Abstract
This review will describe the toxicities associated with the therapeutic administration of cultured immune cells for the treatment of cancer by review of the literature. The toxicities seen are of four types: infection associated with preparative host immunosuppression with chemotherapy prior to cell administration, acute cytokine release by the infused cells, autoimmune complications from attacking “self-antigens” also expressed by some normal tissues, and off-target toxicities where antigens, other than the intended, are attacked. Complications from immunosuppression and cytokine release are often short-lived and currently best addressed by supportive care. Autoimmunity, either “on target, off tumor” or “off target” is the result of the selection of imperfect target antigens. In some cases this can be tolerated because the benefits outweigh the costs. In other cases, alternative target antigens must be found. New strategies targeting viral antigens for virally induced cancers, and antigens encoded by tumor-specific mutations seem to have promise as safe and potentially effective targets for adoptive cell transfer.
A brisk immune response can be a two-edged weapon. Although we are all aware of the benefits of the immune cell defense against pathogens, it is also true that in some cases, severe acute and chronic damage can be inflicted on the host by these same cells. The concept behind adoptive T-cell transfer is to greatly enhance the efficacy of the T-cell repertoire against cancer by administering tumor-reactive T-cells expanded and activated in vitro. So it should be no surprise that toxicities from these cells could also be enhanced. The toxicities encountered are of four types: 1) The host immunosuppression used to enhance function and engraftment of transferred T-cells can have its own side effects, 2) Vigorous T-cell function immediately after administration (or after a brief period of in vivo expansion) can cause acute cytokine toxicity or “cytokine storm”, 3) Expression of the target antigen on some normal tissues can cause an “on target, off tumor” autoimmune injury, and 4) Aberrant reactivities can be introduced into T-cells by modification of their receptors (also designated as “off-target” cross-reactivity). We will discuss the clinical experience and immunological bases for each of these toxicities with attention to management strategies.
In the scenario most familiar to oncologists, we will start with the toxic consequences of host immunosuppression. Murine models have demonstrated that host immunosuppression can enhance the anti-tumor efficacy of adoptively transferred T-cells. This is due to the elimination of suppressor T-regulatory cells, the elaboration of T-cell homeostatic cytokines in response to lymphopenia, the elimination of resident T-cells competing for these trophic cytokines and the increased levels of TLR ligands (such as LPS) associated with immunosuppression (1–3). In humans, pre-conditioning recipients with an immunosuppressive regimen prior to T-cell administration was associated with much greater T-cell survival and complete and durable cancer regressions in patients with melanoma (4). A common regimen to suppress with is a non-myeloablative combination of cyclophosphamide and fludarabine completed at least a day prior to giving T-cells. Patients are completely lymphopenic and neutropenic for a period of approximately 7 days, within which the T-cells are given. With this comes the well-known risks of neutropenic sepsis, anemia and coagulopathy. With the relatively brief period of hematopoietic suppression associated with a single course of Cy-Flu, the main risk has been from sepsis. Standard institutional algorithms to address neutropenic fever are used to minimize this risk. Although most patients need RBC and platelet transfusions, complications from bleeding or anemia are extremely rare. Overall, the mortality from this non-myeloablative hematosuppressive regimen is approximately 1% and constitutes the major component of the risk of death from adoptive T-cell therapy in the NCI Surgery Branch experience (5;6). Additional late complications can come from potential opportunistic infections in the non-neutropenic setting (due to fludarabine-induced prolonged CD4 T-cell depletion), which can be minimalized by adding prophylaxis against pneumocystis and varicella zoster.
When the toxicities specific to T-cell administration itself are considered, the most common is due to cytokine release syndrome (CRS), which in its severe form is called ‘cytokine storm’. As administered T-cells release a variety of cytokines either specifically in response to engaging their cognate antigen or non-specifically due to activation in vitro, one sees a sepsis-like picture with fever, tachycardia, hypotension, vasodilatation and capillary leak. As in sepsis, the precise cytokines involved are likely multiple and may vary from patient to patient. There is no simple correlation of symptom severity with the serum levels of any single cytokine (eg. INF-γ). Mild symptoms respond to anti-pyretic and non-steroidal anti-inflammatory agents. In its most severe form, CRS can progress to shock, multisystem organ failure and even death. The main treatment is supportive because this can be a transient situation due to either discontinuation of a co-administered stimulating cytokine such as IL-2, or because of the self-limited survival in vivo of the majority of highly activated T-cells. In severe cases, vasopressors, intubation and continuous veno-venous hemofiltration will be needed to bridge a patient to recovery. In such cases, a reversible encephalopathy (including coma) often accompanies the failure of other organ systems. The key point is that in the great majority of cases, these manifestations are reversible in the near-term, even when severe. There are no clear predictors of severe cytokine storm and its onset can vary from hours after cell administration to sometimes 5–7 days later. A more rapid onset seems associated with larger cell numbers administered and with the co-administration of IL-2. One cytokine receiving particular attention in patients receiving cells engineered with a chimeric antigen receptor against the B-cell marker CD19 is IL-6. High IL-6 levels have been seen in some patients receiving both T-cells and IL-2 monotherapy, and investigators have reported rapid improvements in fever and hypotension in patients on anti-CD19 CAR protocols after administering the IL-6 receptor blocker, tocilizumab (7). Because the course of cytokine toxicity can be so variable and unpredictable, randomized protocols with tocilizumab may be needed to clearly determine its benefit. Another intervention often considered is the administration of high dose corticosteroids when cytokine storm threatens to become unmanageable. In murine models, corticosteroids can reduce the toxicity of high dose systemic IL-2 but also obviates its anti-tumor efficacy. This led to hesitation in applying it to patients receiving any form of cancer immunotherapy. When the checkpoint blocking antibody, ipilimumab, was initially used, it caused severe colitis and diarrhea in some patients. When this seemed life-threatening (and when some patients showed it could lead to perforation), steroids were given and were beneficial in controlling the colitis (8). Surprisingly, there did not appear to be a discernable impact on the therapeutic efficacy of ipilimumab when steroids were given. As in septic shock, the benefit of steroids in improving survival from the severe cytokine storm associated with T-cell therapy is still controversial. It is also not known if they have a detrimental impact on the efficacy of adoptive T-cell therapy. Another strategy considered is the genetic introduction of a ‘suicide gene’ into T-cells to kill them in vivo in the event of severe toxicity (9). There is no consensus as to which gene to use and whether this will work in the time scale and magnitude required after cytokine storm has commenced. This toxicity is typically self-limited as highly activated T-cells have short survival in vivo and supportive measures are the mainstay of management. Overall, the CRS associated with adoptive cell therapy is quite reminiscent of that seen with high-dose IL-2 administration, and experience gained from giving IL-2 will be useful in managing it.
The most striking toxicities specific to T-cell adoptive therapy are those resulting from a direct T-cell attack on normal tissues. This can take the form of “on target, off tumor” autoimmunity, or “off target” autoimmunity. When the target of anti-tumor T-cells is a normal, non-mutated antigen, the possibility of autoimmune complications exists from expression on some normal tissue. If the normal tissue is a vital one, then unacceptable toxicity could be seen. Yet some normal tissues can tolerate a degree of auto-immune attack and shared antigens on these tissues can still be targeted effectively on cancer cells. When tumor associated antigens were first identified by expression cloning, abundant proteins associated with specific tissues and their unique functions were highly represented. The melanocyte/melanoma antigens related to the production of melanin were identified and targeted with PBL transduced with high avidity T-cell receptors recognizing these proteins, particularly MART-1 and gp100(10). Sequelae attributable to normal melanocyte injury were seen in a high percentage of patients. Most patients developed severe rashes and subsequent vitiligo. In addition, some patients also had uveitis with impaired vision and decreased hearing presumably due to autoimmunity against pigmented cells in the stria vascularis of the inner ear (11;12). Both of these toxicities responded to topical corticosteroids (drops or trans-tympanic injection respectively). No such acute toxicities were seen when the same antigens were targeted with a variety of vaccines, most likely attesting to the relative potencies of T-cell transfer and vaccination. Interestingly, when tumor infiltrating lymphocytes from melanomas are expanded, they often contain some anti-MART or anti-gp100 reactivity but infrequently cause the sequelae described above with TCR-engineered PBL. This is perhaps a reflection of the relative avidity of the TCRs in these two T-cell populations, although other explanations are possible.
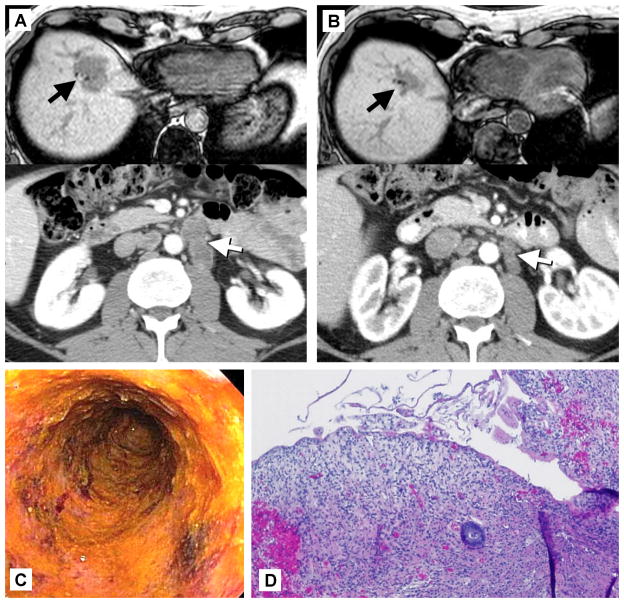
Similar scenarios have also been seen when other normal antigens were targeted. In the earliest studies giving CAR-engineered PBL to patients with renal cancer, the antigen targeted was the carbonic anhydrase IX molecule (CAIX), which is upregulated when the Von Hippel-Lindau tumor suppressor gene is defective as in most clear cell renal cancers. These patients developed hepatotoxicity and CAIX was shown to be expressed by biliary epithelium (13;14). When PBL engineered with an anti-CEA T-cell receptor (of murine origin) were given to patients with colorectal cancer, one patient had tumor regression, but all three had severe diarrhea and colitis documented by endoscopy (Figure 1)(15). This seems to be attributable to CEA expression on some normal crypts in the colon and to a lesser degree throughout the GI tract. The importance of the liver, colon, skin, eyes and inner ear limits the utility of adoptive T-cell therapy against the antigens described, but in another situation, the induced autoimmunity has been acceptable. CD19 is a cell surface antigen expressed by pre-B cells and B-cells, both normal and malignant. Several groups have developed CARs based on anti-CD19 monoclonal antibodies and CD3-zeta and incorporating a variety of different co-stimulatory domains as well (16–18). These have shown very encouraging efficacy against a variety of CD-19+ lymphomas and leukemias, but also induce normal B-cell aplasia (19). Although normal plasma cells do not express CD19, the lack of pre-B cells and mature B-cells can eventually lead to decreased Ig levels and the inability to mount new Ig responses. Yet this has proven quite tolerable with infection surveillance and periodic IgG administration. Furthermore, in some patients, this B-cell aplasia is not permanent and they recover their normal B-cell counts within months after treatment without necessarily suffering tumor relapse. For this particular antigen, the risk-benefit ratio seems in favor of anti-cancer benefit over on-target, off-tumor toxicity. One unusual toxicity in the anti-CD19 CAR trials that is not clearly understood is an unusual neurotoxicity manifested by aphasia, ataxia, some degree of catatonia occasionally progressing to coma or seizures (20). In all but the rare fatal cases, this also appears to be reversible and self-limited. What is not clear is whether it is an on-target, off tumor toxicity or some unusual cytokine-mediated phenomenon. Clear expression of CD19 in the affected brain areas has not been shown, although there are some data that suggest that trace CD19 can be detected in some brains. Typically some CAR-expressing cells can be found in the CSF in patients with neurotoxicity, but MRI scans are usually normal. This toxicity can be quite neurologically focal and is not seen with other types of adoptive T-cell therapy or with high-dose cytokine therapy, both arguing that it is not simply cytokine mediated.
Figure 1.
Regression of colon cancer and severe colitis induced by the adoptive transfer of T-cells genetically engineered with a T-cell receptor recognizing carcinoembryonic antigen (CEA). A) Colonoscopy 10 days after T-cell transfer showing loss of epithelium and granulomatous tissue. B) Biopsy 7 days after T-cell transfer showing loss of colonic epithelium and severe inflammatory colitis. C) Pre-treatment images showing liver metastasis and enlarged periaortic lymph nodes. D) Regression of metastases 4 months after T-cell transfer
The last, and perhaps most difficult type of toxicity is off-target toxicity. This occurs when the receptor-transduced T-cell population unexpectedly attacks an antigen other than the intended one. It can result from cross-reactivity of the receptor with a somewhat similar epitope structure in another antigen or it could theoretically occur when a heterodimeric TCR is introduced into a recipient T-cell with its own endogenous TCR and the alpha and beta chains of the two receptors “cross-pair” forming new, unpredictable hybrid TCR heterodimers. Several examples of the first scenario have been observed and elucidated in clinical trials. An HLA-A0201-restricted alpha-beta TCR against the tumor-germline antigen MAGE-A3 was isolated which targeted the epitope KVAELVHFL. When placed into autologous PBL and given with lymphodepletion and IL-2, nine patients with MAGE-A3+ cancers were treated and there were four partial responses and one complete response that is still sustained over four years later (21). Two patients developed obtundation, initially attributed to cytokine toxicity or a history of multiple resected brain metastases and whole brain irradiation, but they did not recover and one experienced seizures as well. A third patient had mental status changes and seizures but recovered. Autopsy showed diffuse white matter destruction, gliosis and CD8+ T-cell infiltrates in the patients who died. Although no expression of MAGE-A3 in the brain could be detected by a variety of techniques, clear expression of the related protein MAGE-A12 was seen. This antigen contains the epitope KMAELVHFL, which binds avidly to HLA-A0201 and was efficiently cross-recognized by the anti-MAGE-A3 TCR. A second example was seen when an affinity modified TCR against an HLA-A0101-presented MAGE-A3 epitope was used (22). A native human TCR against MAGE-A3 was structurally modified using phage display to enhance its avidity. The modified receptor contained four amino acid substitutions in the CDR2-alpha region. When PBL transduced with this modified TCR were used in patients, two developed cardiogenic shock and died. Eventually it was shown that the enhanced receptor (but not the wild type TCR) cross-recognized the cardiomyocyte protein Titin (23). Titin contained an HLA-A1 binding epitope ESDPIVAQY, similar enough to the target MAGE-A3 epitope, EVDPIGHLY, to be recognized by the affinity-enhanced TCR, despite only sharing 5 of 9 amino acid residues. This illustrates the increased risk of unpredictable self-reactivity from using any TCR that has not been vetted by thymic selection before release into the peripheral T-cell repertoire. This risk extends not only to structurally modified TCRs, but also TCRs raised in HLA transgenic mice (although there is still substantial homology between the murine and human proteomes which will be protective). The surprisingly low degree of homology between the MAGE-A3 and Titin epitopes shows that it may be impossible to anticipate all potential cross-reactivities and Phase I cell dose escalation and a high degree of suspicion may be the only ways to minimize this risk. Another source of potential off-target reactivity is when TCR transduced PBL simultaneously express their native TCR. This can result in the formation of hybrid TCR alpha-beta dimers which, by chance, might recognize self-antigens as they have not been subjected to negative selection in the thymus. Although not encountered in TCR-engineered human ACT trials, an example has been demonstrated in a murine model. Immunosuppressed mice given T-cells transduced with the anti-ovalbumin TCR, OT-1, suffered a lethal graft-versus-host type of toxicity that was not seen when OT-1 transgenic lymphocytes (with only one TCR) were transferred (24). It could also be induced when only the TCR-alpha (or beta) from OT-1 was introduced into the transferred lymphocytes, supporting a role for mixed dimers using that single OT-1 TCR chain. Although specific auto-antigens recognized by mixed dimers were not identified, this toxicity was reduced when RNAi was used to knockdown the endogenous TCRs. A variety of clinical approaches to preventing TCR mixed dimer formation are being developed, including using partially murine TCRs, suppressing endogenous TCR expression and chemical modifications which specifically augment the pairing of the transduced alpha and beta chains (25–27).
The power of T-cell adoptive transfer is both its biggest advantage and its biggest danger. Vaccine strategies to induce anti-tumor T-cell responses have been very non-toxic, but that is most often due to the very weak responses they generate. The ability of adoptively transferred T-cells to target and attack even tiny, remote deposits of antigen-expressing target cells is evidenced both by the complete and durable regressions achieved in patients with metastatic cancer (28)] and the highly selective or localized consequences of some of the unintentional autoimmune toxicities. Ultimately, these issues are best addressed by finding tumor associated target antigens that are highly tumor-specific. One of the only such antigens successfully targeted in patients has been the germline-tumor antigen NY-ESO-1(29). Despite achieving high rates of objective response in patients with metastatic melanoma and synovial sarcoma, no evidence of autoimmunity was seen (30). The class of tumors caused by oncoviruses also opens up the possibility of targeting viral epitopes expressed after transformation, which should also not be associated with autoimmunity. TIL against human papilloma virus-associated cervical cancer have been shown to contain T-cells reactive with the E6 and E7 oncoproteins of HPV and their therapeutic administration has been able to induce tumor regression (31). Perhaps the safest and most universal class of target antigens will prove to be those generated by tumor specific genomic mutations; termed ‘neoantigens’. Recent data show that there are often T-cell responses to these neoantigens in the tumor infiltrating lymphocytes from melanoma (32), perhaps explaining how melanoma TIL can achieve complete tumor regression in the absence of autoimmunity. Furthermore, the TIL from other tumors can also contain such T-cell reactivities, and in one case, the adoptive transfer of those T-cells were therapeutically effective (33). One would predict that native T-cells (or TCRs) against ‘driver’ neoantigens could be used clinically in adoptive transfer protocols and would be associated with the lowest probability of antigen-escape relapses or autoimmunity, perhaps representing the ‘ideal’ target antigens.
Acknowledgments
Funding: National Institutes of Health
References
- 1.Paulos CM, Wrzesinski C, Kaiser A, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–7. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley ME, Gross CA, Somerville RP, et al. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J Clin Oncol. 2013;31:2152–9. doi: 10.1200/JCO.2012.46.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–9. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Straathof KC, Spencer DM, Sutton RE, Rooney CM. Suicide genes as safety switches in T lymphocytes. Cytotherapy. 2003;5:227–30. doi: 10.1080/14653240310001497. [DOI] [PubMed] [Google Scholar]
- 10.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–46. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh S, Karne NK, Kerkar SP, et al. Ocular and systemic autoimmunity after successful tumor-infiltrating lymphocyte immunotherapy for recurrent, metastatic melanoma. Ophthalmology. 2009;116:981–9. doi: 10.1016/j.ophtha.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seaman BJ, Guardiani EA, Brewer CC, et al. Audiovestibular dysfunction associated with adoptive cell immunotherapy for melanoma. Otolaryngol Head Neck Surg. 2012;147:744–9. doi: 10.1177/0194599812448356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamers CH, Sleijfer S, van SS, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21:904–12. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 15.Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620–6. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh H, Manuri PR, Olivares S, et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–71. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan RA, Chinnasamy N, Abate-Daga D, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36:133–51. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–71. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cameron BJ, Gerry AB, Dukes J, et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013;5:197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendle GM, Linnemann C, Hooijkaas AI, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16:565–70. 1p. doi: 10.1038/nm.2128. [DOI] [PubMed] [Google Scholar]
- 25.Bunse M, Bendle GM, Linnemann C, et al. RNAi-mediated TCR knockdown prevents autoimmunity in mice caused by mixed TCR dimers following TCR gene transfer. Mol Ther. 2014;22:1983–91. doi: 10.1038/mt.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Provasi E, Genovese P, Lombardo A, et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med. 2012;18:807–15. doi: 10.1038/nm.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuball J, Dossett ML, Wolfl M, et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109:2331–8. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YT, Boyer AD, Viars CS, Tsang S, Old LJ, Arden KC. Genomic cloning and localization of CTAG, a gene encoding an autoimmunogenic cancer-testis antigen NY-ESO-1, to human chromosome Xq28. Cytogenet Cell Genet. 1997;79:237–40. doi: 10.1159/000134734. [DOI] [PubMed] [Google Scholar]
- 30.Robbins PF, Kassim SH, Tran TL, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21:1019–27. doi: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevanovic S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33:1543–50. doi: 10.1200/JCO.2014.58.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins PF, Lu YC, El-Gamil M, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19:747–52. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–5. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]