Summary
Phosphoenolpyruvate carboxykinase (PEPCK) is well known for its role in gluconeogenesis. However, PEPCK is also a key regulator of TCA cycle flux. The TCA cycle integrates glucose, amino acid and lipid metabolism depending on cellular needs. In addition, biosynthetic pathways crucial to tumor growth require the TCA cycle for the processing of glucose and glutamine derived carbons. We show here an unexpected role for PEPCK in promoting cancer cell proliferation in vitro and in vivo by increasing glucose and glutamine utilization toward anabolic metabolism. Unexpectedly, PEPCK also increased the synthesis of ribose from non-carbohydrate sources, such as glutamine, a phenomenon not previously described. Finally, we show that the effects of PEPCK on glucose metabolism and cell proliferation are in part mediated via activation of mTORC1. Taken together, these data demonstrate a role for PEPCK that links metabolic flux and anabolic pathways to cancer cell proliferation.
Introduction
Reprogramming of cellular metabolism in cancer cells is crucial for maintaining biosynthetic demands, proliferation, energy and reducing equivalents for macromolecular synthesis. Many studies have focused on addiction to either glucose or glutamine as a basis for cancer therapy (DeBerardinis et al., 2008a; Deberardinis et al., 2008b; Gatenby and Gillies, 2004). However, studies are beginning to suggest that these are not universal features in cancer and that cancer cells display metabolic flexibility(Lim et al., 2014; Marin-Valencia and DeBerardinis, 2011). For example when glutamine utilization is inhibited, cells adapt by switching to glucose as a nutrient source. Conversely, when glucose utilization is blocked, cells increase their utilization of glutamine or other nutrient sources (Choo et al., 2010; Le et al., 2012; Lim et al., 2014). This enables cancer cells to adapt metabolically to proliferate and survive stress associated with reduced nutrient availability to satisfy bioenergetic and anabolic demands. Therefore, targeting the ability of cancer cells to utilize glucose and glutamine would provide a significant therapeutic advantage.
Phosphoenolpyruvate carboxykinase (PEPCK) is the rate-limiting enzyme of gluconeogenesis in the liver and kidney. Following the conversion of amino acids and other non-carbohydrate sources to oxaloacetate (OAA) in the TCA cycle, PEPCK catalyzes the conversion of OAA into phosphoenolpyruvate (PEP). PEP is then converted to glucose via a series of enzymes of glycolysis and several unique enzymes. Despite this well-known role of PEPCK, studies in mice using 13C stable isotope tracer studies demonstrate an even more important role for PEPCK in regulating TCA cycle flux (Burgess et al., 2007). For example, reducing PEPCK more than 90% leads to a similar reduction in TCA cycle flux; gluconeogenesis is reduced by only 40%. In addition, it remains unclear whether PEPCK promotes gluconeogenesis in intestinal epithelium (Previs et al., 2009).
The TCA cycle is a central hub of carbon metabolism coordinating the metabolism of glucose, glutamine, other amino acids, respiration, and biosynthetic pathways such as lipogenesis and nucleic acid synthesis. The TCA cycle occurs in the mitochondria and although the Warburg effect was thought to be at odds with oxidative metabolism, mitochondrial function is actually required for transformation and tumor growth. Therefore the TCA cycle represents a nexus point of cancer cell metabolism. The important role that PEPCK plays in regulating the TCA cycle coupled with the requirement of tumor cells to coordinate the use of glucose and glutamine prompted us to determine the role of PEPCK in colorectal cancer.
RESULTS
PEPCK expression is elevated in colorectal cancer
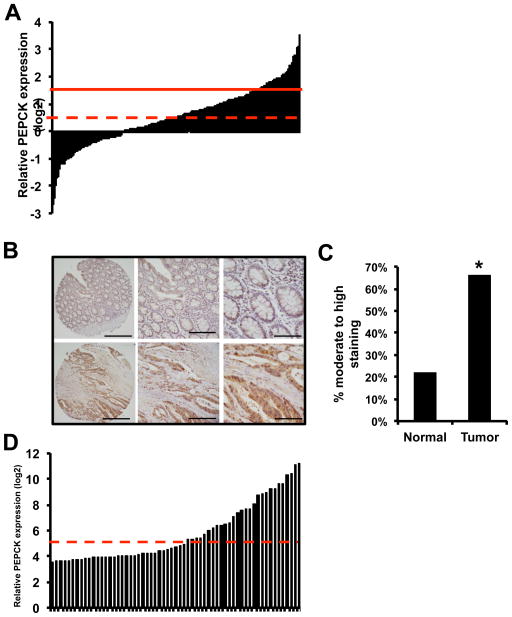
In an effort to determine the role of PEPCK in cancer we examined two different databases for the expression of PEPCK in cancer cell lines derived from different tissues. There was variability between different tissues, colorectal cancer cell lines consistently appeared to have higher expression of PEPCK compared to other cancer types(Figure S1A and S1B). Next we examined the CBioPortal for expression of PEPCK in colon cancer samples. Figure 1A shows that PEPCK was amplified or overexpressed in ~17% of colon derived tumors (Figure 1A). We also used a tissue microarray composed of normal and colon derived tumor tissue to determine the expression of PEPCK in colon cancer. Almost 50% of tumors had moderate to high PEPCK expression, compared to ~20% of non-tumor tissue (Figure 1B and 1C). Furthermore, when including tissues that express PEPCK at low, medium or high expression, over 80% of tumors and 78% of non-tumor tissue expressed PEPCK (not statistically significant)(Figure S1C). There did not appear to be a relationship between tumor grade and expression of PEPCK. There are two isoforms of PEPCK, cytosolic PEPCK (PEPCK1, PCK1, which we refer to as PEPCK) and a mitochondrial isoform of PEPCK (PEPCK2 or PCK2). Recent studies show that PEPCK2 also promotes cell proliferation (Leithner et al., 2014; Mendez-Lucas et al., 2014). Therefore we also examined the CBioPortal for the expression of PEPCK2. Less than 3% of colon cancers had increased expression of PEPCK2, and there were no amplifications (Figure S1D). Given these results and previous studies showing that the cytosolic form of PEPCK regulates TCA cycle flux we focused on the cytosolic form of PEPCK.
Figure 1. PEPCK expression is increased in colon derived tumors and cell lines.
A) CBioPortal data base analysis for PEPCK expression in 198 patient samples. Solid line indicates z=2, dashed line indicates mean.
B) Representative IHC of PEPCK in normal and tumor tissue from colon TMA and the percent of moderate to high PEPCK staining in normal and tumor tissue.
D) PEPCK expression of cell lines from a variety of tissues analyzed using the CBioPortal database. p <0.0002. Solid line indicates z=2, dashed line indicates mean. See also Figure S1
PEPCK regulates the TCA cycle and cell proliferation
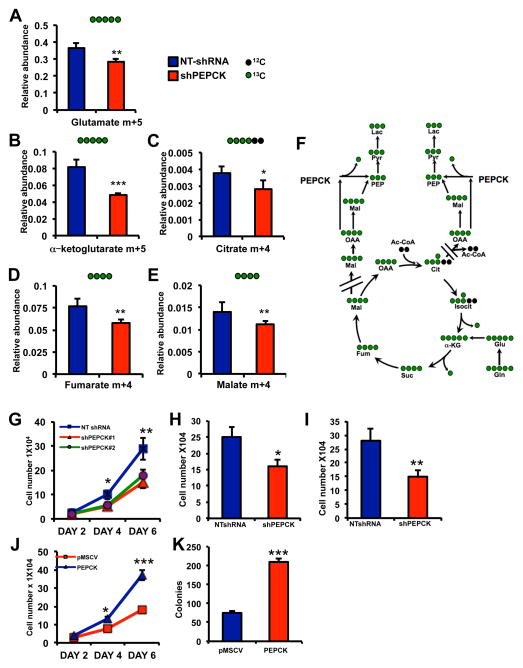
Next we sought to identify colon cancer-derived cell lines expressing PEPCK expression. For initial studies we used the Colo205 cell line, which expresses abundant levels of PEPCK (Figure 1D and S1E). We knocked down PEPCK in the Colo205 cells using different shRNA’s (Figure S2A) and observed increased OAA and decreased in PEP confirming functional knockdown of PEPCK (Figure S2B and S2C). Glutamine is the main non-carbohydrate nutrient source for cancer cells (DeBerardinis and Cheng, 2010). Furthermore, the utilization of glutamine for energy, lactate and macromolecule biosynthesis requires metabolism of glutamine-derived carbons through the TCA cycle. Next we used uniformly labeled [U-13C5-] glutamine to determine whether PEPCK knockdown alters the relative abundance of TCA cycle intermediates derived from glutamine. The abundance of m+5 labeled glutamate; the first product of glutaminolysis from [U-13C5-] glutamine, was reduced in the PEPCK knockdown cells (Figure 2A). The relative abundance of m+5 α-ketoglutarate (αKG), the first TCA cycle intermediate generated from glutaminolysis, was decreased more than 40% in the PEPCK knockdown cells (Figure 2B). In general there was a decrease in the relative abundance of αKG isotopomers, suggesting that PEPCK was reducing flux as previously shown (Burgess et al., 2007)(Figure 2SD). Similarly, there was a decrease in m+4 labeling of citrate, fumarate, and malate. We also observed decreases in the relative abundance of isotopomers of these TCA cycle intermediates further supporting an effect of PEPCK on TCA cycle flux (Figure S2D–G). Taken together these data demonstrate that PEPCK is regulating glutamine utilization and flux through the TCA cycle as previously described(Burgess et al., 2007). Interestingly, despite the role of PEPCK in gluconeogenesis, we did not observe conversion of 13C glutamine into glucose in either the non-target control or PEPCK knockdown cells (data not shown). This is in line with previous studies showing that PEPCK plays a greater role in regulating the TCA cycle than gluconeogenesis and that the intestines are not a source of gluconeogenesis under normal conditions (Previs et al., 2009; Yang et al., 2009). In line with these observations, G6Pase, which converts glucose-6-phosphate (G6P) to glucose, was not detectable by RTPCR or western blotting (data not shown). Although we did not observe 13C labeled glucose (i.e. gluconeogenesis), we did observe 13C labeled G6P. There was a trend towards reduced 13C labeled G6P in PEPCK knockdown cells, but it was not statistically significant (Figure S2H). However, the relative abundance of 13C labeled glycolytic intermediates, phosphoenolpyruvate and pyruvate were significantly reduced in the PEPCK knockdown cells (Figure S2I and S2J)
Figure 2. PEPCK regulates flux through the TCA cycle and tumor cell proliferation.
NT-shRNA and shPEPCK Colo205 cells were incubated with 13C glutamine and GC/MS performed for, A) m+5 labeled glutamate, or m+4 labeled B) αketoglutarate, C) citrate and D) fumarate and E) malate.
F) Schematic for conversion of [U5] 13C glutamine into various metabolites.
G) Proliferation of NT-shRNA and shPEPCK stable knock down Colo205 cells over 6 days.
H) Proliferation of NTshRNA and shPEPCK Colo205 cells after 6 days.
I) Proliferation of Moser human CRC cells with stable knockdown of PEPCK after 6 days.
J) Proliferation and K) clonogenic survival of control and PEPCK overexpressing HT29 cells. n=3 ± SD. * p < 0.05, **p < 0.01, ***p < 0.005. See also Figure S2.
Next we examined the effect of altering PEPCK levels affects cell proliferation. Loss of PEPCK led to an approximately 40% decrease in cell number using distinct shRNAs in the Colo205 cell line (Figure 2G–2H and Figure S2K). We also knocked down PEPCK in the Moser human colon cancer cell line, which express PEPCK, albeit lower levels than Colo205’s, using a distinct shRNA (Figure S1E and S2L inset). Knocking down PEPCK significantly reduced proliferation and clonogenic survival in the Moser cells despite already its lower PEPCK expression (Figure 2I and S2L). This suggests that inhibiting PEPCK can reduce growth even in cancer cells without abundant PEPCK. We examined whether the effect of PEPCK on cell proliferation is specific for colon cancer derived cells. We used the Fao rat hepatoma cell line, which expresses abundant levels of PEPCK (Scott et al., 1998). Knocking down PEPCK in the Fao cell line (using a rat specific shRNA) reduced cell proliferation as evidenced by decreased clonogenic growth (Figure S2M). We also sought to determine whether PEPCK was sufficient to promote cell proliferation. We used HT29 cells, which do not express PEPCK and generated stable cell lines expressing PEPCK (S1E and S2N). Ectopic expression of PEPCK significantly increased cell proliferation (Figure 2J). Likewise, clonogenic growth was increased almost 3-fold (Figure 2K).
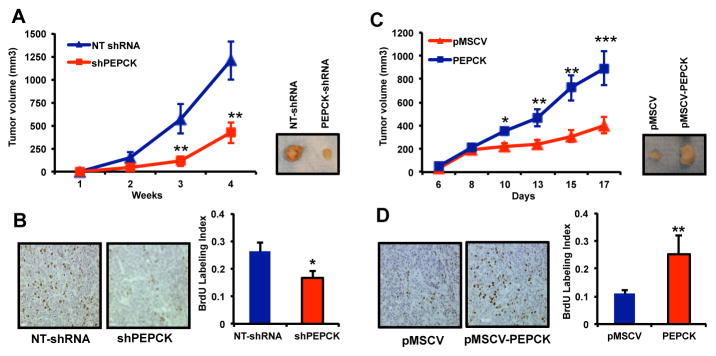
Next we examined the effect of PEPCK on tumor growth in vivo. Knocking down PEPCK reduced the growth of tumors by almost 70% (Figure 3A) compared to control NTshRNA cells. This was associated with a decrease in proliferation as determined by a reduction in BrdU positive nuclei (Figure 3B). In contrast, tumors overexpressing PEPCK grew over 3 times as large as the vector control HT29 cells (Figure 3C) and showed increased in BrdU incorporation (Figure 3D).
Figure 3. PEPCK promotes tumor growth in vivo.
A) Tumor growth of Colo205 NT-shRNA and Colo205 PEPCK-shRNA xenografts.
B) BrdU positive nuclei per field in xenografts from non-target and PEPCK knockdown cell lines.
C) Tumor growth of HT29 pMSCV and HT29-PEPCK xenografts.
D) BrdU positive nuclei per field in xenografts from pMSCV control and PEPCK overexpressing HT29 cell lines. N=8 ± SD. * p < 0.05, ** p < 0.01.
PEPCK promotes glutamine utilization
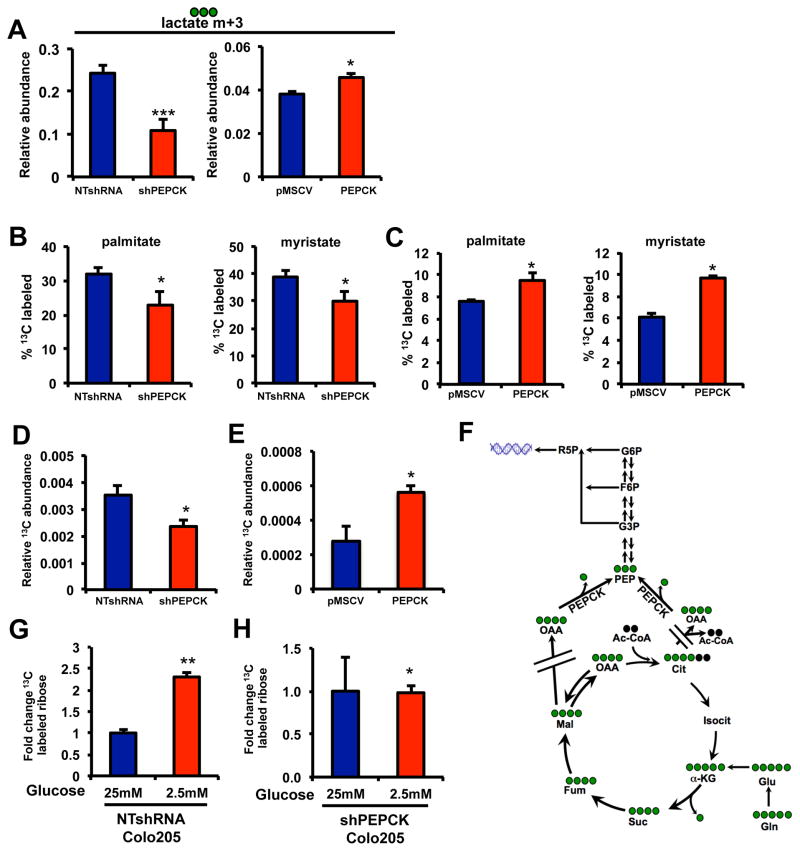
The ability of PEPCK to promote anaplerosis of glutamine into TCA cycle intermediates described above, prompted us to determine how PEPCK affects glutamine beyond the TCA cycle using [U-13C5-] glutamine. The generation of lactate is an important metabolite of cancer cells (DeBerardinis et al., 2008a; Gatenby and Gillies, 2004). For many years it was believed that lactate was derived from glucose. It is now appreciated that tumor cells generate a fraction of their lactate pools from glutamine via flux through the TCA cycle (DeBerardinis et al., 2007). A small percent of lactate was derived from glutamine (<5%, Figure S3A). However, loss of PEPCK led to a decrease in the relative abundance of m+3 labeled lactate as well as the percent of labeled lactate (Figure 4A and S3A). Conversely, overexpression of PEPCK led to an increase in the relative abundance of 13C labeled lactate and a small but significant increase in the fractional labeling of lactate from glutamine (Figure 4A and Figure S3B). Although lactate is usually considered a gluconeogenic substrate, these studies show that PEPCK can actually promote lactate production from glutamine. Hence in addition to the malate shuttle, PEPCK provides another route for lactate production from glutamine. Importantly, the increase in lactate derived from glutamine further demonstrates that PEPCK is increasing TCA cycle flux.
Figure 4. PEPCK promotes glutamine utilization towards lipogenesis and ribose synthesis.
A) 13C lactate levels in media (m+3) in NT and shPEPCK Colo205 cells and pMSCV and PEPCK overexpressing HT29 cells cultured with 13C glutamine.
Percent of 13C labeled palmitate and myristate from B) NT or PEPCK knockdown Colo205 cells, or C) pMSCV and PEPCK overexpressing cells.
D) Relative abundance of 13C labeled ribose derived from 13C glutamine in NT and shPEPCK Colo205 cells.
E) Relative abundance of 13C labeled ribose derived from glutamine from HT29 pMSCV or HT29 PEPCK cells.
F) Pathway for ribose synthesis from glutamine.
Fold change in 13C labeled ribose from 13C glutamine was determined in G) Non target or H) PEPCK knockdown Colo205 after culturing cells in media with reduced glucose levels.
N = 3 ± SD. * p < 0.05, ** p < 0.01, ***p < 0.001. See also Figure S3.
PEPCK promotes anabolic metabolism
Our initial studies show that PEPCK knockdown reduced the relative abundance of labeled glutamine-derived citrate. Citrate is a precursor for de novo lipid synthesis, another common feature of many tumors (Menendez and Lupu, 2007). Glutamine is incorporated into fatty acid pools in proliferating cells after being processed through the TCA cycle (DeBerardinis et al., 2008a; Deberardinis et al., 2008b) (Figure S3C and S3D). This prompted us to examine the effect of PEPCK on fatty acid synthesis in the Colo205 cells. Almost 30% of the palmitate, and its chain shortened product, myristate, were 13C labeled indicating significant amounts of basal anabolic use of glutamine by Colo205 cells for lipid synthesis (Figure 4B). Knocking down PEPCK significantly decreased 13C incorporation into palmitate and myristate. To confirm that PEPCK is sufficient to promote lipogenesis, we examined the incorporation of 13C from glutamine into palmitate and myristate in cells overexpressing PEPCK. Basal incorporation of glutamine into palmitate and myristate were ~7% and 6% respectively, with the remainder coming from non-labeled sources. However, 13C labeled palmitate and myristate were increased ~30% and 60%, respectively, in cells overexpressing PEPCK (Figure 4C).
The presence of glutamine derived G6P and other glycolytic intermediates promoted us to address the fate of these metabolites. Another branch point in glycolysis/gluconeogenesis is the pentose phosphate pathway (PPP), which plays a critical role in cancer proliferation (Tong et al., 2009). The PPP generates ribose for nucleic acid synthesis and NADPH for biosynthetic and antioxidant pathways. It is well established that glutamine provides the amine groups for purine and pyrimidine’s (Salzman et al., 1958). However, its role in ribose synthesis has not been previously described. In non-target shRNA Colo205 cells, ~2.5% of the ribose was derived from glutamine (Figure S3E). The remaining unlabeled ribose most likely reflects glucose derived ribose and existing pools. Knocking down PEPCK led to a 30% decrease in the relative abundance of 13C ribose and fractional labeling derived from glutamine (Figure 4E and S3E). We also examined the effect of PEPCK on ribose production in two additional knockdown cell lines using 14C glutamine. Incorporation of glutamine-derived carbons into the ribose/RNA fraction was decreased in PEPCK knockdown cells. (Figure S3F and S3G). Conversely, overexpression of PEPCK increased the relative abundance and fractional labeling of 13C ribose derived from glutamine (Figure 4E and Figure S3H). This was a surprising finding since the incorporation of non-carbohydrate sources such as glutamine into ribose and especially promotion of this process by PEPCK has not been previously described (Figure 4F).
The levels of glutamine-derived ribose do not make up a significant fraction of the ribose pool under the cell culture conditions we used (<5%, Figure S3E and S3H). The majority of ribose is derived from glucose. However, physiological glucose levels are ~5 mM and tumor cells are often under conditions of hypoglycemia due to increased glucose utilization coupled with disorganized microvasculature (Gatenby and Gillies, 2004). As a result glucose levels in tumors can be reduced almost 90% compared to surrounding normal tissues(Hirayama et al., 2009). This would require cancer cells to synthesize ribose via alternative sources. Glutamine concentrations in tumors are similar to normal surrounding tissue despite the dependence of cancer cells on glutamine (Hirayama et al., 2009). Therefore, we examined the incorporation of 13C glutamine into ribose under reduced glucose conditions. In the NT cells expressing PEPCK, reducing glucose caused 13C glutamine derived 13C ribose to increase almost 3 fold (Figure 4G). In contrast, relative levels of labeled ribose derived from glutamine carbons did not change in the PEPCK knockdown cells (Figure 4H). These data demonstrates that PEPCK enables cells to utilize the carbons from glutamine as an additional source of ribose for nucleic acid synthesis, especially when glucose levels are limiting. It also suggests a coordinated role for glutamine in nucleic acid synthesis, whereby glutamine contributes the amine groups of bases and also the carbon backbone of the ribose group.
We also established xenografts in mice with tumor cells expressing PEPCK or with PEPCK knockdown to determine the role of PEPCK on glutamine metabolism in vivo. Similar to our in vitro studies, there was reduced abundance of 13C citrate (i.e. derived from [U-13C5-] glutamine. Furthermore, we observed reduced enrichment of 13C labeled into palmitate. Likewise, the relative abundance of labeled ribose, which actually made up a greater fraction of labeled ribose than in vitro, was also reduced (Figure S3G–I and data not shown). Thus, despite the well-known gluconeogenic roles of PEPCK, these data demonstrate that PEPCK promotes anabolic metabolism with increased lipid and nucleic acid synthesis from glutamine.
PEPCK protects cells against inhibition of glutaminolysis
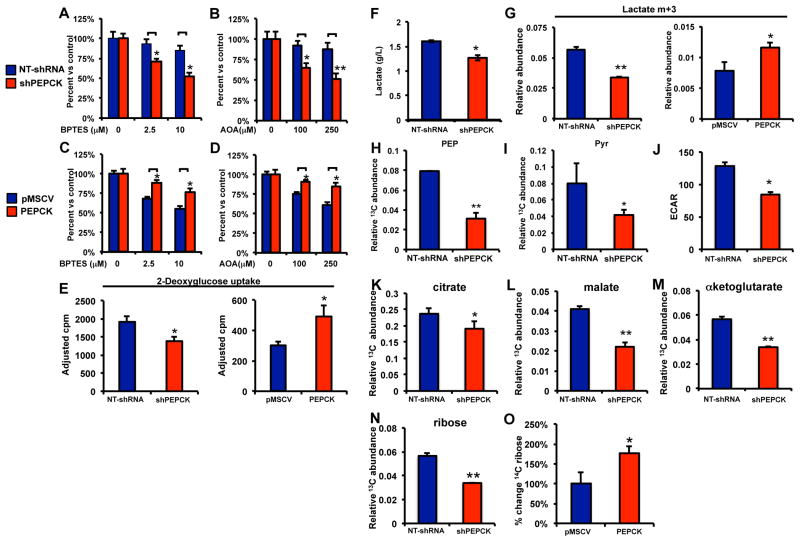
Although glutamine is not considered an essential amino acid, it becomes conditionally essential during periods of increased proliferation and growth(Lacey and Wilmore, 1990). This prompted us to determine if inhibiting glutaminolysis blocks the growth promoting effects of PEPCK. We inhibited glutaminolysis with aminooxyacetate (AOA) or BPTES (DeBerardinis et al., 2007; Robinson et al., 2007). Surprisingly, inhibiting glutaminolysis in NT Colo205 cells expressing endogenous PEPCK had only a modest affect on cell proliferation (Figure 5A and 5B). In contrast, the PEPCK knockdown cells appeared more sensitive to inhibition of glutaminolysis with cell numbers reduced ~25–40% compared to NT Colo205 cells. Similarly, BPTES and AOA had a much greater effect on cell number in HT29-pMSCV, which lack PEPCK, compared to the HT29 expressing PEPCK cells (Figure 5C–5D). Therefore, while PEPCK promotes glutamine utilization, the presence of PEPCK appears to protect cells against reduced glutaminolysis. This suggests that PEPCK can promote the utilization of additional/alternative nutrients. This might be especially important in the tumor microenvironment where nutrients supplies are often limited.
Figure 5. PEPCK promotes glucose utilization.
A and B) Cell proliferation in NTshRNA and shPEPCK Colo205 or C and D) pMSCV or PEPCK expressing HT29 cells following treatment with A and C) BPTES or B and D) AOA for 48 hr.
E) 14C 2-deoxyglucose uptake in PEPCK knockdown (Colo205) and PEPCK overexpressing (HT29) cell lines.
F) Media lactate from NTshRNA and shPEPCK cells determined using a Nova Bioanalyzer.
G) Relative abundance of 13C m+3 labeled lactate from NTshRNA and shPEPCK Colo205 cells and pMSCV or PEPCK expressing HT29 cells.
Relative abundance of 13C labeled H) phosphoenolpyruvate or I) pyruvate from NTshRNA and shPEPCK Colo205 cells.
J) Glycolysis as determined by ECAR using a Seahorse Bioanalyzer. N=10 ± SD.
Relative abundance of 13C labeled K) citrate, L) malate or M) αketoglutarate from NTshRNA and shPEPCK Colo205 cells.
Relative abundance of 13C labeled ribose derived from 13C glucose in N) NTshRNA and shPEPCK Colo205 cells or O) 14C labeled RNA/ribose from 14C glucose in pMSCV or PEPCK expressing HT29 cells. N=3 ± SD, * p < 0.05. See also Figure S5.
PEPCK promotes glucose utilization
Glucose is an important nutrient in tumor metabolism and one of the main carbon sources for nucleic acid and fatty acid synthesis (DeBerardinis et al., 2008a; Deberardinis et al., 2008b; Hatzivassiliou et al., 2005). In addition, a recent study demonstrated a role for PEPCK in promoting glucose metabolism during the metastases of melanoma cells(Li et al., 2015). Therefore, we examined 14C 2-deoxyglucose uptake into cells with gain and loss of PEPCK. Despite the well-known role of PEPCK in glucose production, we observe an increase in 14C 2-deoxyglucose uptake into cells expressing higher levels of PEPCK (Figure 5E). Next we wanted to determine the fate of the increased glucose uptake and whether PEPCK was affecting glycolysis.
Lactate production from glucose is one of the hallmarks of cancer cell metabolism. We initially examined the total concentration of lactate in the media of NT and shPEPCK cells using a NOVA biochemical analyzer. Lactate levels were decreased ~20% in the PEPCK knockdown cells (Figure 5F). This coincided with an increase in pH due to the decrease in lactate (Figure S4A). Next we incubated Colo205 NTshRNA and shPEPCK cells with uniformly labeled 13C glucose to determine if PEPCK affected the levels of glycolytic intermediates specifically from glucose. The relative abundance of m+3 lactate in the media, the main lactate species derived from [U6]-13C glucose via glycolysis, was reduced 40% in the shPEPCK cell line (Figure 5G). Similarly, m+3 lactate abundance was increased about 30% in cells overexpressing PEPCK (Figure 5G). Several other glycolytic intermediates, PEP and pyruvate were also reduced in the PEPCK knockdown cells, reinforcing the ability of PEPCK to promote glycolysis (Figure 5H and 5I). We also used the Seahorse Bioanalyzer to determine if PEPCK was affecting glycolysis, using ECAR as a measure of glycolysis. Basal ECAR was significantly lower in the PEPCK knockdown cells (Figure 5J). Next we examined whether PEPCK had an effect on the flux of glucose-derived carbons into the TCA cycle. The relative abundance of citrate, malate and αKG were reduced in the PEPCK knockdown cells (Figure 5K–5M). We observed a similar effect of PEPCK knockdown on the incorporation of 13C carbon from glucose into lactate, pyruvate and citrate in the Fao cells following PEPCK knockdown (Figure S4B–D). This further supports the ability of PEPCK to promote both glycolysis and increased flux of glucose into the TCA cycle.
Glucose is one of the most important substrates for ribose production in cells. This prompted us to examine how PEPCK affects ribose synthesis. Almost 50% of the ribose was derived from the 13C glucose tracer in NT Colo205 cells, with the remainder coming from existing pools or the salvage pathway (Figure S4E). However, in PEPCK knockdown cells there was a significant reduction in the relative abundance of 13C labeled ribose and fractional labeling (Figure 5N and S4E). We observed a similar reduction in 13C ribose abundance in the Fao PEPCK knockdown cell line (Figure S4F). We also incubated HT29 cells overexpressing PEPCK with 14C glucose to determine if PEPCK was sufficient to promote RNA/ribose synthesis. Indeed, overexpression of PEPCK increased ribose synthesis from 14C glucose (Figure 5O). Next we wanted to determine whether PEPCK could promote glucose metabolism in vivo. Following establishment of PEPCK expressing and PEPCK knockdown xenografts, mice were administered a bolus of 13C glucose. There was a significant reduction in the relative abundance of lactate and citrate in PEPCK knockdown tumors compared to non-target PEPCK expressing tumors (Figure S4G and S4H).
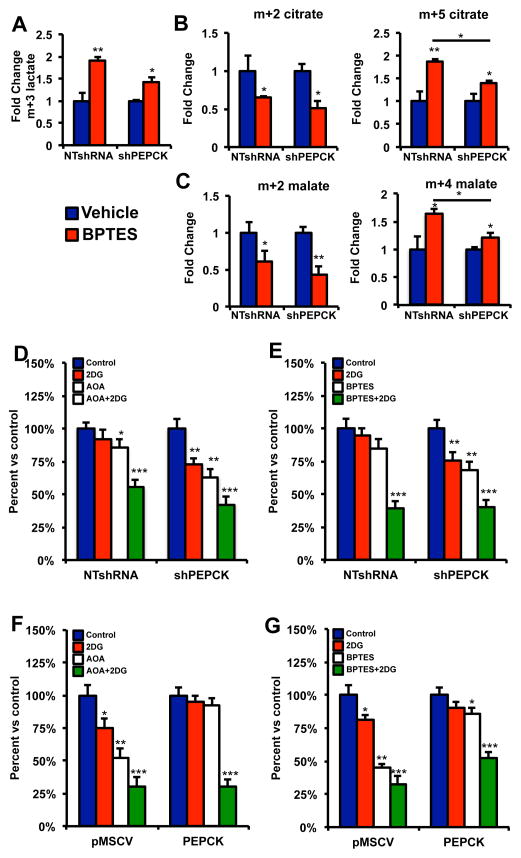
Our data shows that PEPCK expressing cells are able to survive better in the presence of glutaminolysis inhibition. Our date suggests that this is due to increased glucose utilization. Therefore we wanted to determine whether PEPCK promotes glucose utilization to a greater degree when glutamine utilization is inhibited. We treated cells with BPTES in the presence of [U6]-13C glucose and examined changes in glucose utilization. As shown in Figure 6A, in the presence of BPTES, media lactate increased about 2-fold in the NTshRNA PEPCK expressing cells most likely due to increased glucose utilization. In contrast, media lactate only increased ~ 50% in the PEPCK knockdown cells.
Figure 6. PEPCK promotes glucose utilization when glutamine utilization is inhibited.
A) Relative abundance of m+3 media lactate from NTshRNA and shPEPCK Colo205 cells following incubation with 13C glucose in the absence and presence of 10 μM BPTES.
B) Relative abundance of m+2 and m+5 citrate from NTshRNA and shPEPCK Colo205 cells following incubation with 13C glucose in the absence and presence of 10 μM BPTES.
C) Relative abundance of m+2 and m+4 malate from NTshRNA and shPEPCK Colo205 cells following incubation with 13C glucose in the absence and presence of 10 μM BPTES.
D and E) Colo205 NT-shRNA and Colo205 PEPCK-shRNA or F and G) HT29-pMSCV and HT29-PEPCK cells were treated with 2.5 mM 2DG alone or in combination with D and F) 200 μM AOA or E and G) BPTES for two days and cell number determined. N=3 ± SD. * p < 0.05, ** p < 0.01
We did not see a significant change in total labeled citrate following treatment with BPTES in the NTshRNA cells and a small decrease in the shPEPCK cells (data not shown). However, m+2 labeled citrate was reduced in the presence of BPTES (Figure 6B). This is most likely due to the fact that OAA (four unlabeled carbons) derived from amino acids such as glutamine is reduced due to inhibition of glutaminolysis. Therefore, cells are unable to use citrate synthase to condense 13C labeled AcCoA (m+2) with unlabeled OAA (m+0) to generate m+2 citrate. We then examined the levels of m+5 citrate, which would result from 13C labeled AcCoA (m+2) condensing with M+3 OAA. Treatment of cells with BPTES in the presence of 13C glucose led to a >2 fold increase in m+5 labeled citrate, which was blunted in the PEPCK knockdown cells. The labeling of malate reinforces these data. Labeling of malate via acetyl Co would lead to m+2 malate. However, m+4 malate would be derived from m+5 citrate. Indeed, we see more m+4 malate following treatment with BPTES in the PEPCK expressing cells, whereas it was not induced in the knockdown cells (Figure 6C). There are two main routes by which m+3 labeled OAA could be generated. Pyruvate carboxylase can convert mitochondrial pyruvate to OAA, or PEPCK can convert PEP to OAA. Since both reactions produce the same 13C-labeling pattern from [U-13C] glucose, it is not possible to determine which reaction is responsible. However, we do not see a difference in PC expression, and despite the normal view of PEPCK converting OAA to PEP, the ability of PEPCK to convert PEP to OAA has been shown (Wang et al., 2010).
Our data shows that PEPCK promotes both glutamine and glucose utilization, especially when one of them is limiting as might be found in the tumor microenvironment. Therefore we inhibited glutaminolysis, using AOA or BPTES, or glycolysis using 2DG, alone or in combination. PEPCK expressing cells were less sensitive to growth inhibition by BPTES or AOA alone (Figure 6D–6G). Interestingly, PEPCK expressing cells also appeared resistant to growth inhibition by 2DG. However, inhibition of both glycolysis and glutaminolysis in PEPCK expressing cells had a synergistic effect on growth inhibition. The combination of 2DG with BPTES or AOA in PEPCK knockdown cells or cells without PEPCK did not have a similar synergistic effect. This supports a role for PEPCK in enabling cells to utilize both glucose and glutamine, especially when one of these nutrients is limiting.
PEPCK promotes the activation of mTORC1
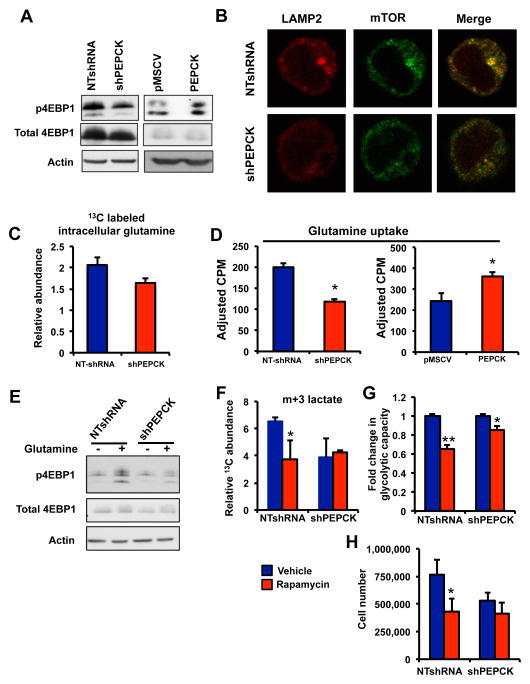
Given the ability of PEPCK to promote glycolysis we examined the expression of several glycolytic genes known to promote tumor cell growth. Overall, we did not observe a difference in expression of any of these genes (Figure S5A and S5B). This raised the question as to how PEPCK regulates glycolysis. mTOR is well known for its role in nutrient sensing and metabolism. Despite the role of mTORC1 and mTORC2 in regulating metabolism, mTORC1 is more responsive to amino acid changes than mTORC2 (Sancak et al., 2008). Amino acids are essential for mTORC1 activation, with leucine and glutamine playing important roles in mTORC1 activation(Csibi et al., 2013; Duran et al., 2012; Jewell et al., 2015). Loss of PEPCK increased mTORC1 activity as judged by phosphorylation of eukaryotic translation initiation factor 4E binding protein 1 (4EBP1), S6kinase (S6K) and ribosomal S6 (S6) (Figure 7A, S5C). Conversely, overexpression of PEPCK led to the activation of mTORC1 as determined by increased phosphorylation of 4EBP1. We did not see any changes to mTORC2 activity as judged by phosphorylation of serine 473 on AKT (Figure S5D). The lysosome plays a major role in mediating the full activation of mTORC1 since localization of mTOR with the lysosome is required for full mTORC1 activation (Sancak et al., 2010). Therefore we investigated whether PEPCK altered the localization of mTOR with the lysosome. In PEPCK expressing cells we observed mTORC1 activation as evidenced by greater mTOR colocalization (yellow) with the lysosomal protein lysosome-associated membrane protein 2 (LAMP2), than in PEPCK knockdown cells (Figure 7B).
Figure 7. PEPCK promotes mTORC1 activation.
A) PEPCK increases phosphorylation of 4EBP1. Immunoblotting for total and phospho4EBP1 was performed for protein of cells following knockdown of PEPCK (shPEPCK) or cells with increased PEPCK expression. Actin was used to control for loading.
B) Immunofluorescence for LAMP2, mTOR or the colocalization (merge, yellow) in Colo205 cells expressing PEPCK (NTshRNA) or with PEPCK knocked down (shPEPCK).
C) PEPCK increases intracellular glutamine levels. Colo205 Non-target and PEPCK knockdown cells were cultured in the presence of [U5]13C glutamine and intracellular glutamine levels determined using GC/MS. See also Figure S6.
D) PEPCK increases glutamine uptake. Colo205 Non-target and PEPCK knockdown cells or HT29 cells with empty vector or PEPCK overexpression were cultured in 14C glutamine and intracellular glutamine uptake determined.
E) Glutamine is responsible for the PEPCK mediated activation of mTORC1. Cells were cultured Colo205 Non-target and PEPCK knockdown cells were cultured in media containing glutamine or without glutamine overnight. Proteins were isolated and immunoblotting performed for total and phospho4EBP1.
F–H) mTORC1 mediates the effects of PEPCK on glycolysis and proliferation.
F) Colo205 Non-target and PEPCK knockdown cells were cultured in the presence of 13C glucose and the presence or absence of 10 nM rapamycin. The relative abundance of lactate m+3 was monitored by GC/MS.
G) Colo205 Non-target and PEPCK knockdown cells were seeded in Seahorse Flux plates and treated with 10nM rapamycin and glycolytic capacity determined. N=10 ± SD.
H) Colo205 Non-target and PEPCK knockdown cells were seeded and then treated with rapamycin for 48 hr and cell number determined using a an automated cell counter.
N=3 ±SD unless otherwise noted. * p < 0.05, ** p < 0.001.
We also examined the metabolism of 13C leucine in cells with altered PEPCK. However, we do not see a difference in leucine metabolism into the TCA cycle in cells expressing PEPCK compared to knockdown cells (Figure S5E). Next we wanted to determine if inhibiting glutamine utilization affected mTORC1 activation. Surprisingly, treatment of cells with BPTES or another inhibitor (Compound 968) had no effect on mTOR activation (Figure S5F and data not shown). Therefore, glutaminolysis does not appear responsible for mTORC1 activation. A more recent study showed that intracellular glutamine could activate mTORC1 (Jewell et al., 2015). Therefore we examined glutamine levels in cells following incubation with [U5]-13C glutamine. The abundance of intracellular 13C labeled glutamine was greater in cells expressing PEPCK compared to PEPCK knockdown cells (Figure 7C). Similarly, 14C glutamine uptake was reduced in PEPCK knockdown cells, whereas glutamine uptake was increase in cells overexpressing PEPCK (Figure 7D).
In order to determine whether glutamine is responsible for PEPCK mediated mTORC1 activation cells were cultured in the presence and absence of glutamine. The absence of glutamine decreased mTORC1 activation in general. However, mTORC1 activation was blunted to a much greater extent in PEPCK expressing cells compared to PEPCK knockdown cells in the absence of glutamine (Figure 7E and S5G). Therefore, these data suggest that PEPCK induced glutamine utilization does not affect mTORC1 activity; rather its ability to increase cellular glutamine levels appears responsible for mTORC1 activation.
Next we wanted to determine whether the activation of mTORC1 in PEPCK expressing cells was mediating the observed effects on metabolism and proliferation. Initially we examine the effect of rapamycin on glutamine metabolism. Despite previous studies showing that inhibition of mTOR decreases glutaminolysis, in our cells, we did not see an effect as determine by changes in the relative abundance of 13C αKG and glutamate (Figure S5H and S5I). Next we examined the effect of mTOR inhibition on PEPCK induced glucose utilization. Unlike glutamine utilization, rapamycin treatment appeared to decreased glucose metabolism. The relative abundance of 13C labeled lactate in the media in PEPCK expressing cells was decreased following treatment with rapamycin (Figure 7F). By contrast we did not see a further reduction in relative abundance of 13C labeled lactate in the PEPCK knockdown cells treated with rapamycin. This effect was blunted in the PEPCK knockdown cells. To further corroborate these studies we examined the effect of rapamycin on glycolytic capacity using the Seahorse Bioanalyzer. Rapamycin decreased glycolytic capacity in the NTshRNA Colo205 cells about 40%(Figure 7G). In contrast, in the PEPCK knockdown cells rapamycin decreased glycolytic capacity was reduced only ~ 20%.
Finally, we wanted to determine whether the effect of PEPCK on cell proliferation involved mTORC1. NTshRNA and shPEPCK cells were incubated in the absence and presence of rapamycin for 48 hr and cell number determined. Cell number was reduced almost 50% in NTshRNA cells treated with rapamycin compared to vehicle treated NTshRNA cells(Figure 7H). In contrast, there was a small but insignificant decrease in cell number in PEPCK knockdown cells following treatment with rapamycin. Therefore, mTORC1 activation by PEPCK appears to increase glucose metabolism and proliferation, but does not appear to alter glutamine metabolism.
DISCUSSION
PEPCK is most well known for its role in GNG. However, its presence in non-GNG tissues suggests alternative roles for PEPCK. Indeed, the role of PEPCK in the intestinal epithelium is controversial (Previs et al., 2009). The intestinal epithelium appears to utilize glutamine for energy rather than GNG by oxidizing glutamine into the TCA cycle, a process that would be promoted by PEPCK(Yang et al., 2009). These studies highlight a role for PEPCK in driving cell growth by promoting anabolic metabolism and metabolic flexibility. PEPCK coordinates the ability of cells to take up and metabolize both glucose (glycolysis) and glutamine (glutaminolysis) for energy and anabolic purposes, features of tumor cells that require the TCA cycle.
Anabolic metabolism is recognized as crucial for tumor cell proliferation (Deberardinis et al., 2008b). We show that PEPCK promotes the utilization of glucose and glutamine for anabolic metabolism, including fatty acid and nucleic acids. Two of the main anabolic pathways driving tumor cell proliferation are lipogenesis and ribose synthesis. In order for cells to convert glucose or glutamine to fatty acids, they must enter the TCA cycle and be converted to citrate. Citrate then leaves the mitochondria via the citric acid transporter and is cleaved to OAA and acetyl CoA by ATP citrate lyase. Acetyl CoA is then used in the synthesis of a variety of lipids. De novo lipogenesis from glucose and glutamine play crucial roles in cancer supporting not only generation of biomass, but also bioactive lipids and lipid molecules driving signal transduction (Louie et al., 2013; Menendez and Lupu, 2007). Lipids can also serve as energy source allowing cancer cells to survive when under nutrient deprived conditions (Zaugg et al., 2011). Therefore the ability PEPCK to promote lipid synthesis helps to coordinate a key feature of cancer metabolism.
The pentose phosphate pathway is responsible for ribose synthesis. Ribose synthesis serves two important roles. The oxidative branch of the PPP generates ribose and NADPH. NADPH is used as a biosynthetic precursor and antioxidant, protecting cancer cells from excessive ROS. The non-oxidative branch of the PPP generates ribose. These two sources of ribose can then be incorporated into nucleic acid for RNA and DNA synthesis. Glucose is the primary substrate for ribose synthesis. Surprisingly, we observed that loss of PEPCK reduced the incorporation of glucose into ribose. Even more surprisingly, we observed a role for PEPCK in promoting ribose synthesis from glutamine. Although the synthesis of ribose from glutamine and its regulation by PEPCK was an unexpected finding, the ability of PEPCK to promote ribose synthesis from glutamine is not a far divergence from its classic metabolic role. PEPCK promotes the entry of carbons from non-carbohydrate sources into the glycolytic pathway via their conversion to PEP from OAA. Once PEP is generated, it is reasonable that carbons derived from PEP can enter the pentose phosphate pathway (Figure 4G). A role for glutamine as the amine donor in purine synthesis and carbon donor in pyrimidine synthesis is well established (Salzman et al., 1958). Therefore the ability to synthesize ribose from glutamine or even other glucogenic amino acids would provide additional sources for RNA and DNA synthesis.
Recent studies show that tumor cells display a high degree of metabolic flexibility. Therefore, a fascinating observation in our studies was the ability of PEPCK to promote the use of multiple carbon sources. This becomes especially important when the reduced nutrient availability of the tumor microenvironment is taken into account. This effect became evident when we inhibited glutaminolysis. PEPCK expressing cells showed increased survival by increasing glucose utilization to meet their metabolic needs. Conversely, when glucose utilization was inhibited, cells expressing PEPCK were able to survive due to increased glutamine utilization. This is particularly relevant to the tumor microenvironment since glucose levels in tumors are ~1/10th the level found in serum or normal tissue (Hirayama et al., 2009). Indeed, we observed greater incorporation of glutamine into ribose when glucose levels were reduced in PEPCK expressing cells. Therefore, PEPCK can promote metabolic flexibility by promoting the use of glutamine and perhaps other non-carbohydrate sources for energy and anabolic metabolism, especially when nutrients are limiting as is often seen in the tumor microenvironment.
We found PEPCK overexpressed or amplified in almost 20% of colorectal cancers from the CBioPortal. While the mechanism for overexpression is not clear, PEPCK is located on chromosome 20q13, which is frequently amplified in several epithelial derived cancers (Hidaka et al., 2000; Iwabuchi et al., 1995; Tanner et al., 1994). The significance of PEPCK amplification in colon cancer is underscored by our observation that increased 20q13 copy number drives higher expression of PEPCK in colon cancer, similarly to that observed with ZNF217, a known oncogenic driver gene of this region (Quinlan et al., 2007)(Figure S1F and S1G). This suggests that PEPCK might also be contributing the oncogenic role of 20q13 amplification. Although previous studies showed that PEPCK levels were reduced in colon-derived cancers, only a few cell lines or tumors were examined(Blouin et al., 2010). In contrast we examined almost 200 colon tumors from the CBioPortal and about 100 colon tumors by IHC in the TMA.
There are two isoforms of PEPCK, cytosolic PEPCK and a mitochondrial isoform of PEPCK, which appear to have a comparable function (PEPCK2 or PCK2). We focused on the cytosolic isoform PEPCK since it is inducible, and as we show here, overexpressed in a significant number of colon cancers. Recent studies do show that PEPCK2 affects cell proliferation (Leithner et al., 2014; Mendez-Lucas et al., 2014). These effects where primarily seen under conditions of nutrient deprivation or stress pathway activation. Furthermore, the significance of those studies is also unclear since less than 3% of colon cancers have increased expression of PEPCK2, and there were no amplifications. It remains to be seen whether PEPCK and PEPCK2 have similar effects on metabolism.
One our unexpected findings was the ability of PEPCK to increase glycolysis. Our data suggests two potential related mechanisms. Cells must maintain the TCA cycle in order to survive. PEPCK is one of the main cataplerotic enzymes of the TCA cycle and will promote the net efflux of TCA cycle intermediates. In order for the TCA cycle to function and hence cell survival, anaplerotic reactions are required to compensate for the cataplerotic action of PEPCK. The siphoning off of intermediates and their incorporation into anabolic molecules (such as lipids and ribose) further reduces carbon availability to replenish the TCA cycle. Therefore cells will increase the oxidative and anaplerotic entry of glucose into the TCA cycle. Support for the glycolytic flux is supported by our data showing that when glutamine anaplerosis is blocked, PEPCK expressing cells increase glycolysis and glucose utilization into the TCA cycle.
While mass action may explain in part the increased glucose flux, another surprising finding was the ability of PEPCK to regulate mTORC1 activity. Although glutaminolysis can activate mTORC1, inhibiting glutamine metabolism did not affect mTORC1 activity nor blunt the PEPCK mediated increase in mTORC1 activity. Our data suggests that regulation of mTORC1 activity by PEPCK is at the level of intracellular glutamine levels. PEPCK increased intracellular glutamine levels and removal of glutamine from the media blunted the effect of PEPCK on mTORC1 activation. This is in line with a recent study showing that glutamine itself can activate mTORC1 (Jewell et al., 2015). Currently we are investigating how PEPCK increases intracellular glutamine levels, leading to mTORC1 activation.
Tumor cells are under significant nutrient stress. The metabolic flexibility of tumor cells to utilize different nutrients provides them with a strong advantage in the face of changing nutrient environment to meet bioenergetic, anabolic and signaling needs. The TCA cycle represents a key nodal point in metabolism linking cellular carbohydrate, amino acid, and bioenergetics, with anabolic and catabolic pathways. This enables cancer cells to promote metabolic flexibility and sustain tumor growth. PEPCK is a key enzyme regulating TCA cycle flux. In summary, our studies show that PEPCK promotes anabolic metabolism from glucose and glutamine to support tumor growth. In addition, we demonstrate a role for PEPCK in activating mTORC1 in part via its ability to regulate amino acid metabolism.
EXPERIMENTAL PROCEDURES
Cell culture
All cancer cell lines were obtained from ATCC except the Moser colon cancer cell line, which were a gift from Dr. Bruce Spiegelman. Additional information can be found in the supplemental experimental procedures.
Analysis of PEPCK expression in human tumors
Expression profiling array data from a published study was used to analyze the PEPCK expression in multiple tumors. Immunohistochemistry was performed on a commercial TMA by the Stony Brook Histopathology Core. Additional information can be found in supplementary experimental procedures.
Growth Assay
Cells were seeded at 10,000–25,000 cells/well in 6-well plates, and allowed to grow at 37°C, 5% CO2. For clonogenic assay, cells were seeded at 2000–5000 cells/well in 6-well plates and allowed to grow at 37°C, 5% CO2. Cells were fixed and stained using crystal violet and colony counting performed using ImageJ. Additional information can be found in supplementary experimental procedures.
Animal work
Animal experiments were performed according to procedures approved by the University of Maryland, Baltimore and Stony Brook University IACUC. Additional information can be found in supplementary experimental procedures.
13C tracer studies
Cells were seeded in triplicate into 100mm or 60 mm dishes and allowed to grow overnight. [U5-13C5] glutamine or [U6-13C6]-glucose (Cambridge Isotope Labs) were used as tracers since they provide excellent analysis of overall central carbon metabolism and in particular the TCA cycle (Metallo et al., 2009). Additional information can be found in supplementary experimental procedures.
Gas Chromatography/Mass Spectrometry (GC/MS)
Mass spectral data were obtained on the HP5973 mass selective detector connected to an HP6890 gas chromatograph. Additional information can be found in supplementary experimental procedures.
Supplementary Material
Acknowledgments
We thank Ms Mallory for IHC work and Stony Brook Biorepository Core. Richard Hanson (OBM) for the PEPCK construct. This work was supported by NIH Grants CA169919 and DK064685, Maryland CRF and Stony Brook Research Foundation to GDG.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online.
AUTHOR CONTRIBUTIONS
EM, RD, KB; Conducted and helped design experiments and were involved with the writing of the manuscript. OU, BJH, AR, CG, WJL, JS Conducted experiments. LB; Analysis of metabolic data and manuscript preparation. WT Pathological analysis. KS; Pathological analysis, tumor scoring and involvement with manuscript preparation. RSP, SD, RD Assisted with analysis and interpretation of gene expression data. RJD Helped design metabolic studies and was involved in manuscript preparation. JMR MBB, Helped with the drug design studies and other aspects of medicinal chemistry. KKW Helped with interpretation of data and guidance on studies. GDG; Oversaw direction of project, conducted experiments, interpretation of data and wrote manuscript.
The authors declare they have no conflict of interest related to the work presented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blouin JM, Bortoli S, Nacfer M, Collinet M, Penot G, Laurent-Puig P, Forest C. Down-regulation of the phosphoenolpyruvate carboxykinase gene in human colon tumors and induction by omega-3 fatty acids. Biochimie. 2010;92:1772–1777. doi: 10.1016/j.biochi.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, Magnuson MA. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 2007;5:313–320. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo AY, Kim SG, Vander Heiden MG, Mahoney SJ, Vu H, Yoon SO, Cantley LC, Blenis J. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol Cell. 2010;38:487–499. doi: 10.1016/j.molcel.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibi A, Fendt SM, Li C, Poulogiannis G, Choo AY, Chapski DJ, Jeong SM, Dempsey JM, Parkhitko A, Morrison T, et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell. 2013;153:840–854. doi: 10.1016/j.cell.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008a;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008b;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran RV, Oppliger W, Robitaille AM, Heiserich L, Skendaj R, Gottlieb E, Hall MN. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell. 2012;47:349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nature reviews. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Hidaka S, Yasutake T, Takeshita H, Kondo M, Tsuji T, Nanashima A, Sawai T, Yamaguchi H, Nakagoe T, Ayabe H, et al. Differences in 20q13.2 copy number between colorectal cancers with and without liver metastasis. Clin Cancer Res. 2000;6:2712–2717. [PubMed] [Google Scholar]
- Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009;69:4918–4925. doi: 10.1158/0008-5472.CAN-08-4806. [DOI] [PubMed] [Google Scholar]
- Iwabuchi H, Sakamoto M, Sakunaga H, Ma YY, Carcangiu ML, Pinkel D, Yang-Feng TL, Gray JW. Genetic analysis of benign, low-grade, and high-grade ovarian tumors. Cancer Res. 1995;55:6172–6180. [PubMed] [Google Scholar]
- Jewell JL, Kim YC, Russell RC, Yu FX, Park HW, Plouffe SW, Tagliabracci VS, Guan KL. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science. 2015;347:194–198. doi: 10.1126/science.1259472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey JM, Wilmore DW. Is glutamine a conditionally essential amino acid? Nutr Rev. 1990;48:297–309. doi: 10.1111/j.1753-4887.1990.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leithner K, Hrzenjak A, Trotzmuller M, Moustafa T, Kofeler HC, Wohlkoenig C, Stacher E, Lindenmann J, Harris AL, Olschewski A, et al. PCK2 activation mediates an adaptive response to glucose depletion in lung cancer. Oncogene. 2014 doi: 10.1038/onc.2014.47. [DOI] [PubMed] [Google Scholar]
- Lim JH, Luo C, Vazquez F, Puigserver P. Targeting Mitochondrial Oxidative Metabolism in Melanoma Causes Metabolic Compensation through Glucose and Glutamine Utilization. Cancer Res. 2014;74:3535–3545. doi: 10.1158/0008-5472.CAN-13-2893-T. [DOI] [PubMed] [Google Scholar]
- Louie SM, Roberts LS, Mulvihill MM, Luo K, Nomura DK. Cancer cells incorporate and remodel exogenous palmitate into structural and oncogenic signaling lipids. Biochim Biophys Acta. 2013;1831:1566–1572. doi: 10.1016/j.bbalip.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Valencia I, DeBerardinis RJ. Targeting the metabolic flexibility of cancer cells: straighten up and die right. Cell Cycle. 2011;10:188. [PubMed] [Google Scholar]
- Mendez-Lucas A, Hyrossova P, Novellasdemunt L, Vinals F, Perales JC. Mitochondrial PEPCK is a Pro-Survival, ER-Stress Response Gene Involved in Tumor Cell Adaptation to Nutrient Availability. J Biol Chem. 2014 doi: 10.1074/jbc.M114.566927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature reviews. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- Previs SF, Brunengraber DZ, Brunengraber H. Is there glucose production outside of the liver and kidney? Annu Rev Nutr. 2009;29:43–57. doi: 10.1146/annurev-nutr-080508-141134. [DOI] [PubMed] [Google Scholar]
- Quinlan KG, Verger A, Yaswen P, Crossley M. Amplification of zinc finger gene 217 (ZNF217) and cancer: when good fingers go bad. Biochim Biophys Acta. 2007;1775:333–340. doi: 10.1016/j.bbcan.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Robinson MM, McBryant SJ, Tsukamoto T, Rojas C, Ferraris DV, Hamilton SK, Hansen JC, Curthoys NP. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) Biochem J. 2007;406:407–414. doi: 10.1042/BJ20070039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman NP, Eagle H, Sebring ED. The utilization of glutamine, glutamic acid, and ammonia for the biosynthesis of nucleic acid bases in mammalian cell cultures. J Biol Chem. 1958;230:1001–1012. [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DK, O’Doherty RM, Stafford JM, Newgard CB, Granner DK. The repression of hormone-activated PEPCK gene expression by glucose is insulin-independent but requires glucose metabolism. J Biol Chem. 1998;273:24145–24151. doi: 10.1074/jbc.273.37.24145. [DOI] [PubMed] [Google Scholar]
- Tanner MM, Tirkkonen M, Kallioniemi A, Collins C, Stokke T, Karhu R, Kowbel D, Shadravan F, Hintz M, Kuo WL, et al. Increased copy number at 20q13 in breast cancer: defining the critical region and exclusion of candidate genes. Cancer Res. 1994;54:4257–4260. [PubMed] [Google Scholar]
- Tong X, Zhao F, Thompson CB. The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr Opin Genet Dev. 2009;19:32–37. doi: 10.1016/j.gde.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, et al. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science. 2010;327:1004–1007. doi: 10.1126/science.1179687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Kalhan SC, Hanson RW. What is the metabolic role of phosphoenolpyruvate carboxykinase? J Biol Chem. 2009;284:27025–27029. doi: 10.1074/jbc.R109.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg K, Yao Y, Reilly PT, Kannan K, Kiarash R, Mason J, Huang P, Sawyer SK, Fuerth B, Faubert B, et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 2011;25:1041–1051. doi: 10.1101/gad.1987211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.