regulator gene that codes for a transcription factor that plays a role in cell cycle progression. The transfection allows for obtaining of immortalized cells, but does not alter the fundamental characteristics of these MSCs [123]. In this case, a library of various types of exosomal carriers for different drug formulations could be developed in the future, and stored in stock for emergency situations. Finally, exosomes may be isolated from other sources (bovine milk, crushed grapes, etc.), purified, loaded with a drug and used for oral or intranasal administration.
In fact, exosomes have already been approved for use in several clinical trials, and our experience with exosome-based therapies in humans is rapidly expanding [124]. In particular, exosomes were puri-fied from monocyte cultures from 15 patients with advanced metastatic melanoma. The good manufacturing procedures (GMP) process allowed harvesting of about 5 × 1014 exosomal MHC class II carriers. Then, the exosomes were loaded with melanoma antigen ex vitro and administered in an autologous fashion in an attempt to promote anti-melanoma immunity via therapeutic vaccination. It was reported that patients well-tolerated repeated administration of autologous exosomes for up to 21 months [125]. In a similar trial, non-small-cell-carcinoma lung cancer patients were injected with autologous exosomes weekly for 4 weeks, and similar low level immune responses were observed [126]. Finally, ascites-derived exosomes in combination with the granulocyte-macrophage colony-stimulating factor (GM-CSF) were utilized in the immunotherapy of colorectal cancer (CRC). A total of 40 patients with advanced CRC were enrolled in this clinical trial and received from 100 to 500 μg of exosomal formulations [127]. The exosome-based therapies were reported to be safe, feasible, and efficient in induction of antigen-specific T lymphocyte response, however, several technical obstacles remain, which must be overcome.
Finally, exosomes derived from grapes will be evaluated for cancer treatment in the clinical trial initiated at the James Graham Brown Cancer Center (NCT01294072). In this study, plant-derived exosomes are loaded with a low molecular-weight anti-inflammatory agent, curcumin, and administered orally into patients with colorectal cancer. The use of plant-derived nanocarriers may solve one of the main problems in the field of exosome-based drug delivery: the isolation and purification of exosomes in sufficient quantities for therapeutic applications.
6. Conclusion
Drug-loaded exosomes may well serve as a next generation drug delivery mechanism that combines nanoparticle size with non-cytotoxic effects, a high drug carrying capacity, and a low immunogenic profile. Future investigations will focus on the production of large amounts of well-characterized exosomal carriers with high loading capacity. Indeed, exosomes should be able to carry a substantial amount of therapeutic cargo to qualify as drug delivery vehicles. Further tailoring exosomes can provide biologically-active carriers that may be modified in accordance to the disease and produce cytotoxic (for cancer treatment), or neuroprotective (for the treatment of neurodegenerative disorders) effects, enhancing the therapeutic outcomes. The fulfillment of these objectives will set a stage for the key steps of the subsequent industrial development of these novel therapeutic interventions including scaling up and quality control of production, rigorous pharmacokinetic and toxicological studies and, eventually, clinical testing.
Indeed, some technological, functional and safety features of exosomal-based drug formulations are yet to be addressed. Deficiencies in our knowledge of the molecular mechanisms of exosomes biogenesis, and a lack of methods to interfere with the packaging of cargo or with vesicle release still hampers identification of their physiological relevance in vivo. Certainly, the complexity of these therapeutic interventions is challenging, yet they promise an unparalleled efficacy in the treatment of many life-threatening conditions, including those lacking effective pharmacotherapy.
Acknowledgments
This study was supported by the United States National Institutes of Health grants 1RO1 NS057748 (to EVB).
References
- 1.Peng Q, Zhang S, Yang Q, Zhang T, Wei XQ, Jiang L, Zhang CL, Chen QM, Zhang ZR, Lin YF. Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials. 2013;43:8521–8530. doi: 10.1016/j.biomaterials.2013.07.102. [DOI] [PubMed] [Google Scholar]
- 2.Beckman JS, Minor RL, Jr., White CW, Repine JE, Rosen GM, Freeman BA. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J. Biol. Chem. 1988;263:6884–6892. [PubMed] [Google Scholar]
- 3.Yoshida K, Burton GF, McKinney JS, Young H, Ellis EF. Brain and tissue distribution of polyethylene glycol-conjugated superoxide dismutase in rats. Stroke. 1992;23:865–869. doi: 10.1161/01.str.23.6.865. [DOI] [PubMed] [Google Scholar]
- 4.Veronese FM, Caliceti P, Schiavon O, Sergi M. Polyethylene glycol-superoxide dismutase, a conjugate in search of exploitation. Adv. Drug Deliv. Rev. 2002;54:587–606. doi: 10.1016/s0169-409x(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 5.Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, van Der Meer JW, Corstens FH, Boerman OC. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J. Pharmacol. Exp. Ther. 2000;292:1071–1079. [PubMed] [Google Scholar]
- 6.Ishida T, Maeda R, Ichihara M, Irimura K, Kiwada H. Accelerated clearance of PEGylated liposomes in rats after repeated injections. J. Control. Release. 2003;88:35–42. doi: 10.1016/s0168-3659(02)00462-5. [DOI] [PubMed] [Google Scholar]
- 7.Ishida T, Kashima S, Kiwada H. The contribution of phagocytic activity of liver macrophages to the accelerated blood clearance (ABC) phenomenon of PEGylated liposomes in rats. J. Control. Release. 2008;126:162–165. doi: 10.1016/j.jconrel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong JK, Hempel G, Koling S, Chan LS, Fisher T, Meiselman HJ, Garratty G. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110:103–111. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 9.Garratty G. Modulating the red cell membrane to produce universal/stealth donor red cells suitable for transfusion. Vox Sang. 2008;94:87–95. doi: 10.1111/j.1423-0410.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- 10.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006:1–29. doi: 10.1002/0471143030.cb0322s30. (Chapter 3, Unit 3 22) [DOI] [PubMed] [Google Scholar]
- 11.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 12.Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mager I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CE, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 14.Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J. Cell Sci. 2005;118:3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 16.Nolte-'t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–1981. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 17.Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015;40:82–88. doi: 10.1016/j.semcdb.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Song J, Chen X, Wang M, Xing Y, Zheng Z, Hu S. Cardiac endothelial cell-derived exosomes induce specific regulatory B cells. Sci. Rep. 2014;4:7583. doi: 10.1038/srep07583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skogberg G, Lundberg V, Berglund M, Gudmundsdottir J, Telemo E, Lindgren S, Ekwall O. Human thymic epithelial primary cells produce exosomes carrying tissue-restricted antigens. Immunol. Cell Biol. 2015:1–8. doi: 10.1038/icb.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benito-Martin A, Di Giannatale A, Ceder S, Peinado H. The new deal: a potential role for secreted vesicles in innate immunity and tumor progression. Front. Immunol. 2015;6:66. doi: 10.3389/fimmu.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcus ME, Leonard JN. FedExosomes: engineering therapeutic biological nanoparticles that truly deliver. Pharmaceuticals (Basel) 2013;6:659–680. doi: 10.3390/ph6050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol. 2015;1295:179–209. doi: 10.1007/978-1-4939-2550-6_15. [DOI] [PubMed] [Google Scholar]
- 23.Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, Cheng HH, Arroyo JD, Meredith EK, Gallichotte EN, Pogosova-Agadjanyan EL, Morrissey C, Stirewalt DL, Hladik F, Yu EY, Higano CS, Tewari M. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. U. S. A. 2014;111:14888–14893. doi: 10.1073/pnas.1408301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Pol E, Coumans FA, Grootemaat AE, Gardiner C, Sargent IL, Harrison P, Sturk A, van Leeuwen TG, Nieuwland R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J. Thromb. Haemost. 2014;12:1182–1192. doi: 10.1111/jth.12602. [DOI] [PubMed] [Google Scholar]
- 25.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Thery C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aalberts M, van Dissel-Emiliani FM, van Adrichem NP, van Wijnen M, Wauben MH, Stout TA, Stoorvogel W. Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biol. Reprod. 2012;86:82. doi: 10.1095/biolreprod.111.095760. [DOI] [PubMed] [Google Scholar]
- 27.Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, Nolte-'t Hoen EN, Piper MG, Sivaraman S, Skog J, Thery C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor DD, Shah S. Methods of isolating extracellular vesicles impact downstream analyses of their cargoes. Methods. 2015 doi: 10.1016/j.ymeth.2015.02.019. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 29.Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, Le Pecq JB. Production and characterization of clinical grade exosomes derived from dendritic cells. J. Immunol. Methods. 2002;270:211–226. doi: 10.1016/s0022-1759(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 30.Muller L, Hong C-S, Stolz DB, Watkins SC, Whiteside TL. Isolation of biologically-active exosomes from human plasma. J. Immunol. Methods. 2014;411:55–65. doi: 10.1016/j.jim.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor DD, Zacharias W, Gercel-Taylor C. Exosome isolation for proteomic analyses and RNA profiling. Methods Mol. Biol. 2011;728:235–246. doi: 10.1007/978-1-61779-068-3_15. [DOI] [PubMed] [Google Scholar]
- 32.Liga A, Vliegenthart ADB, Oosthuyzen W, Dear JW, Kersaudy-Kerhoas M. Exosome isolation: a microfluidic road-map. Lab Chip. 2015;15:2388–2394. doi: 10.1039/c5lc00240k. [DOI] [PubMed] [Google Scholar]
- 33.Chen C, Skog J, Hsu C-H, Lessard RT, Balaj L, Wurdinger T, Carter BS, Breakefield XO, Toner M, Irimia D. Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip. 2010;10:505–511. doi: 10.1039/b916199f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He M, Crow J, Roth M, Zeng Y, Godwin AK. Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology†Author contributions: M. H., Y. Z. and A. K. G. conceived research; M. H. and Y. Z. designed and fabricated the devices; M. H., Y. Z., J. C., and M. R. performed the research; M. H., Y. Z., and A. K. G. analyzed the data; M. H., Y. Z., and A. K. G. wrote the manuscript. ‡Electronic supplementary information (ESI) available. Lab Chip. 2014;14:3773–3780. doi: 10.1039/c4lc00662c. http://dx.doi.org/10.1039/c4lc00662c (Click here for additional data file) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Im H, Shao H, Park YI, Peterson VM, Castro CM, Weissleder R, Lee H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 2014;32:490–495. doi: 10.1038/nbt.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaidyanathan R, Naghibosadat M, Rauf S, Korbie D, Carrascosa LG, Shiddiky MJA, Trau M. Detecting exosomes specifically: a multiplexed device based on alternating current electrohydrodynamic induced nanoshearing. Anal. Chem. 2014;86:11125–11132. doi: 10.1021/ac502082b. [DOI] [PubMed] [Google Scholar]
- 37.Davies RT, Kim J, Jang SC, Choi E-J, Gho YS, Park J. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip. 2012;12:5202–5210. doi: 10.1039/c2lc41006k. [DOI] [PubMed] [Google Scholar]
- 38.Sparks DL, Phillips MC. Quantitative measurement of lipoprotein surface charge by agarose gel electrophoresis. J. Lipid Res. 1992;33:123–130. [PubMed] [Google Scholar]
- 39.Wang Z, Wu H.-j., Fine D, Schmulen J, Hu Y, Godin B, Zhang JXJ, Liu X. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip. 2013;13:2879–2882. doi: 10.1039/c3lc41343h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol. Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 42.Johnstone RM. The Jeanne Manery-Fisher Memorial Lecture 1991. Maturation of reticulocytes: formation of exosomes as a mechanism for shedding membrane proteins. Biochem. Cell Biol. 1992;70:179–190. doi: 10.1139/o92-028. [DOI] [PubMed] [Google Scholar]
- 43.Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 44.Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes: fit to deliver small RNA. Commun. Integr. Biol. 2010;3:447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 46.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 47.Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q, Cao X. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur. J. Immunol. 2006;36:1598–1607. doi: 10.1002/eji.200535501. [DOI] [PubMed] [Google Scholar]
- 48.Yang C, Kim SH, Bianco NR, Robbins PD. Tumor-derived exosomes confer antigen-specific immunosuppression in a murine delayed-type hypersensitivity model. PLoS One. 2011;6:e22517. doi: 10.1371/journal.pone.0022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le MT, Hamar P, Guo C, Basar E, Perdigao-Henriques R, Balaj L, Lieberman J. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J. Clin. Invest. 2014;124:5109–5128. doi: 10.1172/JCI75695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris DA, Patel SH, Gucek M, Hendrix A, Westbroek W, Taraska JW. Exosomes released from breast cancer carcinomas stimulate cell movement. PLoS One. 2015;10:e0117495. doi: 10.1371/journal.pone.0117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lv MM, Zhu XY, Chen WX, Zhong SL, Hu Q, Ma TF, Zhang J, Chen L, Tang JH, Zhao JH. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumour Biol. 2014;35:10773–10779. doi: 10.1007/s13277-014-2377-z. [DOI] [PubMed] [Google Scholar]
- 52.Prokopi M, Kousparou CA, Epenetos AA. The secret role of microRNAs in cancer stem cell development and potential therapy: a notch-pathway approach. Front. Oncol. 2014;4:389. doi: 10.3389/fonc.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao L, Liu W, Xiao J, Cao B. The role of exosomes and “exosomal shuttle microRNA” in tumorigenesis and drug resistance. Cancer Lett. 2015;356:339–346. doi: 10.1016/j.canlet.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 54.Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015;16:24–43. doi: 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 56.Romagnoli GG, Zelante BB, Toniolo PA, Migliori IK, Barbuto JA. Dendritic cell-derived exosomes may be a tool for cancer immunotherapy by converting tumor cells into immunogenic targets. Front. Immunol. 2014;5:692. doi: 10.3389/fimmu.2014.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qazi KR, Gehrmann U, Domange Jordo E, Karlsson MC, Gabrielsson S. Antigenloaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood. 2009;113:2673–2683. doi: 10.1182/blood-2008-04-153536. [DOI] [PubMed] [Google Scholar]
- 58.Kaur S, Singh SP, Elkahloun AG, Wu W, Abu-Asab MS, Roberts DD. CD47-dependent immunomodulatory and angiogenic activities of extracellular vesicles produced by T cells. Matrix Biol. 2014;37:49–59. doi: 10.1016/j.matbio.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Grein SG, Nolte-'t Hoen EN. “Small Talk” in the innate immune system via RNA-containing extracellular vesicles. Front. Immunol. 2014;5:542. doi: 10.3389/fimmu.2014.00542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J. Immunol. 2013;191:6250–6260. doi: 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruno S, Collino F, Deregibus MC, Grange C, Tetta C, Camussi G. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 2013;22:758–771. doi: 10.1089/scd.2012.0304. [DOI] [PubMed] [Google Scholar]
- 62.Morelli AE. The immune regulatory effect of apoptotic cells and exosomes on dendritic cells: its impact on transplantation. Am. J. Transplant. 2006;6:254–261. doi: 10.1111/j.1600-6143.2005.01197.x. [DOI] [PubMed] [Google Scholar]
- 63.Colino J, Snapper CM. Dendritic cell-derived exosomes express a Streptococcus pneumoniae capsular polysaccharide type 14 cross-reactive antigen that induces protective immunoglobulin responses against pneumococcal infection in mice. Infect. Immun. 2007;75:220–230. doi: 10.1128/IAI.01217-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schnitzer JK, Berzel S, Fajardo-Moser M, Remer KA, Moll H. Fragments of antigen-loaded dendritic cells (DC) and DC-derived exosomes induce protective immunity against Leishmania major. Vaccine. 2010;28:5785–5793. doi: 10.1016/j.vaccine.2010.06.077. [DOI] [PubMed] [Google Scholar]
- 65.Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 66.Zonneveld MI, Brisson AR, van Herwijnen MJ, Tan S, van de Lest CH, Redegeld FA, Garssen J, Wauben MH, Nolte-'t Hoen EN. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. J. Extracell. Vesicles. 2014;3 doi: 10.3402/jev.v3.24215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, Buzas EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 69.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M, Ochiya T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep. 2013;3:1197. doi: 10.1038/srep01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xin H, Li Y, Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front. Cell. Neurosci. 2014;8:377. doi: 10.3389/fncel.2014.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ilmer M, Vykoukal J, Recio Boiles A, Coleman M, Alt E. Two sides of the same coin: stem cells in cancer and regenerative medicine. FASEB J. 2014;28:2748–2761. doi: 10.1096/fj.13-244640. [DOI] [PubMed] [Google Scholar]
- 74.Emanueli C, Shearn AI, Angelini GD, Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vasc. Pharmacol. 2015;71:24–30. doi: 10.1016/j.vph.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang L, Ma W, Ma Y, Feng D, Chen H, Cai B. Exosomes in mesenchymal stem cells, a new therapeutic strategy for cardiovascular diseases? Int. J. Biol. Sci. 2015;11:238–245. doi: 10.7150/ijbs.10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 2014;114:333–344. doi: 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- 77.Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014;2:606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aminzadeh MA, Tseliou E, Sun B, Cheng K, Malliaras K, Makkar RR, Marban E. Therapeutic efficacy of cardiosphere-derived cells in a transgenic mouse model of non-ischaemic dilated cardiomyopathy. Eur. Heart J. 2015;36:751–762. doi: 10.1093/eurheartj/ehu196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 80.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 81.Lusardi TA, Murphy SJ, Phillips JI, Chen Y, Davis CM, Young JM, Thompson SJ, Saugstad JA. MicroRNA responses to focal cerebral ischemia in male and female mouse brain. Front. Mol. Neurosci. 2014;7:11. doi: 10.3389/fnmol.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu FJ, Lim KY, Kaur P, Sepramaniam S, Armugam A, Wong PT, Jeyaseelan K. microRNAs involved in regulating spontaneous recovery in embolic stroke model. PLoS One. 2013;8:e66393. doi: 10.1371/journal.pone.0066393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res. Ther. 2014;5:76. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ding Y, Wang J, Stierhof YD, Robinson DG, Jiang L. Unconventional protein secretion. Trends Plant Sci. 2012;17:606–615. doi: 10.1016/j.tplants.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 85.Regente M, Pinedo M, Elizalde M, de la Canal L. Apoplastic exosome-like vesicles: a new way of protein secretion in plants? Plant Signal. Behav. 2012;7:544–546. doi: 10.4161/psb.19675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, Xiang X, Deng ZB, Wang B, Zhang L, Roth M, Welti R, Mobley J, Jun Y, Miller D, Zhang HG. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013;21:1345–1357. doi: 10.1038/mt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalani A, Kamat PK, Chaturvedi P, Tyagi SC, Tyagi N. Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sci. 2014;107:1–7. doi: 10.1016/j.lfs.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kalani A, Tyagi A, Tyagi N. Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Mol. Neurobiol. 2014;49:590–600. doi: 10.1007/s12035-013-8544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang HG. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, Nilsson J, Lotvall J, Kim YK, Gho YS. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 92.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 93.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol. Ther. 2015;23:812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S. Exosome delivered anticancer drugs across the blood–brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015;32:2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Waldenstrom A, Genneback N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One. 2012;7:e34653. doi: 10.1371/journal.pone.0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.El Andaloussi S, Lakhal S, Mager I, Wood MJ. Exosomes for targeted siRNA delivery across biological barriers. Adv. Drug Deliv. Rev. 2013;65:391–397. doi: 10.1016/j.addr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 97.Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012;21:R125–R134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 98.Lai CP, Breakefield XO. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front. Physiol. 2012;3:228. doi: 10.3389/fphys.2012.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lai RC, Yeo RW, Tan KH, Lim SK. Exosomes for drug delivery — a novel application for the mesenchymal stem cell. Biotechnol. Adv. 2012;31:543–551. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 100.Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kooijmans SA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJ, Schiffelers RM, Raemdonck K, Vader P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release. 2013;172:229–238. doi: 10.1016/j.jconrel.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 102.Wahlgren J, De LKT, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, Valadi H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40:e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van der Meel R, Fens MH, Vader P, van Solinge WW, Eniola-Adefeso O, Schiffelers RM. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J. Control. Release. 2014;195:72–85. doi: 10.1016/j.jconrel.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 104.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J. Control. Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jamur MC, Oliver C. Permeabilization of cell membranes. Methods Mol. Biol. 2010;588:63–66. doi: 10.1007/978-1-59745-324-0_9. [DOI] [PubMed] [Google Scholar]
- 107.Pascucci L, Cocce V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, Vigano L, Locatelli A, Sisto F, Doglia SM, Parati E, Bernardo ME, Muraca M, Alessandri G, Bondiolotti G, Pessina A. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J. Control. Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 108.Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J. Biol. Chem. 2012;287:15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee J, Kim J, Jeong M, Lee H, Goh U, Kim H, Kim B, Park JH. Liposome-based engineering of cells to package hydrophobic compounds in membrane vesicles for tumor penetration. Nano Lett. 2015;15:2938–2944. doi: 10.1021/nl5047494. [DOI] [PubMed] [Google Scholar]
- 110.Zhao Y, Haney MJ, Gupta R, Bohnsack JP, He Z, Kabanov AV, Batrakova EV. GDNF-transfected macrophages produce potent neuroprotective effects in Parkinson's disease mouse model. PLoS One. 2014;9:e106867. doi: 10.1371/journal.pone.0106867. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Haney MJ, Suresh P, Zhao Y, Kanmogne GD, Kadiu I, Sokolsky-Papkov M, Klyachko NL, Mosley RL, Kabanov AV, Gendelman HE, Batrakova EV. Blood-borne macrophage-neural cell interactions hitchhike endosome networks for cell-based nanozyme brain delivery. Nanomedicine (Lond.) 2012;7:815–833. doi: 10.2217/nnm.11.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, Delcayre A, Le Pecq JB, Combadiere B, Amigorena S, Thery C. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res. 2008;68:1228–1235. doi: 10.1158/0008-5472.CAN-07-3163. [DOI] [PubMed] [Google Scholar]
- 113.Haney MJ, Zhao Y, Harrison EB, Mahajan V, Ahmed S, He Z, Suresh P, Hingtgen SD, Klyachko NL, Mosley RL, Gendelman HE, Kabanov AV, Batrakova EV. Specific transfection of inflamed brain by macrophages: a new therapeutic strategy for neurodegenerative diseases. PLoS One. 2013;8:e61852. doi: 10.1371/journal.pone.0061852. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Maguire CA, Balaj L, Sivaraman S, Crommentuijn MH, Ericsson M, Mincheva-Nilsson L, Baranov V, Gianni D, Tannous BA, Sena-Esteves M, Breakefield XO, Skog J. Microvesicle-associated AAV vector as a novel gene delivery system. Mol. Ther. 2012;20:960–971. doi: 10.1038/mt.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pan Q, Ramakrishnaiah V, Henry S, Fouraschen S, de Ruiter PE, Kwekkeboom J, Tilanus HW, Janssen HL, van der Laan LJ. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi) Gut. 2012;61:1330–1339. doi: 10.1136/gutjnl-2011-300449. [DOI] [PubMed] [Google Scholar]
- 117.Liu Y, Zhao L, Li D, Yin Y, Zhang CY, Li J, Zhang Y. Microvesicle-delivery miR-150 promotes tumorigenesis by up-regulating VEGF, and the neutralization of miR-150 attenuate tumor development. Protein Cell. 2013;4:932–941. doi: 10.1007/s13238-013-3092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nordin JZ, Lee Y, Vader P, Mager I, Johansson HJ, Heusermann W, Wiklander OP, Hallbrink M, Seow Y, Bultema JJ, Gilthorpe J, Davies T, Fairchild PJ, Gabrielsson S, Meisner-Kober NC, Lehtio J, Smith CI, Wood MJ, Andaloussi SE. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine. 2015;11:879–883. doi: 10.1016/j.nano.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 119.Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2012;65:336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 120.Ban JJ, Lee M, Im W, Kim M. Low pH increases the yield of exosome isolation. Biochem. Biophys. Res. Commun. 2015;461:76–79. doi: 10.1016/j.bbrc.2015.03.172. [DOI] [PubMed] [Google Scholar]
- 121.Portner R, Nagel-Heyer S, Goepfert C, Adamietz P, Meenen NM. Bioreactor design for tissue engineering. J. Biosci. Bioeng. 2005;100:235–245. doi: 10.1263/jbb.100.235. [DOI] [PubMed] [Google Scholar]
- 122.Muller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat. Rev. Neurosci. 2006;7:75–84. doi: 10.1038/nrn1829. [DOI] [PubMed] [Google Scholar]
- 123.Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo AB, Padmanabhan J, Lee CN, de Kleijn DP, Lim SK. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J. Transl. Med. 2011;9:47. doi: 10.1186/1479-5876-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mignot G, Roux S, Thery C, Segura E, Zitvogel L. Prospects for exosomes in immunotherapy of cancer. J. Cell. Mol. Med. 2006;10:376–388. doi: 10.1111/j.1582-4934.2006.tb00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J. Transl. Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]