Abstract
Hydrogels have emerged as promising scaffolds in regenerative medicine for the delivery of biomolecules to promote healing. However, increasing evidence suggests that the context that biomolecules are presented to cells (e.g., as soluble verses tethered signals) can influence their bioactivity. A common approach to deliver biomolecules in hydrogels involves physically entrapping them within the network, such that they diffuse out over time to the surrounding tissues. While simple and versatile, the release profiles in such system are highly dependent on the molecular weight of the entrapped molecule relative to the network structure, and it can be difficult to control the release of two different signals at independent rates. In some cases, supraphysiologically high loadings are used to achieve therapeutic local concentrations, but uncontrolled release can then cause deleterious off-target side effects. In vivo, many growth factors and cytokines are stored in the extracellular matrix (ECM) and released on demand as needed during development, growth, and wound healing. Thus, emerging strategies in biomaterial chemistry have focused on ways to tether or sequester biological signals and engineer these bioactive scaffolds to signal to delivered cells or endogenous cells. While many strategies exist to achieve tethering of peptides, protein, and small molecules, this review focuses on photochemical methods, and their usefulness as a mild reaction that proceeds with fast kinetics in aqueous solutions and at physiological conditions. Photo-click and photo-caging methods are particularly useful because one can direct light to specific regions of the hydrogel to achieve spatial patterning. Recent methods have even demonstrated reversible introduction of biomolecules to mimic the dynamic changes of native ECM, enabling researchers to explore how the spatial and dynamic context of biomolecular signals influences important cell functions. This review will highlight how two photochemical methods have led to important advances in the tissue regeneration community, namely the thiol-ene photo-click reaction for bioconjugation and photocleavage reactions that allow for the removal of protecting groups. Specific examples will be highlighted where these methodologies have been used to engineer hydrogels that control and direct cell function with the aim of inspiring their use in regenerative medicine.
Keywords: thiol-ene, hydrogel, patterning, regenerative medicine, photo-cage, immobilization
Graphical Abstract
1. Introduction
Scaffolding matrices have emerged as promising platforms for the delivery of therapeutics and cells in vivo [1]. Matrices loaded with releasable biomolecules can be implanted in vivo to promote tissue regeneration by signaling to endogenous cells or to cells delivered along with the matrix [2]. A common strategy for controlling the release of biomolecules involves their physical entrapment within scaffolding matrices [3–5], causing many high molecular weight molecules, such as cytokines and growth factors, to slowly diffuse from the matrix where they can direct cellular migration, differentiation and proliferation to promote tissue regeneration [6]. For example, Hubbell et al. demonstrated the promotion of bone regeneration in vivo through implantation of a poly(ethylene glycol) (PEG) hydrogel that was designed to elute bone morphogenetic protein 2 (BMP-2) to the surrounding tissues as the gel degraded [7]. Diffusion controlled release systems are versatile and can be used to deliver large doses without any chemical modification of the biological signal. However, there is limited control over the release profile, especially when loading multiple biomolecules, and maintaining a physiologically relevant concentration over extended time periods typically necessitates supraphysiological loading that can lead to deleterious off-target side-effects [8]. Indeed, the high levels of BMP-2 loaded in scaffolds used for regenerating bone tissue via diffusion controlled delivery systems led to severe ectopic bone growth [9]. With the rising use of regenerative medicine inspired therapeutics, there is a significant need for new biomaterials that allow versatile and precise control over the delivery of multiple biomolecules, from small molecules to proteins, in a manner that maintains their activity and ability to signal to targeted cells.
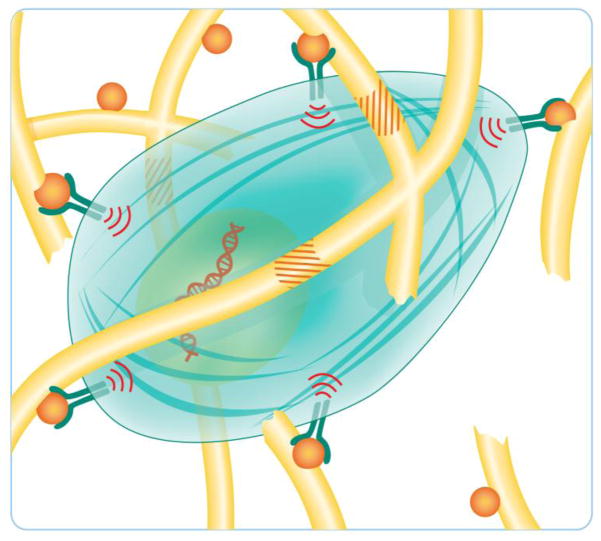
In designing biomaterial platforms to direct cellular function, it is necessary to consider the role that the extracellular matrix (ECM) plays in influencing cells in vivo (Figure 1). Tissues are composed of cells and their associated ECM—the complex, tissue-specific, three-dimensional structure that provides cells with the requisite mechanical and biological signals for healthy function [6]. In native tissues, the ECM sequesters soluble biomolecules [11], a process that functions to shield them from proteolytic degradation [12]. Moreover, the bioactivity of many proteins, such as transforming growth factor-β (TGF-β) and fibroblast growth factor (FGF), is potentiated by affinity binding to the ECM [11–13]. This suggests that cells can interact with biomolecules through the ECM directly and that the context in which a cell interacts with biomolecules can affect its function. Thus, an emerging approach to mimic biomolecule-ECM interactions is to use affinity binding [14, 15] or covalent immobilization [16] of biomolecules to matrices.
Figure 1.
A cell in its microenvironment. ECM proteins (shown as yellow rods) sequester biomolecules, such as growth factors and cytokines (shown as orange spheres). Cells engage these biomolecules through cell surface receptors to elicit signaling. Signaling can lead to cell spreading and proliferation, and the production of enzymes that enable the cell to remodel and degrade the ECM (ECM degradation sequences shown as orange stripes). Figure adapted from Kyburz et al [10].
In addition to how biomolecules are presented to cells, the choice in the biomaterial scaffold itself can influence cellular function. In fact, there is a growing appreciation for the role of biophysical cues, and their potential synergies with biochemical cues, on numerous cell processes [17–19]. One striking example comes from the Blau group, where they demonstrated that muscle satellite stem cells cultured on hydrogels with mechanical properties similar to skeletal muscle engrafted more efficiently and led to significantly more muscle regeneration than those cultured on gels in which the cells could sense the stiffness of the underlying tissue culture polystyrene (TCPS) [20]. Further, the authors discovered that satellite stem cells from aged mice require both the appropriate biophysical (substrate stiffness) and biochemical (mitogen activated protein kinase inhibitor) signals to efficiently reprogram them to a proliferative phenotype capable of muscle repair [21]. Taken together, these results demonstrate how both the context in which biomolecules are presented to cells and the scaffold on which they are presented can affect a biological response.
The emerging role of scaffold interactions with cells and biomolecules have led the field of regenerative medicine to focus on the design of ECM mimics capable of delivering viable cells and biomolecules to promote healing in vivo [22]. Hydrogels hold promise to fulfill these needs because they allow for complete user control over their material properties [23]. Hydrogels are synthetic scaffolds formed by the crosslinking of hydrophilic synthetic polymers or biomacromolecules such as poly(ethylene glycol) [24], poly(vinyl alcohol) [25], collagen [26], hyaluronan [27], and alginate [28] to afford matrices. Many mild crosslinking strategies have been developed, so hydrogels can be synthesized directly in the presence of tissues [29], cells [30], and biologics [7]. Physical or chemical crosslinks render the hydrogels insoluble, but they typically imbibe large amounts of water [31]. The high water content imparts mechanical properties that are similar to many soft tissues and transport properties that are desirable for delivering signals to cells [32]. Numerous material properties can be adjusted by changing the crosslinking density of the polymer network to engineer gels with properties of interest for selected applications [33].
Of the many materials from which a hydrogel can be assembled, there has been significant interest in PEG hydrogels. From an application standpoint, PEG is widely used in clinical medicine, and from a fundamental standpoint, PEG minimizes non-specific adsorption of proteins found in culture media and in vivo [34]. When designing systems to signal to delivered exogenous cells or endogenous cells, these properties are particularly advantageous and afford the user better control over the biological signals that are presented to cells. PEG hydrogels also allow one to directly study the effects of biological signals on cells without confounding results from biologically active hydrogel matrices, such as collagen or hyaluronan.
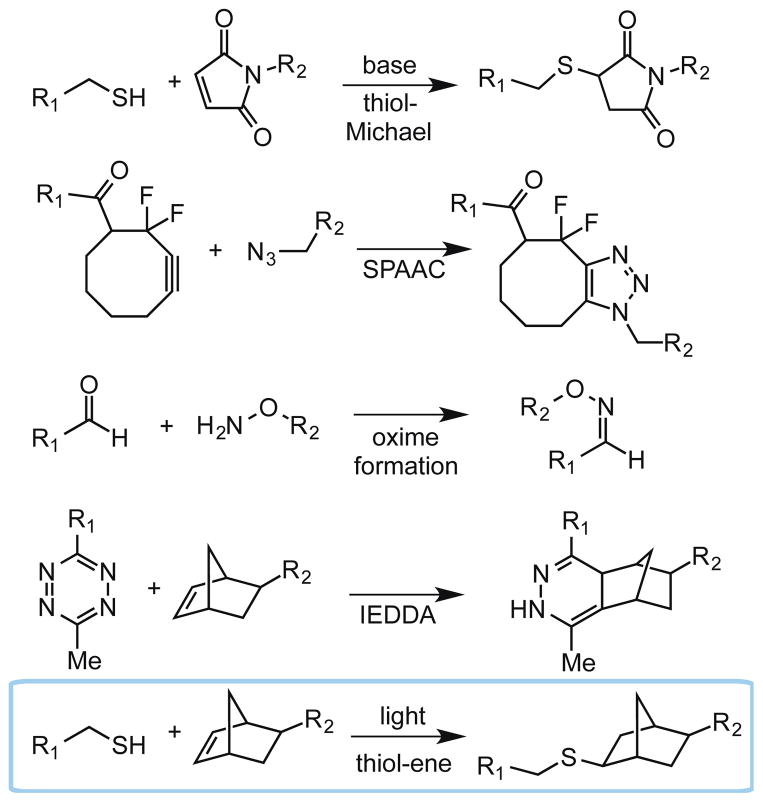
Since PEG itself is bioinert, strategies to functionalize PEG hydrogels are increasingly important and are the focus of this review article. PEG macromolecules are easily amenable to a wide variety of chemistries that can be used to attach biomolecules and impart biological signals within the hydrogel. Specifically, ‘click’ chemistries have been widely adopted within the field of regenerative medicine because they are mild, selective, high yielding, and possess rapid reaction kinetics [35]. Importantly, a subset of click reactions are bioorthogonal (i.e., inert to functional groups found in cells and free of toxic reagents and byproducts), so they can be performed in the presence of cells [36, 37]. The most commonly employed bioorthogonal click reactions include thiol-Michael conjugate addition [38], strain-promoted azide-alkyne cycloaddition (SPAAC) [39], oxime formation [40], and inverse electron demand Diels-Alder (IEDDA) reactions [41] (Figure 2). These click chemistries all occur spontaneously and irreversibly upon addition of biomolecules, and are highly useful for the formation of PEG hydrogels or for post-polymerization modification of hydrogels with pendant biological functional groups. An advantage of bioorthogonal click reactions is the homogenous nature of the reaction throughout the gel with minimal side reactions, but in some experiments and applications, it may be desirable to control the spatial distribution of biomolecules, reversibly tether multiple biomolecules, or release biomolecules at a later time. These caveats are important when considering the fact that the native ECM is highly dynamic and its composition is regularly changing, especially during events such as wound healing [11, 42, 43].
Figure 2.
Bioorthogonal click reactions include the thiol-Michael reaction, strain-promoted azide-alkyne cycloaddition (SPAAC), oxime formation, inverse electron demand Diels Alder (IEDDA) reaction, and the radical-mediated thiol-ene reaction. R1 and R2 can represent either a biomolecule or a hydrogel backbone.
As a result, light-mediated chemistry has emerged as a powerful methodology to incorporate biomolecules within hydrogels with precise user control, and two photochemical methods have recently emerged as leaders in regenerative medicine: thiol-ene photo-click chemistry [44] (Figure 2) and photocleavage reactions [45]. The thiol-ene reaction is a bioorthogonal click reaction between a thiolated molecule and an alkene [31] and photocleavage reactions function to unmask a functionality or release a biomolecule. Upon irradiation, photocleavable cages degrade to expose a reactive handle for subsequent bioconjugation or to restore the bioactivity of a biomolecule. Alternatively, photocleavable linkers degrade to release an immobilized biomolecule. Importantly, since both thiol-ene and photocleavage reactions require light to proceed, only locations in the hydrogel that are exposed to light undergo the photochemical process. Moreover, many wavelengths, light intensities, and irradiation times are cytocompatible and can be used for these reactions to proceed [46]. Thus, using photochemistry, complex patterning of biomolecules can be achieved where traditional bioconjugation strategies fail.
This review will highlight recent examples where photochemistry has been employed to control the presentation of biomolecules within hydrogels to direct cell function. Specifically, we first explore the thiol-ene photo-click reaction and its advantages in allowing patterning of both peptides and proteins. Yet proteins are inherently challenging to tether to hydrogels, especially in radical-mediated processes such as thiol-ene, so we also present specific examples where photocleavage reactions are employed to reveal functionalities for subsequent protein patterning. Finally, we examine how both of these strategies have been modified to achieve reversible patterning of biomolecules within hydrogels. We aim to present a review that inspires the field of regenerative medicine to consider how contextual presentation of biomolecules can influence their function, and we review herein two powerful photochemical methods to control the context in which biomolecules are presented to cells.
2. Strategies in photochemical patterning of biological signals
The context in which a biomolecule is presented to a cell can affect its resultant biological signal. Whether a biomolecule is presented in soluble form or bound to the ECM can potentially affect its rate of internalization (and thus stability) or its ability to cluster cell surface receptors [47]—both of which can alter the observed signal output. Indeed, many growth factors require direct interactions with the ECM for signal transduction. For example, FGF-2 binds to heparan sulfate, a component of the ECM, for bioactivity [12]. Consequently, an emerging strategy in regenerative medicine involves covalently tethering biomolecules to the hydrogel matrix to mimic biomolecule-ECM interactions. In doing so, biomolecules are protected from degradation and delivered to cells in a sustained manner at physiologically relevant concentrations [48]. Significant work has been directed toward tethering peptides and proteins uniformly within hydrogels. In such an approach, proteins are functionalized with a reactive handle and covalently tethered to hydrogels during polymerization or post-polymerization via click chemistry (Figure 2) [28, 49–55]. Still, another way in which the context of biomolecule presentation can be important is in its spatial arrangement within the ECM. Not all cells within a tissue are exposed to the same biochemical signals at any given time [56]. In fact, anisotropic signal presentation in the stem cell niche can direct asymmetric stem cell division [57]. To that end, it is often desirable to control the spatial distribution of biomolecules within hydrogels. Thus, one begins to consider methods to assemble complex biomolecule patterns or concentration gradients within hydrogels to influence specific cell populations without influencing neighboring cell populations. Photochemical reactions have emerged as a means to achieve user-directed covalent immobilization of biomolecules to direct cell function.
2.1 Thiol-ene chemistry: a photo-click reaction
The thiol-ene photo-click reaction was first identified in 1905 [58], but its full utility as a bioorthogonal click reaction was not fully realized until recently. Thiol-ene chemistry meets all the criteria of a click reaction [35], as it involves simple reactive moieties and does not produce any byproducts. It is highly efficient, and can be employed in reactions with a variety of substrates, ranging from small molecule derivatization [59, 60], to bioconjugation chemistry [61], to polymerization [62]. Importantly, the thiol-ene reaction has been demonstrated as bioorthogonal—reactive alkenes are rarely found in nature and native thiols primarily exist as unreactive disulfides or buried within proteins. For these reasons, of all the bioorthogonal photoclick reactions, thiol-ene has been explored most extensively in the biomaterials community. The thiol-ene reaction has been reviewed comprehensively [44, 63–65], but we shall provide herein a brief overview of the reaction with particular emphasis on its utility in regenerative medicine.
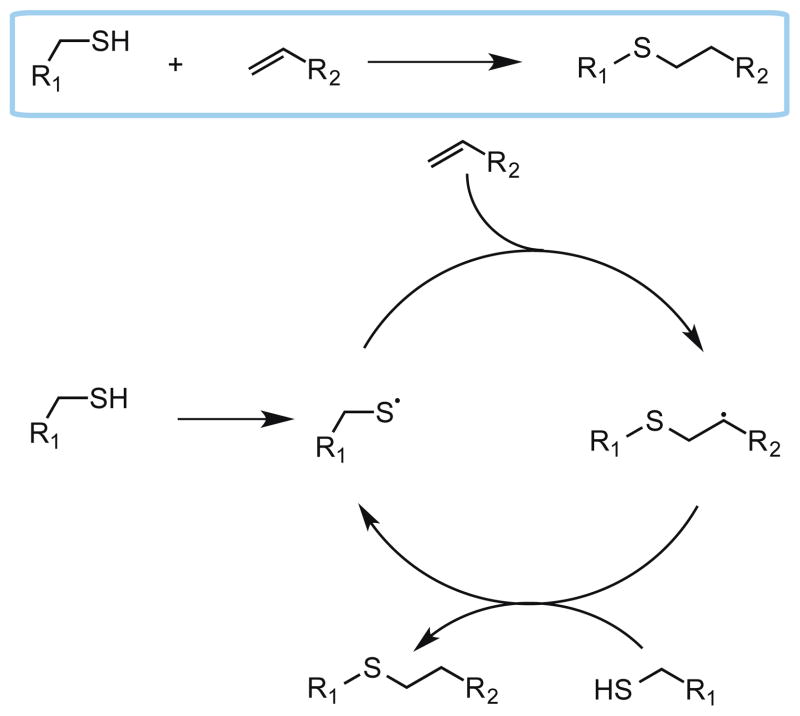
As its name implies, the thiol-ene reaction involves the addition of a thiol to an alkene (“ene”) [63, 64]. It is a radical-mediated process that is initiated by light, heat or redox processes; however light initiation is advantageous because it is cytocompatible. For light-mediated thiol-ene chemistry, the reaction begins with radical initiation upon irradiation to afford a thiyl radical (Scheme 1). Efficient initiation is typically achieved with Norrish type I photoinitiators such as lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) [66] and Irgacure 2959 (for ultraviolet initiation) [28] or Irgacure 819 (for visible light initiation) [67]. Chain transfer involves addition of the thiyl radical to the alkene followed by proton abstraction from a thiol-containing molecule to generate a new thiyl radical. The chain transfer process is highly efficient wherein a single radical causes up to tens of thousands of addition events to occur. Termination occurs rapidly with a bimolecular reaction between any of the radical species generated in the reaction. It is important to note that the efficiency of the thiol-ene reaction relies heavily on the alkene moiety chosen. Electron rich and strained alkenes like vinyl ethers and norbornenes, respectively, react most rapidly toward thiyl radicals whilst electron poor alkenes such as acrylates and maleimides react slowly [68]. The slower free-radical addition of thiyls to acrylates and maleimides should not, however, be confused with the rapid base catalyzed Michael-addition of thiols to acrylates. Indeed, mixed mode polymerizations can occur in photopolymerized thiol-acrylate systems [69]. Thus, for efficient thiol-ene mediated step-growth polymerization and bioconjugation, norbornenes or vinyl ethers should be employed.
Scheme 1.
Mechanism for the photo-click thiol-ene reaction.
Hydrogels are typically assembled using thiol-ene chemistry through the polymerization of multi-armed macromers containing alkene functionalities (typically the highly reactive norbornene [70]) with a dithiol crosslinker (such as a proteolytically degradable peptide to facilitate cell spreading and migration) [27, 71]. Thiol-ene polymerization has a number of advantages over traditional chain growth polymerization reactions. First, thiol-ene polymerization proceeds via step-growth polymerization allowing for the evolution of a more homogenous network structure [62]. It is also not inhibited by the presence of oxygen, which typically leads to longer polymerization times and requires higher radical dosing in traditional radical polymerizations [68]. Furthermore, the thiol-ene reaction also has a significantly lower concentration of free radicals during propagation compared to traditional free radical polymerizations [72]. These combined properties of thiol-ene polymerization act to minimize damage to the encapsulants, and thus polymerization can be performed in the presence of cells, tissues, proteins and other signaling molecules. Perhaps the most significant benefit of the thiol-ene reaction is the ability to utilize photoinitiation in a step-growth polymerization, which allows for control over both the time when and location where polymerization occurs.
The use of photoinitiation can also allow for spatially patterning of thiolated biomolecules. If the gel is polymerized off-stoichiometry (i.e., with excess alkene macromers), unreacted alkenes will be present within the resulting hydrogel. These alkenes can be used for post-polymerization functionalization with thiol-containing biomolecules through a subsequent thiol-ene reaction. Consequently, biomolecules are covalently incorporated within the hydrogel only at locations where light is directed. In doing so, one can assemble complex patterns and concentration gradients of biomolecules within hydrogels. While thiols can be cumbersome to work with due to their propensity to oxidize and form disulfides, these concerns are minimized because disulfides are still reactive in the thiol-ene reaction [73].
Nevertheless, there are some considerations to keep in mind when employing thiol-ene chemistry, especially in the presence of biological systems. First, biomolecules may need to be functionalized with a free thiol to facilitate their attachment to the hydrogel. Modification of biomolecules (especially proteins) can alter or diminish their function. Additionally, the thiol-ene reaction involves irradiation with light and the generation of free-radical species that can potentially lead to cytotoxicity issues [46, 74] and protein damage [72]. If care is taken when selecting the reaction conditions, most deleterious effects can be minimized, rendering the thiol-ene photo-click chemistry a powerful technique to both tether and pattern biomolecules within hydrogels.
2.1.1 Biomolecule patterning with thiol-ene chemistry
The discovery that peptides can mimic certain biological functions of the native ECM proteins from which they were derived has made their use commonplace within regenerative medicine [75]. Modification of hydrogels with these short peptide sequences (typically 3 – 10 amino acids) that are readily synthesized affords the ability to introduce biological function into otherwise biologically inert hydrogels. One has precise control over the biological signals incorporated within the hydrogel because peptides are significantly less complex, and generally more stable [76], than their native protein analogues, which often contain multiple signaling domains [77]. Many biologically active peptides have been developed for use in regenerative medicine [78]. Most commonly employed is the fibronectin-derived RGD peptide sequence. Fibronectin is a ubiquitous protein in the ECM that binds to various integrin receptors and promotes cell adhesion, spreading, and proliferation [79]. Thus, incorporation of RGD into hydrogels allows integrin-mediated cell attachment and improves the survival of anchorage dependent cells in hydrogel environments [80]. Laminin is another protein found extensively in native ECM that plays a role in facilitating cell adhesion, migration and differentiation [81]. The peptide sequences YIGSR [82] and IKVAV [83] have been found to bind cell surface receptors, similarly to fibronectin. These sequences have been widely explored in applications related to neuronal cultures and promoting neurite outgrowth, respectively [84]. Glycosaminoglycan (GAG)-binding peptides have also been identified. For example, Kiessling et al. identified the vitronectin-derived peptide sequence GKKQRFRHRNRKG that binds to GAGs on human embryonic stem cells to influence their differentiation state [85–87]. Peptides can also be employed to sequester signaling molecules within the hydrogel through affinity binding. For example, the peptide WKNFQTI was used to limit the release of its binding partner, monocyte chemotactic protein, from PEG hydrogels [88]. Thus, peptides can be incorporated within hydrogels to facilitate cell attachment, direct cell fate, or control the release of signaling molecules. Numerous ECM-derived peptide epitopes have been identified and excellent reviews on this topic can be found [89, 90].
In 2008, the Anseth lab demonstrated the first example of peptide patterning in hydrogels via the thiol-ene photo-click chemistry [62]. PEG hydrogels were synthesized via an azide-alkyne cycloaddition polymerization to afford hydrogels bearing pendant alkene functionalities. The authors hypothesized that peptides containing cysteine residues can be efficiently patterned through a thiol-ene reaction between the thiol on cysteine and the pendant alkene functionality on the hydrogel. To that end, they synthesized a fluorescently labeled RGDSC peptide, and swelled the hydrogel with a solution of the peptide and a photoinitiator. Mask-based photolithography was employed to perform the thiol-ene reaction at specific locations. Fluorescent patterns were observed suggesting that the thiol-ene reaction was confined to regions that were exposed to light. These findings demonstrated that the thiol-ene reaction could indeed be employed to spatially pattern peptide sequences within hydrogels with micron scale resolution and on time scales requiring only seconds to minutes. Moreover, it validated that incorporating cysteine within peptide sequences is a practical method to generate thiol-containing peptides for patterning.
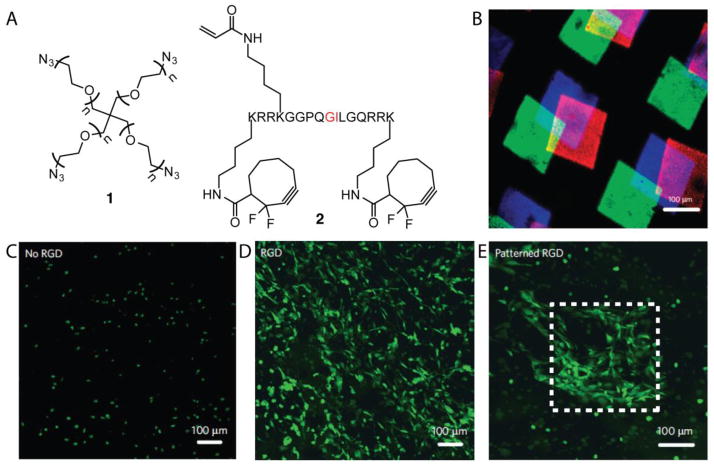
As a bioorthogonal click reaction, the thiol-ene photoclick-chemistry allows for the patterning of biomolecules in the presence of encapsulated cells [91–93]. For example, 4-arm PEG-azide 1 was polymerized via SPAAC with a proteolytically degradable bis-alkyne peptide 2 crosslinker that contained an alkene for subsequent thiol-ene functionalization (Figure 3) [94]. The authors demonstrated that three different fluorescent peptides could be patterned into the gel via thiol-ene chemistry (Figure 3B). Additionally, 3D peptide patterns were generated using multi-photon photolithography. By performing the polymerization in the presence of NIH 3T3 fibroblasts, the cells were encapsulated directly within the hydrogel during polymerization while maintaining high cell viability. Subsequent patterning of RGDSC peptides via thiol-ene chemistry allowed for user-directed cell spreading in specific regions of the gel (Figure 3C–E). Thus, thiol-ene chemistry can be used to pattern multiple peptide patterns within a gel, and importantly, peptide patterns can be generated in the presence of cells to direct their behavior.
Figure 3.
3D hydrogels were generated via SPAAC that contained pendant alkene functionalities for subsequent thiol-ene reactions post polymerization (A). Cell-mediated cleavage of the peptide cross-linker occurs at the residues highlighted in red. Three different fluorescently labeled cysteine-containing peptides were patterned sequentially and imaged via confocal microscopy (B). Three different gels were synthesized containing no RGD (C), uniform RGD (D) or patterned RGD (E) and NIH 3T3 cells expressing GFP were allowed to spread in the gels.
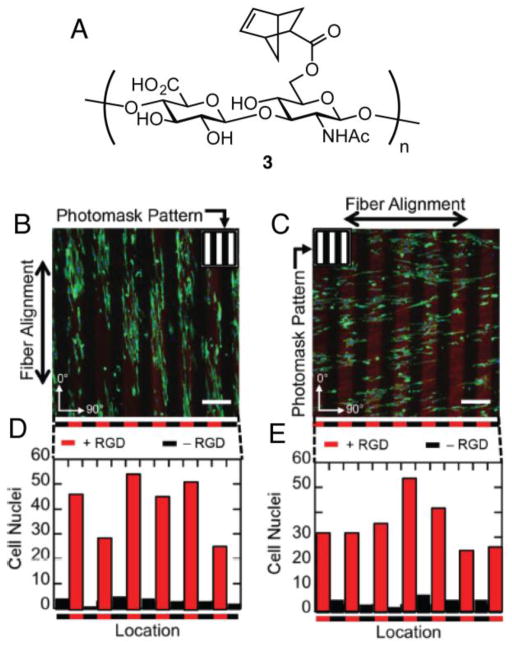
Thiol-ene photopatterning has also been explored in naturally derived hydrogels. Hyaluronic acid (HA) is a polysaccharide often employed in the synthesis of matrices for tissue engineering applications. In one example, norbornene functionalized HA 3 (NorHA) was polymerized via thiol-ene chemistry with dithiol crosslinkers to create hydrogels (Figure 4). Thiolated peptides were patterned into the resulting HA gels through thiol-ene reactions with unreacted, pendant norbornene functional groups [95]. Burdick et al. employed thiol-ene peptide patterning to explore the role of cell alignment in fibrous hydrogels. Covalently crosslinked hydrogel materials are typically amorphous with a molecular size mesh, but native ECM is composed of nanofibrous proteins that can provide structural cues to cells or allow more facile cell spreading and motility [96]. To combine the features of fibrous structure with the mechanical properties of a covalently crosslinked gel, the authors used electrospinning [97]. The resulting gels were photopatterned with 100 μm wide lanes of thiolated RGD using a mask either parallel or antiparallel to the fibers. On hydrogels patterned with RGD parallel to HA fibers, NIH 3T3 fibroblasts elongated in the direction of the hydrogel fibers as expected. Interestingly, gels with RGD patterned antiparallel to HA fibers still showed elongation relative to fiber alignment and not RGD pattern alignment. These data point to the importance of including structural features with spatially localized biochemical features in designing materials for regenerative medicine.
Figure 4.
NorHA 3 was employed by Burdick et al. to generate fibrous hydrogels via electrospinning (A). RGD (red) was photopatterned either parallel (B) or antiparallel to the fibers and NIH 3T3 cells were allowed to adhere to the gels. Cell localization within RGD patterns was quantified (D, E).
While early work focused on the patterning of peptides, thiol-ene chemistry is also beginning to see utility for patterning proteins within hydrogels. In order to ligate proteins to a hydrogel using thiol-ene chemistry, the hydrogel and protein must be functionalized with the appropriate reaction partners. In one scheme, a thiolated protein is reacted with hydrogel functionalized with alkenes via an off-stoichiometric thiol-ene polymerization. If the protein does not natively contain a free sulfhydryl, it can be thiolated using Traut’s reagent (2-iminothiolane), which converts native primary amines to thiols under mild conditions [98]. Alternatively, alkenes can be coupled to proteins through their primary amines so they can react with pendant thiols in hydrogels. Regardless of the approach, the labeling reaction must be optimized to ensure minimal reactive groups are appended to the protein to minimize deleterious effects on bioactivity of labeling.
Alge et al. recently employed thiol-ene chemistry to achieve protein patterning in hydrogels [99]. Hydrogels were synthesized via IEDDA polymerization of 4-arm PEG tetrazene with bis-norbornene functionalized peptide crosslinker. Next, the authors incorporated norbornene-labeled GGKGGC peptide uniformly to install pendant thiols throughout the hydrogel. Two different fluorescently labeled bovine serum albumin (BSA) proteins that had been functionalized with norbornene were patterned within the hydrogel using mask-based photolithography. This work was instrumental in demonstrating that thiol-ene chemistry can be employed to control spatial presentation of proteins within hydrogels. While BSA is not biologically relevant, thiol-ene chemistry has been used to tether growth factors, such as TGF-β, within hydrogels, but without spatial control [50]. Still, these studies suggest that thiol-ene chemistry will be amenable for patterning biologically active proteins within hydrogels.
2.2 Photocleavage reactions
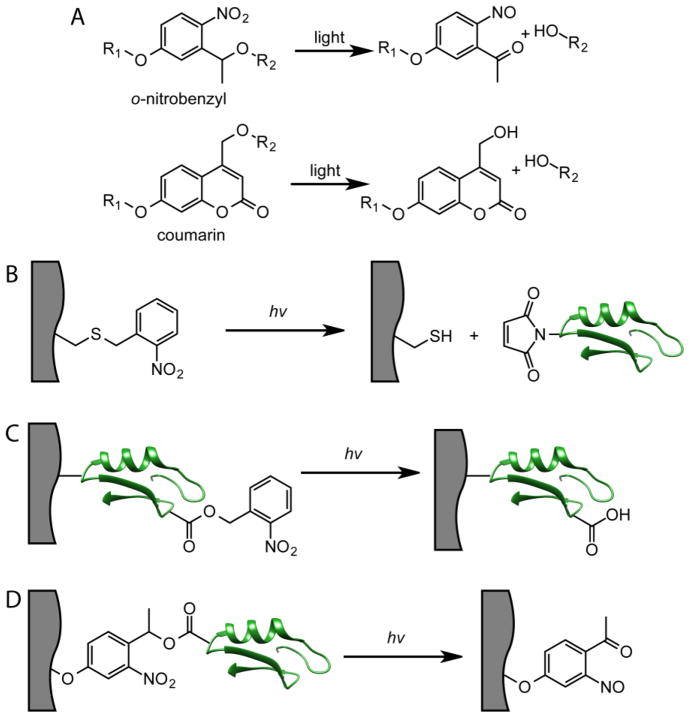
While thiol-ene chemistry is a simple, but powerful, methodology to achieve biomolecule tethering within hydrogels, patterning of proteins introduces additional challenges. Proteins require much longer timescales for diffusion into the hydrogel compared to low molecular weight peptides due to their larger size relative to the mesh of the gel [100]. Thus, sequentially patterning multiple proteins can lead to impractical timescales for creating regenerative biomaterials. Additionally, since thiol-ene bioconjugation proceeds via a radical mechanism, protein damage can be a concern, necessitating an alternative patterning approach. To that end, photocleavage reactions offer a complementary strategy to achieve simultaneous patterning. These reactions involve photocleavable or photocaging groups that undergo degradation upon irradiation with light causing the release of a leaving group, which can be a reactive handle or a biomolecule (Figure 5A) [45]. While they were originally developed for use in organic synthesis, photocleavage reactions have emerged as important tools to achieve biomolecule patterning within hydrogels [101]. In one approach, the photocleavable group acts as a protecting group for a reactive moiety (Figure 5B). For example, they can be used to mask thiol functionalities within hydrogels, which upon irradiation, cleave to expose free thiols that can undergo thiol-Michael click reactions with maleimide-labeled biomolecules [102]. Since this exposure of thiols is a photo-mediated process, the thiol-Michael click reactions occur only in regions that are exposed to light. Alternatively, photocleavable groups can be appended to the biomolecule itself as covalent inhibitors of its function (Figure 5C). While the biomolecule is uniformly presented in the hydrogel, its bioactivity is only restored in irradiated regions where photocleavage occurs [103]. Finally, biomolecules can also be attached to hydrogels using a photocleavable linker that allows for spatially-controlled release of the biomolecule upon irradiation (Figure 5D) [104].
Figure 5.
Methods to achieve patterning using photocleavable groups. A) Generic structures of ONB and coumarin photocleavable groups and their degradation products. B) The photocleavable group functions to mask a reactive handle (in this example, a thiol). Photolysis releases the thiol where it can undergo bioconjugation with a maleimide-labeled biomolecule. C) Photocleavable groups are attached to tethered biomolecules which inhibits their bioactivity. Patterning is achieved by releasing the photocleavable group to restore bioactivity in defined locations within the hydrogels. D) Biomolecules are tethered to hydrogels via a photocleavable group linker. Photolysis releases the biomolecule in defined regions.
Two of the commonly employed photocleavable groups in biomaterials are based on o-nitrobenzyl (ONB) and coumarin moieties. In its simplest form, ONB consists of a 1-nitrobenzyl moiety with a leaving group ortho to the nitro functionality (Figure 5A) [101]. ONBs are highly versatile and have been designed to mask and release carboxylic acids, thiols, alcohols, amines and phosphates for subsequent biomolecule conjugation [45, 105]. Coumarin derivatives contain a leaving group at the 3-position, and generally benefit from electron donating groups located at 6- or 7-position for efficient photolysis [101]. With both photocleavable groups, their absorption maximums can be tuned by altering the electronics of the molecules, and much effort has been directed toward identifying compositions that lead to photocleavage at a variety of wavelengths from near-IR to UV [101].
2.2.1 Patterning using photocleavage reactions
Photocleavage reactions have been used extensively to pattern peptides within hydrogels [102, 103, 106–110]; however, their true utility is in patterning proteins. As previously mentioned, one common approach is to use a photocleavable protecting group to mask a reactive functionality [111, 112]. Upon photocleavage, the reactive group is exposed allowing it to spontaneously react with biomolecules in regions that are irradiated. For example, the Shoichet lab has used this methodology to achieve gradients of proteins within hydrogels [113]. Coumarin-caged thiols within agarose hydrogels were unmasked via photolithography and allowed to react with maleimide-labeled vascular endothelial growth factor 165 (VEGF165). VEGF165 gradients were achieved by controlling the laser power in one direction, and the authors explored whether endothelial cells (ECs) could be directed along the concentration gradient. Interestingly, ECs cultured on VEGF concentration gradients formed tubule-like structures in the direction of the gradient, and the tubules appeared similar to those found in vivo. In a follow-up study, the authors explored how ECs influence retinal stem and progenitor cell (RSPC) proliferation and differentiation [114]. ECs and RSPCs were co-cultured on gradients of VEGF165 where they again formed tubule-like structures. Interestingly, differentiation of RSPCs was inhibited in the presence of ECs, although direct contact was not required, suggesting soluble EC-derived factors influence RSPC fate. These were the first findings that suggest ECs influence RSPC quiescence and could motivate therapeutic strategies for treating retinal degenerative disease.
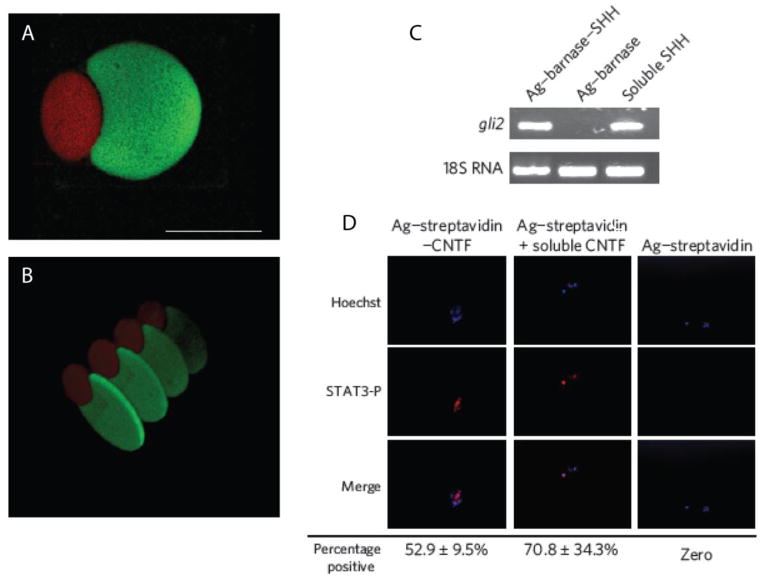
This methodology has also been employed to achieve multi-protein patterning within hydrogels [115]. Coumarin-caged thiols were unmasked in regions such that subsequent thiol-Michael conjugate additions can be performed. Specifically, the authors used two-photon photolithography to unmask thiols in specific regions of the gel, which were then reacted with a maleimide-labeled barnase. After removal of the excess maleimide-labeled barnase, additional thiols were unmasked at separate locations in the gel. A maleimide-streptavidin conjugate was then added and allowed to react at this second location. After washing, complementary barnstar-labeled sonic hedgehog (barnstar-SHH) and biotin-labeled ciliary neurotrophic factor (biotin-CNTF) conjugates were swollen into the gel; barnstar-SHH bound to patterned barnase, while biotin-CNTF bound to patterned streptavidin. Importantly, the proteins were both introduced simultaneously after the patterning and washing steps, greatly reducing the time required for patterning and avoiding protein inactivation and confounding interactions that can result from sequential patterning approaches. Using this multi-step, but highly controlled approach, the authors were able to assemble complex, multi-protein three-dimensional patterns within the hydrogel (Figure 6A, B). The authors verified that the proteins retained their bioactivity upon tethering to the hydrogels using retinal precursor cells (RPCs). For example, the authors assessed the ability of tethered SHH to mediate transcription of the transcription factor Gli2. RPCs were plated on the agarose hydrogels and gli2 mRNA was quantified by RT-PCR (Figure 6C). Similar levels of gli2 mRNA were observed for cells exposed to tethered SHH compared to cells treated with soluble SHH. Similarly, the authors monitored phosphorylated STAT3 by fluorescence microscopy as a readout of CNTF function (Figure 6D). Only cells treated with tethered CNTF or soluble CNTF exhibited staining for phospho-STAT3 suggesting CNTF is active upon tethering to hydrogels. These data demonstrate that photocleavable groups can be used to achieve patterning of bioactive proteins.
Figure 6.
Complex 3D structures of SHH and CNTF were formed using two-photon photolithography in hydrogels viewed from top (A) and side (B). SHH was labeled with a green fluorophore and CNTF was labeled with a red fluorophore. (Scale bar represents 100 μm). C) RPCs upregulate a key SHH signaling mediator, Gli2, in response to immobilized and soluble SHH as analyzed by RT-PCR. D) RCPs stained positive for phospho-STAT3 (red) in response to immobilized and soluble CNTF.
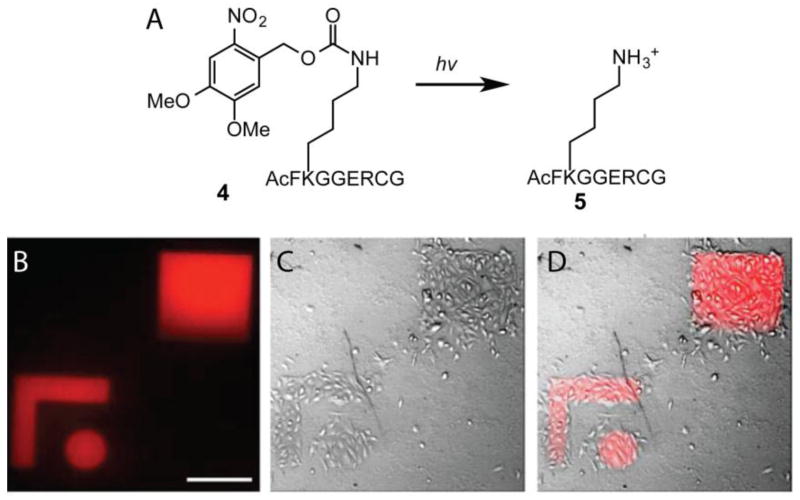
Similarly, photocleavable groups can also be used to mask substrates for an enzyme that mediates ligation of a protein to achieve protein patterning [116]. For example, Lutolf et al. generated PEG hydrogels containing pendant peptide 4 that contained a lysine residue masked by an ONB derivative (Figure 7A) [76]. Photolysis of ONB frees the lysine affording a peptide 5 that is the substrate for transglutaminase factor XIII (FXIIIa), which catalyzes the formation of an isopeptide bond between the N-terminal glutamine of the amino acid sequence NQEQVSPL and a lysine. Thus, the authors postulated that proteins could be linked to the hydrogel via FXIIIa upon photo-uncaging of the lysine on a peptide 4. Indeed, using this methodology, VEGF and Fc-chimera IgG antibody were incorporated within hydrogels with spatial control. To verify that proteins maintained their bioactivity upon patterning via this method, the authors examined the ability of VEGF to mediate cell attachment. Recombinant VEGF fragments were patterned in defined shapes and MSCs were cultured on the gels (Figure 7B–C). Only regions that had been patterned with the VEGF fragments facilitated attachment of the MSCs. These combined studies demonstrate that photocleavage reactions are powerful methodologies to achieve protein patterning within hydrogels with minimal effects on their bioactivities.
Figure 7.
A) Photocaging strategy to unmask the peptide substrate for FXIIIa to perform protein ligations. VEGF fragments (21 kDa) were patterned into hydrogels and MSCs were cultured on top of the hydrogels for 3 h (A C). (Scale bars represent 200 μm)
3. Reversible control over biomolecule patterning
In the previous sections, we have outlined synthetic methods to achieve contextual presentation of biomolecules with respect to their interactions with the ECM, as well as their spatial distribution within matrices. Still, the ECM is highly dynamic and biomolecule expression in the niche varies in response to various external stimuli, which can affect cellular function. For example, a temporally regulated transition from Notch signaling to Wnt signaling is necessary for myogenic differentiation in muscle satellite cells to regenerate muscle tissue [117]. Thus, it is logical to consider the context of reversibility in the presentation of biomolecules. Many of the aforementioned approaches to mediate biomolecule tethering allow for conjugation, and in some cases release, of biological signals, but they are irreversible—in other words, it is not possible to introduce and remove signals multiple times using the same functionalities. Hence, the ability to dynamically control the presentation of biomolecules within hydrogels has been a contemporary topic in the regenerative medicine community [118]. Both thiol-ene chemistry and photocleavage reactions have been adapted to allow for patterning that is both spatial and temporal in nature.
3.1 Thiol-ene mediated reversible biomolecule patterning
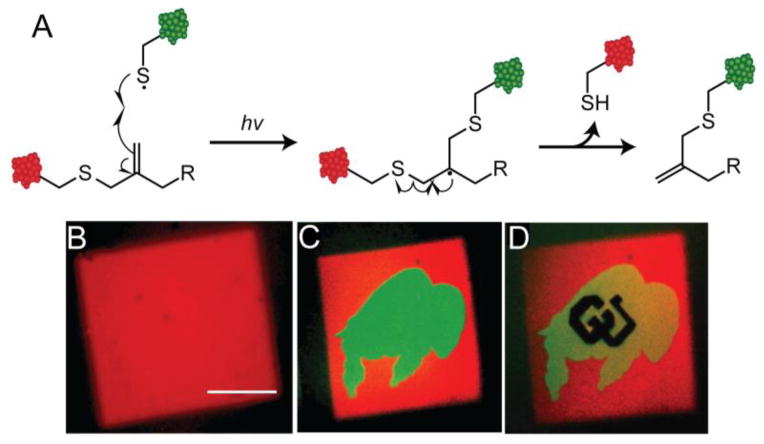
To achieve reversibility in a thiol-ene reaction, the Anseth lab drew inspiration from the chain transfer agents used in reversible addition fragment (RAFT) polymerization [119]. Specifically, the authors postulated that allyl sulfide functional groups would allow for reversible thiol-ene chemistry—attack of a thiyl radical to the allyl sulfide would lead to regeneration of the alkene and release of the previous thiolated molecule (Figure 8A). Thus, allyl sulfide functional groups should theoretically allow for infinite sequential thiol-ene reactions to proceed. To test this hypothesis, PEG hydrogels were synthesized via azide-alkyne cycloaddition polymerization of a 4-arm PEG azide and an allyl sulfide crosslinker. Using multiphoton photolithography, the authors patterned a square of fluorescent RGDS (Figure 8B). RGDS labeled with a different fluorophore was then incorporated and patterned in the arbitrary shape of a buffalo within the previously patterned square, thus exchanging the peptide within these regions (Figure 8C). Finally, a non-fluorescent RGDS peptide was patterned in the shape of ‘CU’ within the buffalo, performing a third exchange of biomolecule (Figure 8D). These data support that the allyl sulfide functionality can be employed to reversibly pattern biomolecules via thiol-ene chemistry within hydrogels. It is likely that this approach can be expanded to achieve reversible peptide and protein patterning to directly exchange the immobilized biochemical signals that cells receive. Allyl sulfide chemistry affords a plethora of opportunities to explore the effects of dynamic biochemical signaling in a more biophysically relevant 3D milieu, where the cell is not polarized as it is on 2D substrates. These studies may lead to a better understanding of the stem cell niche and also influence the regenerative properties of these materials upon transplantation due to the contextual presentation of biophysical and biochemical signals, similar to the findings of the Blau lab [20].
Figure 8.
A) Allyl sulfide strategy to reversibly perform thiol-ene reactions indefinitely to dynamically tether biomolecules. Red CRGDS was patterned via multiphoton patterning to afford a cube (B). Green CRGDS was patterned in the shape of a buffalo to exchange the two peptides (C). Finally, nonfluorescent RGDS was patterned in the shape of ‘CU’ to exchange peptides a second time (D).
3.2 Photocleavage mediated reversible biomolecule patterning
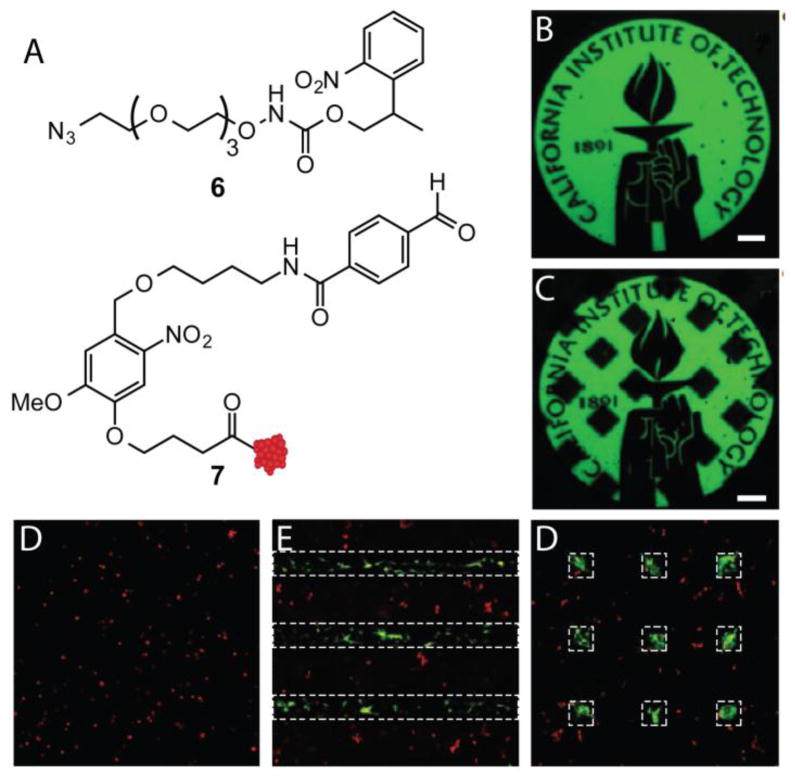
Reversible control over biomolecule patterning with proteins typically involves tethering biomolecules to the hydrogel scaffold via a photocleavable linker (Figure 3C) [104]. Thus, proteins can be patterned with spatial control, and then cleaved and released at a later time point. For example, the Tirrell lab recently exploited ONB functionality to achieve sequential protein patterning [120]. Specifically, the authors generated PEG hydrogels bearing pendant alkoxyamines 6 that are masked by an ONB protecting group (Figure 9A). Multiphoton photolithography was employed to unmask alkoxyamines to react with proteins such as 7 that contained an ONB linker tethered to an aldehyde. This approach allows proteins to be patterned via unmasking of alkoxyamines on the hydrogel, and then released via photolysis of ONB group linking the protein to the hydrogel. Using this strategy, the authors were able to pattern BSA in complex patterns (Figure 9B) and then release BSA from the hydrogel at a later time point (Figure 9C). The authors then explored the role of spatial presentation of vitronectin (VTN) on the osteogenesis of human mesenchymal stem cells (hMSCs). It has previously been shown that hMSCs undergo osteogenesis, as determined by osteocalcin (OC) expression, in 3D environments that allow for cell spreading [27]. The authors postulated that by patterning the adhesive protein VTN in lines, osteogenesis could be induced solely in hMSCs exposed to VTN. Indeed, 3 days after VTN patterning, cells exposed to VTN expressed high levels of OC, while cells outside the VTN patterned regions did not (Figure 9E). VTN was then released at specific regions, and remarkably after 6 days, cells that had previously expressed OC lost expression once VTN was removed (Figure 9F). These data demonstrate that photocleavable groups can be used to not only pattern proteins, but also release them. Moreover, these studies demonstrate that user-directed addition and release of biomolecules can direct cell function.
Figure 9.
SPAAC hydrogels were generated to bear masked alkoxyamine 6. Upon photolysis of ONB, biomolecules can be appended through the gel by aldehyde 7 that has an ONB linker to cleave biomolecules after bioconjugation. Fluorescent BSA resembling structure 7 was patterned within the hydrogel using multiphoton photolithography (B). At a later time point, fluorescent BSA was released from the hydrogel in defined regions (C) (Scale bars represent 100 μm). hMSCs were seeded within SPAAC hydrogels and treated with CellTracker red and stained for OC (green), a marker of osteogenesis. At 1 d, VTN was patterned in lines (shown as white dotted lines) and after 4 d, hMSCs stained for OC in patterned regions (E). VTN was released in regions after 4 d and hMSC OC expression was lost in those regions at 10 d (D).
4. Conclusions and Future Prospects
Hydrogels have emerged as versatile and tailorable scaffolds in regenerative medicine for the delivery of biomolecules to promote healing, and they have played a pivotal role in understanding cellular processes in 3D environments. Indeed, numerous orthogonal chemistries have emerged to achieve complex spatial control over the presentation of biomolecules within hydrogels. Of these, thiol-ene and photocleavage reactions have been highly successful at controlling the 3D presentation of both peptides and proteins in hydrogels to direct cell function. With the ability to integrate biological signals, the field is increasingly focusing on developing chemistries that allow for the presentation of biomolecules with spatial and temporal control (termed ‘4D biology’). To that end, thiol-ene and photocleavage reactions have been adapted to allow for sequential and reversible tethering of biomolecules. Many biological processes are controlled by multiple signals acting in coordination, for example, opposing gradients of chemoattactrants and chemorepellants, sequential changes in adhesive proteins and cytokine signals during differentiation of stem cells, and even dynamic changes in matrix stiffening in combination with inflammatory molecules that accelerate fibrotic diseases. To better understand which signals are important when trying to regenerate tissues or delivery drugs to target the treatment of disease, 4D hydrogel culture systems provide an important tool. However, the multitude of possible variables in the experimental space necessitates the integration of rationale material design with high throughput methods to rapidly create hydrogels with various temporal combinations of biophysical and biochemical cues. The advantage of creating such biomaterial arrays is that they can be easily integrated with existing biological and imaging methods to track and monitor key cellular processes in real time and in response to the matrix environment. While it is not possible, or perhaps even necessary, to recapitulate every aspect of native ECM-cell interactions in vitro, these approaches will be insightful and critical for identifying key biological signal combinations that have either positive or deleterious effects on tissue regeneration, wound healing, and disease progression. As such, findings from such studies should directly impact the design of future material-based therapeutics in regenerative medicine.
Acknowledgments
The authors would like to acknowledge T. Brown and K. A. Kyburz for thoughtful discussion, and E. Kyburz for assistance on figure illustration. Funding for this work was provided in part by the Howard Hughes Medical Institute, and grants from the National Science Foundation (MR1408955) and National Institutes of Health (RO1DE016523).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 2.Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts) J Tissue Eng Regen Med. 2008;2:1–13. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- 3.Leach JB, Schmidt CE. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials. 2005;26:125–135. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Mellott MB, Searcy K, Pishko MV. Release of protein from highly cross-linked hydrogels of poly (ethylene glycol) diacrylate fabricated by UV polymerization. Biomaterials. 2001;22:929–941. doi: 10.1016/s0142-9612(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 5.Lin CC, Metters AT. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv Drug Delivery Rev. 2006;58:1379–1408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Müller R, Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 8.Masters KS. Covalent Growth Factor Immobilization Strategies for Tissue Repair and Regeneration. Macromol Biosci. 2011;11:1149–1163. doi: 10.1002/mabi.201000505. [DOI] [PubMed] [Google Scholar]
- 9.Zwingenberger S, Yao Z, Jacobi A, Vater C, Valladares RD, Li C, Nich C, Rao AJ, Christman JE, Antonios JK, Gibon E, Schambach A, Maetzig T, Goodman SB, Stiehler M. Enhancement of BMP-2 Induced Bone Regeneration by SDF-1α Mediated Stem Cell Recruitment. Tissue Engineering Part A. 2013:131112094536009–131112094536009. doi: 10.1089/ten.tea.2013.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyburz KA, Anseth KS. Synthetic Mimics of the Extracellular Matrix: How Simple is Complex Enough? Ann Biomed Eng. 2015;43:489–500. doi: 10.1007/s10439-015-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 12.Pellegrini L. Role of heparan sulfate in fibroblast growth factor signalling: a structural view. Curr Opin Struct Biol. 2001;11:629–634. doi: 10.1016/s0959-440x(00)00258-x. [DOI] [PubMed] [Google Scholar]
- 13.McCaffrey TA, Falcone DJ, Brayton CF, Agarwal LA, Welt FG, Weksler BB. Transforming growth factor-beta activity is potentiated by heparin via dissociation of the transforming growth factor-beta/alpha 2-macroglobulin inactive complex. J Cell Biol. 1989;109:441–448. doi: 10.1083/jcb.109.1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benoit DSW, Collins SD, Anseth KS. Multifunctional Hydrogels that Promote Osteogenic Human Mesenchymal Stem Cell Differentiation Through Stimulation and Sequestering of Bone Morphogenic Protein 2. Adv Funct Mater. 2007;17:2085–2093. doi: 10.1002/adfm.200700012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CC, Anseth KS. Controlling Affinity Binding with Peptide-Functionalized Poly(ethylene glycol) Hydrogels. Adv Funct Mater. 2009;19:2325–2331. doi: 10.1002/adfm.200900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmökel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17:2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 17.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 18.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate Modulus Directs Neural Stem Cell Behavior. Biophys J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13:645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, Blau HM. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014:1–14. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sant S, Hancock MJ, Donnelly JP, Iyer D, Khademhosseini A. Biomimetic gradient hydrogels for tissue engineering. The Canadian Journal of Chemical Engineering. 2010;88:899–911. doi: 10.1002/cjce.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in Regenerative Medicine. Adv Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proceedings of the National Academy of Sciences. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmedlen RH, Masters KS, West JL. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials. 2002;23:4325–4332. doi: 10.1016/s0142-9612(02)00177-1. [DOI] [PubMed] [Google Scholar]
- 26.Hesse E, Hefferan TE, Tarara JE, Haasper C, Meller R, Krettek C, Lu L, Yaszemski MJ. Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. J Biomed Mater Res A. 2010;94:442–449. doi: 10.1002/jbm.a.32696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12:458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruise GM, Hegre OD, Lamberti FV, Hager SR, Hill R, Scharp DS, Hubbell JA. In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes. Cell Transplant. 1999;8:293–306. doi: 10.1177/096368979900800310. [DOI] [PubMed] [Google Scholar]
- 30.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 31.DeForest CA, Anseth KS. Advances in Bioactive Hydrogels to Probe and Direct Cell Fate. Annu Rev Chem Biomol Eng. 2012;3:421–444. doi: 10.1146/annurev-chembioeng-062011-080945. [DOI] [PubMed] [Google Scholar]
- 32.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 33.Anseth KS, Bowman CN, Brannon-Peppas L. Mechanical properties of hydrogels and their experimental determination. Biomaterials. 1996;17:1647–1657. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- 34.Gombotz WR, Guanghui W, Horbett TA, Hoffman AS. Protein adsorption to poly(ethylene oxide) surfaces. J Biomed Mater Res. 1991;25:1547–1562. doi: 10.1002/jbm.820251211. [DOI] [PubMed] [Google Scholar]
- 35.Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 36.McKay CS, Finn MG. Click Chemistry in Complex Mixtures: Bioorthogonal Bioconjugation. Chem Biol. 2014;21:1075–1101. doi: 10.1016/j.chembiol.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sletten EM, Bertozzi CR. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew Chem Int Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nair DP, Podgórski M, Chatani S, Gong T, Xi W, Fenoli CR, Bowman CN. The Thiol-Michael Addition Click Reaction: A Powerful and Widely Used Tool in Materials Chemistry. Chem Mater. 2014;26:724–744. [Google Scholar]
- 39.Agard NJ, Prescher JA, Bertozzi CR. A Strain-Promoted [3 + 2] Azide–Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J Am Chem Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 40.Grover GN, Lam J, Nguyen TH, Segura T, Maynard HD. Biocompatible Hydrogels by Oxime Click Chemistry. Biomacromolecules. 2012;13:3013–3017. doi: 10.1021/bm301346e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blackman ML, Royzen M, Fox JM. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels–Alder Reactivity. J Am Chem Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voog J, Jones DL. Stem Cells and the Niche: A Dynamic Duo. Cell Stem Cell. 2010;6:103–115. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behonick DJ, Werb Z. A bit of give and take: the relationship between the extracellular matrix and the developing chondrocyte. Mech Dev. 2003;120:1327–1336. doi: 10.1016/j.mod.2003.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoyle CE, Bowman CN. Thiol-Ene Click Chemistry. Angew Chem Int Ed. 2010;49:1540–1573. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- 45.Pelliccioli AP, Wirz J. Photoremovable protecting groups: reaction mechanisms and applications. Photochem Photobiol Sci. 2002;1:441–458. doi: 10.1039/b200777k. [DOI] [PubMed] [Google Scholar]
- 46.Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed. 2000;11:439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 47.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci. 2000;113:1677–1686. doi: 10.1242/jcs.113.10.1677. [DOI] [PubMed] [Google Scholar]
- 48.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J Royal Soc Interface. 2010;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCall JD, Luoma JE, Anseth KS. Covalently tethered transforming growth factor beta in PEG hydrogels promotes chondrogenic differentiation of encapsulated human mesenchymal stem cells. Drug Deliv and Transl Res. 2012;2:305–312. doi: 10.1007/s13346-012-0090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sridhar BV, Doyle NR, Randolph MA, Anseth KS. Covalently tethered TGF- β1 with encapsulated chondrocytes in a PEG hydrogel system enhances extracellular matrix production. J Biomed Mater Res. 2014;102:4464–4472. doi: 10.1002/jbm.a.35115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo Y, Shoichet MS. Light-Activated Immobilization of Biomolecules to Agarose Hydrogels for Controlled Cellular Response. Biomacromolecules. 2004;5:2315–2323. doi: 10.1021/bm0495811. [DOI] [PubMed] [Google Scholar]
- 52.Wang P, Na Z, Fu J, Tan CYJ, Zhang H, Yao SQ, Sun H. Microarray immobilization of biomolecules using a fast trans-cyclooctene (TCO)-tetrazine reaction. Chem Commun. 2014;50:11818–11821. doi: 10.1039/c4cc03838j. [DOI] [PubMed] [Google Scholar]
- 53.Stefonek-Puccinelli TJ, Masters KS. Co-Immobilization of Gradient-Patterned Growth Factors for Directed Cell Migration. Ann Biomed Eng. 2008;36:2121–2133. doi: 10.1007/s10439-008-9581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu L, Ratner BD, Sage EH, Jiang S. Endothelial Cell Migration on Surface-Density Gradients of Fibronectin, VEGF, or Both Proteins. Langmuir. 2007;23:11168–11173. doi: 10.1021/la701435x. [DOI] [PubMed] [Google Scholar]
- 55.Halstenberg S, Panitch A, Rizzi S, Hall H, Hubbell JA. Biologically Engineered Protein -graft-Poly(ethylene glycol) Hydrogels: A Cell Adhesive and Plasmin-Degradable Biosynthetic Material for Tissue Repair. Biomacromolecules. 2002;3:710–723. doi: 10.1021/bm015629o. [DOI] [PubMed] [Google Scholar]
- 56.Murry CE, Keller G. Differentiation of Embryonic Stem Cells to Clinically Relevant Populations: Lessons from Embryonic Development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Knoblich JA. Mechanisms of Asymmetric Stem Cell Division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 58.Posner T. Beiträge zur Kenntniss der ungesättigten Verbindungen. II. Ueber die Addition von Mercaptanen an ungesättigte Kohlenwasserstoffe. Ber Dtsch Chem Ges. 1905;38:646–657. [Google Scholar]
- 59.Dondoni A, Massi A, Nanni P, Roda A. A New Ligation Strategy for Peptide and Protein Glycosylation: Photoinduced Thiol-Ene Coupling. Chem Eur J. 2009;15:11444–11449. doi: 10.1002/chem.200901746. [DOI] [PubMed] [Google Scholar]
- 60.Tyson EL, Niemeyer ZL, Yoon TP. Redox Mediators in Visible Light Photocatalysis: Photocatalytic Radical Thiol Ene Additions. The Journal of organic chemistry. 2014;79:1427–1436. doi: 10.1021/jo500031g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trang VH, Valkevich EM, Minami S, Chen YC, Ge Y, Strieter ER. Nonenzymatic Polymerization of Ubiquitin: Single-Step Synthesis and Isolation of Discrete Ubiquitin Oligomers. Angew Chem Int Ed. 2012;51:13085–13088. doi: 10.1002/anie.201207171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Polizzotti BD, Fairbanks BD, Anseth KS. Three-Dimensional Biochemical Patterning of Click-Based Composite Hydrogels via Thiolene Photopolymerization. Biomacromolecules. 2008;9:1084–1087. doi: 10.1021/bm7012636. [DOI] [PubMed] [Google Scholar]
- 63.Hoyle CE, Lowe AB, Bowman CN. Thiol-click chemistry: a multifaceted toolbox for small molecule and polymer synthesis. Chem Soc Rev. 2010;39:1355–1334. doi: 10.1039/b901979k. [DOI] [PubMed] [Google Scholar]
- 64.Lowe AB. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym Chem. 2010;1:17–36. [Google Scholar]
- 65.Dondoni A. The Emergence of Thiol-Ene Coupling as a Click Process for Materials and Bioorganic Chemistry. Angew Chem Int Ed. 2008;47:8995–8997. doi: 10.1002/anie.200802516. [DOI] [PubMed] [Google Scholar]
- 66.Fairbanks BD, Schwartz MP, Bowman CN, Anseth KS. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials. 2009;30:6702–6707. doi: 10.1016/j.biomaterials.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ibrahim A, Stefano LD, Tarzi O, Tar H, Ley C, Allonas X. High-Performance Photoinitiating Systems for Free Radical Photopolymerization. Application to Holographic Recording. Photochem Photobiol. 2013;89:1283–1290. doi: 10.1111/php.12132. [DOI] [PubMed] [Google Scholar]
- 68.Hoyle CE, Lee TY, Roper T. Thiol-enes: Chemistry of the past with promise for the future. J Polym Sci A Polym Chem. 2004;42:5301–5338. [Google Scholar]
- 69.Salinas CN, Anseth KS. Mixed Mode Thiol–Acrylate Photopolymerizations for the Synthesis of PEG–Peptide Hydrogels. Macromolecules. 2008;41:6019–6026. [Google Scholar]
- 70.Cramer NB, Reddy SK, O’Brien AK, Bowman CN. Thiol–Ene Photopolymerization Mechanism and Rate Limiting Step Changes for Various Vinyl Functional Group Chemistries. Macromolecules. 2003;36:7964–7969. [Google Scholar]
- 71.Sawicki LA, Kloxin AM. Design of thiol-ene photoclick hydrogels using facile techniques for cell culture applications. Biomater Sci. 2014;2:1612–1626. doi: 10.1039/c4bm00187g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCall JD, Anseth KS. Thiol Ene Photopolymerizations Provide a Facile Method To Encapsulate Proteins and Maintain Their Bioactivity. Biomacromolecules. 2012;13:2410–2417. doi: 10.1021/bm300671s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fairbanks BD, Singh SP, Bowman CN, Anseth KS. Photodegradable, Photoadaptable Hydrogels via Radical-Mediated Disulfide Fragmentation Reaction. Macromolecules. 2011;44:2444–2450. doi: 10.1021/ma200202w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26:1211–1218. doi: 10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 75.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 76.Mosiewicz KA, Kolb L, van der Vlies AJ, Martino MM, Lienemann PS, Hubbell JA, Ehrbar M, Lutolf MP. In situ cell manipulation through enzymatic hydrogel photopatterning. 2013;12:1072–1078. doi: 10.1038/nmat3766. [DOI] [PubMed] [Google Scholar]
- 77.Grant DS, Tashiro K, Segui-Real B, Yamada Y, Martin GR, Kleinman HK. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989;58:933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- 78.Tam RY, Owen SC, Shoichet MS. Bio-inspired Materials for Biomedical Engineering. John Wiley & Sons, Inc; 2014. ECM-Inspired Chemical Cues: Biomimetic Molecules and Techniques of Immobilization; pp. 5–24. [Google Scholar]
- 79.Pearlstein E, Gold LI, Garcia-Pardo A. Fibronectin: A review of its structure and biological activity. Mol Cell Biochem. 1980;29:103–128. doi: 10.1007/BF00220304. [DOI] [PubMed] [Google Scholar]
- 80.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 81.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 82.Graf J, Ogle RC, Robey FA, Sasaki M, Martin GR, Yamada Y, Kleinman HK. A pentapeptide from the laminin B1 chain mediates cell adhesion and binds the 67,000 laminin receptor. Biochemistry. 1987;26:6896–6900. doi: 10.1021/bi00396a004. [DOI] [PubMed] [Google Scholar]
- 83.Tashiro K, Sephel GC, Weeks B, Sasaki M, Martin GR, Kleinman HK, Yamada Y. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J Biol Chem. 1989;264:16174–16182. [PubMed] [Google Scholar]
- 84.Schense JC, Bloch J, Aebischer P, Hubbell JA. Enzymatic incorporation of bioactive peptides into fibrin matrices enhances neurite extension. Nat Biotech. 2000;18:415–419. doi: 10.1038/74473. [DOI] [PubMed] [Google Scholar]
- 85.Wrighton PJ, Klim JR, Hernandez BA, Koonce CH, Kamp TJ, Kiessling LL. Signals from the surface modulate differentiation of human pluripotent stem cells through glycosaminoglycans and integrins. Proc Natl Acad Sci U S A. 2014;111:18126–18131. doi: 10.1073/pnas.1409525111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klim JR, Li L, Wrighton PJ, Piekarczyk MS, Kiessling LL. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat Meth. 2010;7:989–994. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Musah S, Morin SA, Wrighton PJ, Zwick DB, Jin S, Kiessling LL. Glycosaminoglycan-Binding Hydrogels Enable Mechanical Control of Human Pluripotent Stem Cell Self-Renewal. ACS Nano. 2012;6:10168–10177. doi: 10.1021/nn3039148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin CC, Boyer PD, Aimetti AA, Anseth KS. Regulating MCP-1 diffusion in affinity hydrogels for enhancing immuno-isolation. Journal of controlled release : official journal of the Controlled Release Society. 2010;142:384–391. doi: 10.1016/j.jconrel.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boontheekul T, Mooney DJ. Protein-based signaling systems in tissue engineering. Curr Opin Biotechnol. 2003;14:559–565. doi: 10.1016/j.copbio.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 90.Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 91.DeForest CA, Sims EA, Anseth KS. Peptide-Functionalized Click Hydrogels with Independently Tunable Mechanics and Chemical Functionality for 3D Cell Culture. Chem Mater. 2010;22:4783–4790. doi: 10.1021/cm101391y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS. A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Adv Mater. 2009;21:5005–5010. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.AMK, Sawicki Lisa A. Design of thiol ene photoclick hydrogels using facile techniques for cell culture applications. Biomater Sci. 2014;2:1612–1626. doi: 10.1039/c4bm00187g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater. 2009;8:659–664. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gramlich WM, Kim IL, Burdick JA. Synthesis and orthogonal photopatterning of hyaluronic acid hydrogels with thiol-norbornene chemistry. Biomaterials. 2013;34:9803–9811. doi: 10.1016/j.biomaterials.2013.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wade RJ, Burdick JA. Engineering ECM signals into biomaterials. Mater Today. 2012;15:454–459. [Google Scholar]
- 97.Wade RJ, Bassin EJ, Gramlich WM, Burdick JA. Nanofibrous Hydrogels with Spatially Patterned Biochemical Signals to Control Cell Behavior. Adv Mater. 2015;27:1356–1362. doi: 10.1002/adma.201404993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bernkop-Schnürch A. Thiolated polymers—thiomers: synthesis and in vitro evaluation of chitosan 2-iminothiolane conjugates. Int J Pharm. 2003;260:229–237. doi: 10.1016/s0378-5173(03)00271-0. [DOI] [PubMed] [Google Scholar]
- 99.Alge DL, Azagarsamy MA, Donohue DF, Anseth KS. Synthetically Tractable Click Hydrogels for Three-Dimensional Cell Culture Formed Using Tetrazine Norbornene Chemistry. Biomacromolecules. 2013;14:949–953. doi: 10.1021/bm4000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lustig SR, Peppas NA. Solute diffusion in swollen membranes. IX. Scaling laws for solute diffusion in gels. J Appl Polym Sci. 1988;36:735–747. [Google Scholar]
- 101.Bao C, Zhu L, Lin Q, Tian H. Building Biomedical Materials using Photochemical Bond Cleavage. Adv Mater. 2015;27:1647–1662. doi: 10.1002/adma.201403783. [DOI] [PubMed] [Google Scholar]
- 102.Luo Y, Shoichet MS. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat Mater. 2004;3:249–253. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- 103.Wirkner M, Weis S, San Miguel V, Álvarez M, Gropeanu RA, Salierno M, Sartoris A, Unger RE, Kirkpatrick CJ, del Campo A. Photoactivatable Caged Cyclic RGD Peptide for Triggering Integrin Binding and Cell Adhesion to Surfaces. ChemBioChem. 2011;12:2623–2629. doi: 10.1002/cbic.201100437. [DOI] [PubMed] [Google Scholar]
- 104.DeForest CA, Anseth KS. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nature Chem. 2011;3:925–931. doi: 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bley F, Schaper K, Görner H. Photoprocesses of molecules with 2-nitrobenzyl protecting groups and caged organic acids. Photochem Photobiol. 2008;84:162–171. doi: 10.1111/j.1751-1097.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 106.Musoke-Zawedde P, Shoichet MS. Anisotropic three-dimensional peptide channels guide neurite outgrowth within a biodegradable hydrogel matrix. Biomed Mater. 2006;1:162–169. doi: 10.1088/1748-6041/1/3/011. [DOI] [PubMed] [Google Scholar]
- 107.Petersen S, Alonso JM, Specht A, Duodu P, Goeldner M, del Campo A. Phototriggering of Cell Adhesion by Caged Cyclic RGD Peptides. Angew Chem Int Ed. 2008;47:3192–3195. doi: 10.1002/anie.200704857. [DOI] [PubMed] [Google Scholar]
- 108.Ohmuro-Matsuyama Y, Tatsu Y. Photocontrolled Cell Adhesion on a Surface Functionalized with a Caged Arginine-Glycine-Aspartate Peptide. Angew Chem Int Ed. 2008;47:7527–7529. doi: 10.1002/anie.200802731. [DOI] [PubMed] [Google Scholar]
- 109.Gu Z, Tang Y. Enzyme-assisted photolithography for spatial functionalization of hydrogels. Lab Chip. 2010;10:1946–1947. doi: 10.1039/c001335h. [DOI] [PubMed] [Google Scholar]
- 110.Li Y, San BH, Kessler JL, Kim JH, Xu Q, Hanes J, Yu SM. Non-Covalent Photo-Patterning of Gelatin Matrices Using Caged Collagen Mimetic Peptides. Macromol Biosci. 2014;15:52–62. doi: 10.1002/mabi.201400436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tischer T, Claus TK, Bruns M, Trouillet V, Linkert K, Rodriguez-Emmenegger C, Goldmann AS, Perrier S, Börner HG, Barner-Kowollik C. Spatially Controlled Photochemical Peptide and Polymer Conjugation on Biosurfaces. Biomacromolecules. 2013;14:4340–4350. doi: 10.1021/bm401274v. [DOI] [PubMed] [Google Scholar]
- 112.Owen SC, Fisher SA, Tam RY, Nimmo CM, Shoichet MS. Hyaluronic Acid Click Hydrogels Emulate the Extracellular Matrix. Langmuir. 2013;29:7393–7400. doi: 10.1021/la305000w. [DOI] [PubMed] [Google Scholar]
- 113.Aizawa Y, Wylie R, Shoichet M. Endothelial cell guidance in 3D patterned scaffolds. Adv Mater Weinheim. 2010;22:4831–4835. doi: 10.1002/adma.201001855. [DOI] [PubMed] [Google Scholar]
- 114.Aizawa Y, Shoichet MS. The role of endothelial cells in the retinal stem and progenitor cell niche within a 3D engineered hydrogel matrix. Biomaterials. 2012;33:5198–5205. doi: 10.1016/j.biomaterials.2012.03.062. [DOI] [PubMed] [Google Scholar]
- 115.Wylie RG, Ahsan S, Aizawa Y, Maxwell KL, Morshead CM, Shoichet MS. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat Mater. 2011;10:799–806. doi: 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- 116.Griffin DR, Borrajo J, Soon A, Acosta-Vélez GF, Oshita V, Darling N, Mack J, Barker T, Iruela-Arispe ML, Segura T. Hybrid Photopatterned Enzymatic Reaction (HyPER) for in Situ Cell Manipulation. ChemBioChem. 2014;15:233–242. doi: 10.1002/cbic.201300687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 118.Peerani R, Zandstra PW. Enabling stem cell therapies through synthetic stem cell niche engineering. J Clin Investig. 2010;120:60–70. doi: 10.1172/JCI41158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gandavarapu NR, Azagarsamy MA, Anseth KS. Photo-Click Living Strategy for Controlled, Reversible Exchange of Biochemical Ligands. Adv Mater. 2014;26:2521–2526. doi: 10.1002/adma.201304847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.DeForest CA, Tirrell DA. A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nat Mater. 2015;14:523–531. doi: 10.1038/nmat4219. [DOI] [PubMed] [Google Scholar]