Abstract
Recently, immunotherapy for cancer has begun to garner traction with encouraging results in a number of malignancies. Included within this arena has been the genetic engineering of autologous T cells with chimeric antigen receptors (CAR) against tumor target ligands. The majority of this experience has included the use of CAR T cells directed against CD19 for B cell hematologic malignancies. The most striking efficacy to date with CAR T cells directed against CD19 has been in relapsed and refractory B cell acute lymphoblastic leukemia (B-ALL), with the overwhelming majority of patients experiencing complete remissions. Additionally, single-center and largely early phase studies have demonstrated responses in patients with varying histologies of relapsed and refractory B cell non-Hodgkin lymphoma (B-NHL). The favorable response rates seen with this technology have been tempered by the high-risk of toxicity, particularly in the form of cytokine-release syndrome (CRS) and neurotoxicity. Agents such as tocilizumab and corticosteroids have been used to treat these toxicities. The current state-of-the-science includes: strategies to circumvent and treat toxicity, manufacturing and study of later generation CAR constructs with the intention of improving efficacy and development of CARs against other tumor targets for both hematologic and solid tumor malignancies. The observation of an early efficacy signal assures further integration and development of this modality into future immunotherapeutic strategies for various cancers.
Keywords: Chimeric Antigen Receptor, Immunotherapy, Cancer
The last few years have seen outstanding results from clinical trials evaluating gene-engineered autologous T cells for cancer. More than just a new drug class, gene-targeted T cells represent a “living drug” with the potential to eradicate widespread cancer and provide long-term protection in the form of immunologic memory. As with any new therapeutic drug class there are critical issues to understand when applying this therapy to patients and to assist in its further clinical development. We will briefly present the historical background and review the remarkable clinical success of this novel cell therapy.
The Chimeric Antigen Receptor
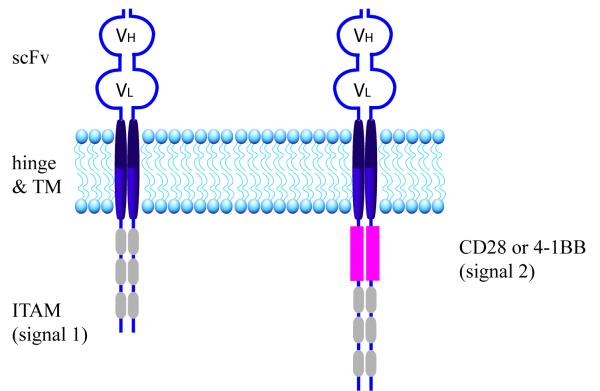
The chimeric antigen receptor[1] (CAR) is a hybrid molecule composed of antigen-binding domains fused to T cell activation and costimulatory domains (Figure 1). The antigen-binding domain of an antibody is recreated in the form of a single chain variable fragment (scFv). It is expressed on the cell surface and anchored to the cell membrane via hinge and transmembrane domains, usually from CD8 or immunoglobulin proteins. The intracellular portion of the CAR is composed of CD3ζ immunoreceptor tyrosine-based activation motif (ITAM) domains that support T cell activation, signaling, and cytotoxicity when the scFv binds its antigen. Therefore, T cells genetically modified to express this hybrid protein are endowed with a new antigen-specificity in addition to the antigen-specificity encoded by the endogenous T cell receptor.
Figure 1. The Chimeric Antigen Receptor.
On the left is a 1st generation CAR composed of an scFv (binds antigen), hinge and transmembrane (TM) domains (anchors the CAR in the cell membrane), and the three intracellular ITAM motifs of CD3ζ (signal 1 that mediates T cell activation). On the right is a 2nd generation CAR, which also includes the intracellular domain of an immunoreceptor, such as CD28 or 4-1BB, that mediates co-stimulation (signal 2).
The CAR design of scFv, hinge, transmemembrane, and CD3ζ is considered to be 1st generation (Figure 1). T cells modified with 1st generation CARs were quite effective in vitro, but had limited expansion and persistence in vivo that did not provide protection against tumor growth in animal models.[1] This limited efficacy was overcome due to insights from T cell biology. For full activation and persistence of endogenous T cells, 2 signals are needed: one through CD3ζ and the second through the CD28 costimulatory receptor. CAR researchers intuited that a chimeric receptor composed of a CD3ζ activation domain paired with a costimulatory domain could improve in vivo T cell function (Figure 1). Indeed, this is what was observed when T cells modified with 2nd generation CARs were infused into immune deficient mice. Importantly, researchers determined that CD28 was not the only costimulatory domain that enhanced CAR T cell function; CD27, OX40, or 41BB also enhance CAR T cell in vivo function when paired with CD3ζ activation.[2-6] These pre-clinical results demonstrating increased persistence, expansion, and tumor protection of 2nd generation CAR T cells were later confirmed by a Phase I trial comparing 1st generation CAR T cells and 2nd generation CAR T cells adoptively transferred into patients with B cell malignancies.[7] This clinical trial served as the critical rationale for further evaluating 2nd generation CAR T cell in patients.
The Phase I experience with CAR T cells as a cancer immunotherapy
B-cell non-Hodgkin’s Lymphoma (B-NHL)
While many CARs had undergone preclinical development and validation, the initial clinical evaluation focused on CD19-targeted CAR T cells because the CD19 B cell antigen was deemed a safe target. This is due to the limited expression pattern of CD19, which is restricted to the B cell lineage, so the potential of on-target off-tumor complications would be limited to B cell aplasia. The earliest trials focused on indolent histology-B-NHL such as chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and the results were disappointing with sporadic objective responses.[7-9] However, these trials did advance the clinical application of CAR T cells by establishing critical factors required for optimal CAR T cell function, which were then adapted by the next iteration of trials targeting CD19. In the first clinical experience of CAR T cell for patients with follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL), Jensen et al[9] demonstrated that electroporation of T cells and the subsequent long-term culture resulted in poorly functional CAR T cells and nearly all trials subsequently have used viral transduction as their mode of gene transfer with this 1st generation construct. This group was also the first to demonstrate the immune-mediated host rejection of “foreign” CARs, which could limit the persistence and long-term efficacy of CAR T cell therapy and is being addressed by humanizing murine-based scFv’s. Brentjens et al [8] from the Memorial Sloan Kettering Cancer Center (MSKCC) group targeted patients’ T cells to CD19 with a 2nd generation CAR that included CD3ζ and CD28 intracellular domains. All but one of the treated adults had relapsed/refractory CLL. Their report established that conditioning chemotherapy was required for optimal CAR T cell function. Patients treated with conditioning chemotherapy followed by CD19-targeted CAR T cells had better outcomes, including stable disease and objective response, and the CAR T cells persisted for up to two months and expanded to comprise up to 5% of peripheral blood cells, while CAR T cells were undetectable in patients treated without conditioning chemotherapy. Savoldo et al [7] reported a substantial improvement in expansion and persistence with 2nd generation vs 1st generation CAR T cells when infused into patients with B-NHL. They determined that 2nd generation CAR T cells could be detected up to 6 months post infusion, versus 2 weeks for 1st generation CAR T cells, and expanded up to a maximum that was 60x greater than 1st generation CAR T cells. However, there were no objective responses noted in any of the treated patients.
Recently, the group at the National Cancer Institute (NCI) has published the largest cohort of B-NHL patients treated with CD19-targeted CAR T cells preceded by chemotherapy consisting of cyclophosphamide 60-120 mg/kg and fludarabine at a total dose of 125 mg/m2 with the purpose of lympho-depletion.[10] Six of 7 patients with DLBCL responded with either a complete (CR) or partial remission and all 6 patients with indolent histology B-NHL responded (PR or CR). The longest responses were 1 year in a DLBCL patient and 2 years in a patient with indolent B-NHL. The CAR T expansion peaked from 7-17 days post infusion. The investigators chose to lower the dose of CAR T cells from 5 ×106/kg to 1 × 106/kg due to toxicity manifested predominately by the cytokine-release syndrome (CRS). Thirteen of 15 patients experienced ≥ grade 3 toxicity. In a follow-up study presented at the American Society of Hematology (ASH) meeting in 2014, the NCI investigators studied lower doses of conditioning chemotherapy (cyclophosphamide 900 mg/m2 and fludarabine 90 mg/m2) and noted less toxicity related to severe CRS.[11]
Investigators from the University of Pennsylvania (UPENN) recently presented updated results of a phase IIa study treating chemotherapy refractory FL, mantle cell lymphoma (MCL) and DLBCL patients with CAR T cells.[12] The UPENN 2nd generation CAR incorporates a 4-1BB co-stimulatory domain. Patients received physician’s choice conditioning chemotherapy prior to administration of CAR T cells. Of 12 evaluable patients treated with DLBCL, 2 patients had a CR and 4 had a PR to CAR T cells with an overall response rate (ORR) of 50%. The longest responder is in continuous remission > 1 year following CAR-T treatment. All 7 patients with FL responded, with longest remission being > 1 year post-treatment. One of 2 patients with MCL experienced a response with short (< 2 months) follow-up thus far. Twelve non-hematologic ≥ grade 3 toxic events were observed with 4 of these events related to CRS or neurotoxicity. At the same meeting, investigators from the Fred Hutchinson Cancer Research Center group updated their study of CAR T cells, which include 4-1BB co-stimulation, for refractory B-cell malignancies.[13] The novelty of this study is that the CAR T product is composed of a fixed 1:1 ratio of CD8+ T central memory cells to CD4+ T cells based on encouraging data from animal models. Seven of 13 B-NHL patients responded (CR n=1, PR n=6) with no episodes of severe CRS. Responses appeared to correlate with peak expansion and persistence of CAR T in this interim analysis.
Lastly, the group from the Memorial Sloan Kettering Cancer Center (MSKCC) is currently evaluating CAR T cells in consolidation for high-risk relapsed/refractory DLBCL/aggressive histology B-NHL in partial remission after salvage chemotherapy following a HDT-ASCT.[14] The intention of this approach is to deliver the cellular immune therapy in a maximally lympho-ablative setting immediately following HDT-ASCT for potential optimization of CAR T cell expansion and efficacy. The second generation CAR T construct includes a CD28 co-stimulatory molecule. In an update presented at the 2015 ASCO meeting, 4 of 10 evaluable patients were in continuous remission at a median of 14 months, and 2 patients in CR at nearly 2 years, following study treatment.[14] All but one patient developed febrile neutropenia, as is expected post HDT. The most common ≥ grade 3 toxicity was reversible neurotoxicity in 7/11 patients. The investigators have attributed these toxicities to CRS related to CAR T cells.
Results from early stage clinical trials have demonstrated that CD19-targeted CAR T cells are an active therapy for NHL. Longer follow-up is needed to determine the durability of the remissions and there is a need for improvement in the CR rate. Multiple groups are evaluating how to improve this therapy by further optimizing conditioning chemotherapy, T cell subsets, combinatorial regimens, and even CAR design. While multiple agents have been recently approved for relapsed NHL, the large numbers of patients that relapse after or don’t respond to these agents are still in need of novel therapies.
B cell acute lymphoblastic leukemia (B-ALL)
Early evaluation in patients with NHL provided a proof-of-principle for CD19-targeted CAR T cells as a cancer immunotherapy. Therefore, Phase I trials were developed to evaluate the safety and efficacy of CD19-targeted CAR T cells against B-ALL.[15-19] There were concerns about the feasibility of targeting an acute leukemia, such as B-ALL, with a gene-engineered cell therapy. It was unknown if a sufficient number of T cells could be collected from patients with high blast counts or pancytopenic from disease or high-doses of frequent, multi-agent chemotherapeutic regimens. Furthermore, multiple high-dose chemotherapy regimens could potentially affect the function of collected T cells. Another major concern was that the time required to collect, genetically modify, and expand CD19-targeted CAR T cells would be too long and result in patients dying due to progression of disease before they could be treated on trial. B-ALL remains one of the few oncologic diagnoses that require hospitalization and urgent treatment to prevent death. Indeed, the overall survival of adults with relapsed/refractory B-ALL is a few months.[20, 21] Lastly, it was unknown if the adoptive transfer of CAR T cells could eradicate a rapidly proliferating acute leukemia. However, the efforts of multiple independent groups have demonstrated that B-ALL is uniquely sensitive to the adoptive transfer of 2nd generation CD19-targeted CAR T cells and none of the concerns regarding the feasibility of CAR T cells as a cancer therapy were realized.
The Sadelain and Brentjens group at MKSCC[15, 16], were the first to publish their experience targeting B-ALL with CD19-targeted CAR T cells. The exciting results from the MSKCC trial were later confirmed by studies from the Mackall group at the NCI[19] and the June group at UPENN[17, 18], suggesting that the first clinical indication for a gene-modified cell therapy for a cancer is on the horizon. There are differences to highlight among the various clinical trial designs and therapeutic products that provide important insights about this cancer immunotherapy. The MSKCC group has detailed their efforts including adults with relapsed/refractory B-ALL (n=16), while the UPENN and NCI groups have treated mostly children (n=21 for NCI, n=25 for UPENN) with a few adults (n=5 for UPENN). [15-19] CAR T cells were produced by viral transduction, either using gammaretroviral (NCI and MSKCC) or lentiviral (UPENN) vectors, followed by expansion with CD3/CD28 beads. Both the NCI (0.03 to 3 × 106 CAR T cells/kg) and the UPENN (0.8 to 21 × 106 CAR T cells/kg) groups evaluated ranges of CAR T cell doses, while the MSKCC group evaluated one dose level (3×106 CAR T cells/kg). While all the CARs are 2nd generation CARs they differ through their co-stimulatory intracellular domain, CD28 (NCI and MSKCC) or 41BB (UPENN), and scFv, FMC63 (NCI and UPENN) or SJ25C1 (MSKCC). In addition, patients were treated with a fixed conditioning regimen consisting of either fludarabine and cyclophosphamide (NCI) or cyclophosphamide alone (MSKCC), or a physician’s choice regimen (UPENN). Despite these differences the outcomes were remarkably similar.[22-25]
The reports from these groups clearly establish the feasibility of collecting T cells from patients with an acute leukemia, genetically modifying the T cells to target CD19, and expanding them to a sufficient number in a timely fashion. The required T cell dose was achieved in all but 2 of the 57 treated patients.[15-19] This is further supported by the intent to treat analysis provided by the NCI[19], which demonstrated that every patient enrolled on their trial (n=21) was successfully infused with CAR T cells. The expected CR rate after salvage chemotherapy for patients with relapsed/refractory B-ALL is approximately 20-30%.[21] However, the CR rate after CD19-targeted CAR T cell infusion was remarkably high in all the trials and without precedent: NCI (67%), MSKCC (88%), and UPENN (90%).[15-19] Furthermore, reports from the recent ASH 2014 and ASCO 2015 meetings confirm that CR rates remain high (67-91%) as the number of patients (n=103) treated has increased and other medical centers have opened their own CD19-targeted CAR T cell trials for B-ALL.[22-25] Furthermore, these are high-quality remissions as reflected by the absence of disease by flow cytometry or immunoglobulin deep sequencing, with molecular CR (CRm) being reported in 57% of patients by the NCI[19] and 75% by MSKCC[16]. This suggests that these remissions will be durable and early follow-up data are supportive with event-free and/or disease-free survivals at 6 months ranging from 67 to 78%.[17-19]
Correlative analyses performed by the three groups have revealed important observations. The NCI group reported that CAR T cell expansion correlated with response, which is intuitive because en mass activation of CD19-targeted CAR T cells should result in expansion and tumor eradication.[19] While expansion is correlated with response, no such correlation has been reported with CAR T cell persistence, albeit with limited follow-up. The CD28 -containing 2nd generation CARs used by MSKCC and the NCI persist for < 2-3 months and a contraction occurs after the loss of normal and malignant B cells, which models a normal immune response.[15, 16, 19] In contrast, about two-thirds of patients treated on the UPENN trial have CAR T cell persistence > 4 months.[17, 18] It is uncertain if this enhanced persistence is due to the lentiviral modification of T cells or the use of the 41BB co-stimulatory domain in their 2nd generation CARs. It is also unknown if this enhanced persistence will result in more durable remissions than those seen in the MSKCC and/or NCI treated patients. Despite the impressive initial responses there is some concern regarding the potential for immune escape as a source for relapse. Reports of CD19-negative relapses in patients treated with CD19-targeted CAR T cells have been published[17, 18], and one group[25] presented results that estimate CD19-negative escape may account for half of all relapses. Finally, all groups investigating CD19-targeted CAR T cells have also reported that CRS correlates with pre-treatment disease burden. Understanding the biology of the CRS and developing effective clinical management schemes are now the main limiting factors for the successful clinical adaptation of CAR T cells to a wider population of cancer patients.
The Cytokine Release Syndrome
CD19-targeted CAR T cells infused into patients with relapsed/refractory B-ALL, and to a lesser extent NHL result in a constellation of clinical signs and symptoms that are likely a by-product of en masse T cell activation. The diagnostic hallmark of this syndrome is the secretion of cytokines, although no consistent pattern of up-regulated cytokines is evident, probably reflecting the personalized nature of this therapy, since every CAR T cell product is different. On average, within 1-5 days of CAR T cell infusion patients develop fevers, with some being high-grade (>40°C), that persist despite treatment with anti-pyretics.[15-19] Over the ensuing days these patients develop varying levels of hypoxia, hypotension, and tachycardia. These toxicities can be self-limiting and require only monitoring with minimal support such as fluids and supplemental oxygen by nasal cannula. However, some patients require aggressive support such as vasopressors and/or mechanical ventilation, as well as therapies directed to attenuating the CRS.
While cytokines are largely the cause for the clinically worrisome toxicities, they also represent a potential target for attenuation of the CRS. Corticosteroids and cytokine blocking proteins, such as tocilizumab and etanercept, have been used with success at ameliorating the CRS.[15-19] Treatment of patients with these agents results in rapid (within a few hours) resolution of the toxicities from the CRS. However, early evidence suggests that some of the CRS interventions may affect CAR T cell efficacy. The MSKCC group determined that steroid treatment was lymphotoxic, resulting in no CAR T cell expansion presumably due to CAR T cell death.[15, 16] Importantly, they reported no such lymphotoxic CAR T cell effect after single agent tocilizumab treatment. In fact, CAR T cells were detected at 5x greater levels in the BM of CRS patients given supportive care alone or treated with tocilizumab versus steroids.[15, 16] This lack of expansion impacts clinical outcome since all the patients treated with steroids in the MSKCC cohort developed early relapses. While cytokine blocking agents do not appear to affect CAR T cell expansion, it is unknown if they may limit long-term CAR T cell efficacy, which will only be revealed when there is a long enough follow-up to assess durability of remissions in patients treated with these agents.
Patients in all three trials also developed neurologic toxicities after CAR T cell infusion.[15-19] These can range from mild complications, such as confusion, to severe complications such as seizures and/or obtundation requiring intubation and mechanical ventilation. In some patients the toxicities progressed with the patients first developing mild confusion, progressing to delirium, aphasia, and ultimately resulting in obtundation and/or seizures. The mechanism behind these toxicities remains in question although there is evidence to implicate CAR T cells. First of all, widespread activation of T cells can mediate a similar constellation of symptoms as reported by clinical trials using anti-CD28 or blinatumomab antibodies, which both activate T cells en masse.[26, 27] Furthermore, the majority of the neurotoxicities occur in patients with severe CRS. All three groups have documented infiltration of CAR T cells into the cerebrospinal fluid.[16, 18, 19] It is uncertain if infiltration of the CAR T cells is antigen-specific or a by-product of inflammation. While the CRS and neurotoxicities are concerning it is re-assuring that all groups have reported full resolution of symptoms and no long-lasting deficits.
Conclusions
The success of CD19-targeted CAR T cells against B-ALL has established the potential for CAR T cells as a cancer immunotherapy. The main focus over the next several years will involve confirming the clinical efficacy of this therapy for B-ALL to garner the first clinical indication for a gene-engineered cell immunotherapy. However, there will also be new trials evaluating CARs against a number of other malignancies to establish its utility as a general cancer therapy. Early results targeting solid tumors have demonstrated safety but limited efficacy suggesting that adapting this therapy to other cancers will require additional genetic modifications of the CAR or the CAR T cells that will utilize advances in CAR design.
Contributor Information
Marco L Davila, Moffitt Cancer Center.
Craig Sauter, Memorial Sloan Kettering Cancer Center.
Renier Brentjens, Memorial Sloan Kettering Cancer Center.
References
- 1.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nature reviews Cancer. 2003 Jan;3(1):35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 2.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003 Mar;9(3):279–86. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 3.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004 Apr;18(4):676–84. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 4.Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006 Nov 15;66(22):10995–1004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 5.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004 Jan 1;172(1):104–13. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 6.Song DG, Ye Q, Poussin M, Harms GM, Figini M, Powell DJ., Jr. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood. 2012 Jan 19;119(3):696–706. doi: 10.1182/blood-2011-03-344275. [DOI] [PubMed] [Google Scholar]
- 7.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011 May 2;121(5):1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011 Nov 3;118(18):4817–28. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010 Sep;16(9):1245–56. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma and Indolent B-Cell Malignancies Can Be Effectively Treated With Autologous T Cells Expressing an Anti-CD19 Chimeric Antigen Receptor. J Clin Oncol. 2014 Aug 25; doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochenderfer JN, Somerville R, Lu L, Iwamoto A, Yang JC, Klebanoff C, et al. Anti-CD19 CAR T Cells Administered after Low-Dose Chemotherapy Can Induce Remissions of Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma. Blood. 2014;124(21):550. [Google Scholar]
- 12.Schuster SJ, Svoboda J, Nasta S, Porter DL, Mato A, Shah GD, et al. Phase IIa trial of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed or refractory CD19+ lymphomas. J Clin Oncol. 2015;33(suppl) abstract 8516. [Google Scholar]
- 13.Turtle CJ, Berger C, Sommermeyer D, Budiarto T, Hanafi L, Melville K, et al. Immunotherapy with CD19-specific chimeric antigen receptor (CAR)-modified T cells of defined subset composition. J Clin Oncol. 2015;33(suppl) abstract 3006. [Google Scholar]
- 14.Sauter CS, Riviere I, Bernal Y, Wang X, Purdon T, Yoo S, et al. Phase I trial of 19-28z chimeric antigen modified T cells (19-28z CAR-T) post-high dose therapy and autologous stem cell transplant (HDT-ASCT) for relapsed and refractory (rel/ref) aggressive B-cell non-Hodgkin lymphoma (B-NHL) J Clin Oncol. 2015;33(suppl) abstract 8515. [Google Scholar]
- 15.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013 Mar 20;5(177):177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014 Feb 19;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013 Apr 18;368(16):1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014 Oct 16;371(16):1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2014 Oct 10; doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007 Feb 1;109(3):944–50. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien S, Schiller G, Lister J, Damon L, Goldberg S, Aulitzky W, et al. High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome-negative acute lymphoblastic leukemia. J Clin Oncol. 2013 Feb 20;31(6):676–83. doi: 10.1200/JCO.2012.46.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JH, Riviere I, Wang X, Bernal Y, Purdon T, Halton E, et al. Efficacy and safety of CD19-targeted 19-28z CAR modified T cells in adult patients with relapsed or refractory B-ALL. ASCO Meeting Abstracts; 2015 May 18.2015. p. 7010. [Google Scholar]
- 23.Turtle CJ, Berger C, Sommermeyer D, Budiarto T, Hanafi L-A, Melville K, et al. Immunotherapy with CD19-specific chimeric antigen receptor (CAR)-modified T cells of defined subset composition. ASCO Meeting Abstracts; 2015 May 18.2015. p. 3006. [Google Scholar]
- 24.Lee DW, Stetler-Stevenson M, Sabatino M, Yuan C, Fry TJ, Shah NN, et al. Intent-to-Treat Results of a Phase I Trial of CD19 Chimeric Antigen Receptor Engineered T Cells Using a Consistent Treatment Regimen Reveals a 67% Complete Response Rate in Relapsed, Refractory Acute Lymphoblastic Leukemia. 2014.
- 25.Grupp SA, Maude SL, Shaw P, Aplenc R, Barrett DM, Callahan C, et al. T Cells Engineered with a Chimeric Antigen Receptor (CAR) Targeting CD19 (CTL019) Have Long Term Persistence and Induce Durable Remissions in Children with Relapsed, Refractory ALL. 2014.
- 26.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006 Sep 7;355(10):1018–28. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 27.Teachey DT, Rheingold SR, Maude SL, Zugmaier G, Barrett DM, Seif AE, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013 Jun 27;121(26):5154–7. doi: 10.1182/blood-2013-02-485623. [DOI] [PMC free article] [PubMed] [Google Scholar]