Abstract
Introduction
Stroke is a major public health concern worldwide given the associated morbidity and mortality. Smoking is a risk factor for stroke, but the relationship between secondhand smoke (SHS) exposure and stroke has been inconsistent to date. The aim of the current study was to examine the association of SHS exposure and risk of stroke and its subtypes (ischemic and hemorrhagic stroke) among nonsmokers.
Methods
Demographic and clinical characteristics were compared by SHS exposure status for black and white nonsmokers aged ≥45 years in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study in 2014. Hazard ratios (HRs) and corresponding 95% CIs were calculated by Cox proportional hazards models to assess the relationship between SHS exposure and stroke risk.
Results
Of the 21,743 participants (38% African American, 45% male), SHS exposure in the past year was reported by 23%. Compared with those without SHS exposure, exposed participants were more likely to be female, white, younger, and reside with a smoker (all p<0.001). A total of 428 incident strokes were observed from April 2003 to March 2012 during a mean follow-up of 5.6 years. The risk of overall stroke was increased 30% among those with SHS exposure after adjustment for other stroke risk factors (95% CI=2%, 67%). This relationship appeared to be driven by ischemic strokes.
Conclusions
SHS exposure is independently associated with an increased risk of stroke. Future studies are needed to confirm these findings and examine the role of long-term effects of SHS exposure on stroke outcomes.
Introduction
In 2010, stroke and ischemic heart disease accounted for one in four deaths globally, an increase from one in five deaths two decades prior.1 Stroke is the fourth leading cause of death in the U.S., responsible for one of every 19 deaths.2,3 It is also a leading cause of disability.4 Nearly 800,000 people in the U.S. suffer a stroke each year, the large majority of whom are ischemic.2 In addition, stroke incidence remains very high among African Americans, with risk of first stroke twice that of whites.5
Risk factors for stroke include smoking,6 hypertension,7 and atrial fibrillation.8 Stroke risk is reduced by lowering blood pressure (BP) levels to <120/80 mm/Hg8,9 and through physical activity.10,11 Previous studies reported a relationship between secondhand smoke (SHS) exposure and increased risk of stroke.12–21 However, not all studies have found an association.14,21–23 Whincup et al.23 evaluated the relationship between high versus low cotinine levels and stroke in male nonsmokers (hazard ratio [HR]=0.77, 95% CI=0.42, 1.41) and never smokers (HR=2.16, 95% CI=0.80, 5.80) and found no association with stroke after multivariable adjustment.
Exposure to SHS is concerning, as 18% of the U.S. adult population currently smokes.24 Furthermore, elevated serum cotinine levels (≥0.05 ng/mL) were observed among 40% of nonsmoking adults participating in the 1999–2010 National Health and Nutrition Examination Survey, with exposure at work reported by 14% and at home by 6%.25 Compared with individuals without environmental tobacco smoke (ETS) exposure, the risk of mortality from coronary heart disease was 25%–35% higher for those with low ETS exposure by an earlier review.26 Development of carotid artery intimal-medial thickness, a risk factor for stroke,27 has been associated with short- (3 years) and long-term (10 years) SHS exposure.28,29 Proposed mechanisms for how SHS exposure increases stroke risk include increased thrombogenicity and higher fibrinogen levels, lower high-density lipoprotein (HDL) cholesterol levels, carboxyhemoglobinemia, hemodynamic and autonomic effects, acute endothelial dysfunction, oxidative stress, and progression of atherosclerosis due to 1,3-butadiene or other toxic compounds found in cigarette smoke.6,30,31
The aim of the current study was to examine the association between SHS exposure and risk of stroke and stroke subtypes (ischemic and hemorrhagic stroke) among nonsmokers after accounting for stroke risk factors and to evaluate potential confounding factors of any observed association. The potential relationships between SHS exposure and stroke were investigated by testing the hypotheses that: (1) higher prevalence of SHS exposure is associated with increased incidence of stroke; and (2) higher prevalence of SHS exposure is associated with increased incidence of stroke subtypes.
Methods
Study Design
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study is a national, population-based, longitudinal study investigating cardiovascular disease events and mortality endpoints among white (55%) and African American (45%) adults aged ≥45 years.32 The cohort includes participants (55% female) from the 48 contiguous U.S. states. The study received IRB approval from all participating institutions, and study participants provided written informed consent. The methods were published previously.32
Briefly, demographic characteristics, SES, and medical history were obtained and a risk factor evaluation carried out (including smoking status and SHS exposure) during a baseline telephone interview conducted between 2003 and 2007. In-home examinations followed and involved physical, clinical, and laboratory measurements including height, weight, BP, lipid profiles, glucose, and an electrocardiogram (ECG). Participants are called every 6 months for follow-up, with the main goals of ascertaining potential stroke events and cognitive impairment.
Measures
At baseline, current smoking was defined as active smoking among those who smoked >100 cigarettes in their lifetime. Former smoking was defined as non-current use and smoking >100 cigarettes in their lifetime. Pack years smoked was calculated for former smokers by multiplying the number of packs smoked per day by the years of smoking. The remainder of participants was classified as nonsmokers. Current smokers were excluded from the analysis. SHS exposure was assessed by duration (Does anyone living with you smoke cigarettes regularly?) and frequency (During the past year, about how many hours PER WEEK, on the average, were you in close contact with people when they were smoking? For example, in your home, in a car, at work, or other close quarters?). The primary exposure, exposure to SHS, was defined as >1 hour per week in close contact with a smoker(s).28,33 Thus, individuals reporting 0–1 hour of SHS exposure per week were characterized as not exposed. Continuous SHS exposure was truncated to 168 hours for 17 individuals who reported exposure exceeding the maximum number of hours in a week.
Race was self-reported as African American or white. Stroke belt region was defined as residing within a region of the country with excess stroke mortality spanning eight southeastern states of Alabama, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, and Arkansas.34 Education was dichotomized as high school or less versus greater than high school, and annual income was categorized as <$20,000, $20,000–$35,000, $35,000–$75,000, ≥$75,000, or refused to report, and dichotomized as <$35,000 versus ≥$35,000 in the model.
Alcohol consumption was categorized as never versus ever based on self-report. Self-reported exercise was characterized as none or exercising “enough to work up a sweat” one to three times per week or four or more times per week, with further categorization for the model into none versus one or more time per week.
Baseline BMI was calculated as kg/m2 using measured weight (kg) and height (m) and was categorized as underweight (<18.5), normal (18.5–24.9), overweight (25–29.9), or obese (>30).35 Waist circumference (cm) was measured, and serum chemistries, C-reactive protein (CRP) (mg/L), and white blood cell count were measured using standard methods at the University of Vermont in Burlington, Vermont.36 Diabetes was defined as fasting glucose ≥126 mg/dL, non-fasting glucose ≥200 mg/dL, or self-reported use of diabetic medication or insulin. Left ventricular hypertrophy (LVH) was classified according to the Sokolow–Lyon LVH limb lead criteria.37 Dyslipidemia was defined as total cholesterol ≥240 mg/dL, low-density lipoprotein ≥160 mg/dL, HDL ≤40, or self-reported use of lipid-lowering medication.
A trained technician measured systolic blood pressure (SBP) and diastolic blood pressure (DBP) at the in-home visit using a standard protocol. Hypertension was defined as SBP ≥140, DBP ≥90, or by self-reported current use of antihypertensive medication. History of heart disease was determined by self-reported diagnoses of myocardial infarction (MI), coronary artery bypass graft, bypass, angioplasty or stenting, or by evidence of MI on ECG. Atrial fibrillation was determined by ECG evidence as recorded during the in-home visit or by self-report. Factors included in the Framingham Stroke Risk Score were age, diabetes mellitus, history of cardiovascular disease, atrial fibrillation, current antihypertensive medication use, SBP, and LVH.38
Fatal and non-fatal incident stroke occurring after the baseline survey was the primary outcome measure among those stroke-free at baseline. Events reported during follow-up as potential stroke, transient ischemic attack (TIA), death, hospitalization, or emergency department visit for brain aneurysm, brain hemorrhage, stroke symptoms, or unknown reason prompted a request for medical records. Those deemed potential strokes after nurse review were centrally adjudicated by physicians. For deaths without medical records, death certificates were examined and adjudicated, and interviews with surviving family members undertaken. Stroke was defined in four ways:
medical records confirmed focal neurological deficit for ≥24 hours;
medical records confirmed focal/nonfocal neurologic deficit with imaging verification;
stroke as underlying cause of death available from National Death Index (NDI) (ICD-10: I60–I67, I69, I672, I678–I679, G450–453 and G46) without medical record; and
probable stroke determination made during review of proxy interview.
Although the overall analysis included all four definitions of stroke, the subtype analysis did not include NDI and exit interview defined stroke, owing to unavailability of stroke subtype information.
Statistical Analysis
Demographic and clinical characteristics were summarized overall and by SHS exposure. Differences between SHS exposure groups were tested using chi-square tests, t-tests, and the Mann–Whitney U test. Crude incidence rates and corresponding 95% CIs were estimated for stroke and its subtypes by SHS exposure. Cox proportional hazards models were performed to determine whether the risk of stroke and stroke subtypes differed by SHS exposure. Survival times were censored at first stroke event (date of stroke), date of death occurring prior to stroke, or date of the last completed follow-up. Univariate models were fit, followed by models with adjustment for demographics (age, race, sex, and the age–race interaction) and SES factors (income and educational level), then with the addition of smoking history and Framingham Stroke Risk Score factors, then with the addition of CRP, and finally with the addition of BMI and lifestyle/behavioral factors (alcohol use and exercise). An age–race interaction was identified by earlier REGARDS analyses and defined a priori. Models were fit for all participants, then separately for never smokers and former smokers to further explore the relationship between SHS exposure and risk of stroke and stroke subtypes. Overall models with adjustment beyond demographics and SES factors also adjusted for smoking history (former versus never). Models for former smokers with adjustment beyond demographics and SES factors additionally adjusted for pack years smoked. The proportional hazards assumption was examined using log–log plots and was met for any stroke event and ischemic stroke but was not met for every stratum of hemorrhagic stroke. Statistical analyses were performed in 2014 using SPSS, version 21.39
Results
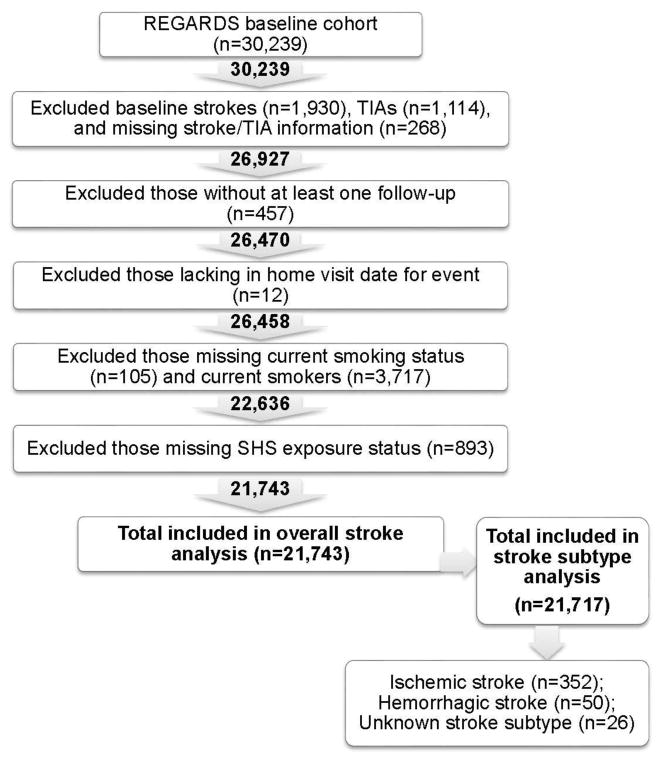
The REGARDS parent study included a total of 30,239 individuals, of whom 21,743 were included in the overall stroke analysis and 21,717 were included in stroke subtype analyses (based on exclusion of 26 individuals missing stroke subtype information). Figure 1 shows a flow diagram for inclusion of participants.
Figure 1.
Participant selection criteria.
Table 1 presents demographic and clinical characteristics stratified by SHS exposure. Of 21,743 participants, 23% (n=5,081) reported SHS exposure in the past year and 77% (n=16,662) reported no SHS exposure. Individuals reporting SHS exposure were significantly younger (62.9 [SD=8.7] vs 65.6 [SD=9.5] years) and more likely to be white (56% vs 44%), female (53% vs 47%), and with lower education levels (all p<0.001) than those without exposure. Certain clinical characteristics and lifestyle/behavioral factors differed among those with SHS exposure and those without SHS exposure. However, history of cardiovascular disease, atrial fibrillation, LVH, Framingham Stroke Risk Score, and region of residence (belt versus non-belt) did not differ significantly between the two groups.
Table 1.
Demographic and Clinical Characteristics of REGARDS Participants by Exposure to Secondhand Smoke (n=21,743)
| Characteristic | SHS Exposure a (n =5,081) n (%), mean + SD, median (range) | No SHS Exposure b (n=16,662) n (%), mean + SD, median (range) | p-value |
|---|---|---|---|
| Age (years) | 62.9 + 8.7 | 65.6 + 9.5 | <0.001 |
| 45–64 | 3,023 (60) | 7,874 (47) | <0.001 |
| >65 | 2,058 (41) | 8,788 (53) | |
| African-American | 2,231 (44) | 6,106 (37) | <0.001 |
| Female | 2,675 (53) | 9,283 (56) | <0.001 |
| Region | |||
| Belt | 2,861 (56) | 9,187 (55) | 0.14 |
| Non-belt | 2,220 (44) | 7,475 (45) | |
| Education | |||
| <High school | 577 (11) | 1,629 (10) | <0.001 |
| High school graduate | 1,472 (29) | 3,893 (23) | |
| Some college | 1,434 (28) | 4,348 (26) | |
| >College graduate | 1,598 (31) | 6,783 (41) | |
| Income, $ | |||
| <20,000 | 865 (17) | 2,430 (15) | <0.001 |
| 20,000–34,000 | 1,258 (25) | 3,804 (23) | |
| 35,000–74,000 | 1,695 (33) | 5,231 (31) | |
| >75,000 | 769 (15) | 3,173 (19) | |
| Refused | 494 (10) | 2,024 (12) | |
| Alcohol consumption | |||
| None | 2,890 (57) | 10,511 (63) | <0.001 |
| Moderate | 1,850 (36) | 5,408 (32) | |
| Heavy | 244 (5) | 496 (3) | |
| Smoking history | |||
| Never | 2,413 (48) | 9,251 (56) | <0.001 |
| Former | 2,668 (53) | 7,441 (45) | |
| Reside with regular smoker | 1,216 (24) | 938 (6) | <0.001 |
| Exercise | |||
| None | 1,533 (30) | 5,277 (32) | 0.01 |
| 1–3 times/wk | 1,973 (39) | 6,065 (36) | |
| >4 times/wk | 1,507 (30) | 5,113 (31) | |
| Atrial fibrillation | 403 (8) | 1302 (8) | 0.80 |
| BMI (kg/m2) | 30.1 + 6.1 | 29.3 + 6.1 | <0.001 |
| Underweight | 27 (1) | 126 (1) | <0.001 |
| Normal | 923 (18) | 3,930 (24) | |
| Overweight | 1,873 (37) | 6,266 (38) | |
| Obese | 2,212 (44) | 6,246 (37) | |
| Waist circumference (cm) | 98.1 + 15.8 | 95.6 + 15.5 | <0.001 |
| Cardiovascular disease history | 772 (15) | 2,628 (16) | 0.33 |
| Diabetes | 1,115 (22) | 3,142 (19) | <0.001 |
| C-reactive protein (mg/L), (IQR) | 2.3 (4.1) | 2.0 (3.7) | <0.001 |
| White blood cell count | 5.9 + 5.0 | 5.7 + 2.1 | 0.048 |
| Systolic blood pressure (mmHg) | 128 + 17 | 127 + 16 | <0.001 |
| Hypertension | 3,021 (59) | 9,279 (56) | <0.001 |
| Hypertension, SR | 2,987 (59) | 9,107 (55) | <0.001 |
| Dyslipidemia | 2,919 (57) | 9,223 (55) | 0.01 |
| Antihypertensive medication | |||
| Ever | 2,809 (55) | 8,587 (52) | <0.001 |
| Current | 2,634 (52) | 8,079 (48) | <0.001 |
| Framingham Stroke Risk Score | 5.6 (0.5 – 82.9) | 6.2 (0.5 – 93.9) | 0.70 |
| Left ventricular hypertrophy | 517 (10.3) | 1,621 (9.9) | 0.38 |
Note: Boldface indicates statistical significance (p<0.05).
SHS, secondhand smoke; $, U.S. dollar; wk, week; IQR, interquartile range; SBP, systolic blood pressure; DPB, diastolic blood pressure; SR, self-reported; med, medication; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Secondhand smoke exposure was defined as >1 hour per week in close contact with a smoker(s) in the past year.
No secondhand smoke exposure was defined as 0 to 1 hour per week in close contact with a smoker(s) in the past year.
Demographic and clinical characteristics of former and never smokers were further examined by SHS status (Appendix Table 1). Of the 5,081 participants reporting SHS exposure, 53% (n=2,668) were former smokers. Forty-four percent (n=7,411) of those without SHS exposure (n=16,662) were former smokers. Associations between SHS exposure and risk factors were similar for never and former smokers reporting SHS exposure but differed among those without SHS exposure.
This analysis included events from April 2003 to March 2012. The average follow-up time for all participants was 5.6 (SD=1.9) years (median, 6.0; range, 0–9), and 428 stroke events were observed (352 ischemic, 50 hemorrhagic, and 26 strokes of unknown subtype). Stroke incidence rates are presented by SHS exposure in Table 2.
Table 2.
Stroke Incidence by Exposure to Secondhand Smoke
| No. of Events | Cumulative Person-Years | Event Rate/1,000 Person- Years (95% CI) | |
|---|---|---|---|
| Any stroke a | 428 | 121,231 | 3.53 (3.20, 3.87) |
| SHS exposure | 111 | 28,359 | 3.91 (3.22, 4.68) |
| No SHS exposure | 317 | 92,872 | 3.41 (3.05, 3.80) |
| Ischemic stroke | 352 | 121,231 | 2.90 (2.61, 3.21) |
| SHS exposure | 90 | 28,359 | 3.17 (2.55, 3.86) |
| No SHS exposure | 262 | 92,872 | 2.82 (2.49, 3.17) |
| Hemorrhagic stroke | 50 | 121,231 | 0.41 (0.31, 0.53) |
| SHS exposure | 12 | 28,359 | 0.42 (0.29, 0.55) |
| No SHS exposure | 38 | 92,872 | 0.41 (0.29, 0.55) |
SHS, secondhand smoke
Twenty-six strokes were of unknown subtype.
We hypothesized that higher prevalence of SHS exposure in the past year would be associated with increased risk of stroke and its subtypes. Crude and adjusted HRs for all nonsmoking participants and for never and formers smokers are displayed in Table 3. In unadjusted models, the risk of stroke did not differ significantly between those exposed to SHS and those without exposure. However, after adjustment for demographic and SES covariates, SHS exposure was associated with increased risk of stroke (HR=1.31, 95% CI=1.03, 1.65) among nonsmoking participants. Further adjustment did not change the HRs meaningfully, suggesting these factors were not confounders, although the association was no longer significant in the final multivariable model.
Table 3.
Association of Secondhand Smoke Exposure and Stroke among All Participants and Never and Former Smokers
| Any Stroke | Secondhand smoke exposure (>1 hour/week over past year) | |||||
|---|---|---|---|---|---|---|
| No. of Events | Model 1 Unadjusted | Model 2 a | Model 3 b | Model 4 c | Model 5 d | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Overall e | 428 | 1.16 (0.93, 1.44) | 1.31 (1.03, 1.65) | 1.30 (1.02, 1.67) | 1.31 (1.02, 1.69) | 1.27 (0.98, 1.64) |
| Never smokers | 217 | 1.07 (0.77, 1.48) | 1.32 (0.94, 1.87) | 1.28 (0.84, 1.84) | 1.28 (0.89, 1.86) | 1.17 (0.80, 1.72) |
| Former smokers f | 211 | 1.22 (0.91, 1.63) | 1.30 (0.94, 1.80) | 1.33 (0.95, 1.87) | 1.36 (0.96, 1.93) | 1.37 (0.97, 1.95) |
| Ischemic Stroke | Secondhand smoke exposure (>1 hour/week over past year) | |||||
|---|---|---|---|---|---|---|
| No. of Events | Model 1 Unadjusted | Model 2 a | Model 3 b | Model 4 c | Model 5 d | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Overall e | 352 | 1.13 (0.89, 1.44) | 1.29 (1.00, 1.68) | 1.29 (0.98, 1.69) | 1.28 (0.97, 1.69) | 1.21 (0.91, 1.61) |
| Never smokers | 184 | 1.11 (0.78, 1.57) | 1.63 (0.94, 1.98) | 1.32 (0.89, 1.96) | 1.31 (0.87, 1.95) | 1.17 (0.77, 1.78) |
| Former smokersf | 168 | 1.15 (0.82, 1.60) | 1.25 (0.87, 1.79) | 1.27 (0.87, 1.86) | 1.26 (0.85, 1.87) | 1.26 (0.85, 1.87) |
| Hemorrhagic Stroke | Secondhand smoke exposure (>1 hour/week over past year) | |||||
|---|---|---|---|---|---|---|
| No. of Events | Model 1 Unadjusted | Model 2 a | Model 3 b | Model 4 c | Model 5 d | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Overall e | 50 | 1.04 (0.55, 2.00) | 0.84 (0.38, 1.85) | 0.86 (0.39, 1.91) | 0.94 (0.42, 2.09) | 0.95 (0.42, 2.12) |
| Never smokers | 23 | 0.81 (0.28, 2.39) | 0.89 (0.29, 2.72) | 0.94 (0.31, 2.89) | 1.04 (0.34, 3.24) | 1.03 (0.33, 3.22) |
| Former smokers f | 27 | 1.18 (0.52, 2.69) | 0.80 (0.26, 2.45) | 0.77 (0.25, 2.37) | 0.84 (0.27, 2.61) | 0.89 (0.28, 2.76) |
HR, hazard ratio
Model 2: Adjusted for demographics (age, race, sex, age*race), SES factors (education [<high school vs. >high school] and income [<$35,000 vs. >$35,000]).
Model 3: Adjusted for demographics, SES factors, and Framingham Stroke Risk Score factors (diabetes, cardiovascular disease history, atrial fibrillation, current antihypertensive medication use, systolic blood pressure [SBP] and left ventricular hypertrophy).
Model 4: Adjusted for demographics, SES factors, Framingham Stroke Risk Score factors, and C-reactive protein (CRP).
Model 5: Adjusted for demographics, SES factors, Framingham Stroke Risk Score factors, BMI and lifestyle/behavioral factors (physical activity [none vs. >1 time per week], and weekly alcohol consumption [ever vs. never]).
Overall models 3–5 also adjusted for smoking history (former vs. never).
Models 3–5 for former smokers adjusted for pack years smoked.
Next, the relationship between SHS exposure and stroke subtype was assessed. Upon adjustment for demographic and SES covariates, the HR for ischemic stroke was 1.29 (95% CI=1.00, 1.68). The risk of ischemic stroke with SHS exposure remained non-significantly elevated for all nonsmokers, never smokers, and former smokers for the remaining models. There was no association between SHS exposure and hemorrhagic stroke.
Additional models were performed including SHS exposure as a continuous variable. Trends for any stroke were similar to those observed when dichotomizing SHS exposure, although an association was observed for hemorrhagic stroke only when including SHS exposure continuously (Appendix Table 2).
Discussion
The main findings of this study were that the risk of stroke was 30% higher among those reporting SHS exposure than those not reporting such exposure, and appeared to be driven by ischemic strokes. This finding existed independent of demographics, SES factors, smoking history, Framingham Stroke Risk factors, and CRP concentration, and although there was no confounding by lifestyle factors, statistical significance was lost after adjustment for these.
These findings are consistent with prior studies, meta-analyses, and government reports demonstrating increased stroke risk among nonsmokers exposed to SHS.14–16,18,20,21,40 One meta-analysis observed a non-linear dose–response relationship between SHS and stroke.18 Specifically, the risk of stroke was highest for those exposed to 40 cigarettes per day compared with those without exposure (HR=1.56, 95% CI=1.25, 1.96), although an association was also present for five cigarettes per day.18 A U.S. study of never smokers found an association between 20 hours or more of SHS exposure per week at home and stroke overall (relative risk [RR]=1.42, 95% CI=1.08, 1.88) and among women.14 An increased risk of stroke was observed for never smokers (HR=1.42, 95% CI=1.05, 1.93), former smokers (HR=1.72, 95% CI=1.33, 2.22), and men and (cigarette-smoking) women with spouses who smoke.12,40 Therefore, several studies have reported associations between SHS exposure and stroke.
However, not all studies have replicated the association between SHS exposure and stroke.14,21–23 A 2004 British study comparing high (2.8–14 ng/mL) versus low (<0.7 ng/mL) serum cotinine levels among male nonsmokers observed a non-significantly decreased risk of stroke after covariate adjustment (HR=0.77, 95% CI=0.42, 1.41) and a non-significantly increased risk for never smokers (HR=2.16, 95% CI=0.80, 5.80).23 SHS exposure, assessed by cotinine levels (0.71–15 ng/mL vs ≤0.05 ng/mL cotinine levels), was not associated with stroke among nonsmokers or when excluding former smokers in another British study.41 Considering subtypes, a meta-analysis found no association for SHS exposure and hemorrhagic stroke (RR=0.97, 95% CI=0.68, 1.37).21 SHS exposure was also not associated with hemorrhagic stroke among nonsmokers (OR=0.9, 95% CI=0.6, 1.5) or past smokers (OR=1.2, 95% CI=0.8, 2.0) in a 2004 Australian study.22 No associations were observed between SHS and ischemic stroke for exposure to 1–19 hours per week at home (RR=0.95, 95% CI=0.73, 1.22), among men exposed to 20 hours or more per week at home, or for exposure outside of the home.14
To summarize, previous studies suffer from limitations in that few were prospective, adjustment for potential confounders has varied, stroke and SHS exposure have not been consistently defined, measurement and sources of SHS exposure have differed, stroke subtypes have not always been assessed, and some studies have been underpowered by inadequate sample size. Four prospective studies reported an overall direct association12,14,23,40 and three reported a null association,14,41 although one only included men.23
Exposure to SHS is causally associated with coronary heart disease morbidity and mortality among nonsmokers,42 and the CDC states that the evidence for stroke is only suggestive of a relationship.43 SHS exposure has been linked to subclinical vascular disease, as determined by coronary flow velocity reserve, coronary artery dilation, systemic atherosclerosis, aortic pressure diameter relation, and damage to the brachial artery.28,29,44–48
An association was found between SHS exposure and stroke following adjustment for covariates. This negative confounding effect may indicate the potential for confounding of SHS exposure with stroke risk factors. When comparing the factors individually in Model 2, an increased risk of stroke was found after adjustment for age (HR=1.44, 95% CI=1.16, 1.80). Exercise and alcohol were included separately in Model 5 and the risk of stroke was increased only following adjustment for exercise (HR=1.30, 95% CI=1.01, 1.68).
Strengths of the current study include the population-based sample of a large, prospectively followed, well-characterized cohort that includes a large proportion of African Americans and physician-adjudicated incident strokes. Furthermore, the 23% prevalence of SHS exposure found by our study is similar to that of U.S. nonsmokers in 2011–2012 (25.3%).49
Limitations
Limitations must also be considered, which include lack of cotinine measures to validate SHS exposure and use of self-reports to classify prevalent stroke and TIA. Statistical power to examine the risk of SHS exposure by stroke subtype may have been limited. Although no association was observed between SHS exposure and hemorrhagic stroke, the proportional hazards assumption was not met for every stratum, which may have increased the probability of type II error. The null finding may be due to the small number of hemorrhagic strokes in our cohort. Lifetime SHS exposure was also unavailable.
Conclusions
Exposure to SHS is associated with increased risk of stroke. Our findings suggest the possibility for adverse health outcomes such as stroke among nonsmokers exposed to SHS, and add to the body of evidence supporting stricter smoking regulations. Future research will need to investigate the role of cardiovascular disease risk factors in the association and explore potential exposure to additional environmental variables, such as ambient air pollutants, in relation to stroke.
Supplementary Material
Acknowledgments
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke (NINDS), NIH, and DHHS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or NIH. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at www.regardsstudy.org.
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. http://dx.doi.org/10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. http://dx.doi.org/10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4):1–117. [PubMed] [Google Scholar]
- 4.U. S. Burden of Disease Collaborators. The state of U.S. health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. http://dx.doi.org/10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleindorfer D, Broderick J, Khoury J, et al. The unchanging incidence and case-fatality of stroke in the 1990s: a population-based study. Stroke. 2006;37(10):2473–2478. doi: 10.1161/01.STR.0000242766.65550.92. http://dx.doi.org/10.1161/01.STR.0000242766.65550.92. [DOI] [PubMed] [Google Scholar]
- 6.Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. 2010;8(7):917–932. doi: 10.1586/erc.10.56. http://dx.doi.org/10.1586/erc.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whisnant JP. Modeling of risk factors for ischemic stroke. The Willis Lecture. Stroke. 1997;28(9):1840–1844. doi: 10.1161/01.str.28.9.1840. http://dx.doi.org/10.1161/01.STR.28.9.1840. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.str.22.8.983. http://dx.doi.org/10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290(8):1049–1056. doi: 10.1001/jama.290.8.1049. http://dx.doi.org/10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 10.Lee IM, Hennekens CH, Berger K, Buring JE, Manson JE. Exercise and risk of stroke in male physicians. Stroke. 1999;30(1):1–6. doi: 10.1161/01.str.30.1.1. http://dx.doi.org/10.1161/01.STR.30.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Sacco RL, Gan R, Boden-Albala B, et al. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke. 1998;29(2):380–387. doi: 10.1161/01.str.29.2.380. http://dx.doi.org/10.1161/01.STR.29.2.380. [DOI] [PubMed] [Google Scholar]
- 12.Glymour MM, Defries TB, Kawachi I, Avendano M. Spousal smoking and incidence of first stroke: the Health and Retirement Study. Am J Prev Med. 2008;35(3):245–248. doi: 10.1016/j.amepre.2008.05.024. http://dx.doi.org/10.1016/j.amepre.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Shu XO, Yang G, et al. Association of passive smoking by husbands with prevalence of stroke among Chinese women nonsmokers. Am J Epidemiol. 2005;161(3):213–218. doi: 10.1093/aje/kwi028. http://dx.doi.org/10.1093/aje/kwi028. [DOI] [PubMed] [Google Scholar]
- 14.Iribarren C, Darbinian J, Klatsky AL, Friedman GD. Cohort study of exposure to environmental tobacco smoke and risk of first ischemic stroke and transient ischemic attack. Neuroepidemiology. 2004;23(1–2):38–44. doi: 10.1159/000073973. http://dx.doi.org/10.1159/000073973. [DOI] [PubMed] [Google Scholar]
- 15.Bonita R, Duncan J, Truelsen T, Jackson RT, Beaglehole R. Passive smoking as well as active smoking increases the risk of acute stroke. Tob Control. 1999;8(2):156–160. doi: 10.1136/tc.8.2.156. http://dx.doi.org/10.1136/tc.8.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You RX, Thrift AG, McNeil JJ, Davis SM, Donnan GA. Ischemic stroke risk and passive exposure to spouses’ cigarette smoking. Melbourne Stroke Risk Factor Study (MERFS) Group. Am J Public Health. 1999;89(4):572–575. doi: 10.2105/ajph.89.4.572. http://dx.doi.org/10.2105/AJPH.89.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Y, Lam TH, Jiang B, et al. Passive smoking and risk of peripheral arterial disease and ischemic stroke in Chinese women who never smoked. Circulation. 2008;118(15):1535–1540. doi: 10.1161/CIRCULATIONAHA.108.784801. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.784801. [DOI] [PubMed] [Google Scholar]
- 18.Oono IP, Mackay DF, Pell JP. Meta-analysis of the association between secondhand smoke exposure and stroke. J Public Health (Oxf) 2011;33(4):496–502. doi: 10.1093/pubmed/fdr025. http://dx.doi.org/10.1093/pubmed/fdr025. [DOI] [PubMed] [Google Scholar]
- 19.He Y, Jiang B, Li LS, et al. Secondhand smoke exposure predicted COPD and other tobacco-related mortality in a 17-year cohort study in China. Chest. 2012;142(4):909–918. doi: 10.1378/chest.11-2884. http://dx.doi.org/10.1378/chest.11-2884. [DOI] [PubMed] [Google Scholar]
- 20.Wilson N, Thomson G. Still dying from second-hand smoke at work: a brief review of the evidence for smoke-free workplaces in New Zealand. N Z Med J. 2002;115(1165):U240. [PubMed] [Google Scholar]
- 21.Lee PN, Forey BA. Environmental tobacco smoke exposure and risk of stroke in nonsmokers: a review with meta-analysis. J Stroke Cerebrovasc Dis. 2006;15(5):190–201. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.002. http://dx.doi.org/10.1016/j.jstrokecerebrovasdis.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Anderson CS, Feigin V, Bennett D, et al. Active and passive smoking and the risk of subarachnoid hemorrhage: an international population-based case-control study. Stroke. 2004;35(3):633–637. doi: 10.1161/01.STR.0000115751.45473.48. http://dx.doi.org/10.1161/01.STR.0000115751.45473.48. [DOI] [PubMed] [Google Scholar]
- 23.Whincup PH, Gilg JA, Emberson JR, et al. Passive smoking and risk of coronary heart disease and stroke: prospective study with cotinine measurement. BMJ. 2004;329(7459):200–205. doi: 10.1136/bmj.38146.427188.55. http://dx.doi.org/10.1136/bmj.38146.427188.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agaku IT, King BA, Dube SR CDC. Current cigarette smoking among adults - United States, 2005–2012. MMWR Morb Mortal Wkly Rep. 2014;63(2):29–34. [PMC free article] [PubMed] [Google Scholar]
- 25.Gan WQ, Mannino DM, Jemal A. Socioeconomic disparities in secondhand smoke exposure among US never-smoking adults: the National Health and Nutrition Examination Survey 1988–2010. Tob Control. 2014 doi: 10.1136/tobaccocontrol-2014-051660. http://dx.doi.org/10.1136/tobaccocontrol-2014-051660. [DOI] [PubMed]
- 26.Howard G, Thun MJ. Why is environmental tobacco smoke more strongly associated with coronary heart disease than expected? A review of potential biases and experimental data. Environ Health Perspect. 1999;107(Suppl 6):853–858. doi: 10.1289/ehp.99107s6853. http://dx.doi.org/10.1289/ehp.99107s6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103. http://dx.doi.org/10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 28.Howard G, Wagenknecht LE, Burke GL, et al. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA. 1998;279(2):119–124. doi: 10.1001/jama.279.2.119. http://dx.doi.org/10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- 29.Diez-Roux AV, Nieto FJ, Comstock GW, Howard G, Szklo M. The relationship of active and passive smoking to carotid atherosclerosis 12–14 years later. Prev Med. 1995;24(1):48–55. doi: 10.1006/pmed.1995.1007. http://dx.doi.org/10.1006/pmed.1995.1007. [DOI] [PubMed] [Google Scholar]
- 30.Penn A, Snyder CA. 1,3 Butadiene, a vapor phase component of environmental tobacco smoke, accelerates arteriosclerotic plaque development. Circulation. 1996;93(3):552–557. doi: 10.1161/01.cir.93.3.552. http://dx.doi.org/10.1161/01.CIR.93.3.552. [DOI] [PubMed] [Google Scholar]
- 31.Rogers RL, Meyer JS, Shaw TG, Mortel KF, Hardenberg JP, Zaid RR. Cigarette smoking decreases cerebral blood flow suggesting increased risk for stroke. JAMA. 1983;250(20):2796–2800. http://dx.doi.org/10.1001/jama.1983.03340200030024. [PubMed] [Google Scholar]
- 32.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. http://dx.doi.org/10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 33.McClure LA, Murphy HL, Roseman J, Howard G, Malarcher A. Regional and racial differences in smoking and exposure to secondhand smoke: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Prev Chronic Dis. 2011;8(5):A108. [PMC free article] [PubMed] [Google Scholar]
- 34.Lanska DJ, Kuller LH. The geography of stroke mortality in the United States and the concept of a stroke belt. Stroke. 1995;26(7):1145–1149. doi: 10.1161/01.str.26.7.1145. http://dx.doi.org/10.1161/01.STR.26.7.1145. [DOI] [PubMed] [Google Scholar]
- 35.CDC. Healthy Weight - it’s not a diet, it’s a lifestyle! About BMI for Adults. 2011 www.cdc.gov/healthyweight/assessing/bmi/adult_BMI/index.html.
- 36.Gillett SR, Boyle RH, Zakai NA, McClure LA, Jenny NS, Cushman M. Validating laboratory results in a national observational cohort study without field centers: the Reasons for Geographic and Racial Differences in Stroke cohort. Clin Biochem. 2014;47(16–17):243–246. doi: 10.1016/j.clinbiochem.2014.08.003. http://dx.doi.org/10.1016/j.clinbiochem.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37(2):161–186. doi: 10.1016/0002-8703(49)90562-1. http://dx.doi.org/10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 38.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318. doi: 10.1161/01.str.22.3.312. http://dx.doi.org/10.1161/01.STR.22.3.312. [DOI] [PubMed] [Google Scholar]
- 39.IBM Corp. IBM SPSS Statistics for Windows. 22.0. Armonk, NY: IBM Corp; 2012. [Google Scholar]
- 40.Qureshi AI, Suri MF, Kirmani JF, Divani AA. Cigarette smoking among spouses: another risk factor for stroke in women. Stroke. 2005;36(9):e74–e76. doi: 10.1161/01.STR.0000177475.30281.7f. http://dx.doi.org/10.1161/01.STR.0000177475.30281.7f. [DOI] [PubMed] [Google Scholar]
- 41.Jefferis BJ, Lawlor DA, Ebrahim S, et al. Cotinine-assessed second-hand smoke exposure and risk of cardiovascular disease in older adults. Heart. 2010;96(11):854–859. doi: 10.1136/hrt.2009.191148. http://dx.doi.org/10.1136/hrt.2009.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.U.S. DHHS. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. U.S. DHHS; Washington, D.C: 2006. [PubMed] [Google Scholar]
- 43.CDC; National Center for Chronic Disease Prevention and Health Promotion; Office on Smoking and Health (U.S.) How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: CDC; 2010. [PubMed] [Google Scholar]
- 44.Stefanadis C, Dernellis J, Toutouzas P. Mechanical properties of the aorta determined by the pressure-diameter relation. Pathol Biol (Paris) 1999;47(7):696–704. [PubMed] [Google Scholar]
- 45.Otsuka R, Watanabe H, Hirata K, et al. Acute effects of passive smoking on the coronary circulation in healthy young adults. JAMA. 2001;286(4):436–441. doi: 10.1001/jama.286.4.436. http://dx.doi.org/10.1001/jama.286.4.436. [DOI] [PubMed] [Google Scholar]
- 46.Sumida H, Watanabe H, Kugiyama K, Ohgushi M, Matsumura T, Yasue H. Does passive smoking impair endothelium-dependent coronary artery dilation in women? J Am Coll Cardiol. 1998;31(4):811–815. doi: 10.1016/s0735-1097(98)00010-2. http://dx.doi.org/10.1016/S0735-1097(98)00010-2. [DOI] [PubMed] [Google Scholar]
- 47.Raitakari OT, Adams MR, McCredie RJ, Griffiths KA, Celermajer DS. Arterial endothelial dysfunction related to passive smoking is potentially reversible in healthy young adults. Ann Intern Med. 1999;130(7):578–581. doi: 10.7326/0003-4819-130-7-199904060-00017. http://dx.doi.org/10.7326/0003-4819-130-7-199904060-00017. [DOI] [PubMed] [Google Scholar]
- 48.Lekakis J, Papamichael C, Vemmos C, et al. Effect of acute cigarette smoking on endothelium-dependent brachial artery dilatation in healthy individuals. Am J Cardiol. 1997;79(4):529–531. doi: 10.1016/s0002-9149(96)00805-3. http://dx.doi.org/10.1016/S0002-9149(96)00805-3. [DOI] [PubMed] [Google Scholar]
- 49.Homa DM, Neff LJ, King BA, et al. Vital signs: disparities in nonsmokers’ exposure to secondhand smoke - United States, 1999–2012. MMWR Morb Mortal Wkly Rep. 2015;64(4):103–108. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.