Abstract
One-component nanomedicine (OCN) represents an emerging class of therapeutic nanostructures that contain only one type of chemical substance. This one-component feature allows for fine-tuning and optimization of the drug loading and physicochemical properties of nanomedicine in a precise manner through molecular engineering of the underlying building blocks. Using a precipitation procedure or effective molecular assembly strategies, molecularly crafted therapeutic agents (e.g. polymer-drug conjugates, small molecule prodrugs, or drug amphiphiles) could involuntarily aggregate, or self-assemble into nanoscale objects of well-defined sizes and shapes. Unlike traditional carrier-based nanomedicines that are inherently multicomponent systems, an OCN does not require the use of additional carriers and could itself possess desired physicochemical features for preferential accumulation at target sites. We review here recent progress in the molecular design, conjugation methods, and fabrication strategies of OCN, and analyze the opportunities that this emerging platform could open for the new and improved treatment of devastating diseases such as cancer.
Graphical abstract
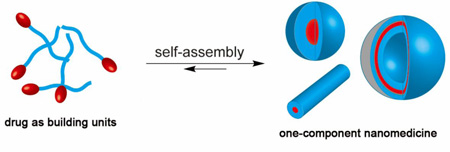
One-component nanomedicine (OCN) represents an emerging class of therapeutic nanostructures that contain only one type of chemical substance.
1. Introduction
Using discrete nanostructures to deliver pharmaceutically active compounds offers possibilities for both improved treatment efficacy and reduced side effects. In this approach, water insoluble/sensitive drugs, when loaded within a nanocarrier, could be made to have increased in vivo solubility/stability, prolonged circulation time, and enhanced accumulation at disease sites. With the protection of the carrier, the loaded drugs are not expected to interface with the biological environments before their release to the surroundings of their molecular targets. It is the physicochemical properties of the carrier, rather than the molecular characteristics of the drug to be delivered, that determine the pharmacokinetic profiles and biodistribution of the resulting nanomedicine [1]. Therefore, manipulating the size, shape, and surface chemistry of the carriers presents a logical way to gain control over the nanomedicine’s circulation and targeting fates, and thus to increase the drug’s therapeutic index [2]. Over the past three decades, many carrier-based drug delivery platforms have been developed, including liposomes [3–5], polymeric nanoparticles [6–10], dendrimers [11–14], inorganic nanoparticles [15–18], protein analogous micelles [19–22], nanodiamonds [23–27], albumin-bound nanoparticles [28–30], and molecularly targeted nanoparticles [31–35]. These carrier-based nanomedicines are inherently multicomponent systems that contain well-defined nanostructures as the delivery vehicle, one or more active pharmaceutical ingredients (APIs) as the therapeutic agent, and sometimes stealth and/or bioactive moieties to prolong circulation and to facilitate preferential accumulation at target sites. In most cases, each component is developed individually, and then combined to form a nanomedicine through a series of formulation procedures and conjugation methods. Although many of these nano-formulated medicines have shown much improved in vivo efficacy relative to that of the free drugs in animal models, further optimization of these nanomedicines to achieve the desired pharmacokinetic profile has proven challenging due to the interdependence of each individual component that often causes unpredictable and inconsistent formulation outcomes. In sharp contrast to the development of a great diversity of nanostructure platform technologies, only a select few have shown superior advantages over the drug formulations currently being used in clinic so as to receive approval by the Food and Drug Administration (FDA) [36–40]. This difficulty in improving and optimizing nanomedicine formulations is regarded as one of the major hurdles for the development of clinically useful nanomedicines for more effective cancer treatments. One possible solution could be to blur the line between the carrier and the drug by optimizing the nanomedicine construct as one integral component [37].
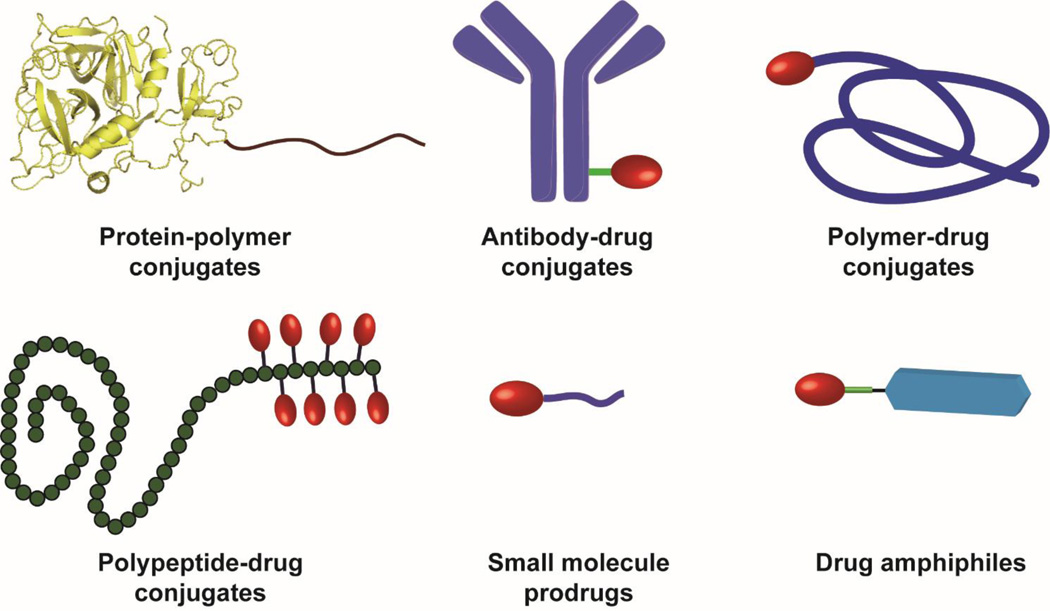
A rapidly growing interest in the nanomedicine community over the past five years has been the use of drug molecules to directly create well-defined nanostructures [41–49]. These drug-made nanostructures are essentially one-component nanomedicines (OCNs) because they contain only one type of chemical substance. Therefore, through molecular design of the building units, one could potentially gain control over the structural features and physicochemical properties of the resulting nanomedicine. Fig. 1 lists six different types of potential molecular building units for the construction of OCN, including protein-polymer conjugates [50], antibody-drug conjugates [51], polymer-drug conjugates [44, 52], polypeptide-drug conjugates [42], small molecule prodrugs [43, 53], and drug amphiphiles [41, 45], all of which involve chemical conjugation of an auxiliary segment to the API. The purpose of incorporating the additional segment is in some cases to improve water solubility and stability, to help evade immune surveillance, to enhance their accumulation at the disease site, and in other cases to direct the conjugates to assemble into nanoscale objects. Given that protein drugs and antibodies themselves are well-defined nanoscale objects, their assemblies into higher level structures are often not pursued, although there has been some interesting work on the self-assembling protein polymers [54, 55]. The radius of gyration for water-soluble polymer-drug conjugates also falls into the range of nanoscale [56], however, they are not shape-persistent nanostructures due to frequent changes in chain conformations in response to surroundings and flow conditions [57, 58]. In this review, we focus our discussion primarily on OCN formed by aggregation or assembly of rationally designed polymer-drug conjugates, polypeptide-drug conjugates, amphiphilic prodrugs, and drug amphiphiles. In particular, we will emphasize aspects central to the rational design, conjugation chemistry and fabrication strategies for developing OCNs, and analyze the unique opportunities that this emerging area could bring into the field of targeted drug delivery.
Fig. 1.
Schematic illustration of six different types of molecular building units that can be potentially used to create one-component nanomedicines (OCNs). Protein-polymer conjugates and antibody-drug conjugates can be regarded as OCN alone due to their well-defined three dimensional nanostructures. Polymer-drug conjugates and polypeptide-drug conjugates consisting of hydrophobic drugs and hydrophilic polymer have the potential to self-assemble or aggregate into nanostructures under appropriate conditions. Small molecule prodrugs can be made into nanoscale objects via either the nanoprecipitation procedure or the assembly approach. Drug amphiphiles are rationally designed self-assembling drug-peptide conjugates that can spontaneously associate into discrete, stable, well-defined nanostructures with a high and quantitative drug loading in aqueous solutions.
In general, OCNs offer two unique/advantageous properties in comparison to traditional carrier-based nanomedicine: 1) OCN allows for a precise control over the drug loading at the individual particle level because the nanostructures formed have exactly the same drug content as that of the individual conjugate. This feature eliminates completely the drug loading variation among different particles. High drug loading capacity can also be realized (up to 100% if the nanostructure is made of free drugs [59]); 2) The physicochemical and structural features of the OCN construct can be tuned by simply optimizing the molecular design of the building units. OCN also makes it possible to manipulate the drug packing order within the nanostructures, affording an additional means to tune drug release rate, because the degree of packing order is expected to correlate with drug release rate. Essentially for OCN, the drug loading, circulation fates, and drug release are all encoded within the chemical structure of the building unit. With the advancement of modern synthetic chemistry and purification techniques, these building units can be made with high precision in terms of molecular weight and chain architectures, particularly for small molecule drug conjugates that can be purified using HPLC to contain monodisperse compounds of the same mass. Therefore, OCN design has a great potential to standardize the mass production of nanomedicine, and their reproducibility would accelerate direct knowledge transfer across research laboratories, thus enabling a rapid knowledge accumulation within the community.
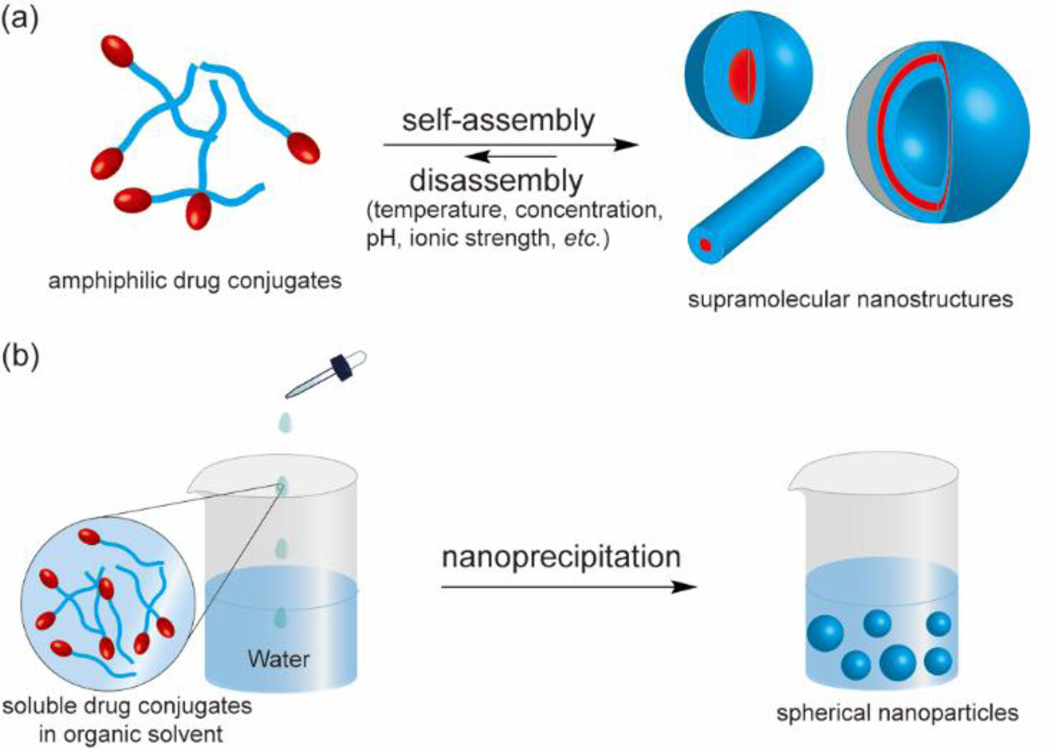
Molecular assembly [60–63] and nanoprecipitation [64–67] are two most common approaches to convert molecular units into nanoscale objects (Fig. 2). The molecular assembly strategy takes advantage of the amphiphilic feature of the building unit for self-aggregation in aqueous solution. If designed correctly, amphiphilic drug conjugates could spontaneously assemble above the critical micelle (assembly) concentration (CMC/CAC) into nano-sized architectures assuming a wide variety of sizes and shapes. The eventual structural features are determined by both the molecular design and the assembly conditions (e.g. pH, temperature, concentration, ionic strength, the presence of other molecules, and kinetic pathways). In contrast, the molecular characteristics of the building units play little role in the size and shape of nanostructures prepared using the nanoprecipitation approach, which, to a large extent, are determined by the preparation procedures, such as conjugate concentration, solvent choices, titration or mixing rate, and agitation. In this method, the molecular building unit is first dissolved in an organic solvent, and the homogenous solution is then mixed with water to force the originally soluble molecules to aggregate into spherical objects with part of the hydrophilic segment displayed on the surface. The organic solvent will be later removed to form a dispersion of spherical nanoparticles in aqueous solution. An important feature to distinguish self-assembled nanostructures from those prepared by nanoprecipitation is that the former is often in a dynamic equilibrium with the unassembled molecules in solution and possesses a high degree of internal order and well-characterized surface chemisty, while the latter comprises disordered, kinetically trapped chains that are forced together.
Fig. 2.
Schematic illustration of using the assembly strategy (a) and the nanoprecipitation method (b) to construct OCN. In the assembly approach, amphiphilic drug conjugates spontaneously associate into nano-sized structures of various sizes and shapes in aqueous solution (a), whereas in the nanoprecipitation method therapeutic conjugates are forced to aggregate into spherical nanoparticles upon mixing of water with an organic solvent containing the conjugate (b).
2. Molecular building blocks for One-component nanomedicine
2.1 Protein conjugates
Many protein-based drugs have been used in the clinic and currently enjoy unprecedented recognition of their therapeutic potential [68, 69]. Therapeutic proteins permit an individualized treatment approach that supports a specifically targeted therapeutic process. As a result of their three-dimensional conformation, proteins themselves are well-defined nanostructures with both functions of targeting and self-delivery. However, protein drugs commonly suffer from their pharmacokinetic and pharmacological drawbacks such as short circulating half-lives, potential immunogenicity, instability against proteolytic degradation, and sometimes low water solubility [70]. Conjugation of the protein with a biologically inert polymer is a clinically well-proven method to improve its chemical stability while retaining the biological function [71]. Since Abuchowski and coworkers reported the success of covalent attachment of polyethylene glycol (PEG) to bovine serum albumin (a process known as PEGylation), tremendous efforts have been devoted into the study of therapeutic protein-polymer conjugates [50, 72]. PEG-protein conjugates have gained particular attention because of PEG’s ability to protect against protein enzymatic degradation and also to bypass the surveillance of the reticuloendothelial system (RES) [73]. This successful strategy has led to a number of FDA approved protein conjugates, including PEG-adensoine deaminase (Adagen®), PEG-asparaginase (Oncaspar®), PEG-interferon α-2a (Pegasys®), PEG-granulocyte colony-stimulating factor (Neulasta®), and also many in clinical trials [69, 74–76].
Antibody-drug conjugates (ADCs) are an emerging class of anticancer therapeutics that combine the specificity of monoclonal antibodies and the efficacy of small-molecule therapeutics [51, 77, 78]. The development of monoclonal antibodies specific to tumor antigens makes it possible to enhance the selectivity of anti-cancer drugs via targeted delivery. Since the inception of first-generation ADCs with doxorubicin, methotrexate and cisplatin, it has taken decades for researchers to find the optimized combination of antibody targeting agent, linker, and cytotoxic drug that could ferry a therapeutic level of ADC to the tumor site. The FDA approval of brentuximab vedotin (Adcetris®) in 2011 and ado-trastuzumab emtansine (Kadcyla®) in 2013 has inspired many researchers to this area, leading to an accelerated clinical testing of ADCs [79, 80].
There have also been some interest in designing protein conjugates that can self-assemble or aggregate into higher level nanostructures [81–90]. Nolte and coworkers synthesized a giant amphiphile using the enzyme lipase B from Candida antarctica as the headgroup and a maleimide-functionalized polystyrene of 40 repeat units as the hydrophobic tail [91]. These bioconjugates were found to assemble into micrometer long rods, or bundles of rods, in aqueous solution. Recently, this strategy has been utilized by several other research laboratories to create a variety of self-assembled morphologies [86, 92–94]. For example, Velonia and co-workers reported the synthesis of a bionanoreactor of BSA-polystyrene conjugates via protein-initiated atom transfer radical polymerization (ATRP) [95]. In their work, a maleimide-capped ATRP initiator was first coupled to BSA, which was further used as a macroinitiator to polymerize styrene monomers using copper-mediated ATRP. The resultant amphiphilic protein conjugates can assemble into spherical aggregates ranging from 20 nm to 100 nm. Very recently, MacKay and co-workers reported the self-assembly of a series of protein-polymer conjugates into nano-sized structures using the unique thermal responsive properties of elastin-like polypeptides (ELPs) [54, 55, 96, 97]. In their typical design, the ELP gene was ligated to the encoding sequence of the selected protein, and the fusion protein constructs were expressed in BLR E. coli and purified using ELP-mediated phase separation. Above the critical temperature, the expressed protein polymers can aggregate into spherical, worm-like, or vesicular nanostructures in aqueous solution. In one example, they demonstrated that fusion of an anti-CD20 single chain protein to a soluble ELP G(VPGAG)192Y could assemble into worm-like nanostructures, which could significantly slow down the tumor growth rate compared with a chimeric protein Rituximab [96].
2.2 Polymer-drug conjugates
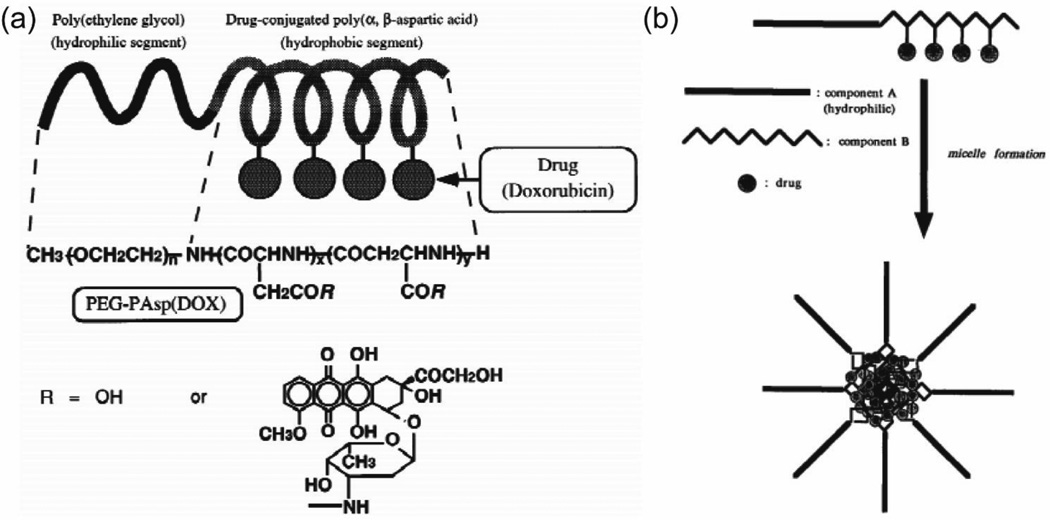
A popular strategy throughout the history of developing nanomedicine is the rational design of polymer-drug conjugates [69, 73, 98–101]. Pioneering work on the conjugation of hydrophobic drugs to a hydrophilic polymer was initially intended to take advantage of the relatively larger size of the polymer to avoid quick renal clearance and to improve the drug’s water solubility, thus leading to enhanced tumor accumulation [102]. Kataoka group reported a micelle-forming block copolymer-drug conjugates system with doxorubicin and poly(ethylene glycol)-poly(α,β-aspartic acid) (PEG-b-PAsp) (Fig. 3) [44, 52, 103–106]. PEG-b-PAsp was first synthesized as a macromolecular precursor for further conjugation with DOX, which was covalently introduced into the side chain of the PAsp segment with an approximately 50% occupancy rate, through a condensation reaction between the carboxylic groups of the PAsp and the primary-amino-group of DOX. Hydrophobicity and π-π interactions among DOX molecules act together to stabilize the nanostructures in aqueous solution. Since the hydrophilic polymers are well displayed on the micellar surface that well segregate the core from the external environment, they serve the roles of preventing premature chemical degradation of the loaded drug and also of reducing the possibility for RES uptake during the blood circulation. This shielding also allows for regulating the molecular affinity interaction between nanostructures and cell membrane, resulting in adjustable intracellular drug release [107]. It was also found that these micelles can be further stabilized via physical encapsulation of free DOX into the core. These DOX micelles demonstrated prolonged circulation time in the blood due to reduced uptake by the RES, and their accumulation in the solid tumor was proposed through the enhanced permeability and retention (EPR) effect [108, 109]. In another example, Allen and co-workers conjugated docetaxel (DTX) to the hydrophobic side of poly(ethylene glycol)-b-poly(ε -caprolactone) (PEG-b-PCL) copolymers [110]. The micelles formed by the DTX-conjugated PEG-b-PCL copolymer can also be used to encapsulate more DTX in the core. The addition of the DTX was found to lower the CMC in aqueous solution thus improving the stability of nanostructures. With the enhanced interactions between DTX and the micellar core, the release of physically encapsulated DTX from PEG-b-PCL-DTX micelles showed a slower rate than the release from PEG-b-PCL micelles. These findings suggest that the self-assembled polymer drug conjugates show some advantageous properties over the unassembled ones.
Fig. 3.
Illustration of Kataoka’s molecular design of doxorubicin-conjugated poly(ethylene glycol)–poly(α,β-aspartic acid) block copolymer (a) and the formation of micelles by self-assembly of the drug conjugate in water (b). (adapted from ref. [44, 104])
To avoid premature disassembly of supramolecular nanostructures upon plasma dilution, chemical crosslinking of the molecular building units that are held together through non-covalent interactions appears to be an effective strategy to improve their stability at low concentrations. Wooley and co-workers pioneered the concept of shell crosslinked knedel-like (SCK) nanoparticles to “fix” the self-assembled nanostructures and exploited these chemically stabilized carriers to deliver a variety of therapeutic agents [111–114]. Zhang et al. synthesized poly(ethylene oxide)-b-polyphosphoester-based paclitaxel drug conjugates (PEO-b-PPE-g-PTX) in a two-step manner using organocatalyst-promoted ring-opening-polymerization followed by click reaction-based conjugation of a PTX prodrug. The PEO-b-PPE-g-PTX formed well-defined core–shell nanoparticles in aqueous solution, containing ultra-high levels of drug loading [115]. In the development of second generation core-shell nanoparticles, Wooley Lab incorporated acid-labile β-thiopropionate linkages at the grafted side chain to afford a pH-sensitive release for PTX [116]. Compared to the first generation version without the β-thiopropionate linker, the PEO-b-PPE-g-PTX G2 revealed faster hydrolytic release kinetics for free PTX at the acidic pH, showing enhanced in vitro cytotoxicity and reduced IC50 values in two tested cancer cell lines.
Since the size and shape of supramolecular nanostructures are sensitive to the chemical composition of both the hydrophilic and hydrophobic segments, tuning either segment could potentially change the morphology of the nanostructure [60, 62, 63, 117–119]. Shen and co-workers reported that varying the number of hydrophobic drug molecules linked to the hydrophilic PEG can produce nanostructures with different size and shape, but with the same surface chemistry [120]. Multiple conjugation sites for camptothecin (CPT) linkage were enabled through the use of dendritic polylysine. When the number of CPTs was increased to four, the morphological transformation of nanostructures was observed to occur from spheres to rods of 60 nm in diameter and 500 nm in length.
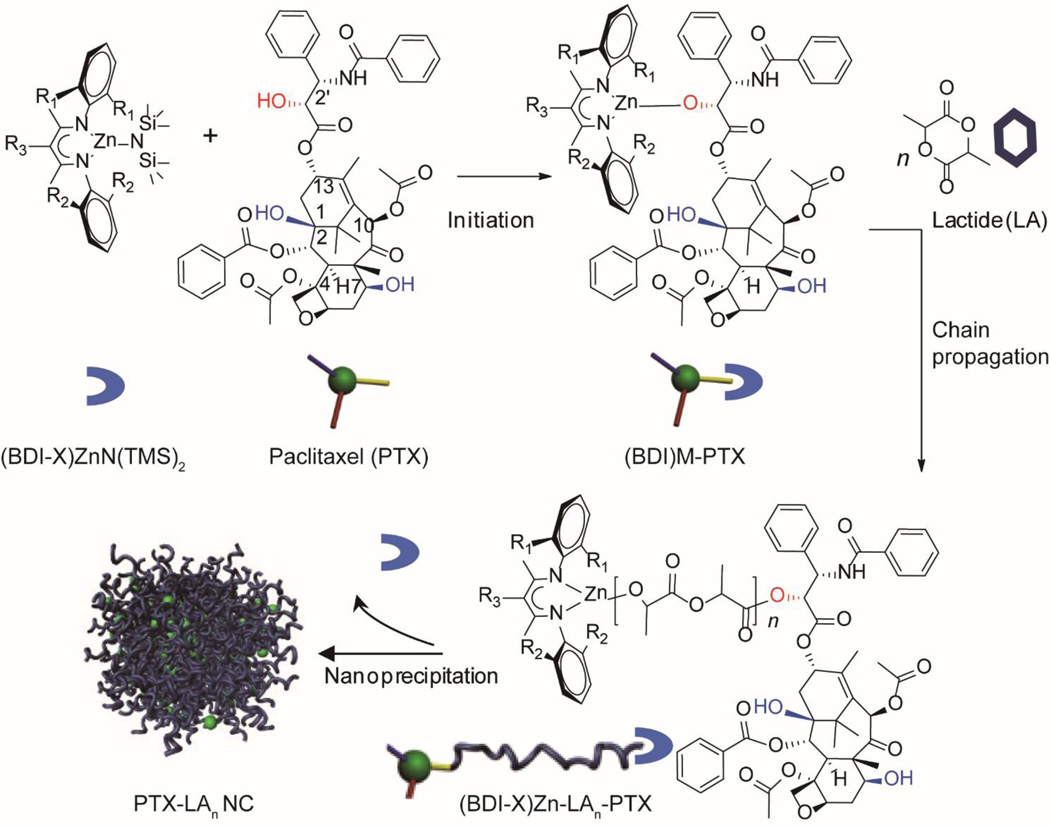
Another emerging strategy to synthesize polymer-drug conjugates is to use hydrophobic drugs as initiators for the polymerization [121–124]. Cheng and co-workers reported the method of drug-initiated polymerization and formulation of nanoconjugates as delivery vehicles [125, 126]. Paclitaxel was first used as a drug initiator to formulate PTX-polylactide (PLA) conjugates via living polymerization (Fig. 4) [127]. Since PTX has three hydroxyl groups at C2´, C1, and C7 positions, it could potentially initiate LA polymerization at three different sites. However, due to the steric hindrance and bulky metal catalyst, polymerization of LA was preferentially initiated at the 2´-OH group of PTX. This strategy was further extended to construct doxorubicin-polylactide (DOX-PLA) conjugates via ring-opening polymerization (ROP) [128]. Doxorubicin also contains multiple types of conjugation groups to initiate the ROPs. By using a particular catalyst, the 14-hydroxyl group can serve as the initiation site for chemoselective incorporation of DOX into PLA without protecting other free amine and hydroxyl groups. In these two cases, the one-pot polymerizations can be completed within hours to generate conjugates with well-controlled molecular weight and low polydispersity, with nearly 100% drug-loading efficiency and 100% LA conversion. These PLA-drug conjugates can be further formulated using the nanoprecipitation method to form uniform particles less than 100 nm and 150 nm in size. And the nanoparticle size can be fine-tuned by adjusting the conjugate concentration in a water-miscible organic solvent or by choosing different solvents such as DMF, THF or acetone. This drug-initiated polymerization approach provides an innovative way to design polymer-drug conjugates with improved control over the drug loading and nanoparticle size.
Fig. 4.
Illustration of Cheng’s PTX-initiated lactic acid (LA) polymerization for regio- and chemo-selective PTX-PLA conjugates using (BDI)ZnN(TMS)2 as the catalyst, and the formation of well-defined drug nanoparticles using the nanoprecipitation method.
2.3 Polypeptide-drug conjugates
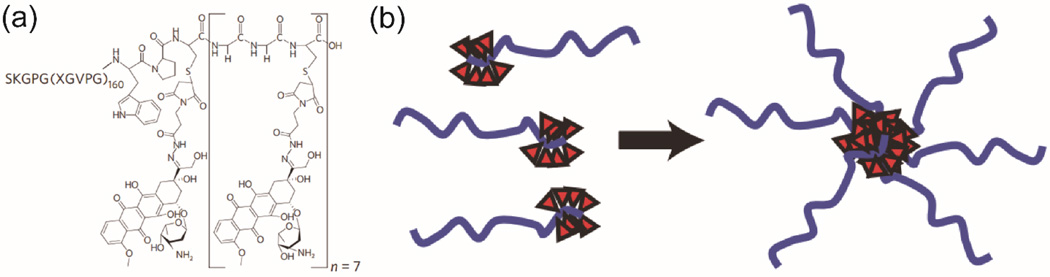
Polypeptide-drug conjugates, although less studied relative to polymer-drug conjugates, represent a very promising drug delivery platform [129–131]. Polypeptidic materials could be designed to possess metabolizable and biodegradable features, bioinspired functionalities, and stimuli-responsive properties. Polypeptides containing amino acids with functionalizable side chains (e.g. lysine, glutamic acids, cysteine, serine) can be used to conjugate with drug molecules to improve the drug’s bioavailability and efficacy [132–134]. In some cases, amphiphilic polypeptide-drug conjugates exhibited the ability to aggregate into nanostructures under physiologically relevant conditions [42, 135]. Chilkoti and co-workers reported the conjugation of multiple hydrophobic doxorubicin molecules to artificial recombinant chimeric polypeptides (CPs) containing an elastin-like segment by covalently linking to a short cysteine-rich segment, and this conjugation led to formation of sub-100-nm-sized, near-monodisperse nanoparticles (Fig. 5) [42]. They further demonstrated a modular platform for self-assembly of CPs by direct linkage of fourteen different reactive maleimide-based hydrophobic molecules [136]. It was found that the drug’s octanol–water distribution coefficient determines the tendency of aggregation, and a critical threshold of hydrophobicity must be met to trigger the self-assembly of resulting conjugates into nanoparticles. As a result of thermal responsive feature of ELPs, the polypeptide-drug conjugates could undergo an inverse phase transition thus enabling a nanoparticle-to-aggregate morphology transformation within a particular temperature range. To take advantage of the thermal responsive property transition between 37°C and 42°C, McDaniel et al. developed an analytical model that predicts Tt in terms of CP sequence, chain length, and solution concentration, and produced thermal responsive DOX-loaded nanoparticles with controlled properties [137]. They reported that, by externally heating the tumor to 42 °C, tumor targeting can be achieved using “heat seeking” DOX-loaded polypeptide nanoparticles. After thermally cycling the tumor, the nanoparticles will experience a reversible micelle-to-aggregate phase separation in tumor vasculature, and accumulate largely at tumor sites. In their more recent work, they demonstrated that the CP-DOX nanoparticles can strongly inhibit metastasis and improve the survival of mice in two syngeneic metastatic cell lines [138]. Their work provides an insightful and inspiring strategy to design conjugation-triggered polypeptide assembly, and the assembled polypeptide-drug nanoparticles can serve as a new class of drug delivery vehicles to carry chemotherapeutics to solid tumors.
Fig. 5.
(a) A representative molecular design of Chilkoti’s polypeptide-drug conjugate, where DOX was conjugated to ELPs via the Cys residues by a heterobifunctional linker. (b) Schematic illustration of conjugation-triggered molecular assembly into drug-containing nanoparticles (adapted from ref. [42]).
2.4 Polymerizable prodrugs
Post-functionalization of hydrophilic polymers or polypeptides with hydrophobic drugs has been widely used as the primary strategy in the synthesis of polymer-drug conjugates [42, 44, 52]. However, this popular strategy may suffer from some potential drawbacks, such as low yield due to multi-step reactions involved, limited control of the conjugation sites, and relatively low drug loading [139–141]. To overcome these limitations, direct polymerization of polymerizable drugs has been pursued. For example, Uhrich and co-workers used the conventional condensation polymerization to create biodegradable polyprodrugs [142–144]. However, only a very limited number of drugs can be directly polymerized, and the polymerized products typically do not carry assembling potential, and may still need delivery vehicles to maximize their efficacy. Another approach is to design and synthesize prodrugs containing polymerizable groups [145–147]. After carefully designed polymerization, the eventual drug-containing polymers could form self-assembled nanostructures. Liu and co-workers reported hierarchical structures assembled from polymerized reduction-cleavable camptothecin (CPT) prodrug [148]. By tuning co-solvent types, compositions, and water addition rates, the polyprodrug amphiphiles can self-assemble into four types of uniform nanostructures including spheres, large compound vesicles, smooth disks, and staggered lamellae with identical chemical compositions. Their in vitro and in vivo results suggested that the staggered lamellae are superior to the other three nanostructures. Very recently, Chilkoti and co-workers used the ring-opening polymerization to homo or hetero-polymerize both CPT and chlorambucil (CL) prodrugs, initiated by a PEG macroinitiator [139]. These amphiphilic diblock copolymers can spontaneously self-assemble into nanoparticles, where drugs are sequestered in the core that is coated with a PEG corona. The drugs can be triggered to release in response to a physiologically relevant stimulus. This work demonstrated the general rules of converting functionalizable drugs to polymerizable prodrugs, clearly showing that the versatility of ROP method for polymerization or copolymerization of structurally diverse drugs. The drug loading in this case can be controlled by adjusting the monomer(s)/initiator feed ratio, and drug release rate can be tuned by the choice of different linkers.
Brush polymers could be made multivalent and polymer itself is within nanoscopic range, which make them attractive for drug delivery applications [149]. Johnson and co-workers reported the synthesis of multi-drug-loaded nanoscopic brush-arm star polymers (BASP) by the “brush-first” ring-opening metathesis polymerization (ROMP), carrying precise molar ratios of three anticancer drugs: doxorubicin, camptothecin, and cisplatin [150]. Norbornene-terminated macromonomeric drug conjugates were introduced as building blocks for the parallel construction of a series of multi-drug-loaded nanoparticles, followed by in situ cross-linking with a bis-norbornene drug containing derivative. They demonstrated orthogonally triggered release of three drugs via hydrolysis, redox and UV irradiation respectively. Along the same line, they synthesized a novel hydrolytically labile DOX-conjugated BASP (DOX-BASP) using acid-cleavable cross-linker that degrades and releases therapeutic drugs efficiently [151]. This kind of PEGylated drug-BASPs feature a unimolecular micelle-like structure with readily tunable core and shell functionality.
2.5 Small molecule prodrugs
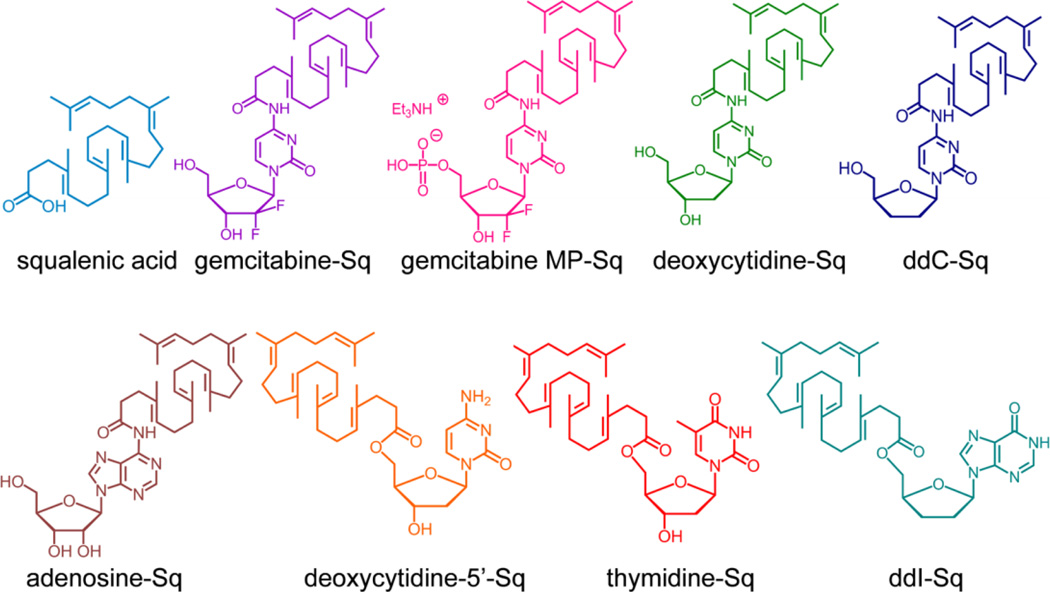
The emergence and development of small molecule prodrugs has been primarily focused on improving the drug’s water solubility, bioavailability, chemical stability, and in some cases brain permeability and targeting, and also on addressing the multiple drug resistance mechanisms [152–154]. These pharmacologically inactive or less active drug derivatives are typically not designed to assemble into nanostructures. Recently, there have been some efforts to use these well-designed small molecule prodrugs as building blocks to create nanoscale objects. In a pioneering study, Couvreur and co-workers reported the concept of “squalenoylation” by utilizing anticancer or antiviral compounds to construct nanoparticles [43]. A series of squalene-based nucleolipids were synthesized by grafting squalenic acid onto the nucleobase or the 5´ position of the sugar moiety (Fig. 6) [155]. Nanoprecipitation of squalene-drug bioconjugates, such as nucleoside analogues, paclitaxel, penicillin and doxorubicin conjugates, yielded nanoparicles of 100 to 300 nm in size with very low polydispersity [53, 156–159]. They discovered that nanoparticles made of gemcitabine-squalene (Gem-Sq) bioconjugates actually enter the cell via a passive diffusion rather than endocytosis [160]. The Gem-Sq nanoparticles could act as a reservoir to control and prolong the release of the bioactive gemcitabine, exhibiting longer t1/2 than that of the free gemcitabine [161]. It was also found that conjugation of gemcitabine to squalene reduces the metabolism of gemcitabine into its inactive difluoro-deoxyuridine metabolite. This “squalenoylation” strategy was further extended to create doxorubicin derivatives, forming “loop-train” structures with an average diameter of 130 nm [53]. Their SQ-DOX nanoparticles displayed comparable in vitro cytotoxicity against cancer cells, but largely improved their in vivo efficacy relative to free doxorubicin.
Fig. 6.
Chemical structures of squalenic acid and squalene-based nucleolipids. Squalenoylation was achieved by directly grafting squalenic acid onto anticancer or antiviral compounds (adapted from ref. [155]).
It is known that lipid-like molecules could potentially self-assemble into vesicular structures allowing for encapsulation of both hydrophilic and hydrophobic drugs. To take advantages of the assembly properties of lipids while increasing the drug loading content, Shen et al. introduced the concept of using hydrophobic drug CPT to replace the fatty acid(s) in phospholipids to form phospholipid analogues [162]. A short nonionic oligo (ethylene glycol) (OEG) chain with eight repeat units was selected as the water soluble segment to maximize the drug loading content and also to lower the CMC for improved stability. Two CPT molecules were linked to one OEG chain via a biodegradable β-thioester bond. They found that the resultant lipid-mimic amphiphiles could be formulated to form stable 100–200nm liposome-like nanocapsules with a CPT loading content as high as 58 wt % in aqueous solution. These nanocapsules can act as carriers to deliver DOX. Along the same lines, Yan and co-workers reported an amphiphilic drug–drug conjugate (ADDC) by directly connecting the hydrophilic anticancer drug irinotecan (Ir) to the hydrophobic anticancer drug chlorambucil (Cb) via a hydrolyzable ester linker [163]. In this system, the amphiphilic Ir–Cb conjugate was found to aggregate in water into nanoparticles with a diameter around 80 nm, as a result of further clustering of the Ir–Cb ADDC micelles. Their experimental results revealed that the Ir–Cb ADDC nanoparticles have much longer blood retention time and higher tumor accumulation in comparison to either free Ir or free Cb. The release of both anticancer drugs was triggered by the hydrolysis of ester bond, leading to a simultaneous release of the two drugs and synergistic anticancer activities both in vitro and in vivo.
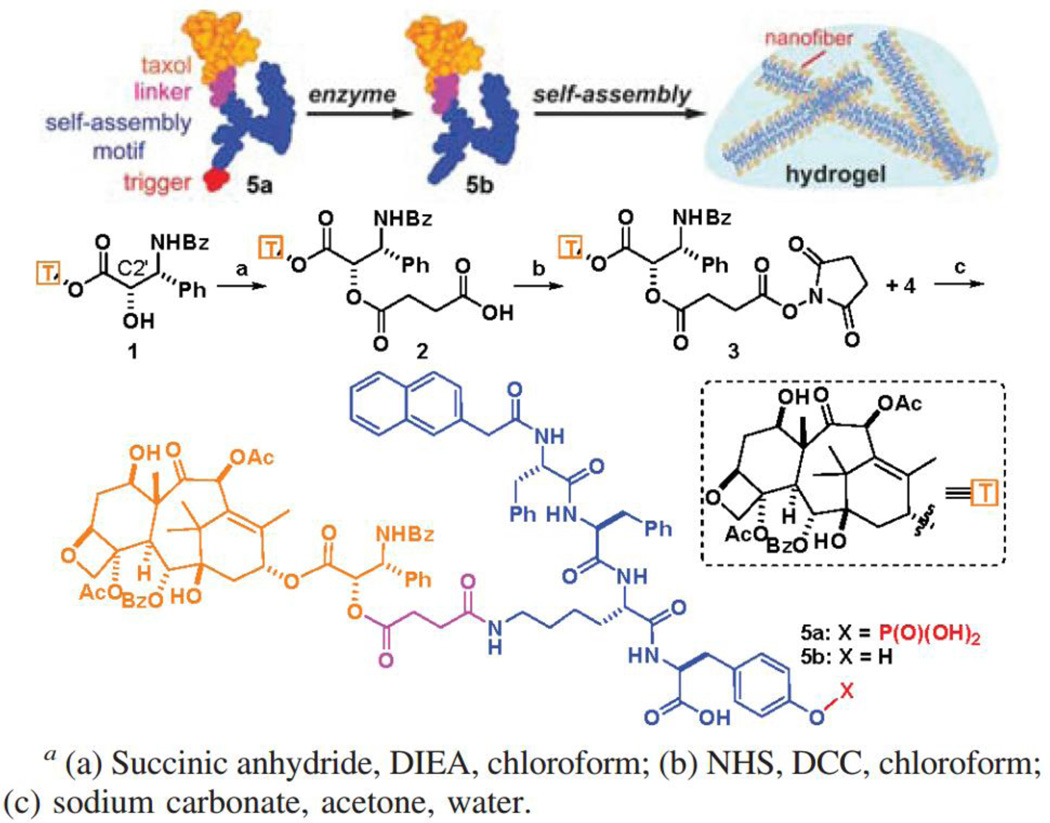
Amphiphilic molecules containing both hydrophilic and hydrophobic segments can associate through non-covalent interactions to form a variety of nano- and microscale structures in aqueous solution [164, 165]. Of particular interest are filamentous assemblies due to their ability to entangle to form supramolecular hydrogels that can be used for the delivery of small molecule drugs, genes, and biologics [166–171]. To achieve precise drug loading content and homogeneous drug distribution within the hydrogel, therapeutic drugs have been directly used to create hydrogels without compromising their efficacies [172–175]. In this supramolecular design, drug release is regulated by both chemical degradation of the linker and the disassembly kinetics of the nanostructures. Xu and co-workers originally converted vancomycin, an essential antibiotics, into a hydrogelator by introducing a pyrene group to the C-terminal [176]. They found that the vancomycin-pyrene hydrogel exhibited unusually high potency against vancomycin-resistant enteroccoci (VRE). Using a similar concept, the Xu Lab further designed PTX-based supramolecular hydrogels that can be triggered to form by specific enzymatic activities [177]. The hydrogel precursor consists of a self-assembling motif, an enzyme-cleavable phosphatase substrate, and a paclitaxel molecule (Fig. 7). In the absence of the phosphatase, the hydrogel precursor (compound 5a) has excellent water stability and stays in the monomeric form, but upon addition of the alkaline phosphatase, nanofibers start to form within 5 min and the solution eventually transforms to a self-supporting hydrogel. Along the same line, olsalazine, an anti-inflammatory prodrug currently used in clinic, was also made into supramolecular hydrogels by conjugation with tripeptide derivatives [178]. These olsalazine-containing hydrogels can be disrupted to release the bioactive drug in a controlled manner. In their recent effort, D-amino acids were used in the design of hydrogels containing naproxen, a nonsteroidal anti-inflammatory drug [179]. It was found that incorporation of D-amino acids led to an unexpected and significant elevation in the selectivity of the conjugate toward COX-2, with only slightly compromised naproxen activity. This strategy of constructing therapeutic supramolecular hydrogelators has been used by several labs to expand the library of drug-based hydrogelators [15, 180, 181]. In particular, Yang and co-workers reported the conjugation of folic acid with hydrophobic therapeutic agents to from stable supramolecular hydrogels [182]. Shi and co-workers reported a supramolecular hydrogel formed by CPT-PEG prodrug self-assembled micelles with the assistance of α–Cyclodextrin (α–CD), and demonstrated that water-soluble 5-Fluorouracil (5-FU) can be loaded into the gel to achieve synergetic cytotoxicity against cancer cells [173].
Fig. 7.
Schematic illustration of Xu’s design of the enzyme-triggered formation of supramolecular hydrogels. This particular precursor consists of a self-assembly promoting motif, an enzyme-cleavable group, and a paclitaxel drug molecule. After the enzymatic reaction, the paclitaxel derivative can spontaneously assemble into nanofibers in water that eventually entangle into a self-supporting hydrogel (adapted from ref. [177]).
2.6 Self-assembling drug amphiphiles
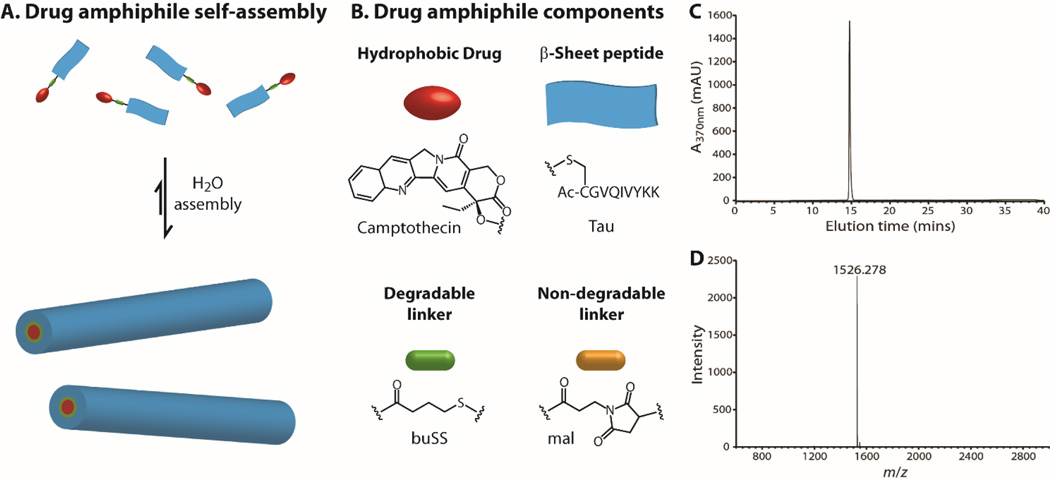
Taking advantages of biological function and assembly potential of peptides, Cui and co-workers developed a novel class of drug amphiphiles (DAs) by covalent linkage of one or more anticancer drugs to a rationally chosen/designed peptide through a biodegradable linker [45, 183–185]. Their core design concept is to consider drugs as molecular building units, not just being functional units. By incorporating a short peptide as both a functionally active and structurally guiding unit, the resulting DAs are amphiphilic prodrugs capable of forming a variety of supramolecular morphologies in aqueous solutions. Fig. 8 illustrates a typical design of camptothecin (CPT) drug amphiphile containing three essential parts: the anticancer drug CPT, a β-sheet-forming peptide sequence derived from the tau protein, and a reducible disulfylbutyrate (buSS) linker. This strategy can be potentially extended to any drugs that possess functionalizable groups such as hydroxyl, amine, thiol, or carboxylic acid. The versatility of the peptide design also allows for facile incorporation of any biologically active sequences such as cell penetrating peptides, tissue penetrating peptides, or tumor targeting peptides. Clearly, the linker could be chosen using hydrolysable, enzymatically degradable, or reducible chemical bonds. Importantly, the small molecule feature makes it possible for HPLC purification to eliminate all the impurities so as to obtain DAs of exactly the same mass. Therefore, the resulting supramolecular nanostructures are essentially one-component nanomedicines with the drug loading precisely defined by chemical structure of the building unit.
Fig. 8.
Schematic illustration of a representative camptothecin drug amphiphile (DA). (A) The self-assembled nanostructures contain the same drug content as that of the individual DA. (B) Illustration of the three key components: the hydrophobic drug CPT, Tau-β-sheet-forming peptide, and the buSS biodegradable linker (or using a non-degradable amine linker). RP-HPLC trace (C) and MALDI-Tof mass spectrum (D) of the mCPT-buSS-Tau DA to reveal high purify and monodispersity in molecular mass.
Cheetham et al. reported the supramolecular assembly of camptothecin DAs into discrete, stable, well-defined nanostructures with a high and quantitative drug loading [45]. The drug content was precisely controlled using the two amine functionalities of the amino acid lysine to create branching points for attaching one, two or four CPT molecules, corresponding to respective high drug loadings of 23%, 31% and 38%. When the number of CPTs was changed from one to four in the DAs, the resulting nanostructures could vary from long filaments to short filaments and then to nanotubes. The short-term stability study showed that dissociation occurred only when the concentrations were diluted to the nano-molar range. Their results revealed that the stability increases with the increasing CPT number, which attributes to the increasing hydrophobicity in combination with possible π–π associative interactions among the CPT units. Their release experiments indicated that the self-assembled nanostructures can serve as reservoirs for long-term supply of monomeric CPT conjugates that can be quickly converted to bioactive CPT in the presence of Glutathione. Both CPT segments and biodegradable linkers are buried in the cores of the assembled nanostructures, and this shielding from the external environment provides an effective means for the controlled release of activated drugs. In the cytotoxicity studies, the DA nanostructures released the bioactive CPT mainly by reductive degradation of the disulfide bond in the presence of intracellular GSH, and showed high potency in different cancer cell lines. Lin et al. expanded this concept to another important anti-cancer drug, paclitaxel [186]. They found that the bulky size of PTX does not prevent the chosen peptide from assembling into one dimensional nanostructures. In vitro study showed that filaments with 41% PTX loading have near identical toxicity to free PTX, indicating that the self-assembling PTX DA does not compromise the drug’s efficacy.
Rational combinations of two or more drugs can potentially generate synergistic effect in reducing side effects associated with high dose of single drugs, and also offer a way to overcome multidrug resistance mechanisms that cancer cells may develop during the course of the treatment. Cheetham et al. reported the synthesis and assembly of a mikto-arm star dual drug amphiphile containing both a bulky PTX and a planar CPT [187]. The two different anti-cancer drugs were conjugated to the terminal lysine of a β-sheet forming peptide (Sup35), and the dual DAs could spontaneously associate into a well-defined filamentous morphology containing a fixed 41% total drug loading. The hetero dual DA was found to effectively release the two anticancer agents, exhibiting superior cytotoxicity against PTX-resistant cervical cancer cells. They believed that delivering two drugs of completely different action mechanisms could increase the possibility of overcoming multi-drug resistance and reduce the possibility for tumor reoccurrence. Similar to synthetic drug amphiphiles, some natural drugs themselves are amphiphilic. Lock et al. reported the self-assembly behavior of methotrexate and folic acid. Their results revealed that folic acid exhibits rich self-assembly behavior via Hoogsteen hydrogen bonding under various solvent conditions, whereas methotrexate is unable to assemble into any well-defined nanostructures under the same conditions, despite their very similar chemical structures [59].
Co-assembly of two or more different amphiphilic molecules is a robust strategy to tune size, shape and surface chemistry of the resulting nanostructures. Mixing of oppositely charged amphiphilic molecules could produce morphologies different from those formed by individual molecules. Lin and co-workers showed that catanionic mixtures of two drug amphiphiles lead to formation of a multiwalled nanotube morphology containing a 36% fixed CPT loading [188]. They found that the molecular mixing ratio, solvent composition, overall drug concentrations, and the molecular design of the DAs are all important experimental parameters contributing to the resulting morphologies. Although systemic delivery of the CPT nanotubes through tail-vein injection did not show any improvement for tumor targeting, direct injection of the fluorescent-labeled nanotubes into the tumor site revealed the significantly enhanced retention time of CPT nanotubes, offering the possibility for sustained release of CPT over a long period of time that would be beneficial for local tumor treatment.
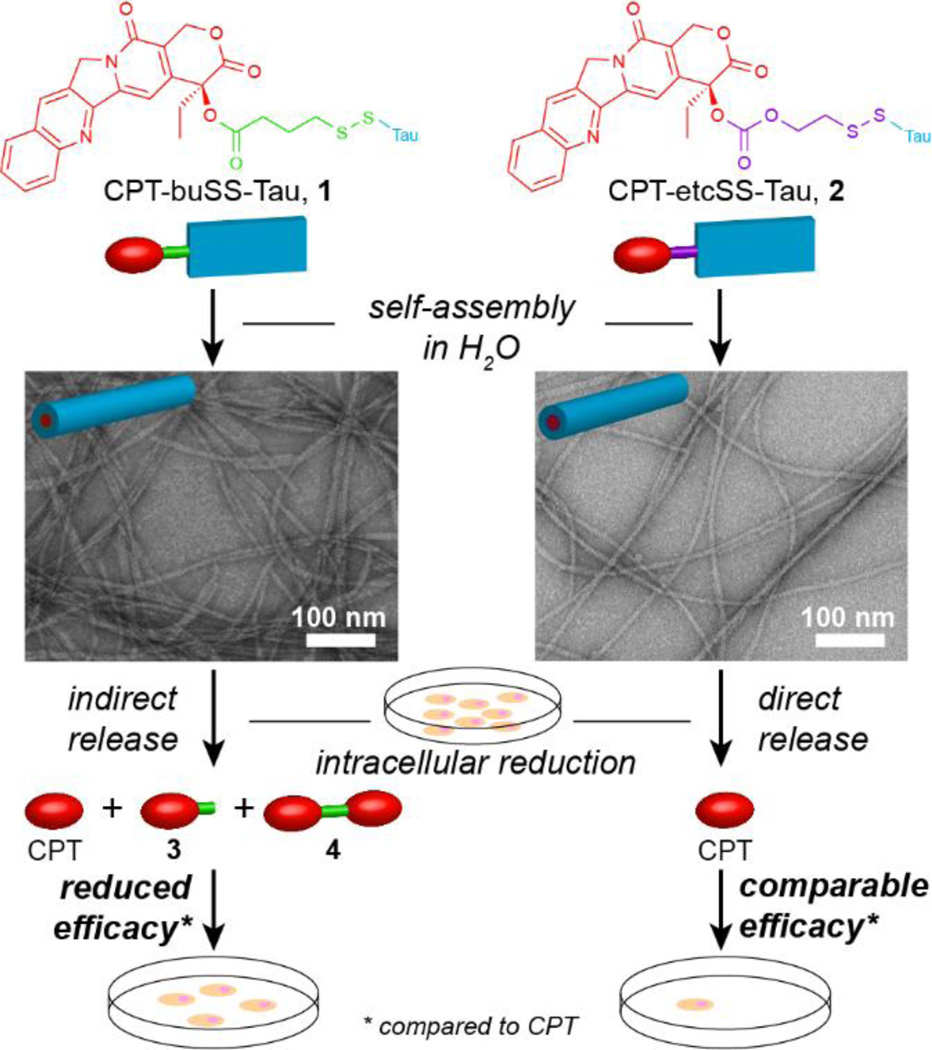
Since the hydrophobic anti-cancer drugs and biodegradable linkers are buried in the cores of nanostructures, the drug release process could be complicated, often involving both the nanostructure dissociation into monomeric units and the chemical breakdown of the linker that bridges the drug with the peptide. Cheetham et al. reported that the choice of the degradable linkers can have a significant impact on the release mechanism of free bioactive CPT from assembled nanostructures (Fig. 9) [189]. They demonstrated that in vitro activity of buSS-linked drug amphiphile is compromised by the nanostructure-promoted formation and protection of CPT-based disulfide dimers due to high local concentration of β-sheet forming aggregates and the hydrophobic nature of CPT. However, these effects can be largely negated by introducing their carbonate-based analogue, which undergoes rapid self-immolation upon reduction and avoids dimerization.
Fig. 9.
Self-assembly of the ester-based CPT-buSS-Tau (1) and carbonate-based CPT-etcSS-Tau (2) drug amphiphiles into filamentous structures and the effect of the linker on the release mechanism of the free drug, CPT, and the subsequent cytotoxicity. Representative TEM images reveal similar morphologies assembled by the two CPT DAs that only differ in the linker design (adapted from ref. [189]).
3. Future prospects
Thirty years of extensive research in nanomedicine has led to significant progress in the development of many nanocarrier-based platform technologies, and accumulated general knowledge in the design of long circulating and better targeted delivery systems. However, the lack of FDA approval of carrier-based nanomedicine underscores the challenges for researchers working in this area. As we reflect on the successes and failures of the past nanomedicine research, it is equally critical for the community to define future directions that promise far more effective cancer chemotherapies. Clearly, many of the well-identified issues in targeted drug delivery should be specifically addressed, including prolonged circulation, tumor accumulation, specific tumor targeting, tumor penetration, intracellular trafficking, drug resistance, and body clearance. Eventually, all the solutions would come to the optimization of the nanosized carriers to tune the key features such as size, shape, surface chemistry, stability, and responsiveness to a particular stimuli. One critical yet historically overlooked area is the optimization of nanomedicine constructs as an integral unit, not just the carrier itself. The multicomponent nature of traditional carrier-based nanomedicines results in inherent difficulties for further improvement due to the interdependence of each unit. The emergence and development of one-component nanomedicines may not provide the final answer, but present a promising alternative approach, because the drug loading, circulation fates, and drug release within the OCN design are all encoded within the chemical structure of the building unit. Through rational design of the therapeutic agents, OCN can be developed with precise drug loading and predictable physicochemical properties. As stated earlier, OCN offers a great potential to standardize the mass production of nanomedicine, and their reproducibility would accelerate direct knowledge transfer across research laboratories, thus enabling a rapid knowledge accumulation within the community.
Supplementary Material
Acknowledgement
We acknowledge financial support from the National Science Foundation (DMR 1255281) and the National Institutes of Health (NIH/1R21CA191740).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 2.Zhang PC, Cheetham AG, Lin YA, Cui H. Self-Assembled Tat Nanofibers as Effective Drug Carrier and Transporter. Acs Nano. 2013;7:5965–5977. doi: 10.1021/nn401667z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 4.Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4:93–97. doi: 10.1186/bcr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaneda Y. Virosomes: evolution of the liposome as a targeted drug delivery system. Adv Drug Deliver Rev. 2000;43:197–205. doi: 10.1016/s0169-409x(00)00069-7. [DOI] [PubMed] [Google Scholar]
- 6.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliver Rev. 2012;64:206–212. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliver Rev. 2012;64:24–36. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 8.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 9.Pridgen EM, Langer R, Farokhzad OC. Biodegradable, polymeric nanoparticle delivery systems for cancer therapy. Nanomedicine-Uk. 2007;2:669–680. doi: 10.2217/17435889.2.5.669. [DOI] [PubMed] [Google Scholar]
- 10.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee CC, MacKay JA, Frechet JMJ, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 12.Majoros IJ, Myc A, Thomas T, Mehta CB, Baker JR. PAMAM dendrimer-based multifunctional conjugate for cancer therapy: Synthesis, characterization, and functionality. Biomacromolecules. 2006;7:572–579. doi: 10.1021/bm0506142. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy TD, Karellas P, Henderson SA, Giannis M, O'Keefe DF, Heery G, Paull JRA, Matthews BR, Holan G. Dendrimers as drugs: Discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol Pharmaceut. 2005;2:312–318. doi: 10.1021/mp050023q. [DOI] [PubMed] [Google Scholar]
- 14.Najlah M, D'Emanuele A. Synthesis of dendrimers and drug-dendrimer conjugates for drug delivery. Curr Opin Drug Disc. 2007;10:756–767. [PubMed] [Google Scholar]
- 15.Yu J, Ha W, Chen J, Shi YP. pH-Responsive supramolecular hydrogels for codelivery of hydrophobic and hydrophilic anticancer drugs. Rsc Adv. 2014;4:58982–58989. [Google Scholar]
- 16.Johannsen M, Gneveckow U, Eckelt L, Feussner A, Waldofner N, Scholz R, Deger S, Wust P, Loening SA, Jordan A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int J Hyperther. 2005;21:637–647. doi: 10.1080/02656730500158360. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy JR, Weissleder R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv Drug Deliver Rev. 2008;60:1241–1251. doi: 10.1016/j.addr.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami T, Tsuchida K. Recent advances in inorganic nanoparticle-based drug delivery systems. Mini-Rev Med Chem. 2008;8:175–183. doi: 10.2174/138955708783498078. [DOI] [PubMed] [Google Scholar]
- 19.Kim WY, Xiao JT, Chaikof EL. Recombinant Amphiphilic Protein Micelles for Drug Delivery. Langmuir. 2011;27:14329–14334. doi: 10.1021/la202864x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marullo R, Kastantin M, Drews LB, Tirrell M. Peptide contour length determines equilibrium secondary structure in protein-analogous micelles. Biopolymers. 2013;99:573–581. doi: 10.1002/bip.22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tirrell M. Modular materials by self-assembly. Aiche J. 2005;51:2386–2390. [Google Scholar]
- 22.Trent A, Marullo R, Lin B, Black M, Tirrell M. Structural properties of soluble peptide amphiphile micelles. Soft Matter. 2011;7:9572–9582. [Google Scholar]
- 23.Chow EK, Zhang XQ, Chen M, Lam R, Robinson E, Huang HJ, Schaffer D, Osawa E, Goga A, Ho D. Nanodiamond Therapeutic Delivery Agents Mediate Enhanced Chemoresistant Tumor Treatment. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001713. [DOI] [PubMed] [Google Scholar]
- 24.Lam R, Ho D. Nanodiamonds as vehicles for systemic and localized drug delivery. Expert Opin Drug Del. 2009;6:883–895. doi: 10.1517/17425240903156382. [DOI] [PubMed] [Google Scholar]
- 25.Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7:11–23. doi: 10.1038/nnano.2011.209. [DOI] [PubMed] [Google Scholar]
- 26.Zhang XQ, Lam R, Xu XY, Chow EK, Kim HJ, Ho D. Multimodal Nanodiamond Drug Delivery Carriers for Selective Targeting, Imaging, and Enhanced Chemotherapeutic Efficacy. Adv Mater. 2011;23:4770-+. doi: 10.1002/adma.201102263. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y, Li J, Li WX, Zhang Y, Yang XF, Chen N, Sun YH, Zhao Y, Fan CH, Huang Q. The Biocompatibility of Nanodiamonds and Their Application in Drug Delivery Systems. Theranostics. 2012;2:302–312. doi: 10.7150/thno.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes E, Ferreira JA, Peixoto A, Lima L, Barroso S, Sarmento B, Santos LL. New trends in guided nanotherapies for digestive cancers: A systematic review. J Control Release. 2015;209:288–307. doi: 10.1016/j.jconrel.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Harries M, Ellis P, Harper P. Nanoparticle albumin-bound paclitaxel for metastatic breast cancer. J Clin Oncol. 2005;23:7768–7771. doi: 10.1200/JCO.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Kratz F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Cho KJ, Wang X, Nie SM, Chen Z, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 32.Lee H, Lytton-Jean AKR, Chen Y, Love KT, Park AI, Karagiannis ED, Sehgal A, Querbes W, Zurenko CS, Jayaraman M, Peng CG, Charisse K, Borodovsky A, Manoharan M, Donahoe JS, Truelove J, Nahrendorf M, Langer R, Anderson DG. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol. 2012;7:389–393. doi: 10.1038/nnano.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J Cell Biol. 2010;188:759–768. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nat Rev Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 35.Su CW, Chiang CS, Li WM, Hu SH, Chen SY. Multifunctional nanocarriers for simultaneous encapsulation of hydrophobic and hydrophilic drugs in cancer treatment. Nanomedicine-Uk. 2014;9:1499–1515. doi: 10.2217/nnm.14.97. [DOI] [PubMed] [Google Scholar]
- 36.Duncan R, Gaspar R. Nanomedicine(s) under the Microscope. Mol Pharmaceut. 2011;8:2101–2141. doi: 10.1021/mp200394t. [DOI] [PubMed] [Google Scholar]
- 37.Grodzinski P, Farrell D. Future Opportunities in Cancer Nanotechnology-NCI Strategic Workshop Report. Cancer Res. 2014;74:1307–1310. doi: 10.1158/0008-5472.CAN-13-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamaly N, Xiao ZY, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venditto VJ, Szoka FC. Cancer nanomedicines: So many papers and so few drugs! Adv Drug Deliver Rev. 2013;65:80–88. doi: 10.1016/j.addr.2012.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin R, Cui HG. Supramolecular nanostructures as drug carriers. Current Opinion in Chemical Engineering. 2015;7:75–83. [Google Scholar]
- 42.MacKay JA, Chen MN, McDaniel JR, Liu WG, Simnick AJ, Chilkoti A. Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat Mater. 2009;8:993–999. doi: 10.1038/nmat2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desmaele D, Gref R, Couvreur P. Squalenoylation: A generic platform for nanoparticular drug delivery. J Control Release. 2012;161:609–618. doi: 10.1016/j.jconrel.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 44.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv Drug Deliver Rev. 2012;64:37–48. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 45.Cheetham AG, Zhang PC, Lin YA, Lock LL, Cui HG. Supramolecular Nanostructures Formed by Anticancer Drug Assembly. J Am Chem Soc. 2013;135:2907–2910. doi: 10.1021/ja3115983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delplace V, Couvreur P, Nicolas J. Recent trends in the design of anticancer polymer prodrug nanocarriers. Polym Chem-Uk. 2014;5:1529–1544. [Google Scholar]
- 47.Kataoka K, Kwon GS, Yokoyama M, Okano T, Sakurai Y. Block-Copolymer Micelles as Vehicles for Drug Delivery. J Control Release. 1993;24:119–132. [Google Scholar]
- 48.Coburn JM, Kaplan DL. Engineering Biomaterial-Drug Conjugates for Local and Sustained Chemotherapeutic Delivery. Bioconjugate Chem. 2015;26:1212–1223. doi: 10.1021/acs.bioconjchem.5b00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan XY, Li BB, Lu XG, Jia F, Santori C, Menon P, Li H, Zhang BH, Zhao JJ, Zhang K. Light-Triggered, Self-Immolative Nucleic Acid-Drug Nanostructures. J Am Chem Soc. 2015;137:6112–6115. doi: 10.1021/jacs.5b00795. [DOI] [PubMed] [Google Scholar]
- 50.Pelegri-O'Day EM, Lin EW, Maynard HD. Therapeutic Protein-Polymer Conjugates: Advancing Beyond PEGylation. J Am Chem Soc. 2014;136:14323–14332. doi: 10.1021/ja504390x. [DOI] [PubMed] [Google Scholar]
- 51.Flygare JA, Pillow TH, Aristoff P. Antibody-Drug Conjugates for the Treatment of Cancer. Chem Biol Drug Des. 2013;81:113–121. doi: 10.1111/cbdd.12085. [DOI] [PubMed] [Google Scholar]
- 52.Kwon GS, Kataoka K. Block copolymer micelles as long-circulating drug vehicles. Adv Drug Deliver Rev. 2012;64:237–245. [Google Scholar]
- 53.Maksimenko A, Dosio F, Mougin J, Ferrero A, Wack S, Reddy LH, Weyn AA, Lepeltier E, Bourgaux C, Stella B, Cattel L, Couvreur P. A unique squalenoylated and nonpegylated doxorubicin nanomedicine with systemic long-circulating properties and anticancer activity. P Natl Acad Sci USA. 2014;111:E217–E226. doi: 10.1073/pnas.1313459110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi P, Aluri S, Lin YA, Shah M, Edman M, Dhandhukia J, Cui HG, MacKay JA. Elastin-based protein polymer nanoparticles carrying drug at both corona and core suppress tumor growth in vivo. J Control Release. 2013;171:330–338. doi: 10.1016/j.jconrel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang W, Sreekumar PG, Valluripalli V, Shi P, Wang JW, Lin YA, Cui HG, Kannan R, Hinton DR, MacKay JA. Protein polymer nanoparticles engineered as chaperones protect against apoptosis in human retinal pigment epithelial cells. J Control Release. 2014;191:4–14. doi: 10.1016/j.jconrel.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng LLX, Ivetac A, Chaudhari AS, Van S, Zhao G, Yu L, Howell SB, McCammon JA, Gough DA. Characterization of a Clinical Polymer-Drug Conjugate Using Multiscale Modeling. Biopolymers. 2010;93:936–951. doi: 10.1002/bip.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Polymers for Drug Delivery Systems. Annu Rev Chem Biomol. 2010;1:149–173. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelley EG, Albert JNL, Sullivan MO, Epps TH. Stimuli-responsive copolymer solution and surface assemblies for biomedical applications. Chem Soc Rev. 2013;42:7057–7071. doi: 10.1039/c3cs35512h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lock LL, LaComb M, Schwarz K, Cheetham AG, Lin YA, Zhang PC, Cui HG. Self-assembly of natural and synthetic drug amphiphiles into discrete supramolecular nanostructures. Faraday Discuss. 2013;166:285–301. doi: 10.1039/c3fd00099k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cui HG, Webber MJ, Stupp SI. Self-Assembly of Peptide Amphiphiles: From Molecules to Nanostructures to Biomaterials. Biopolymers. 2010;94:1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 62.Aida T, Meijer EW, Stupp SI. Functional Supramolecular Polymers. Science. 2012;335:813–817. doi: 10.1126/science.1205962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stupp SI, Zha RH, Palmer LC, Cui HG, Bitton R. Self-assembly of biomolecular soft matter. Faraday Discuss. 2013;166:9–30. doi: 10.1039/c3fd00120b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297:967–973. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 65.Barichello JM, Morishita M, Takayama K, Nagai T. Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Dev Ind Pharm. 1999;25:471–476. doi: 10.1081/ddc-100102197. [DOI] [PubMed] [Google Scholar]
- 66.Bilati U, Allemann E, Doelker E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24:67–75. doi: 10.1016/j.ejps.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 67.Govender T, Stolnik S, Garnett MC, Illum L, Davis SS. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J Control Release. 1999;57:171–185. doi: 10.1016/s0168-3659(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 68.Leader B, Baca QJ, Golan DE. Protein therapeutics: A summary and pharmacological classification. Nat Rev Drug Discov. 2008;7:21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 69.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 70.Tong R, Cheng JJ. Anticancer polymeric nanomedicines. Polym Rev. 2007;47:345–381. [Google Scholar]
- 71.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 72.Abuchowski A, Vanes T, Palczuk NC, Davis FF. Alteration of Immunological Properties of Bovine Serum-Albumin by Covalent Attachment of Polyethylene-Glycol. J Biol Chem. 1977;252:3578–3581. [PubMed] [Google Scholar]
- 73.Larson N, Ghandehari H. Polymeric Conjugates for Drug Delivery. Chem Mater. 2012;24:840–853. doi: 10.1021/cm2031569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alconcel SNS, Baas AS, Maynard HD. FDA-approved poly(ethylene glycol)-protein conjugate drugs. Polym Chem-Uk. 2011;2:1442–1448. [Google Scholar]
- 75.Peer D, Karp JM, Hong S, FaroKHzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 76.Pfister D, Morbidelli M. Process for protein PEGylation. J Control Release. 2014;180:134–149. doi: 10.1016/j.jconrel.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 77.Lambert JM. Drug-conjugated monoclonal antibodies for the treatment of cancer. Curr Opin Pharmacol. 2005;5:543–549. doi: 10.1016/j.coph.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 78.Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol. 2010;10:345–352. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- 79.Panowski S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. Mabs-Austin. 2014;6:34–45. doi: 10.4161/mabs.27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agarwal P, Bertozzi CR. Site-Specific Antibody-Drug Conjugates: The Nexus of Biciorthogonal Chemistry, Protein Engineering, and Drug Development. Bioconjugate Chem. 2015;26:176–192. doi: 10.1021/bc5004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Broyer RM, Grover GN, Maynard HD. Emerging synthetic approaches for protein-polymer conjugations. Chem Commun. 2011;47:2212–2226. doi: 10.1039/c0cc04062b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grover GN, Maynard HD. Protein-polymer conjugates: synthetic approaches by controlled radical polymerizations and interesting applications. Curr Opin Chem Biol. 2010;14:818–827. doi: 10.1016/j.cbpa.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hannink JM, Cornelissen JJLM, Farrera JA, Foubert P, De Schryver FC, Sommerdijk NAJM, Nolte RJM. Protein-polymer hybrid amphiphiles. Angew Chem Int Edit. 2001;40:4732-+. [PubMed] [Google Scholar]
- 84.Lee LA, Niu ZW, Wang Q. Viruses and Virus-Like Protein Assemblies-Chemically Programmable Nanoscale Building Blocks. Nano Res. 2009;2:349–364. [Google Scholar]
- 85.Matsumoto NM, Prabhakaran P, Rome LH, Maynard HD. Smart Vaults: Thermally-Responsive Protein Nanocapsules. Acs Nano. 2013;7:867–874. doi: 10.1021/nn3053457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thordarson P, Le Droumaguet B, Velonia K. Well-defined protein-polymer conjugates-synthesis and potential applications. Appl Microbiol Biot. 2006;73:243–254. doi: 10.1007/s00253-006-0574-4. [DOI] [PubMed] [Google Scholar]
- 87.van Rijn P. Polymer Directed Protein Assemblies. Polymers-Basel. 2013;5:576–599. [Google Scholar]
- 88.Boerakker MJ, Hannink JM, Bomans PHH, Frederik PM, Nolte RJM, Meijer EM, Sommerdijk NAJM. Giant amphiphiles by cofactor reconstitution. Angew Chem Int Edit. 2002;41:4239–4241. doi: 10.1002/1521-3773(20021115)41:22<4239::AID-ANIE4239>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 89.Boerakker MJ, Botterhuis NE, Bomans PHH, Frederik PM, Meijer EM, Nolte RJM, Sommerdijk NAJM. Aggregation Behavior of giant amphiphiles prepared by cofactor reconstitution. Chem-Eur J. 2006;12:6071–6080. doi: 10.1002/chem.200600089. [DOI] [PubMed] [Google Scholar]
- 90.Reynhout IC, Cornelissen JJLM, Nolte RJM. Self-assembled architectures from biohybrid triblock copolymers. J Am Chem Soc. 2007;129:2327–2332. doi: 10.1021/ja066790f. [DOI] [PubMed] [Google Scholar]
- 91.Velonia K, Rowan AE, Nolte RJM. Lipase polystyrene giant amphiphiles. J Am Chem Soc. 2002;124:4224–4225. doi: 10.1021/ja017809b. [DOI] [PubMed] [Google Scholar]
- 92.Dirks AJ, Nolte RJM, Cornelissen JJLM. Protein-Polymer Hybrid Amphiphiles. Adv Mater. 2008;20:3953–3957. [Google Scholar]
- 93.Lavigueur C, Garcia JG, Hendriks L, Hoogenboom R, Cornelissen JJLM, Nolte RJM. Thermoresponsive giant biohybrid amphiphiles. Polym Chem-Uk. 2011;2:333–340. [Google Scholar]
- 94.Velonia K. Protein-polymer amphiphilic chimeras: recent advances and future challenges. Polym Chem-Uk. 2010;1:944–952. [Google Scholar]
- 95.Le Droumaguet B, Velonia K. In situ ATRP-Mediated hierarchical formation of giant amphiphile bionanoreactors. Angew Chem Int Edit. 2008;47:6263–6266. doi: 10.1002/anie.200801007. [DOI] [PubMed] [Google Scholar]
- 96.Aluri SR, Shi P, Gustafson JA, Wang W, Lin YA, Cui HG, Liu SL, Conti PS, Li ZB, Hu PS, Epstein AL, MacKay JA. A Hybrid Protein - Polymer Nanoworm Potentiates Apoptosis Better than a Monoclonal Antibody. Acs Nano. 2014;8:2064–2076. doi: 10.1021/nn403973g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Janib SM, Pastuszka MF, Aluri S, Folchman-Wagner Z, Hsueh PY, Shi P, Lin YA, Cui H, MacKay JA. A quantitative recipe for engineering protein polymer nanoparticles. Polym Chem-Uk. 2014;5:1614–1625. doi: 10.1039/C3PY00537B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Conover CD, Greenwald RB, Pendri A, Gilbert CW, Shum KL. Camptothecin delivery systems: enhanced efficacy and tumor accumulation of camptothecin following its conjugation to polyethylene glycol via a glycine linker. Cancer Chemoth Pharm. 1998;42:407–414. doi: 10.1007/s002800050837. [DOI] [PubMed] [Google Scholar]
- 99.Kopecek J, Kopeckova P, Minko T, Lu ZR. HPMA copolymer-anticancer drug conjugates: design, activity, and mechanism of action. Eur J Pharm Biopharm. 2000;50:61–81. doi: 10.1016/s0939-6411(00)00075-8. [DOI] [PubMed] [Google Scholar]
- 100.Singer JW, Bhatt R, Tulinsky J, Buhler KR, Heasley E, Klein P, de Vries P. Water-soluble poly-(L-glutamic acid)-Gly-camptothecin conjugates enhance camptothecin stability and efficacy in vivo. J Control Release. 2001;74:243–247. doi: 10.1016/s0168-3659(01)00323-6. [DOI] [PubMed] [Google Scholar]
- 101.Yu Y, Chen CK, Law WC, Mok J, Zou J, Prasad PN, Cheng C. Well-Defined Degradable Brush Polymer-Drug Conjugates for Sustained Delivery of Paclitaxel. Mol Pharmaceut. 2013;10:867–874. doi: 10.1021/mp3004868. [DOI] [PubMed] [Google Scholar]
- 102.Khandare J, Minko T. Polymer-drug conjugates: Progress in polymeric prodrugs. Prog Polym Sci. 2006;31:359–397. [Google Scholar]
- 103.Yokoyama M, Kwon GS, Okano T, Sakurai Y, Seto T, Kataoka K. Preparation of Micelle-Forming Polymer Drug Conjugates. Bioconjugate Chem. 1992;3:295–301. doi: 10.1021/bc00016a007. [DOI] [PubMed] [Google Scholar]
- 104.Yokoyama M, Miyauchi M, Yamada N, Okano T, Sakurai Y, Kataoka K, Inoue S. Polymer Micelles as Novel Drug Carrier - Adriamycin-Conjugated Poly(Ethylene Glycol) Poly(Aspartic Acid) Block Copolymer. J Control Release. 1990;11:269–278. [PubMed] [Google Scholar]
- 105.Yokoyama M, Miyauchi M, Yamada N, Okano T, Sakurai Y, Kataoka K, Inoue S. Characterization and Anticancer Activity of the Micelle-Forming Polymeric Anticancer Drug Adriamycin-Conjugated Poly(Ethylene Glycol)-Poly(Aspartic Acid) Block Copolymer. Cancer Res. 1990;50:1693–1700. [PubMed] [Google Scholar]
- 106.Harada A, Kataoka K. Supramolecular assemblies of block copolymers in aqueous media as nanocontainers relevant to biological applications. Prog Polym Sci. 2006;31:949–982. [Google Scholar]
- 107.Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: Tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjugate Chem. 2005;16:122–130. doi: 10.1021/bc0498166. [DOI] [PubMed] [Google Scholar]
- 108.Yokoyama M, Fukushima S, Uehara R, Okamoto K, Kataoka K, Sakurai Y, Okano T. Characterization of physical entrapment and chemical conjugation of adriamycin in polymeric micelles and their design for in vivo delivery to a solid tumor. J Control Release. 1998;50:79–92. doi: 10.1016/s0168-3659(97)00115-6. [DOI] [PubMed] [Google Scholar]
- 109.Yokoyama M, Okano T, Sakurai Y, Kataoka K. Improved Synthesis of Adriamycin-Conjugated Poly(Ethylene Oxide) Poly(Aspartic Acid) Block-Copolymer and Formation of Unimodal Micellar Structure with Controlled Amount of Physically Entrapped Adriamycin. J Control Release. 1994;32:269–277. [Google Scholar]
- 110.Mikhail AS, Allen C. Poly(ethylene glycol)-b-poly(epsilon-caprolactone) Micelles Containing Chemically Conjugated and Physically Entrapped Docetaxel: Synthesis, Characterization, and the Influence of the Drug on Micelle Morphology. Biomacromolecules. 2010;11:1273–1280. doi: 10.1021/bm100073s. [DOI] [PubMed] [Google Scholar]
- 111.Li A, Luehmann HP, Sun GR, Samarajeewa S, Zou J, Zhang SY, Zhang FW, Welch MJ, Liu YJ, Wooley KL. Synthesis and In Vivo Pharmacokinetic Evaluation of Degradable Shell Cross-Linked Polymer Nanoparticles with Poly(carboxybetaine) versus Poly(ethylene glycol) Surface-Grafted Coatings. Acs Nano. 2012;6:8970–8982. doi: 10.1021/nn303030t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Samarajeewa S, Shrestha R, Elsabahy M, Karwa A, Li A, Zentay RP, Kostelc JG, Dorshow RB, Wooley KL. In Vitro Efficacy of Paclitaxel-Loaded Dual-Responsive Shell Cross-Linked Polymer Nanoparticles Having Orthogonally Degradable Disulfide Cross-Linked Corona and Polyester Core Domains. Mol Pharmaceut. 2013;10:1092–1099. doi: 10.1021/mp3005897. [DOI] [PubMed] [Google Scholar]
- 113.Zhang FW, Elsabahy M, Zhang SY, Lin LY, Zou J, Wooley KL. Shell crosslinked knedel-like nanoparticles for delivery of cisplatin: effects of crosslinking. Nanoscale. 2013;5:3220–3225. doi: 10.1039/c3nr34320k. [DOI] [PubMed] [Google Scholar]
- 114.Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41:2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang SY, Zou J, Elsabahy M, Karwa A, Li A, Moore DA, Dorshow RB, Wooley KL. Poly(ethylene oxide)-block-polyphosphester-based paclitaxel conjugates as a platform for ultra-high paclitaxel-loaded multifunctional nanoparticles. Chem Sci. 2013;4:2122–2126. doi: 10.1039/C3SC50252J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zou J, Zhang FW, Zhang SY, Pollack SF, Elsabahy M, Fan JW, Wooley KL. Poly(ethylene oxide)-block-Polyphosphoester-graft-Paclitaxel Conjugates with Acid-Labile Linkages as a pH-Sensitive and Functional Nanoscopic Platform for Paclitaxel Delivery. Adv Healthc Mater. 2014;3:441–448. doi: 10.1002/adhm.201300235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pochan DJ, Chen ZY, Cui HG, Hales K, Qi K, Wooley KL. Toroidal triblock copolymer assemblies. Science. 2004;306:94–97. doi: 10.1126/science.1102866. [DOI] [PubMed] [Google Scholar]
- 118.Wang Z, Li YW, Dong XH, Yu XF, Guo K, Su H, Yue K, Wesdemiotis C, Cheng SZD, Zhang WB. Giant gemini surfactants based on polystyrene-hydrophilic polyhedral oligomeric silsesquioxane shape amphiphiles: sequential "click" chemistry and solution self-assembly. Chem Sci. 2013;4:1345–1352. [Google Scholar]
- 119.Zhang LF, Eisenberg A. Multiple Morphologies of Crew-Cut Aggregates of Polystyrene-B-Poly(Acrylic Acid) Block-Copolymers. Science. 1995;268:1728–1731. doi: 10.1126/science.268.5218.1728. [DOI] [PubMed] [Google Scholar]
- 120.Zhou ZX, Ma XP, Jin EL, Tang JB, Sui MH, Shen YQ, Van Kirk EA, Murdoch WJ, Radosz M. Linear-dendritic drug conjugates forming long-circulating nanorods for cancer-drug delivery. Biomaterials. 2013;34:5722–5735. doi: 10.1016/j.biomaterials.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 121.Marsh A, Khan A, Haddleton DM, Hannon MJ. Atom transfer polymerization: Use of uridine and adenosine derivatized monomers and initiators. Macromolecules. 1999;32:8725–8731. [Google Scholar]
- 122.Chen XJ, McRae S, Parelkar S, Emrick T. Polymeric Phosphorylcholine-Camptothecin Conjugates Prepared by Controlled Free Radical Polymerization and Click Chemistry. Bioconjugate Chem. 2009;20:2331–2341. doi: 10.1021/bc900339x. [DOI] [PubMed] [Google Scholar]
- 123.Harrisson S, Nicolas J, Maksimenko A, Bui DT, Mougin J, Couvreur P. Nanoparticles with In Vivo Anticancer Activity from Polymer Prodrug Amphiphiles Prepared by Living Radical Polymerization. Angew Chem Int Edit. 2013;52:1678–1682. doi: 10.1002/anie.201207297. [DOI] [PubMed] [Google Scholar]
- 124.Maksimenko A, Bui DT, Desmaele D, Couvreur P, Nicolas J. Significant Tumor Growth Inhibition from Naturally Occurring Lipid-Containing Polymer Prodrug Nanoparticles Obtained by the Drug-Initiated Method. Chem Mater. 2014;26:3606–3609. [Google Scholar]
- 125.Tong R, Tang L, Ma L, Tu CL, Baumgartner R, Cheng JJ. Smart chemistry in polymeric nanomedicine. Chem Soc Rev. 2014;43:6982–7012. doi: 10.1039/c4cs00133h. [DOI] [PubMed] [Google Scholar]
- 126.Tong R, Gabrielson NP, Fan TM, Cheng JJ. Polymeric nanomedicines based on poly(lactide) and poly(lactide-co-glycolide) Curr Opin Solid St M. 2012;16:323–332. doi: 10.1016/j.cossms.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tong R, Cheng JJ. Paclitaxel-initiated, controlled polymerization of lactide for the formulation of polymeric nanoparticulate delivery vehicles. Angew Chem Int Edit. 2008;47:4830–4834. doi: 10.1002/anie.200800491. [DOI] [PubMed] [Google Scholar]
- 128.Tong R, Cheng JJ. Ring-Opening Polymerization-Mediated Controlled Formulation of Polylactide-Drug Nanoparticles. J Am Chem Soc. 2009;131:4744–4754. doi: 10.1021/ja8084675. [DOI] [PubMed] [Google Scholar]
- 129.MacEwan SR, Chilkoti A. Elastin-Like Polypeptides: Biomedical Applications of Tunable Biopolymers. Biopolymers. 2010;94:60–77. doi: 10.1002/bip.21327. [DOI] [PubMed] [Google Scholar]
- 130.Chilkoti A, Dreher MR, Meyer DE. Design of thermally responsive, recombinant polypeptide carriers for targeted drug delivery. Adv Drug Deliver Rev. 2002;54:1093–1111. doi: 10.1016/s0169-409x(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 131.Bhattacharyya J, Bellucci JJ, Weitzhandler I, McDaniel JR, Spasojevic I, Li XH, Lin CC, Chi JTA, Chilkoti A. A paclitaxel-loaded recombinant polypeptide nanoparticle outperforms Abraxane in multiple murine cancer models. Nat Commun. 2015;6 doi: 10.1038/ncomms8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Meyer DE, Kong GA, Dewhirst MW, Zalutsky MR, Chilkoti A. Targeting a genetically engineered elastin-like polypeptide to solid tumors by local hyperthermia. Cancer Res. 2001;61:1548–1554. [PubMed] [Google Scholar]
- 133.Dreher MR, Raucher D, Balu N, Colvin OM, Ludeman SM, Chilkoti A. Evaluation of an elastin-like polypeptide-doxorubicin conjugate for cancer therapy. J Control Release. 2003;91:31–43. doi: 10.1016/s0168-3659(03)00216-5. [DOI] [PubMed] [Google Scholar]
- 134.Furgeson DY, Dreher MR, Chilkoti A. Structural optimization of a "smart" doxorubicin-polypeptide conjugate for thermally targeted delivery to solid tumors. J Control Release. 2006;110:362–369. doi: 10.1016/j.jconrel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 135.Chilkoti A, Christensen T, MacKay JA. Stimulus responsive elastin biopolymers: applications in medicine and biotechnology. Curr Opin Chem Biol. 2006;10:652–657. doi: 10.1016/j.cbpa.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McDaniel JR, Bhattacharyya J, Vargo KB, Hassouneh W, Hammer DA, Chilkoti A. Self-Assembly of Thermally Responsive Nanoparticles of a Genetically Encoded Peptide Polymer by Drug Conjugation. Angew Chem Int Edit. 2013;52:1683–1687. doi: 10.1002/anie.201200899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McDaniel JR, MacEwan SR, Li XH, Radford DC, Landon CD, Dewhirst M, Chilkoti A. Rational Design of "Heat Seeking" Drug Loaded Polypeptide Nanoparticles That Thermally Target Solid Tumors. Nano Lett. 2014;14:2890–2895. doi: 10.1021/nl5009376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mastria MC Eric M, Mc Daniel Jonathan R, Li Xinghai, Hyun Jinho, Dewhirst Mark W, Chilkoti Ashutosh. Doxorubicin-conjugated polypeptide nanoparticles inhibit metastasis in two murine models of carcinoma. J Control Release. 2015;208:52–58. doi: 10.1016/j.jconrel.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu JY, Liu WE, Weitzhandler I, Bhattacharyya J, Li XH, Wang J, Qi YZ, Bhattacharjee S, Chilkoti A. Ring-Opening Polymerization of Prodrugs: A Versatile Approach to Prepare Well-Defined Drug-Loaded Nanoparticles. Angew Chem Int Edit. 2015;54:1002–1006. doi: 10.1002/anie.201409293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ulbrich K, Subr V, Strohalm J, Plocova D, Jelinkova M, Rihova B. Polymeric drugs based on conjugates of synthetic and natural macromolecules I. Synthesis and physico-chemical characterisation. J Control Release. 2000;64:63–79. doi: 10.1016/s0168-3659(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 141.Ulbrich K, Subr V. Polymeric anticancer drugs with pH-controlled activation. Adv Drug Deliver Rev. 2004;56:1023–1050. doi: 10.1016/j.addr.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 142.Rosario-Melendez R, Harris CL, Delgado-Rivera R, Yu L, Uhrich KE. PolyMorphine: An innovative biodegradable polymer drug for extended pain relief. J Control Release. 2012;162:538–544. doi: 10.1016/j.jconrel.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ouimet MA, Griffin J, Carbone-Howell AL, Wu WH, Stebbins ND, Di R, Uhrich KE. Biodegradable Ferulic Acid-Containing Poly(anhydride-ester): Degradation Products with Controlled Release and Sustained Antioxidant Activity. Biomacromolecules. 2013;14:854–861. doi: 10.1021/bm3018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rosario-Melendez R, Yu WL, Uhrich KE. Biodegradable Polyesters Containing Ibuprofen and Naproxen As Pendant Groups. Biomacromolecules. 2013;14:3542–3548. doi: 10.1021/bm400889a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang N, Chen ZC, Lu D, Lin MF. Controllable selective synthesis of a polymerizable prodrug of cytarabine by enzymatic and chemical methods. Bioorg Med Chem Lett. 2005;15:4064–4067. doi: 10.1016/j.bmcl.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 146.Li X, Wu Q, Lv DS, Lin XF. Controllable synthesis of polymerizable ester and amide prodrugs of acyclovir by enzyme in organic solvent. Bioorgan Med Chem. 2006;14:3377–3382. doi: 10.1016/j.bmc.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 147.Ding Y, Chen WL, Hu JH, Du M, Yang D. Polymerizable disulfide paclitaxel prodrug for controlled drug delivery. Mat Sci Eng C-Mater. 2014;44:386–390. doi: 10.1016/j.msec.2014.08.046. [DOI] [PubMed] [Google Scholar]
- 148.Hu XL, Hu JM, Tian J, Ge ZS, Zhang GY, Luo KF, Liu SY. Polyprodrug Amphiphiles: Hierarchical Assemblies for Shape-Regulated Cellular Internalization, Trafficking, and Drug Delivery. J Am Chem Soc. 2013;135:17617–17629. doi: 10.1021/ja409686x. [DOI] [PubMed] [Google Scholar]
- 149.Johnson JA, Lu YY, Burts AO, Xia Y, Durrell AC, Tirrell DA, Grubbs RH. Drug-Loaded, Bivalent-Bottle-Brush Polymers by Graft-through ROMP. Macromolecules. 2010;43:10326–10335. doi: 10.1021/ma1021506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liao LY, Liu J, Dreaden EC, Morton SW, Shopsowitz KE, Hammond PT, Johnson JA. A Convergent Synthetic Platform for Single-Nanoparticle Combination Cancer Therapy: Ratiometric Loading and Controlled Release of Cisplatin, Doxorubicin, and Camptothecin. J Am Chem Soc. 2014;136:5896–5899. doi: 10.1021/ja502011g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gao AX, Liao LY, Johnson JA. Synthesis of Acid-Labile PEG and PEG-Doxorubicin-Conjugate Nanoparticles via Brush-First ROMP. Acs Macro Letters. 2014;3:854–857. doi: 10.1021/mz5004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mahato R, Tai WY, Cheng K. Prodrugs for improving tumor targetability and efficiency. Adv Drug Deliver Rev. 2011;63:659–670. doi: 10.1016/j.addr.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huttunen KM, Raunio H, Rautio J. Prodrugs-from Serendipity to Rational Design. Pharmacol Rev. 2011;63:750–771. doi: 10.1124/pr.110.003459. [DOI] [PubMed] [Google Scholar]
- 154.Singh Y, Palombo M, Sinko PJ. Recent trends in targeted anticancer prodrug and conjugate design. Curr Med Chem. 2008;15:1802–1826. doi: 10.2174/092986708785132997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lepeltier E, Bourgaux C, Rosilio V, Poupaert JH, Meneau F, Zouhiri F, Lepetre-Mouelhi S, Desmaele D, Couvreur P. Self-Assembly of Squalene-Based Nucleolipids: Relating the Chemical Structure of the Bioconjugates to the Architecture of the Nanoparticles. Langmuir. 2013;29:14795–14803. doi: 10.1021/la403338y. [DOI] [PubMed] [Google Scholar]
- 156.Couvreur P, Stella B, Reddy LH, Hillaireau H, Dubernet C, Desmaele D, Lepetre-Mouelhi S, Rocco F, Dereuddre-Bosquet N, Clayette P, Rosilio V, Marsaud V, Renoir JM, Cattel L. Squalenoyl nanomedicines as potential therapeutics. Nano Lett. 2006;6:2544–2548. doi: 10.1021/nl061942q. [DOI] [PubMed] [Google Scholar]
- 157.Dosio F, Reddy LH, Ferrero A, Stella B, Cattel L, Couvreur P. Novel Nanoassemblies Composed of Squalenoyl-Paclitaxel Derivatives: Synthesis, Characterization, and Biological Evaluation. Bioconjugate Chem. 2010;21:1349–1361. doi: 10.1021/bc100154g. [DOI] [PubMed] [Google Scholar]
- 158.Semiramoth N, Di Meo C, Zouhiri F, Said-Hassane F, Valetti S, Gorges R, Nicolas V, Poupaert JH, Chollet-Martin S, Desmaele D, Gref R, Couvreur P. Self-Assembled Squalenoylated Penicillin Bioconjugates: An Original Approach for the Treatment of Intracellular Infections. Acs Nano. 2012;6:3820–3831. doi: 10.1021/nn204928v. [DOI] [PubMed] [Google Scholar]
- 159.Arias JL, Reddy LH, Othman M, Gillet B, Desmaele D, Zouhiri F, Dosio F, Gref R, Couvreur P. Squalene Based Nanocomposites: A New Platform for the Design of Multifunctional Pharmaceutical Theragnostics. Acs Nano. 2011;5:1513–1521. doi: 10.1021/nn1034197. [DOI] [PubMed] [Google Scholar]
- 160.Bildstein L, Marsaud V, Chacun H, Lepetre-Mouelhi S, Desmaele D, Couvreur P, Dubernet C. Extracellular-protein-enhanced cellular uptake of squalenoyl gemcitabine from nanoassemblies. Soft Matter. 2010;6:5570–5580. [Google Scholar]
- 161.Reddy LH, Khoury H, Paci A, Deroussent A, Ferreira H, Dubernet C, Decleves X, Besnard M, Chacun H, Lepetre-Mouelhi S, Desmaele D, Rousseau B, Laugier C, Cintrat JC, Vassal G, Couvreur P. Squalenoylation favorably modifies the in vivo pharmacokinetics and biodistribution of gemcitabine in mice. Drug Metab Dispos. 2008;36:1570–1577. doi: 10.1124/dmd.108.020735. [DOI] [PubMed] [Google Scholar]
- 162.Shen YQ, Jin EL, Zhang B, Murphy CJ, Sui MH, Zhao J, Wang JQ, Tang JB, Fan MH, Van Kirk E, Murdoch WJ. Prodrugs Forming High Drug Loading Multifunctional Nanocapsules for Intracellular Cancer Drug Delivery. J Am Chem Soc. 2010;132:4259–4265. doi: 10.1021/ja909475m. [DOI] [PubMed] [Google Scholar]
- 163.Huang P, Wang DL, Su Y, Huang W, Zhou YF, Cui DX, Zhu XY, Yan DY. Combination of Small Molecule Prodrug and Nanodrug Delivery: Amphiphilic Drug-Drug Conjugate for Cancer Therapy. J Am Chem Soc. 2014;136:11748–11756. doi: 10.1021/ja505212y. [DOI] [PubMed] [Google Scholar]
- 164.Branco MC, Schneider JP. Self-assembling materials for therapeutic delivery. Acta Biomater. 2009;5:817–831. doi: 10.1016/j.actbio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tu RS, Tirrell M. Bottom-up design of biomimetic assemblies. Adv Drug Deliver Rev. 2004;56:1537–1563. doi: 10.1016/j.addr.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 166.Wang HM, Yang ZM, Adams DJ. Controlling peptide-based hydrogelation. Mater Today. 2012;15:500–507. [Google Scholar]
- 167.Estroff LA, Hamilton AD. Water gelation by small organic molecules. Chem Rev. 2004;104:1201–1217. doi: 10.1021/cr0302049. [DOI] [PubMed] [Google Scholar]
- 168.Appel EA, del Barrio J, Loh XJ, Scherman OA. Supramolecular polymeric hydrogels. Chem Soc Rev. 2012;41:6195–6214. doi: 10.1039/c2cs35264h. [DOI] [PubMed] [Google Scholar]
- 169.Du XW, Zhou J, Xu B. Supramolecular Hydrogels Made of Basic Biological Building Blocks. Chem-Asian J. 2014;9:1446–1472. doi: 10.1002/asia.201301693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Weingarten AS, Kazantsev RV, Palmer LC, McClendon M, Koltonow AR, Samuel APS, Kiebala DJ, Wasielewski MR, Stupp SI. Self-assembling hydrogel scaffolds for photocatalytic hydrogen production. Nat Chem. 2014;6:964–970. doi: 10.1038/nchem.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, Lamm MS, Pochan DJ, Schneider JP. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. P Natl Acad Sci USA. 2007;104:7791–7796. doi: 10.1073/pnas.0701980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhao F, Ma ML, Xu B. Molecular hydrogels of therapeutic agents. Chem Soc Rev. 2009;38:883–891. doi: 10.1039/b806410p. [DOI] [PubMed] [Google Scholar]
- 173.Vemula PK, Wiradharma N, Ankrum JA, Miranda OR, John G, Karp JM. Prodrugs as self-assembled hydrogels: a new paradigm for biomaterials. Curr Opin Biotech. 2013;24:1174–1182. doi: 10.1016/j.copbio.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 174.Vemula PK, Li J, John G. Enzyme catalysis: Tool to make and break amygdalin hydrogelators from renewable resources: A delivery model for hydrophobic drugs. J Am Chem Soc. 2006;128:8932–8938. doi: 10.1021/ja062650u. [DOI] [PubMed] [Google Scholar]
- 175.Vemula PK, Cruikshank GA, Karp JM, John G. Self-assembled prodrugs: An enzymatically triggered drug-delivery platform. Biomaterials. 2009;30:383–393. doi: 10.1016/j.biomaterials.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 176.Xing BG, Yu CW, Chow KH, Ho PL, Fu DG, Xu B. Hydrophobic interaction and hydrogen bonding cooperatively confer a vancomycin hydrogel: A potential candidate for biomaterials. J Am Chem Soc. 2002;124:14846–14847. doi: 10.1021/ja028539f. [DOI] [PubMed] [Google Scholar]
- 177.Gao Y, Kuang Y, Guo ZF, Guo ZH, Krauss IJ, Xu B. Enzyme-Instructed Molecular Self-assembly Confers Nanofibers and a Supramolecular Hydrogel of Taxol Derivative. J Am Chem Soc. 2009;131:13576-+. doi: 10.1021/ja904411z. [DOI] [PubMed] [Google Scholar]
- 178.Li XM, Li JY, Gao YA, Kuang Y, Shi JF, Xu B. Molecular Nanofibers of Olsalazine Form Supramolecular Hydrogels for Reductive Release of an Anti-inflammatory Agent. J Am Chem Soc. 2010;132:17707–17709. doi: 10.1021/ja109269v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li JY, Kuang Y, Gao Y, Du XW, Shi JF, Xu B. D-Amino Acids Boost the Selectivity and Confer Supramolecular Hydrogels of a Nonsteroidal Anti-Inflammatory Drug (NSAID) J Am Chem Soc. 2013;135:542–545. doi: 10.1021/ja310019x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang CB, Bian MJ, Yang ZM. A polymer additive boosts the anti-cancer efficacy of supramolecular nanofibers of taxol. Biomater Sci-Uk. 2014;2:651–654. doi: 10.1039/c3bm60252d. [DOI] [PubMed] [Google Scholar]
- 181.Majumder J, Das MR, Deb J, Jana SS, Dastidar P. beta-Amino Acid and Amino-Alcohol Conjugation of a Nonsteroidal Anti-Inflammatory Drug (NSAID) Imparts Hydrogelation Displaying Remarkable Biostability, Biocompatibility, and Anti-Inflammatory Properties. Langmuir. 2013;29:10254–10263. doi: 10.1021/la401929v. [DOI] [PubMed] [Google Scholar]
- 182.Li XY, Yang CB, Zhang ZL, Wu ZD, Deng Y, Liang GL, Yang ZM, Chen H. Folic acid as a versatile motif to construct molecular hydrogelators through conjugations with hydrophobic therapeutic agents. J Mater Chem. 2012;22:21838–21840. [Google Scholar]
- 183.Chen ZP, Zhang PC, Cheetham AG, Moon JH, Moxley JW, Lin YA, Cui HG. Controlled release of free doxorubicin from peptide-drug conjugates by drug loading. J Control Release. 2014;191:123–130. doi: 10.1016/j.jconrel.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 184.Lock LL, Tang Z, Keith D, Reyes C, Cui HG. Enzyme-Specific Doxorubicin Drug Beacon as Drug-Resistant Theranostic Molecular Probes. Acs Macro Letters. 2015;4:552–555. doi: 10.1021/acsmacrolett.5b00170. [DOI] [PubMed] [Google Scholar]
- 185.Zhang PC, Lock LL, Cheetham AG, Cui HG. Enhanced Cellular Entry and Efficacy of Tat Conjugates by Rational Design of the Auxiliary Segment. Mol Pharmaceut. 2014;11:964–973. doi: 10.1021/mp400619v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lin R, Cheetham AG, Zhang PC, Lin YA, Cui HG. Supramolecular filaments containing a fixed 41% paclitaxel loading. Chem Commun. 2013;49:4968–4970. doi: 10.1039/c3cc41896k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cheetham AG, Zhang P, Lin YA, Lin R, Cui H. Synthesis and self-assembly of a mikto-arm star dual drug amphiphile containing both paclitaxel and camptothecin. J Mater Chem B. 2014;2:7316–7326. doi: 10.1039/C4TB01084A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lin YA, Cheetham AG, Zhang PC, Ou YC, Li YG, Liu GS, Hermida-Merino D, Hamley IW, Cui HG. Multiwalled Nanotubes Formed by Catanionic Mixtures of Drug Amphiphiles. Acs Nano. 2014;8:12690–12700. doi: 10.1021/nn505688b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cheetham AG, Ou YC, Zhang PC, Cui HG. Linker-determined drug release mechanism of free camptothecin from self-assembling drug amphiphiles. Chem Commun. 2014;50:6039–6042. doi: 10.1039/c3cc49453e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.