Abstract
Objective
To evaluate peripheral blood T-helper (TH) cell associated cytokine and chemokine profiles in localized scleroderma (LS), and correlate them with clinical disease features, including disease activity parameters.
Methods
A 29-plex Luminex platform was used to analyze the humoral profile of plasma samples from 69 pediatric LS patients and 71 healthy pediatric controls. Cytokine/chemokine levels were compared between these two groups and within LS patients, focusing on validated clinical outcome measures of disease activity and damage in LS.
Results
Plasma levels of IP-10, MCP-1, IL-17a, IL-12p70, GM-CSF, PDGF-bb, IFN-α2, and IFN-γ were significantly higher in LS compared to healthy controls. Analysis within the LS group demonstrated IP-10, TNF-α and GM-CSF correlated with clinical measures of disease activity. Several cytokines/chemokines correlated with anti-histone antibody, while only a few correlated with positive ANA and single-stranded DNA antibody.
Conclusion
This is the first time that multiple cytokines and chemokines have been examined simultaneously LS. In general, a TH-1 (IFN-γ) and TH-17 (IL-17a) predominance was demonstrated in LS compared to healthy controls. There is also an IFN–γ signature with elevated IP-10, MCP-1 and IFN-γ, which has been previously demonstrated in systemic sclerosis, suggesting a shared pathophysiology. Within the LS patients, those with active disease demonstrated IP-10, TNF-α and GM-CSF, which may potentially serve as biomarkers of disease activity in the clinical setting.
Keywords: localized scleroderma, pediatric rheumatology, cytokines, T-helper cells, Luminex, disease activity
1. INTRODUCTION
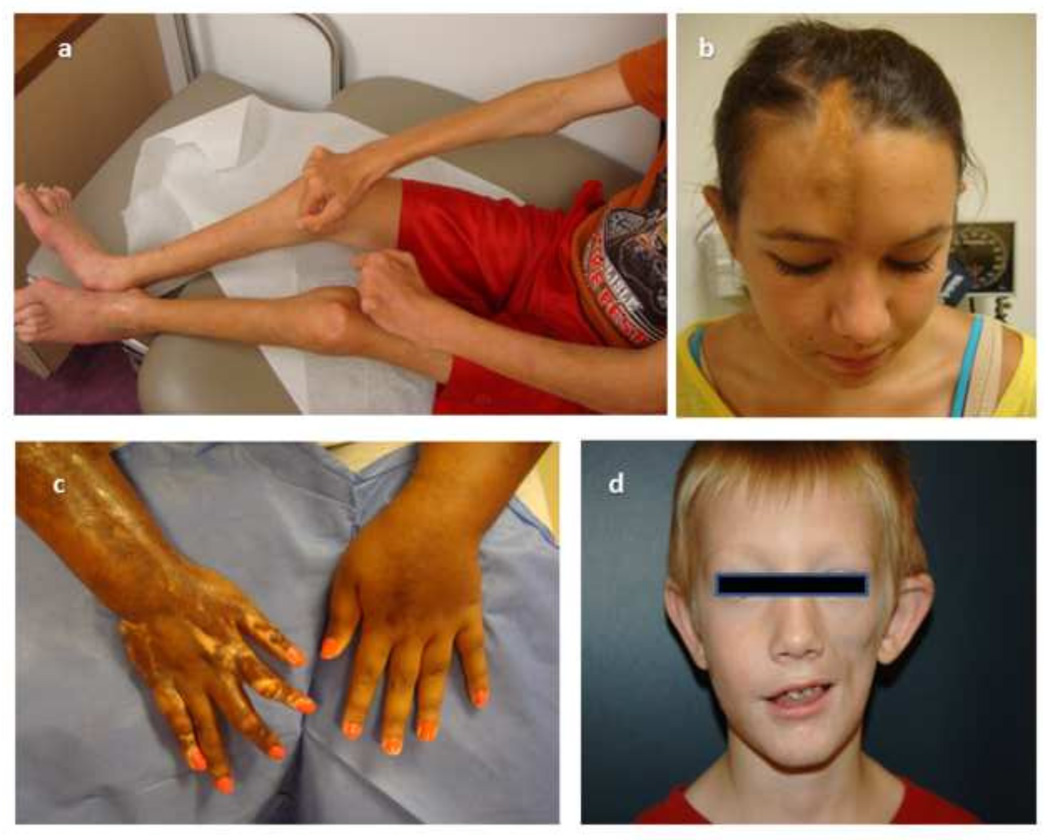
“Scleroderma” encompasses systemic sclerosis (SSc) and localized scleroderma (LS). SSc, which more commonly affects adults, is characterized by skin, vascular and visceral organ fibrosis. In contrast, LS, also known as ‘morphea’, which more commonly affects children, is characterized by fibrosis of skin and underlying tissue without vascular or internal organ involvement [1]. The hallmark of LS is skin lesions with an inflammatory ‘active’ phase demonstrated histologically by a dense dermal and subcutaneous lymphocytic infiltrate causing clinical signs of erythema and edema. This is followed by a ‘damage’ phase characterized by closely packed homogenous dense collagen deposition with no or sparse cellular infiltrate leading to physical examination findings of fibrotic patches or linear bands of skin that are thick, hard and discolored on the face, trunk, and extremities (Fig 1).
Figure 1. Clinical manifestations of localized scleroderma (LS) in children.
Subtypes of LS cause different manifestations of disease involvement and damage; a) pansclerotic morphea, affecting all four extremities and some of trunk b) linear scleroderma of the head, ‘en coup de sabre’, resulting in a deep linear band down the mid forehead with skull depression, c) linear scleroderma extremity, causing elbow, wrist and significant finger joint contracture, d) linear scleroderma of the head, hemifacial atrophy/Parry Romberg, atrophy of left face with associated brainstem lesion.
The fibrosis and resultant atrophy of the skin and underlying connective tissue, including subcutaneous fat, muscle, tendons and bone, cause significant deformity and severe functional impairment in actively growing children [2]. Because juvenile LS presents during development and can persist for years (mean disease duration of 13.5 years [3]) the morbidity, especially growth disturbance and contractures of the limbs, can be quite substantial. A recent review of a prospective cohort of 253 LS patients in the Childhood Arthritis & Rheumatology Research Alliance (CARRA) registry found that 38% had musculoskeletal damage including muscle atrophy, extremity shortening and/or joint contractures, and 25% had limited functional capacity [4]. In long term follow-up of adults with childhood onset LS, 25% reported mild to moderate disability after 20 years [1]. Disease damage is most notable in the linear scleroderma subset of LS, the most common subset in juvenile LS (60%). It involves the limbs in a progressive manner, transversing joints leading to contractures of muscles and tendons and ankylosis of the joints (Fig 1a &1c). In patients with involvement of the scalp and face, in either a linear pattern/grove (‘en coup de sabre’) or hemifacial atrophy pattern (‘Parry Romberg syndrome’) there is an association with neurological manifestations (20%), including chronic headaches, strokes and seizures [5–7].
Understanding the mediators involved and intervening in the active inflammatory phase of the disease, before excessive fibrosis and atrophy occurs, is key to halting this process. The pathogenesis of LS is understudied, and most literature relies on it sharing the clinical feature of skin fibrosis with its ‘companion disease’ SSc. Evaluation of the skin lesions in both LS and SSc show lymphocytes as the predominant cell type in the dermal and subcutaneous infiltrate, with LS demonstrating more intense inflammation [8, 9]. The lymphocytes have been further characterized in SSc, with T lymphocytes (CD4 and CD8) as the predominant cell type in the dermis of adult SSc patients [10, 11]. T cell activation and associated cytokine release are thought to play a pivotal role in SSc pathogenesis through stimulating fibroblasts to promote fibrosis [12, 13]. The CD4+ T-helper (TH) cell lineage and its effector cytokines have been identified in biopsies of skin lesions of adult patients with SSc [10, 14, 15], and in peripheral circulation[16–18] and culture in supernatants of peripheral blood mononuclear cells (PBMC) of SSc patients [19]. TH cells include 3 main effector cell types, TH1, TH2, TH17, each are differentiation states characterized by the predominant cytokines they produce, namely interferon-γ (IFN-γ), interleukin-4 (IL-4) / IL-13 and IL-17, respectively. Many autoimmune diseases, including scleroderma, are thought to be propagated by an imbalance of TH cell subsets and their associated cytokines [10, 14–20, 21].
There is a wide breadth of literature supporting a TH2 cellular and associated cytokine predominance in SSc (reviewed by Hasegawa et al 2012 [13]), with peripheral blood and tissue (skin and lung) derived IL-4 and IL-13 correlating with the degree of skin and lung fibrosis and disease burden [12, 13, 18, 22–26]. Both IL-4 and IL-13 are effector cytokines of the TH2 lineage characterized as pro-fibrotic and anti-inflammatory due to their respective actions as initiators of extracellular matrix production and inhibitors of TH1 function [27]. Therefore, it is suspected that the TH2 cytokine signature in SSc supports the fibrotic component of the disease, with peripheral cytokines serving as prognostic markers in SSc patients [12, 13]. The cytokines associated with a TH1 profile, IL-1, TNF-α and IFN-γ, have been identified as elevated in the peripheral blood of SSc patients compared to healthy controls and their levels decrease overtime, signifying their elevation during earlier, active disease [28]. The ‘hallmark’ effector cytokine of TH17 cells, IL-17a, has been demonstrated in significant amounts in the skin, lungs and sera of adult SSc patients during the early, more active, stages of the disease [16]. These findings suggest that TH17 and TH1 cells may contribute to the cellular inflammation in SSc via production of associated pro-inflammatory cytokines [13].
The literature available regarding the potential role of TH cells and associated cytokines in localized scleroderma is much more limited, with only a few studies evaluating TH cell associated cytokines in the peripheral blood of LS patients [20, 21, 29–33] (Table 1). Three of these studies included TH1 and TH17 associated cytokines, IL-1β, TNF-α, IL-17a, and IL-23 , with the latter 3 cytokines correlating inversely with disease duration and IL-1β associating with active disease state [21, 32, 33]. Two studies evaluating sera levels of TH2 associated cytokines, IL-4 and IL-13, demonstrated clinical correlation to disease burden in LS via ‘lesion load’, both total number of lesions and presence of generalized plaque morphea subtype (defined as >3 large lesions in > 2 anatomic areas) [20, 21]. These findings support our hypothesis discussed in our prior review of the literature that LS is likely a predominately TH1 disease in the inflammatory/early stages whereas it is likely a TH2 driven process in the later, fibrotic stages [29].
Table 1.
T-helper cell associated cytokines and chemokines evaluated in localized scleroderma (LS)
| Reference | No. of LS patients |
Subtypes | Cytokine | Specimen | Cytokine level* |
Disease features associated with cytokine presence |
|---|---|---|---|---|---|---|
| Danczak-Pazdrowska CEJI 2012 | 41 | Plaque (20) Generalized (14) Linear (7) |
IL-1β | Plasma (ELISA) IL-1β gene in PBMC and skin (real time PCR) |
Elevated in LS plasma and gene expression PBMC |
ANA titer and active disease state correlated only in linear scleroderma subgroup to IL- 1β in PBMC |
| Danczak-Pazdrowska AMS 2012 | 41 | Plaque (20) Generalized (14) Linear (7) |
IL-17a | Plasma (ELISA) IL-17a and IL-23 gene in PBMC and skin (real-time PCR) |
IL-17a gene elevated in LS PBMC and decreased in skin |
IL-17a in PBMC negative correlation to disease duration of entire LS group, and to the LoSSI in GM subtype |
| IL-23 | IL-23 gene elevated in LS PBMC |
IL-17a in skin negative correlation ANA titer in GM subgroup |
||||
| IL-23 in PBMC negative correlation to disease duration of entire LS group, and to the LoSSI in plaque and GM subtypes |
||||||
| Ihn 1995 | 48 | Linear (22) Generalized (16) Plaque (10) |
IL-2 IL-4 IL-6 |
Serum (ELISA) | IL-2, IL-4, and IL-6 elevated in LS |
Presence of IL-2, IL-4, and IL-6 correlated to one another and had higher frequency of detection in GM subtype IL-2 correlated with RF positivity IL-4 and IL-6 correlated with AHA positivity |
| Ihn 1994 | 48 | Linear (22) Generalized (16) Plaque (10) |
IL-8 | Serum (ELISA) | IL-8 elevated in LS |
Higher detection frequency in generalized and linear subtypes |
| Hasegawa 2003 | 45 | Linear (22) Generalized (12) Plaque (11) |
IL-13 | Serum (ELISA) |
IL-13 and TNF-α elevated in LS |
IL-13 correlated with number of lesions Higher detection of IL-13 frequency in GM and plaque subtypes |
| TNF-α | TNF-α correlated with IL-6, AHA, and anti-ssDNA presence, number of linear lesions, and frequency of muscle involvement Higher levels of TNF-α correlated with shorter disease duration Higher detection frequency of TNF-α in GM and linear subtypes |
compared to healthy controls
RT PCR – real time polymerase chain reaction
PBMC – peripheral blood mononuclear cells
LoSSI – localized scleroderma severity score index
GM – generalized morphea
ANA – anti-nuclear antibody
AHA – anti-histone antibody
Anti-ssDNA – anti-single-stranded DNA antibody
In regard to these prior studies in LS (Table 1), three or fewer serum cytokines were evaluated at a time, limiting the ability to compare across the different TH cell associated cytokine profiles in tandem, none of the studies were specifically conducted in a pediatric population and few used validated outcome measures in LS. Therefore, this study was designed to examine a more global array of TH1, TH2 and TH17 associated cytokines and chemokines in a well-established pediatric-onset LS cohort, and to compare them to validated clinical parameters in LS. Examining for the presence of TH -associated cytokines in the peripheral blood of LS subjects in parallel with detailed clinical information is one of the first steps to investigate their potential role in localized scleroderma as clinically actionable biomarkers as well as biomarkers reflecting pathogenesis, which may direct the path to the identification of new therapeutic targets.
2. METHODS
2.1. Study participants and controls
Plasma samples were obtained from pediatric LS patients and healthy pediatric controls using two separate processes. Healthy plasma control samples were obtained through an IRB approved protocol to collect and store de-identified excess clinical samples obtained from well-child visits for routine pediatric care (typically in tandem with routine lead toxicity and hemoglobin screening). These subjects are clinically screened for any acute illnesses (fever, infection or trauma) and chronic illnesses. An honest broker provided basic demographic information including age and sex. Blood samples and concurrent clinical data of LS subjects were collected prospectively during their clinical visits to the Pediatric Scleroderma Clinic at the Children’s Hospital of Pittsburgh of UPMC (CHP) through the National Registry of Childhood Onset Scleroderma Database (NRCOS; IRB protocol approved since 2003). Consent and assent were obtained from parents and their children prior to initiating research activities. All LS patients were diagnosed clinically by a pediatric rheumatologist (KST) and were further classified by disease subtype according to the Padua criteria [34].
For all subjects, plasma was separated from whole blood by low speed centrifugation at 4°C within 24 hours of blood collection and stored in 200 µL aliquots at −80°C.
2.2. Clinical measures
LS patients were evaluated by their treating physician (KST) using two validated clinical activity measures, the Physician Global Assessment of Disease Activity (PGA-A) and the modified Localized Scleroderma Severity Index (mLoSSI) [35]. PGA-A is graded on a 100-mm visual analog scale (VAS), accounting for cutaneous variables including new lesions within the previous month, enlargement of any existing lesion within the past month, erythema/violaceous color at the lesion border, and skin thickening/induration at the lesion border [35]. The mLoSSI includes the sum of three separate scores from the following domains summed over 18 cutaneous body sites: erythema (none, pink, red, dark red), skin thickness (none, mild, moderate, marked), and new lesion/lesion extension (absent, present) [35]. LS patients were considered to have active disease if they had both PGA-A and mLoSSI scores greater than zero [36].
Disease damage measures including Physician Global Assessment of Damage (PGA-D) and the Localized Scleroderma Damage Index (LoSDI) were also utilized [37]. PGA-D was reported using a 100-mm VAS scale to reflect both cutaneous (hyper/hypopigmentation, dermal atrophy, subcutaneous atrophy) and extra-cutaneous manifestations (joint contracture, limb-length discrepancy, facial atrophy etc.) [37]. The LoSDI was created using the sum of individual scores for 18 surface body sites and assessed dermal atrophy (none, shiny skin, visible vessels, “cliff drop”), subcutaneous atrophy (none, flat, concave, marked atrophy) and maximum hyperpigmentation/hypopigmentation [37].
Other clinical outcome measures that were collected included the following: disease duration, systemic treatment, disease subtype, presence of extracutaneous manifestations (ECMs), patient measured indicators, traditional inflammatory markers (i.e. – sedimentation rate) and antibody status. The patient reported indicators included were the parent and patient Global Assessment of disease impact (Parent-GA, Patient-GA), which are 0 to 100 VAS scales regarding disease severity (no problem = 0, to very severe problem = 100), and the Childhood Dermatology Life Quality Index (CDLQI). The CDLQI is a reliable and valid measure of quality of life impact in pediatric dermatologic diseases [38], including LS [39]. Patients answer 10 questions, which are summed into an overall score, range 0–30. Higher scores indicate that quality of life has been adversely affected.
Specific antibodies evaluated for this study included anti-nuclear antibodies (ANA), anti-histone antibodies (AHA) and anti-single stranded DNA antibodies (anti-ssDNA). These have been described in LS as reflecting disease severity [40, 41] and have been associated with other cytokines evaluated in LS [20, 21, 33]. Levels of ANA for all LS subjects (healthy controls not tested) were determined using indirect immunofluorescence on HEp-2 cells through the University of Pittsburgh Immunology laboratory. An ANA titer of 1:80 or greater was considered positive. Sera samples meeting the cutoff titer of 1:80 were serially diluted to 1:1280. AHA and anti-ssDNA antibodies were assayed by ELISA through the University of Pittsburgh Immunology laboratory. AHA levels higher than 1.0 U/mL and levels of anti-ssDNA antibodies higher than 19 U/mL were considered positive.
2.3. Cytokine and chemokine analysis
Plasma levels of twenty-nine total TH1, TH2, and TH17-associated cytokines and chemokines were analyzed using Milliplex Luminex xMAP Technology (EMD Millipore, USA) and the Luminex bead immunoassay system (Human Cytokine/chemokine Panel 1 and human TH17 kits; BioRad, Hercules, CA), following the manufacturer’s instructions. These studies were performed at the University of Pittsburgh Cancer Institute (UPCI) Luminex Core Facility. Plasma samples (both LS and healthy control) were undiluted and compared to a High-PMT-Standard Dilution Series (BioRad, Hercules, CA). Samples were run in duplicate, and inter-panel and intra-assay control plasma samples were included to ensure consistency across panels. The cytokines and chemokines included were macrophage inflammatory protein −1α (MIP-1α), interferon gamma-induced protein 10 (IP-10), interleukin −5 (IL-5), IL-4, IL-2, IL-17a, IL-13, IL-12p70, granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor-α (TNF-α), IL-8, IL-6, IL-1β, IL-10, interferon-γ (IFN-γ), IFN-α2, platelet-derived growth factor-bb (PDGF-bb), IL-1α, monocyte chemotactic protein- 1 (MCP-1), vascular endothelial growth factor (VEGF), IL-17f, IL-22, IL-33, IL-21, IL-23, IL-17e, IL-27, IL-31, and IL-28α (general detection limit 3.2 pg/mL – 10,000 pg/mL).
2.4. Data and statistical analysis
All analyses were performed using software (SPSS, Version.20, IBM Corp, Armonk, NY) and R language, version 13.1 (R Core Team, Vienna, Austria). Agreement between duplicate samples was measured using the intra-class correlation coefficient (ICC). Medians and interquartile ranges (IQR) and counts and percentages were used to summarize continuous and categorical variables respectively. Cytokine and chemokine levels in LS patients were compared to levels in healthy controls using Mann-Whitney U tests. Similar analyses were performed on only the LS patients using categorical clinical variables to form subgroups, such as active versus inactive disease and antibody-positive versus negative patients.
Adjusted analyses were also conducted in which the log of each cytokine/chemokine was entered as predictor of disease group adjusted for age at sample collection. To allow for the possibility of a non-linear effect of age on the log-odds of LS, natural cubic splines were used. Models that adjust for sex in addition to age were also fitted but did not alter the results.
Spearman’s correlation was used to examine relationships among cytokines (e.g., IL-4, IL-5 and IL-13 would be expected to correlate since they are thought to demonstrate a TH2 ‘signal’) and between cytokines and continuous outcomes (clinical disease activity, damage and severity measures, and patient-derived outcome measures). All p-values associated with testing the 29 cytokines/chemokines were corrected for False Discovery Rates (FDR) using the Benjamini-Hochberg adjustment (B–H). Alpha levels were set at 0.05 for all analyses and both raw and corrected p-values are reported.
3. RESULTS
3.1 Characteristics of the study cohort
Plasma samples from 69 pediatric LS patients and 71 healthy pediatric controls were included in the analysis. Demographic and clinical data collected on the LS subjects are summarized in Table 2. For LS subjects, the average age at sample collection was 12.5 years (median = 13, IQR = 10 – 16) and the majority of patients were female (67%) and Caucasian (91%). The average age of healthy controls was 5.9 years (median = 3, IQR = 2 – 9) and the majority were also female (54%).
Table 2.
Demographic and clinical features of localized scleroderma patients (n=69)
| Clinical Parameters | |
|---|---|
| Demographics | median (IQR) |
| Age at disease onset (years) | 9.0 (7.0–11.0) |
| Age at sample collection (years) | 13.0 (10.0 – 16.0) |
| Disease duration at sample collection (years) | 3.4 (0.3 – 5.3) |
| LS Subtype | n (%) |
| Linear scleroderma trunk/extremity | 27 (39) |
| Linear scleroderma head (en coup de sabre / Parry Romberg Syndrome) | 14 (20) |
| Mixed morphea | 10 (14) |
| Generalized morphea | 8 (12) |
| Plaque (circumscribed superficial) morphea | 5 (7) |
| Deep (circumscribed deep) morphea | 4 (6) |
| Pansclerotic morphea | 1 (1) |
| Disease Activity Measures | median (IQR) |
| PGA-A* | 0 (0 – 34.0) |
| mLoSSI* | 1.0 (0.0 – 6.0) |
| Disease Damage Measures | |
| PGA-D | 31.0 (21.0 – 39.0) |
| LoSDI | 9.0 (5.0 – 17.5) |
| Parent/Patient Global Assessment | |
| Parent-GA | 15.5 (3.0 – 49.25) |
| Patient-GA | 10.0 (0.0 – 54.0) |
| CDLQI | 2.0 (0.25 – 4.75) |
| Serum Antibody Positivity | n (%) |
| ANA | 22/62 (36) |
| AHA | 19/59 (32) |
| Anti-ssDNA | 17/57 (30) |
| *For those with active disease (n = 30): | median (IQR) |
| PGA-A | 39.0 (28.0–50.0) |
| mLoSSI | 6.0 (3.0–9.0) |
PGA-A, Physician global assessment of activity; mLoSSI, modified Localized Scleroderma Skin Score; PGA-D, Physician Global Assessment of Damage; LoSDI, Localized Scleroderma Damage Index; Parent-GA, parent Global Assessment of disease impact; Patient-GA, patient Global Assessment of disease impact; CDLQI, Childhood Dermatology Life Quality Index; ANA, anti-nuclear antibodies; AHA, anti-histone antibodies; anti-ssDNA, anti-single stranded DNA antibodies
Upon defining LS subjects as active (PGA-A and mLoSSI scores >0), thirty subjects (43%) were classified as having active disease with a median PGA-A = 39.0 and median mLoSSI = 6.0 (Table 2). Most LS patients with active disease (73%, 22/30) were not on systemic medications at the time of blood draw, reflecting that this was their initial rheumatology visit or that they were experiencing a flare of disease off medication. Conversely, the majority of the inactive LS group (34/39, 87%) were on systemic medications at time of blood draw, reflecting disease remission on systemic therapy. Overall, 42 (60%) LS subjects were on standard systemic treatment with methotrexate + prednisone [42–44] at the time of blood draw and had a median treatment duration of 1.4 years (IQR 0.0 – 3.7 years).
3.2 Consistency among Luminex plates
Agreement between duplicate samples was moderate to high (ICC: 0.63 – 0.99) indicating excellent reproducibility and inter-assay controls. Average analyte levels between the duplicate wells were used for further analyses.
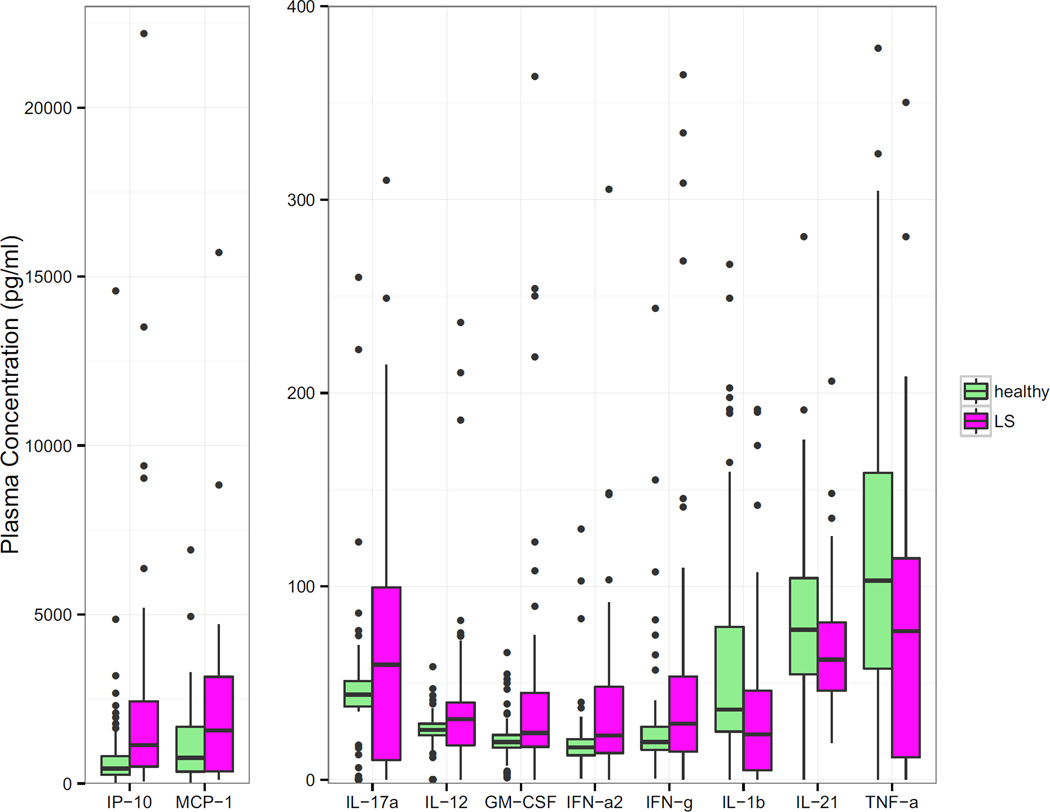
3.3 Healthy vs. LS patient cytokine profiles
The following cytokines and chemokines were found to be elevated in the plasma of pediatric LS patients when compared to healthy pediatric controls: IP-10, MCP-1, IL-17a, IL-12p70, GM-CSF, PDGF-bb, IFN-α2 and IFN-γ (Table 3 & Figure 2). Plasma levels of IL-1β, IL-21 and TNF-α were found to be significantly lower in LS plasma samples than in healthy controls (Table 3 & Figure 2). When correcting for FDR using the Benjamini-Hochberg method and after adjusting for age using logistic models, most of the cytokine and chemokine median levels remained significantly different from controls (Table 3).
Table 3.
Comparison of significantly different cytokine and chemokine levels between localized scleroderma patients and healthy controls (plasma, pg/mL)
| LS plasma levels Median (IQR) n = 69 |
Control plasma levels Median (IQR) n = 71 |
p | *corrected p | |
|---|---|---|---|---|
| Elevated in LS | ||||
| IP-10 ‡€ | 1140.3 (502.3 – 2431.8) | 445.8 (258.5 – 806.2) | <0.001 | <0.001 |
| MCP-1 ‡€ | 1573.0 (362.0 – 3185.5) | 757.0 (349.9 – 1678.3) | 0.004 | 0.024 |
| IL-17a ‡€ | 59.5 (10.2 – 99.5) | 44.0 (37.9 – 51.0) | 0.003 | 0.023 |
| IL-12 ‡ | 31.3 (17.8 – 40.0) | 25.80 (22.9 – 29.0) | 0.002 | 0.017 |
| GM-CSF‡ | 24.1 (17.0 – 45.0) | 19.5 (16.5 – 23.2) | 0.007 | 0.033 |
| IFN-α2 ‡€ | 22.9 (13.8 – 48.0) | 16.8 (12.7 – 20.9) | 0.011 | 0.045 |
| PDGF-bb | 28.5 (16.8 – 2854.9) | 17.0 (15.0 – 25.4) | 0.018 | 0.057 |
| IFN-γ | 29.0 (14.5 – 53.3) | 19.5 (15.5 – 27.4) | 0.040 | 0.107 |
| Decreased in LS | ||||
| IL-lβ ‡ | 23.5 (4.9 – 46.0) | 36.3 (24.9 – 79.0) | 0.002 | 0.017 |
| IL-21 € | 62.2 (46.0 – 81.4) | 77.5 (54.0 – 104.3) | 0.015 | 0.055 |
| TNFα | 76.8 (11.5 – 114.5) | 103.0 (57.5 – 158.8) | 0.039 | 0.107 |
corrected for False Discovery Rates (FDR) by Benjamini-Hochberg (B–H) method
significant after B-H correction
significant after adjusting for age and B-H correction
Figure 2. Plasma cytokine and chemokine level (pg/mL) comparison between pediatric localized scleroderma patients (n=69) and healthy pediatric controls (n=71).
The plasma cytokines and chemokines demonstrated were significantly different between pediatric LS and healthy. PDGF-bb was the only significantly different chemokine not displayed due to outliers, though medians are shown in Table 3.
3.4 Relationships among cytokines
The TH1 analytes (IFN-γ, IL-12p70 and TNF-α) were significantly correlated with each other (rs of 0.49 – 0.74; p <0.001). Similarly, the TH2 analytes (IL-4, −5, and −13) also demonstrated significant positive correlations with each other (rs 0.83 – 0.91; p <0.001). The TH17 analytes (IL-17a, −17e, −17f, −22, −23, −27, −28a, and −31) also showed moderate to high positive correlations with each other (rs 0.57 – 0.74; p <0.001).
3.5 Relationships between cytokine/chemokine levels and LS clinical features
3.5.1. Disease activity
IP-10 was significantly elevated in patients with active disease compared to levels in patients with inactive disease (Table 5). IP-10 was also found to correlate moderately positively with clinical LS disease activity parameters, the PGA-A (rs = 0.45) and the mLoSSI (rs 0.34) (Table 4).Other analytes that were significantly elevated in LS subjects with active compared to inactive disease were GM-CSF and TNF-α (Table 5). Both had fair correlations with the PGA-A (rs = 0.28 and 0.31 respectively), and TNF-α also correlated with the mLoSSI (rs = 0.29) (Table 4). IL-8, IL-13, IL-1β, and IL-10 did not distinguish active from inactive disease, but did significantly correlate with PGA-A and/or mLoSSI clinical parameters (Table 4).
Table 5.
Comparison of significant difference in cytokine and chemokine levels in localized scleroderma with and without additional clinical features (plasma, pg/mL)
| Clinical feature | Plasma level those with feature Median (IQR) |
Plasma levels those without feature Median (IQR) |
p |
*corrected p |
|
|---|---|---|---|---|---|
| Disease status | Active disease (n=30) |
Inactive disease (n=39) |
|||
| IP-10‡ | 2087 (1022.1 – 3080.3) | 880 (450.9 – 1271.6) | 0.001 | 0.028 | |
| GM-CSF | 33.9 (21.8–59.7) | 21.0 (7.0–34.5) | 0.032 | 0.299 | |
| TNF-α | 93.75 (27.4–141.5) | 74.0 (8.9–107.3) | 0.043 | 0.299 | |
| Extracutaneous manifestations (ECM) | |||||
| Present (n = 17) | Absent (n = 52) | ||||
| Musculoskeletal | |||||
| No significant differences in cytokine levels | |||||
| Present (n = 12) | Absent (n = 57) | ||||
| Neurological | |||||
| IL-17a | 30.0 (2.6 – 62.1) | 66.0 (26.0 – 103.0) | 0.024 | 0.422 | |
| IFN-γ | 15.4 (6.8 – 43.5) | 31.0 (17.0 – 57.0) | 0.031 | 0.422 | |
| ¥Traditional inflammatory markers | |||||
| Present (n = 13) | Absent (n = 56) | ||||
| Eosinophilia (> 3%) | |||||
| IL-5 | 22.3 (10.4 – 81.0) | 11.0 (0.1 −28.0) | 0.047 | 0.333 | |
| IL-21 | 77.8 (58.5 – 97.7) | 60 (40.2 – 76.7) | 0.036 | 0.033 | |
| Elevated (n = 5) | Normal (n = 64) | ||||
| Elevated sedimentation rate | |||||
| ( >20 mm/h) | IP-10‡ | 3166.0 (2392.0 – 17840.0) | 983.0 (481.0 – 2074.0) | 0.001 | 0.029 |
| TNF-α | 208.5 (65.2 – 383.6) | 74.0 (9.6 – 113.5) | 0.025 | 0.363 | |
corrected for False Discovery Rates (FDR) by Benjamini-Hochberg (B–H) method
significant after B-H correction
too few patients with abnormal lab values for other traditional markers analyzed: White blood cell count, C-Reactive protein, and Creatine phosphokinase (CPK)
Table 4.
Significant correlations of clinical features with plasma cytokine and chemokine levels in localized scleroderma patients (n = 69)
| Cytokine | Spearman’s rho | p | *corrected p | |
|---|---|---|---|---|
| Disease Activity Measure | ||||
| PGA-A | IP-10 ‡ | 0.45 | <0.001 | 0.005 |
| TNF-α | 0.31 | 0.012 | 0.167 | |
| IL-8 | 0.28 | 0.023 | 0.167 | |
| GM-CSF | 0.28 | 0.023 | 0.167 | |
| mLoSSI | IP-10 | 0.34 | 0.004 | 0.130 |
| TNF-α | 0.29 | 0.018 | 0.187 | |
| IL-8 | 0.25 | 0.038 | 0.187 | |
| IL-10 | 0.26 | 0.031 | 0.187 | |
| IL-13 | 0.24 | 0.049 | 0.187 | |
| IL-lβ | 0.25 | 0.045 | 0.187 | |
| Disease Damage Measure | ||||
| PGA-D | No significant correlations | |||
| LoSDI | IL-17a | −0.31 | 0.011 | 0.190 |
| IL-4 | −0.29 | 0.019 | 0.190 | |
| IL-6 | −0.28 | 0.020 | 0.190 | |
| IL-17f | −0.27 | 0.030 | 0.197 | |
| Patient Assessment Measure | ||||
| Parent-GA | IP-10 ‡ | 0.40 | 0.002 | 0.042 |
| IL-5 ‡ | 0.38 | 0.003 | 0.042 | |
| IL-12 ‡ | 0.36 | 0.004 | 0.043 | |
| IL-13 | 0.32 | 0.014 | 0.086 | |
| IL-2 | 0.31 | 0.015 | 0.086 | |
| Patient-GA | IP-10 | 0.31 | 0.016 | 0.451 |
| CDLQI | IL-12 | 0.29 | 0.040 | 0.400 |
| IL-8 | 0.28 | 0.045 | 0.400 | |
corrected for False Discovery Rates (FDR) by Benjamini-Hochberg (B–H) method
significant after B-H correction
PGA-A, Physician global assessment of activity; mLoSSI, modified Localized Scleroderma Skin Score; PGA-D, Physician Global Assessment of Damage; LoSDI, Localized Scleroderma Damage Index; Parent-GA, parent Global Assessment of disease impact; Patient-GA, patient Global Assessment of disease impact; CDLQI, Childhood Dermatology Life Quality Index
3.5.2. Disease damage
Disease damage parameters, PGA-D and LoSDI, were studied as outcome measures reflecting the level of cutaneous and global LS disease damage respectively. There were no significant correlations with any chemokine/cytokine levels and PGA-D. However, the LoSDI had significant negative correlations with cytokines IL-4, IL-6, IL-17a and IL-17f (Table 4).
3.5.3 Extracutaneous manifestations
All ECMs were recorded as per Zulian et al. [45] who studied the prevalence and clinical features of ECMs in a large cohort (750) of children with juvenile LS. The most common in our cohort were musculoskeletal and neurological, as found previously, and were used for the subanalysis. Musculoskeletal (MSK) involvement, defined as joint contracture, arthralgia, arthritis, leg length discrepancy and gait abnormality secondary to LS, was documented in 17 (25%) of the LS subjects. Comparison of cytokine levels between those with and without MSK involvement did not reveal any significant differences (Mann Whitney U, p <0.05 raw and p (B–H)). Neurologic involvement, defined as headache, peripheral neuropathy, brain lesions, and seizure associated with LS, was documented in 18 (26%) of the subjects (those with CNS involvement with linear head involvement and those with peripheral neuropathy associated with linear limb involvement). Comparing LS subjects with neurological involvement to those without, two cytokines were significantly lower in those with neurological manifestations, IL-17a and INF-γ (Table 5).
3.5.4. Patient and parent assessment
The relationships of cytokine and chemokine levels to validated patient-derived outcome measures, Parent-GA, Patient-GA and CDLQI, were evaluated. IP-10 had the strongest (moderate) correlation to the Parent-GA and Patient-GA (Table 4). IL-12p70 positively correlated with both the Parent-GA and CDLQI. IL-2, IL-5 and. IL-13 had independent positive correlations with the Parent-GA, while IL-8 independently correlated with the CDLQI (Table 4).
3.5.5. Antibody positivity
Cytokine and chemokine levels were analyzed between antibody positive and negative LS groups (Table 2 describes number of Ab positive patients). Patients with positive AHA exhibited significantly elevated levels of IL-1α, IL-1β, IL-2, IL-4, IL-10, IL-12p70, IL-13, IL-17a, IL-17e, IL-17f, IL-22, IL-23, IL-31, IL-33, IFN-γ, IFN-α2, GM-CSF and VEG-F (corrected p-value <0.05). IFN-α2 and IL-33 were significantly elevated in anti-ssDNA positive patients (raw p <0.05, corrected p values not significant). IL-17a and IL-8 levels were significantly lower in ANA positive patients (raw p = 0.04, corrected p values not significant).
We also evaluated AHA and anti-ssDNA status in relation to clinical disease activity in two manners, one by dichotomizing data into positive antibody (yes/no) and active disease status (yes/no) and performing Chi-square analyses, and the other by comparing Ab level to mLoSSI and PGA-A as graded measures using Mann Whitney U analyses. We did not find any significant differences between those with active and inactive disease in regard to presence or level of antibody positivity (data not shown).
3.5.6 Traditional serologic markers
Comparison of cytokine levels between those LS subjects with and without the following elevated general parameters of inflammation: sedimentation rate, C-Reactive Protein (CRP), White Blood Count (WBC) and Creatine Phosphokinase (CPK) were examined (Mann Whitney U, p <0.05 raw and p (B–H)) (Table 5). Eosinophilia was observed in 13 LS subjects and was associated with higher levels of IL-5 and IL-21. Elevated sedimentation rate (ESR) was observed in 5 LS subjects and was associated with elevated IP-10 and TNF-α levels (Table 5). Elevated WBC, CRP and CPK was only observed in 2 LS subjects each, therefore statistical analysis was not feasible to compare those with and without elevation of these laboratory parameters.
3.5.7 Systemic medication
The cytokine levels were compared between all patients on systemic medications (n=42) and those off systemic medication (n=27) at the time of blood draw, regardless of disease activity status. Most cytokines (24/29) were significantly elevated in patients off medications compared to those on medications (p (B–H) < 0.05); the exceptions were GM-CSF, INF-α2, VEGF, IL-21 and IL-28. The majority of those off medications (22/27) were categorized in the active group.
3.5.8. Other clinical features
There were no significant differences in cytokine levels between male and female LS patients. The great majority of cytokines/chemokines (22/29) significantly correlated (corrected p <0.05) in a negative fashion with disease duration (rs −0.30 to −0.50). The median disease duration at sample collection was 3.4 years, IQR 0.3 to 5.3 years (Table 2). When disease duration was categorized using the date of LS onset to time of sample collection into < 2 years, 2–4 years and >4 years, there was an impressive decrease (median 5 fold, IQR (3.5 – 27.5) in cytokine/chemokine level noted in the group with disease duration >4 years (data not shown). Many cytokines and chemokines (19/29) had significant negative correlations with age at blood sample (rs −0.26 to −0.38). We were unable to further analyze cytokine levels in relationship to LS subtype due to small numbers within each LS subtype.
4. DISCUSSION
4.1
This study was designed to investigate numerous TH associated cytokines and chemokines in tandem between LS and healthy subjects, and investigate relations within the LS subjects to disease activity and other clinical parameters. Overall findings support a ‘proinflammatory’ TH-1 and TH-17 predominance, with the TH-1 effector cytokines IFN-γ and IL-12 and the classic TH-17 effector cytokine, IL-17a, elevated in LS compared to controls. Within LS we analyzed several different features, including disease activity status, correlation with disease activity and damage parameters, and association of cytokines/chemokines with extracutanoeus manifestations, traditional serologic markers of disease activity and antibodies associated with LS. These findings, although limited to sample sizes within the subgroups, support IP-10 as a predominant chemokine reflecting disease activity supported by its associations with multiple disease activity features, such as activity status, mLoSSI, PGA-A and elevated sedimentation rate. It was also one of the few chemokines that correlated with the patients’ assessment of disease impact. We have previously demonstrated IP-10 in active LS inflammatory infiltrates of the dermis by immunohistochemistry[30]. IP-10 may prove as a serological marker of disease activity in the future. Other potential candidates would be TNF-α and GM-CSF due to their association with disease activity state, global and cutaneous activity parameters (PGA-A, mLoSSI) and sedimentation rate.
Comparing these findings to the literature, in regard to SSc and LS, there are some expected and unexpected findings. Traditionally, SSc is thought of as a ‘pro-fibrotic’ “TH-2” driven disease, mostly through its association with sclerosis, with IL-4 and IL-13 as predominant cytokines of interest (see Introduction). Conversely, though LS does have a fibrotic or sclerotic component, several groups support the presence of a more robust inflammatory component at the skin level than SSc [8, 9]. There are two histopathological reviews of the skin comparing LS to SSc skin that support this. Although many characteristics of the skin findings overlap, the degree of cellular infiltrate was noted in these two blinded studies to be more substantial and a differentiating feature of LS [8, 9]. There was more of an inflammatory infiltrate in LS despite both LS and SSc specimens having a similar degree of sclerosis documented, signifying that LS may be more ‘pro-inflammatory’ even when features of disease damage are present, such as dermal sclerosis and collagen bundle deposition.
This ‘pro-inflammatory’ cytokine signature was demonstrated in the peripheral blood when comparing LS patients and healthy controls showing that IP-10, MCP-1, IL-17a, IL-12p70, GM-CSF, PDGF-bb, IFN-α2, and IFN-γ were elevated in patients. Increased IL-12p70, IFN-γ and IP-10 signify a TH-1 profile. IL-12 promotes TH-1 differentiation leading to production of IFN-γ that in turn induces expression of the IFNγ-inducible IP-10. In scleroderma lesions, IP-10 is likely produced by a variety of cells (fibroblasts, endothelial cells, etc.) that attract TH-1 cells to inflammatory sites in a positive feedback loop [46]. Therefore, although not a traditional ‘Th-1 effector cytokine’, IP-10 is a chemokine associated with a Th-1 or ‘pro-inflammatory’ signal, which had the strongest positive correlations to clinical disease activity parameters as mentioned above. The combination of IP-10, MCP-1 and IFN-γ elevation in LS also supports the concept of an “interferon-γ signal”, another ‘pro-inflammatory’ signal, which has been recently reported in other autoimmune diseases, including the earlier active phase of SSc [47]. TH-17 effector cytokines, such as IL-17a, are recognized as predominant ‘pro-inflammatory’ cytokines in rheumatoid arthritis and psoriasis with recent medications targeted towards these cytokines effectively treating active inflammatory disease with less damage accrual [48]. We demonstrated elevated IL-17a levels in LS subjects compared to healthy controls, suggesting a TH −17 signal. When comparing to clinical parameters, we found a negative correlation between IL-17 and IL-17f and the LoSDI as well as negative correlation with disease duration, signifying that higher levels are likely demonstrated in earlier, more active disease. This observation is supported by the negative correlation between disease duration and IL-17a gene expression in adult LS PBMCs in another study [32]. Danczak-Pazdrowska et al. evaluated IL-17a in adult LS subjects and found increased expression of IL-17a genes in PBMC by real time PCR compared to controls. The protein expression of IL-17a in the skin has not been evaluated in LS, though it has been supported in SSc studies [16].
Those cytokines and chemokines associated with active disease likely reflect the inflammatory state and could be promoting a TH1/TH17 profile. IP-10 acts through the receptor CXCR3 to attract TH-1 cells to inflammatory sites in the skin [49]. GM-CSF is secreted by activated T cells, among other cell types, and can induce either a TH1 (IFN-γ) or TH2 (IL-4, IL-5) response, depending on the environment [50], whereas TNF-α is an effector cytokine affiliated with both TH1 and TH17 lineages [13]. It is noteworthy that although TNF-α levels were lower in the total LS group (n = 69) compared to healthy controls in the cross-sectional analysis, comparison of active and inactive LS subgroups showed TNF-α to be elevated in those with active disease. TNF-α levels also correlated positively with the clinical activity measures mLoSSI and PGA-A, and a traditional serologic marker, sedimentation rate. Hasegawa et al. found that disease duration was shorter in LS patients with elevated serum TNF-α, consistent with a contribution of TNF-α to the active inflammatory state [21]. TNF-α has been demonstrated in the skin of active LS/morphea lesions in a case report of pre- and post-infliximab therapy and decreased after anti-TNF-α therapy[51]. Traditional TH-2 cytokines, IL-4, IL-5 and IL-13, were not significantly elevated in LS compared to healthy controls, nor strongly correlated with disease activity measures. This is contrary to general findings in SSc, which typically support a predominant TH2 profile in the peripheral blood, skin and lungs of SSc patients [18, 25, 52]. This TH2 profile might signify a later fibrotic phase of SSc.
Regarding auto-antibodies, AHA positivity correlated with elevated levels of numerous cytokines and chemokines, perhaps signifying general autoimmunity/active disease state in AHA positive subjects. AHA has shown correlations with IL-4, IL-6 and TNF-α in prior studies [20, 21], is generally correlated with clinical characteristics of disease severity, such as total number of lesions and total body areas affected, and is more commonly seen in the generalized morphea subtype of LS [41]. The negative correlation between ANA positivity and IL-17a level in our cohort and the Danczak-Pazdrowska LS cohort are of unclear importance [32].
Elevation of traditional inflammatory serologic markers in LS is not common [45] as seen in this cohort with less than 20% demonstrating any elevation of WBC, Eosinophil count, ESR, CRP or CPK, supporting the need for additional biomarker investigation. IP-10 and TNF-α were notably elevated in those with elevated ESR, further supporting their reflection of disease activity, and as expected, IL-5 was elevated in those with Eosinophilia.
Further analyzing LS subjects by the presence or absence of the main ECMs associated with this condition, MSK or neurological involvement, did not demonstrate many cytokine differences, and two ‘pro-inflammatory’ cytokines were found to be lower in those with neurological ECMs. The caveat is that many of these subjects had more chronic ECM changes, such as white matter brain lesions and joint contractures from long-standing disease and resultant fibrosis. In a larger cohort analysis, further sub-dividing those with ‘active’ and ‘inactive’ ECMs may yield additional information.
4.2 Limitations
Due to the limited availability of healthy plasma specimens, the pediatric controls were not age matched to LS subjects. The controls were younger on average. To control for this difference, cytokine/chemokine analyses between healthy and LS subjects were adjusted for age (reported above) and most cytokine/chemokine levels remained significantly different. Of note, the cytokine/chemokine relationship with age was negative for most mediators, signifying higher levels in younger children, both control and LS subjects. Therefore, finding significant elevations of several cytokines/chemokines in LS (who were older children on average compared to healthy controls) suggests true relationships.
The sample size in general limited our ability to fully analyze subgroup comparisons within LS, such as disease subtype, abnormal traditional inflammatory markers, and antibody positivity. Another limitation of this study was our inability to include a well-established profibrotic cytokine in scleroderma, TGF-β, since it was not compatible with the Luminex platform we used. Future studies should include TGF-β and other T-regulatory associated cytokines.
4.3 Future investigations
Additional study is needed to identify the cellular source of the predominant cytokines in LS through flow cytometry analyses and examining LS skin for the expression of these cytokines, especially during the active phase of disease. In addition, longitudinal serological analyses of cytokines/chemokines as LS transitions between active and inactive disease will also be informative regarding the use of certain cytokines or groups of cytokines as predictors of disease activity or treatment response.
5. CONCLUSION
This is the first comprehensive TH-associated cytokine and chemokine (TH1, TH2, TH17) peripheral blood analysis in localized scleroderma, and specifically in a pediatric LS cohort. To our knowledge, this is the first time 19 of the 29 cytokines/chemokines have been analyzed in LS (pediatric or adult onset). The cytokine/chemokine data are further enriched by the ability to directly compare them to clinical measures of disease activity, damage and severity captured at the same visit as the blood draw through the patients’ enrollment in the NRCOS. The patients included in this study were representative of those described in other large pediatric LS cohorts with predominance of females, Caucasians and the linear scleroderma subtype [5, 53], enhancing the generalizability of the results.
Overall findings in this study suggest a constellation of ‘pro-inflammatory’ signals, TH-1, TH-17 and INF-γ , are more predominant in LS than SSc, its ‘companion disease’, perhaps indicating LS is more driven by an inflammatory rather than fibrotic component. This may explain why more traditional anti-rheumatic agents in rheumatology practice have not been very successful in treating SSc while agents such as methotrexate and corticosteroids are able to halt and partially reverse some disease aspects in LS. Specific cytokines identified with disease activity in LS were IP-10, GM-CSF and TNF-α. Further analyses of these cytokines and their role in LS may be particularly useful since active disease is the most logical stage for intervention to prevent cumulative damage. Targeting these cytokines/chemokines or their associated drivers may yield effective therapies for LS with potentially less side effects than traditional MTX and CS combination.
ACKNOWLEDGEMENTS
Other Acknowledgments
Authors thank Jane Rasmussen and the staff of the Specimen Processing Laboratory of Children’s Hospital of Pittsburgh for their assistance in collection of de-identified excess clinical specimens from routine well-child visits; and Robert Mueller, Jeffrey Dvergsten, and Kristy Huysman for the processing and cataloguing of specimens. Authors would also like to thank the Luminex Core Facility Services at the University of Pittsburgh Cancer Institute (UPCI) for their assistance with plasma analysis. We would like to thank Thaschawee Arkachaisri, MD for establishing the pediatric scleroderma clinic and registry at the University of Pittsburgh. Additionally, we would like to thank Mary Lucas, Cody Caplinger and Christina Mihok for their assistance with organizing and maintaining the National Registry of Childhood Onset Scleroderma Database. Lastly, we would like to thank our patients for enrolling into the NRCOS, allowing the comparison of clinical and serological data. For those with photographs included, written informed consent was obtained from the parents for publication of this manuscript.
Source of Support
This study was made possible by funding through the Nancy Taylor Foundation for Chronic Diseases Inc. (KST and AV), the NIAMS Mentored Patient Oriented Research Award, NIH Grant No. K23 AR059722 (KT), and the NIAMS Mid-Career Investigator Award in Patient-Oriented Research, NIH Grant No. K24 AR060297 (CFB). We appreciate their continued support.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- LS
Localized scleroderma
- SSc
Systemic sclerosis
- CHP
Children’s Hospital of Pittsburgh of UPMC
- TH
T-helper
- MIP-1α
macrophage inflammatory protein -1α
- IP-10
Interferon-gamma inducible protein-10
- IL
interleukin
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- TNF-α
tumor necrosis factor–α
- IFN-γ
Interferon-gamma
- PDGF-bb
platelet-derived growth factor-bb
- MCP-1
monocyte chemotactic protein- 1
- VEGF
vascular endothelial growth factor
- PBMC
peripheral blood mononuclear cells
- NRCOS
National registry of childhood onset scleroderma
- IRB
Institutional review board
- PGA-A
Physician global assessment of activity
- mLoSSI
modified Localized Scleroderma Severity Index
- VAS
visual analog scale
- PGA-D
Physician Global Assessment of Damage
- LoSDI
Localized Scleroderma Damage Index
- ANA
anti-nuclear antibodies
- AHA
anti-histone antibodies
- anti-ssDNA
anti-single stranded DNA antibodies
- Parent-GA
parent Global Assessment of disease impact
- Patient-GA
patient Global Assessment of disease impact
- CDLQI
Childhood Dermatology Life Quality Index
- ECM
Extracutaneous Manifestations
- ICC
Intra-class correlation coefficient
- IQR
interquartile ranges
- FDR
False Discovery Rates (FDR)
- B-H
Benjamini-Hochberg adjustment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s Contributions:
KST participated in the design and coordination of the study, obtained and analyzed clinical data and drafted and edited the manuscript. KK organized samples for Luminex processing through the core facility, paired both experimental and clinical data and drafted and edited the manuscript. CK performed the initial statistical analysis of clinical and laboratory data associated with the plasma samples and assisted in drafting and editing of the manuscript. JY performed the corrected and adjusted analysis of the data and assisted in drafting and editing the manuscript. KM assisted in the review of literature, additional clinical feature analysis and assisted in drafting and editing the manuscript. AV contributed to the acquisition and analysis of healthy control specimens and editing the manuscript. TM and CFB as mentors participated in the design of the study, analysis of experimental data and contributed to drafting and editing the manuscript. All authors read and approved the final manuscript to be published in Seminars in Arthritis & Rheumatism.
Contributor Information
Katherine Kurzinski, Email: katherine.kurzinski@gmail.com.
Christina Kelsey, Email: cek53@pitt.edu.
Jonathan Yabes, Email: yabesjg@upmc.edu.
Kelsey Magee, Email: mageeke@upmc.edu.
Abbe N. Vallejo, Email: andv26@pitt.edu.
Thomas Medsger, Jr., Email: tam8@pitt.edu.
Carol A. Feghali-Bostwick, Email: feghalib@musc.edu.
References
- 1.Peterson LS, Nelson AM, Su WP, Mason T, O'Fallon WM, Gabriel SE. The epidemiology of morphea (localized scleroderma) in Olmsted County 1960–1993. The Journal of rheumatology. 1997;24(1):73–80. [PubMed] [Google Scholar]
- 2.Zulian F. New developments in localized scleroderma. Current opinion in rheumatology. 2008;20(5):601–607. doi: 10.1097/BOR.0b013e328309a5eb. [DOI] [PubMed] [Google Scholar]
- 3.Marzano AV, Menni S, Parodi A, Borghi A, Fuligni A, Fabbri P, Caputo R. Localized scleroderma in adults and children. Clinical and laboratory investigations on 239 cases. European journal of dermatology : EJD. 2003;13(2):171–176. [PubMed] [Google Scholar]
- 4.wu EYRE, Torok KS, Li SC, Fuhlbrigge RC CARRANet Investigators. Description of the Localized Scleroderma Subgroup of CARRAnet Registry. Arthritis Rheum. 2011;63(Supplement):S787–S788. [Google Scholar]
- 5.Zulian F, Athreya BH, Laxer R, Nelson AM, Feitosa de Oliveira SK, Punaro MG, Cuttica R, Higgins GC, Van Suijlekom-Smit LW, Moore TL, et al. Juvenile localized scleroderma: clinical and epidemiological features in 750 children. An international study. Rheumatology (Oxford) 2006;45(5):614–620. doi: 10.1093/rheumatology/kei251. [DOI] [PubMed] [Google Scholar]
- 6.Sommer A, Gambichler T, Bacharach-Buhles M, von Rothenburg T, Altmeyer P, Kreuter A. Clinical and serological characteristics of progressive facial hemiatrophy: a case series of 12 patients. J Am Acad Dermatol. 2006;54(2):227–233. doi: 10.1016/j.jaad.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Falanga V, Medsger TA, Jr, Reichlin M, Rodnan GP. Linear scleroderma. Clinical spectrum, prognosis, and laboratory abnormalities. Annals of internal medicine. 1986;104(6):849–857. doi: 10.7326/0003-4819-104-6-849. [DOI] [PubMed] [Google Scholar]
- 8.Succaria F, Kurban M, Kibbi AG, Abbas O. Clinicopathological study of 81 cases of localized and systemic scleroderma. Journal of the European Academy of Dermatology and Venereology : JEADV. 2013;27(2):e191–e196. doi: 10.1111/j.1468-3083.2012.04581.x. [DOI] [PubMed] [Google Scholar]
- 9.Torres JE, Sanchez JL. Histopathologic differentiation between localized and systemic scleroderma. The American Journal of dermatopathology. 1998;20(3):242–245. doi: 10.1097/00000372-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Roumm AD, Whiteside TL, Medsger TA, Jr, Rodnan GP. Lymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlations. Arthritis Rheum. 1984;27(6):645–653. doi: 10.1002/art.1780270607. [DOI] [PubMed] [Google Scholar]
- 11.Kalogerou A, Gelou E, Mountantonakis S, Settas L, Zafiriou E, Sakkas L. Early T cell activation in the skin from patients with systemic sclerosis. Annals of the rheumatic diseases. 2005;64(8):1233–1235. doi: 10.1136/ard.2004.027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baraut J, Michel L, Verrecchia F, Farge D. Relationship between cytokine profiles and clinical outcomes in patients with systemic sclerosis. Autoimmunity reviews. 2010;10(2):65–73. doi: 10.1016/j.autrev.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa M, Takehara K. Potential immunologic targets for treating fibrosis in systemic sclerosis: a review focused on leukocytes and cytokines. Seminars in arthritis and rheumatism. 2012;42(3):281–296. doi: 10.1016/j.semarthrit.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Keystone EC, Lau C, Gladman DD, Wilkinson S, Lee P, Shore A. Immunoregulatory T cell subpopulations in patients with scleroderma using monoclonal antibodies. Clinical and experimental immunology. 1982;48(2):443–448. [PMC free article] [PubMed] [Google Scholar]
- 15.Inoshita T, Whiteside TL, Rodnan GP, Taylor FH. Abnormalities of T lymphocyte subsets in patients with progressive systemic sclerosis (PSS, scleroderma) The Journal of laboratory and clinical medicine. 1981;97(2):264–277. [PubMed] [Google Scholar]
- 16.Kurasawa K, Hirose K, Sano H, Endo H, Shinkai H, Nawata Y, Takabayashi K, Iwamoto I. Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum. 2000;43(11):2455–2463. doi: 10.1002/1529-0131(200011)43:11<2455::AID-ANR12>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 17.Matsushita T, Hasegawa M, Hamaguchi Y, Takehara K, Sato S. Longitudinal analysis of serum cytokine concentrations in systemic sclerosis: association of interleukin 12 elevation with spontaneous regression of skin sclerosis. The Journal of rheumatology. 2006;33(2):275–284. [PubMed] [Google Scholar]
- 18.Needleman BW, Wigley FM, Stair RW. Interleukin-1, interleukin-2, interleukin-4, interleukin-6, tumor necrosis factor alpha, and interferon-gamma levels in sera from patients with scleroderma. Arthritis Rheum. 1992;35(1):67–72. doi: 10.1002/art.1780350111. [DOI] [PubMed] [Google Scholar]
- 19.Valentini G, Baroni A, Esposito K, Naclerio C, Buommino E, Farzati A, Cuomo G, Farzati B. Peripheral blood T lymphocytes from systemic sclerosis patients show both Th1 and Th2 activation. Journal of clinical immunology. 2001;21(3):210–217. doi: 10.1023/a:1011024313525. [DOI] [PubMed] [Google Scholar]
- 20.Ihn H, Sato S, Fujimoto M, Kikuchi K, Takehara K. Demonstration of interleukin-2, interleukin-4 and interleukin-6 in sera from patients with localized scleroderma. Archives of dermatological research. 1995;287(2):193–197. doi: 10.1007/BF01262331. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa M, Sato S, Nagaoka T, Fujimoto M, Takehara K. Serum levels of tumor necrosis factor and interleukin-13 are elevated in patients with localized scleroderma. Dermatology. 2003;207(2):141–147. doi: 10.1159/000071783. [DOI] [PubMed] [Google Scholar]
- 22.Baraut J, Farge D, Jean-Louis F, Kesmandt H, Durant C, Verrecchia F, Michel L. [Cytokines in systemic sclerosis] Pathologie-biologie. 2012;60(2):127–139. doi: 10.1016/j.patbio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Atamas SP, Yurovsky VV, Wise R, Wigley FM, Goter Robinson CJ, Henry P, Alms WJ, White B. Production of type 2 cytokines by CD8+ lung cells is associated with greater decline in pulmonary function in patients with systemic sclerosis. Arthritis Rheum. 1999;42(6):1168–1178. doi: 10.1002/1529-0131(199906)42:6<1168::AID-ANR13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 24.Parel Y, Aurrand-Lions M, Scheja A, Dayer JM, Roosnek E, Chizzolini C. Presence of CD4+CD8+ double-positive T cells with very high interleukin-4 production potential in lesional skin of patients with systemic sclerosis. Arthritis Rheum. 2007;56(10):3459–3467. doi: 10.1002/art.22927. [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. The Journal of rheumatology. 1997;24(2):328–332. [PubMed] [Google Scholar]
- 26.Fuschiotti P, Medsger TA, Jr, Morel PA. Effector CD8+ T cells in systemic sclerosis patients produce abnormally high levels of interleukin-13 associated with increased skin fibrosis. Arthritis Rheum. 2009;60(4):1119–1128. doi: 10.1002/art.24432. [DOI] [PubMed] [Google Scholar]
- 27.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nature reviews Immunology. 2004;4(8):583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gourh P, Arnett FC, Assassi S, Tan FK, Huang M, Diekman L, Mayes MD, Reveille JD, Agarwal SK. Plasma cytokine profiles in systemic sclerosis: associations with autoantibody subsets and clinical manifestations. Arthritis research & therapy. 2009;11(5):R147. doi: 10.1186/ar2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurzinski K, Torok KS. Cytokine profiles in localized scleroderma and relationship to clinical features. Cytokine. 2011;55(2):157–164. doi: 10.1016/j.cyto.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magee KE, Kelsey CE, Kurzinski KL, Ho J, Mlakar LR, Feghali-Bostwick CA, Torok KS. Interferon-gamma inducible protein-10 as a potential biomarker in localized scleroderma. Arthritis research & therapy. 2013;15(6):R188. doi: 10.1186/ar4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ihn H, Sato S, Fujimoto M, Kikuchi K, Takehara K. Demonstration of interleukin 8 in serum samples of patients with localized scleroderma. Archives of dermatology. 1994;130(10):1327–1328. [PubMed] [Google Scholar]
- 32.Danczak-Pazdrowska A, Kowalczyk M, Szramka-Pawlak B, Gornowicz-Porowska J, Szewczyk A, Silny W, Olewicz-Gawlik A, Molinska-Glura M, Zaba R, Hrycaj P. Interleukin-17A and interleukin-23 in morphea. Archives of medical science : AMS. 2012;8(6):1089–1095. doi: 10.5114/aoms.2012.32421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danczak-Pazdrowska A, Kowalczyk MJ, Szramka-Pawlak B, Gornowicz-Porowska J, Szewczyk A, Silny W, Olewicz-Gawlik A, Molinska-Glura M, Zaba R, Hrycaj P. Interleukin 1 beta in morphea. Cent Eur J Immunol. 2012;37(3):247–252. [Google Scholar]
- 34.Laxer RM, Zulian F. Localized scleroderma. Current opinion in rheumatology. 2006;18(6):606–613. doi: 10.1097/01.bor.0000245727.40630.c3. [DOI] [PubMed] [Google Scholar]
- 35.Arkachaisri T, Vilaiyuk S, Li S, O'Neil KM, Pope E, Higgins GC, Punaro M, Rabinovich EC, Rosenkranz M, Kietz DA, et al. The localized scleroderma skin severity index and physician global assessment of disease activity: a work in progress toward development of localized scleroderma outcome measures. The Journal of rheumatology. 2009;36(12):2819–2829. doi: 10.3899/jrheum.081284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelsey CE, Torok KS. The Localized Scleroderma Cutaneous Assessment Tool: Responsiveness to change in a pediatric clinical population. Journal of the American Academy of Dermatology. 2013;69(2):214–220. doi: 10.1016/j.jaad.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arkachaisri T, Vilaiyuk S, Torok KS, Medsger TA., Jr Development and initial validation of the localized scleroderma skin damage index and physician global assessment of disease damage: a proof-of-concept study. Rheumatology (Oxford) 2010;49(2):373–381. doi: 10.1093/rheumatology/kep361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis-Jones MS, Finlay AY. The Children's Dermatology Life Quality Index (CDLQI): initial validation and practical use. The British journal of dermatology. 1995;132(6):942–949. doi: 10.1111/j.1365-2133.1995.tb16953.x. [DOI] [PubMed] [Google Scholar]
- 39.Das S, Bernstein I, Jacobe H. Correlates of self-reported quality of life in adults and children with morphea. Journal of the American Academy of Dermatology. 2014;70(5):904–910. doi: 10.1016/j.jaad.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arkachaisri T, Fertig N, Pino S, Medsger TA., Jr Serum autoantibodies and their clinical associations in patients with childhood- and adult-onset linear scleroderma. A single-center study. The Journal of rheumatology. 2008;35(12):2439–2444. doi: 10.3899/jrheum.080098. [DOI] [PubMed] [Google Scholar]
- 41.Takehara K, Sato S. Localized scleroderma is an autoimmune disorder. Rheumatology (Oxford) 2005;44(3):274–279. doi: 10.1093/rheumatology/keh487. [DOI] [PubMed] [Google Scholar]
- 42.Torok KS, Arkachaisri T. Methotrexate and corticosteroids in the treatment of localized scleroderma: a standardized prospective longitudinal single-center study. The Journal of rheumatology. 2012;39(2):286–294. doi: 10.3899/jrheum.110210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zulian F, Martini G, Vallongo C, Vittadello F, Falcini F, Patrizi A, Alessio M, La Torre F, Podda RA, Gerloni V, et al. Methotrexate treatment in juvenile localized scleroderma: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2011;63(7):1998–2006. doi: 10.1002/art.30264. [DOI] [PubMed] [Google Scholar]
- 44.Li SC, Torok KS, Pope E, Dedeoglu F, Hong S, Jacobe HT, Rabinovich CE, Laxer RM, Higgins GC, Ferguson PJ, et al. Development of consensus treatment plans for juvenile localized scleroderma: a roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis care & research. 2012;64(8):1175–1185. doi: 10.1002/acr.21687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zulian F, Vallongo C, Woo P, Russo R, Ruperto N, Harper J, Espada G, Corona F, Mukamel M, Vesely R, et al. Localized scleroderma in childhood is not just a skin disease. Arthritis Rheum. 2005;52(9):2873–2881. doi: 10.1002/art.21264. [DOI] [PubMed] [Google Scholar]
- 46.Antonelli A, Ferrari SM, Giuggioli D, Ferrannini E, Ferri C, Fallahi P. Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmunity reviews. 2014;13(3):272–280. doi: 10.1016/j.autrev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Mayes MD, Tan FK, Wu M, Reveille JD, Harper BE, Draeger HT, Gonzalez EB, Assassi S. Correlation of interferon-inducible chemokine plasma levels with disease severity in systemic sclerosis. Arthritis Rheum. 2013;65(1):226–235. doi: 10.1002/art.37742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, Durez P, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Science translational medicine. 2010;2(52):52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 49.Giustizieri ML, Mascia F, Frezzolini A, De Pita O, Chinni LM, Giannetti A, Girolomoni G, Pastore S. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. The Journal of allergy and clinical immunology. 2001;107(5):871–877. doi: 10.1067/mai.2001.114707. [DOI] [PubMed] [Google Scholar]
- 50.Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, Zhang Y, Zhang L, Yuan ZR, Tan HS, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don't know. Cell research. 2006;16(2):126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 51.Diab M, Coloe JR, Magro C, Bechtel MA. Treatment of recalcitrant generalized morphea with infliximab. Archives of dermatology. 2010;146(6):601–604. doi: 10.1001/archdermatol.2010.120. [DOI] [PubMed] [Google Scholar]
- 52.Salmon-Ehr V, Serpier H, Nawrocki B, Gillery P, Clavel C, Kalis B, Birembaut P, Maquart FX. Expression of interleukin-4 in scleroderma skin specimens and scleroderma fibroblast cultures. Potential role in fibrosis. Archives of dermatology. 1996;132(7):802–806. [PubMed] [Google Scholar]
- 53.Christen-Zaech S, Hakim MD, Afsar FS, Paller AS. Pediatric morphea (localized scleroderma): review of 136 patients. Journal of the American Academy of Dermatology. 2008;59(3):385–396. doi: 10.1016/j.jaad.2008.05.005. [DOI] [PubMed] [Google Scholar]