i. Summary
Endogenous estrogens, predominantly 17β-estradiol (E2), mediate various very diverse effects throughout the body in both normal physiology and disease. Actions include development (including puberty) and reproduction as well as additional effects throughout life in the metabolic, endocrine, musculoskeletal, nervous, cardiovascular and immune systems. The actions of E2 have traditionally been attributed to the classical nuclear estrogen receptors (ERα and ERβ) that largely mediate transcriptional/genomic activities. However, more recently the G protein-coupled estrogen receptor GPER/GPR30 has become recognized as an essential mediator of certain, and particularly rapid, signaling events in response to E2. Murine genetic knockout (KO) models represent an important approach to understand the mechanisms of E2 action in physiology and disease. Studies of GPER KO mice over the last years have revealed functions for GPER in the regulation of obesity, insulin resistance and glucose intolerance, among other areas of (patho)physiology. This chapter focuses on methods for the evaluation of metabolic parameters in vivo and ex vivo with an emphasis on glucose homeostasis and metabolism through the use of glucose and insulin tolerance tests, pancreatic islet and adipocyte isolation and characterization.
Keywords: Estrogen, GPER, GPR30, Metabolism, Obesity, Adipose, Adipocyte, Insulin Resistance, Insulin Sensitivity, Glucose Tolerance, Type 2 diabetes
1. Introduction
Obesity and its pathophysiological consequences, including diabetes and cardiovascular diseases, are becoming ever more prevalent, leading to their designation as epidemics (1–3). In mouse models as in humans, there is a clear sex difference with respect to metabolism (4–8). In women and female mice, estrogen (E2) is protective against obesity, diabetes and cardiovascular disease (9, 10). This protection is less evident in males, and is lost in females after menopause or following oophorectomy/ovariectomy. Thus, the function of estrogen and its receptors in regulating metabolism has been of great interest (11); however, with the recent identification of a 7-transmembrane estrogen receptor (GPR30/GPER) (12–14), unrelated to the classical estrogen receptors (ERα and ERβ), many questions arose as to the role of this newest estrogen receptor in the regulation of metabolism (15–18). In addition to selective pharmacological agents (19–21), knockout mice represent an important tool to examine GPER function in vivo. GPER KO mice exhibit increased adiposity and impaired insulin secretion from pancreatic islets (22, 23); however, only female mice are glucose intolerant at six months of age (24), whereas males become progressively glucose intolerant with age (22). These phenotypes suggest that GPER plays an important role in metabolism and merits further investigation.
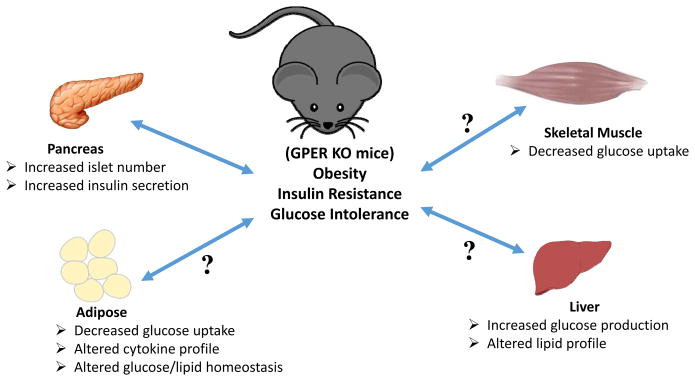
Obesity not only results in increased overall fat but also remodeling of existing adipose tissue. In addition, adipose tissue is not an inert storage tissue, as long thought, but rather an active endocrine gland (25, 26). Furthermore, adipose tissue in the obese exhibits increased leukocyte infiltration that in turn alters the cytokine profiles of the macrophages/adipocytes giving rise to a pro-inflammatory phenotype (27). Increased pro-inflammatory cytokines and circulating lipids (arising from dyslipidemia) result in metabolic dysfunction due to multiple effects on various tissues (Figure 1) (28). In prediabetes, insulin resistance sets in and the insulin responsive tissues, adipose, skeletal muscle and liver, become less responsive to insulin; with adipose and skeletal muscle displaying diminished insulin-stimulated glucose uptake along with increased glucose production from liver. Since the body fails to respond to insulin, in order to maintain normal blood glucose, insulin production and secretion increase. This leads to the compensatory formation of new pancreatic islets but eventually, when the islets are exhausted due to continued overload, normoglycemia cannot be maintained and fasting blood glucose levels rise. To further compound these problems, lipotoxicity interferes with hepatic function and fails to control gluconeogenesis in spite of already high blood glucose levels (29). Usually in a healthy animal, blood glucose changes are highly dynamic and can be measured using very simple and effective methods, such as intraperitoneal glucose tolerance tests (IPGTT) and insulin tolerance tests (IPITT). These tests can reveal the dynamics of blood glucose homeostasis and are important tools to investigate glucose metabolism. As estrogen and its receptors play important roles in glucose and lipid metabolism as well as metabolic dysfunction, employing knockout mice with a deficiency in a single estrogen receptor has been highly informative regarding the roles of individual estrogen receptors. The procedures and assays described in this chapter (Table 1) provide important information regarding normal metabolic function as well as dysfunction under both fasting and non-fasting conditions in the mouse.
Figure 1. Schematic representation of the possible metabolic dysfunctions leading to or resulting from obesity in GPER-deficient mice.
Increased adiposity results in insulin resistance with ensuing glucose intolerance. Obesity may also result in multiple defects in metabolically active tissues such as the pancreas, adipose, liver and muscle. In the pancreas, insulin resistance leads to increased insulin secretion and a compensatory increase in the number of islets, resulting in hyperinsulinemia. In adipose and muscle, insulin resistance may lead to decreased insulin-stimulated glucose uptake, which along with concomitantly increasing hepatic glucose production, leads to hyperglycemia. In addition, altered lipid homeostasis in the adipose and liver lead to hyperlipidemia. Together with hypertension, these pathophysiological metabolic effects (abdominal obesity, elevated fasting plasma glucose, high serum triglycerides, and low high-density cholesterol levels) comprise the condition referred to as metabolic syndrome. Whereas GPER is known to stimulate insulin secretion and survival of pancreatic beta cells, little is known regarding the role(s) of GPER in other insulin-responsive peripheral organs/tissues (as indicated by question marks (?)).
Table 1.
Procedures and assays to study functions of GPER in metabolism.
| Assay | Tissue/Cells | Function |
|---|---|---|
| Adipocyte isolation | Adipose tissue | Metabolic assays/adipokine secretion |
| Adipogenesis | Preadipocytes | Triglyceride accumulation |
| Fasting glucose/insulin | Fasting mouse blood | Insulin resistance |
| Glucose tolerance/insulin tolerance | Fasted mouse blood | Glucose homeostasis |
| Glucose uptake | Adipocytes/adipose tissue | Insulin sensitivity |
| Islet isolation | Pancreas | Insulin secretion |
2. Materials
2.1 Isolation of adipocytes and stromal vascular fraction
Mouse perigonadal fat pads.
Growth medium: DMEM/F12, 10% fetal bovine serum, penicillin/streptomycin/glutamine (100 U/mL; 100 μg/mL and 0.292 mg/mL, respectively).
Krebs Ringer solution, HEPES buffered (KRH buffer): 25 mM HEPES, pH 7.4, 120 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 1.3 mM CaCl2, 1.3 mM KH2PO4.
Collagenase solution (100 mg/mL): 100 mg collagenase per mL KRH buffer.
RBC lysis buffer: 155 mM NH4Cl, 12 mM NaHCO3, 0.1 mM EDTA.
Cell Strainers (40/100/400 μm).
Sterile petri plates.
Sterile 50 mL conical tubes.
Water bath shaker.
Centrifuge and microfuge.
Pentobarbital.
2.2 Differentiation of preadipocytes
3T3-L1 preadipocytes or primary preadipocytes.
Insulin, 10 mg/mL stock (1000X).
3-isobutyl-1-methylxanthine (IBMX), 50 mM stock (100X).
Dexamethasone, 1 mM stock (1000X).
Differentiation medium: DMEM, 10% FBS, Pen/Strep/Glutamine (100 U/mL; 100 μg/mL and 0.292 mg/mL, respectively), 10 μg/mL Insulin, 500 μM IBMX, 1 μM dexamethasone.
2.3 Glucose uptake
Differentiated adipocytes as in 2.2 or minced adipose tissue pieces (ex vivo).
2-deoxy-D-[2,6-3H]-glucose.
2-deoxy-D-[2,6]-glucose.
KRH buffer containing radioactive glucose: KRH buffer, 2-deoxy-D-[2,6-3H]-glucose in 200 μM non-radioactive 2-deoxy-D-[2,6]-glucose.
RIPA buffer: 10 mM TrisHCl, pH 8.0, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl.
Reagents for standard protein assay.
Perchloric acid.
Hydrogen peroxide.
2.4 Glucose and insulin testing
Animal scale.
Mouse restrainer.
Timer
Blood glucose meter (ReliOn Confirm Micro, or similar) and glucose strips (ReliOn, or similar).
Insulin Syringes (½ cc or 1 cc) with 30½ G needle.
Glucose stock (20% w/v in saline).
Insulin (Humulin R, Eli Lilly & Co).
Fine scissors.
Mercodia Ultrasensitive Mouse Insulin Elisa kit (Calibrators, standards, 96-well plate coated with capture antibody, Antibody-enzyme conjugate, Substrate TMB, Stop solution, Wash buffer).
Plasma samples of interest.
Plate reader with capacity to read at absorbance 450 nm.
2.5 Islet isolation
Hank’s Balanced Salt Solution (HBSS).
Collagenase (Fraction V) in HBSS.
DMEM with 3 mM or 11 mM glucose, 10% FBS, Pen/Strep/Glutamine (100 U/mL; 100 μg/mL and 0.292 mg/mL, respectively).
Sodium diatrizoate (such as Histopaque).
3. Methods
3.1 Adipocyte and stromal vascular fraction isolation from adipose tissue
Adipose tissue consists of a variety of cells such as preadipocytes, adipocytes, macrophages, and endothelial cells, etc. To understand the differentiation process of preadipocytes or physiological function of adipocytes, such as cytokine secretion (without the input from macrophages), in response to a specific stimulus or condition, isolation of individual cell types is essential. Ensure that all procedures are carried out in accordance with local institutional policies and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Sacrifice mice by injecting pentobarbital or by cervical dislocation.
Isolate the desired fat pads (perigonadal/subcutaneous/brown) in sterile DMEM/F12 medium.
Mince the tissue in a sterile petri plate and add sterile collagenase solution (~5 mL/g tissue) and transfer to a sterile 50 mL conical tube.
Incubate for 1 h at 37 °C in a shaking water bath at 150 rpm.
After digestion, add an equal volume of cold DMEM/F12 to stop digestion.
Pass the digested fat slurry through a sterile cell strainer (100–400 μm) attached on top of a 50 ml tube by gravity. The undigested tissue on the filter can be discarded.
To the flow through, add 20 mL DMEM/F12.
Centrifuge at 50 × g (or 500 rpm) for 5 min to remove any debris.
After centrifugation, adipocytes will float.
The adipocytes can be collected from the top and transferred to a new tube containing in DMEM/F12 medium supplemented with 10% FBS, Pen/Strep/Glutamine (100 U/mL; 100 μg/mL and 0.292 mg/mL, respectively).
The adipocytes remain floating but can be cultured further for 48–72 hours for various experimental procedures (see Note 1).
Transfer the medium below the floating adipocytes to a new 50 mL tube and centrifuge at 500 × g for 15 min to pellet the stromal vascular (SV) cells.
Remove most of the supernatant.
Resuspend the cells in 10 mL RBC lysis buffer.
Incubate at room temperature for 5 min.
Add an equal volume of growth medium.
Filter the cell suspension through a 40 μm cell strainer into a new tube to remove endothelial cell clumps.
Centrifuge the sample at 500 × g for 5 min.
Remove most of the supernatant and resuspend the pellet in 10 mL growth medium.
Dispense the appropriate number of cells into the desired plate, dish or flask.
3.2 Differentiation of Preadipocytes (3T3-L1 or primary cells)
Preadipocytes can either be cultured from a cell line such as the murine fibroblasts, 3T3-L1 cells committed to an adipocytic fate, or can be isolated from adipose tissue ex vivo as discussed above (in 3.1).
-
1
After the preadipocytes reach confluence (day 0), replace the growth medium and let cells grow for another two days (day 2).
-
2
Induce differentiation by adding differentiation medium for 48 hours (days 3–4).
-
4
Following differentiation, adipocyte maturation is induced in growth medium supplemented with only insulin for another 3 days with medium changes every other day (days 5–7).
-
5
By day 7–10, lipid droplets will be visible in the adipocytes (see Note 2).
-
6
Adipocytes can be used for various assays at any time between 5–10 days following the initiation of differentiation.
3.3 Glucose uptake in adipocytes/adipose tissue
Once preadipocytes are committed to an adipocytic fate, they upregulate and express glucose transporter 4 (GLUT4). This renders them responsive to insulin, which results in the stimulation of glucose uptake and conversion to fat in the presence of insulin and excess glucose. However, under conditions of cellular insulin resistance, adipocytes fail to respond to insulin and exhibit diminished glucose uptake in response to insulin. Glucose uptake is measured in the adipose tissue ex vivo to assess insulin responsiveness.
Once preadipocytes (3T3-L1 or primary cells) are differentiated into adipocytes in 24-well plates, serum starve the cells in KRH buffer for 3 h with appropriate treatments or controls (see Note 3).
Wash the cells with KRH buffer and incubate with 1 mL of KRH supplemented with 100 nM insulin for 15 min (see Note 4).
To the solution above, add 1 μCi 2-deoxy-D-[2,6-3H]-glucose (in non-radioactive 2-deoxy-D-[2,6]-glucose (200 μM, 50 μl total volume) to each well for 10 min.
Wash the cells with KRH buffer 3 times to remove excess tritiated glucose.
Lyse the cells in 1 mL of RIPA buffer and mix an aliquot (800 μL) with scintillation fluid in a vial and leave overnight (see Note 5). Read in a scintillation counter the next day.
Measure the protein content in the RIPA lysate using a standard protein assay.
Normalize the values obtained from the radioactive counts to the protein concentration in the wells.
For glucose uptake in ex vivo adipose tissue, mince the tissue into small pieces and weigh.
Incubate the tissue fragments in KRH buffer for 2 h followed by steps 2 to 4 as above.
After washing, solubilize the tissues in 400 μL perchloric acid and 200 μL H2O2 at 60°C overnight.
After dissolution, place an aliquot in scintillation fluid overnight, then count as in step 3.3.5. Normalize the values to the tissue weight.
3.4 Intraperitoneal glucose (IPGTT) and insulin tolerance test (IPITT)
One of the earliest hallmarks of metabolic dysfunction is impaired glucose tolerance. In the glucose tolerance test, impaired glucose clearance in response to a bolus of glucose reflects either a defect in insulin secretion or ineffective insulin function in peripheral tissues. In contrast, the insulin tolerance test determines whether the metabolic dysfunction is primarily the result of a defect in the pancreatic β-cell function (insulin secretion) or due to insulin resistance in peripheral tissues (insulin responsiveness) (see Notes 6 and 7).
Fast the mice for 4 h prior to the assay. Weigh each mouse and place it in an individual fresh cage with only water (without food) for the 4 h period. Care should be taken to keep the mice calm (see Note 8).
Prior to the assay, calibrate the glucometer as per the manufacturer’s instructions. Although we use a ReliOn glucometer, any other commercial glucometer for human use, such as Accu-Check Active (Roche Diagnostics), One Touch Ultra (LifeScan), etc., will be adequate.
At the start of the experiment, place the mouse in a restrainer and gently nick the tail 1–2 cm from the tip. Discard the first drop of blood and record the basal blood glucose (0 min reading) by inserting the test strip in the glucometer and directly touching it to the drop of blood on the tail (test strip uses about 3–5 μL of blood).
Immediately after the basal glucose recording, inject the mouse intraperitoneally with the glucose solution (2 g/kg body weight).
Proceed with subsequent mice, keeping the gap between mice constant. Take the readings at regular intervals (15, 30, 60 and 120 min) after the injection. The blood glucose first rises rapidly, and then decreases over time due to clearance from the blood (see Notes 9 and 10).
For the insulin tolerance test, mice are similarly prepared as for the GTT, except that instead of injecting glucose, insulin is injected (0.5 U/kg body weight). Subsequently, mice display an initial decrease in the blood glucose that rises back to basal levels over time.
Graph blood glucose readings as a function of time and analyze the area under the curve (AUC) to reveal the nature of any metabolic dysfunction/differences between groups of mice (see Notes 11 and 12).
3.5 Fasting plasma glucose & insulin levels and calculation of insulin resistance index
Fasting levels of glucose reveal whether or not mice are normoglycemic in the resting or basal state. In some instances however, even though mice exhibit normal fasting glucose levels, their fasting insulin levels might be higher due to insulin resistance, where the body requires higher insulin levels to maintain normal blood glucose levels.
For measuring fasting blood glucose, mice are prepared exactly as in 3.4 and fasting blood glucose is measured directly from a blood drop at the tail tip using the glucometer.
For fasting insulin measurements, plasma is separated from the fasting blood (either obtained from terminal sacrifice or tail bleed) and insulin is measured using a mouse ultrasensitive insulin ELISA kit following the manufacturer’s instructions. The assay is typically based on a sandwich ELISA where two antibodies against different epitopes are utilized. The assay range for such a kit is typically 0.025 μg/L to 1.5 μg/L.
Pipet calibration standards, controls and plasma (25 μL each) into microtiter plate wells coated with capture antibody.
Add 100 μL antibody-enzyme conjugate to each well and incubate on a shaker at room temperature (18–25 °C) for 2 h. This antibody is the detection antibody and is conjugated to horseradish peroxidase (HRP).
Wash 3 times with 350 μL wash buffer and add 200 μL substrate 3,3′,5,5′-tetramethylbenzidine (TMB) and incubate at room temperature for 15 min. Stop the reaction by adding 50 μL of stop solution. Mix the plate contents on a shaker for few seconds and place in a plate reader set to measure absorbance at 450 nm.
If samples fall out of range and are saturated, dilute and read again. However, linearity may be compromised through dilution of off-scale samples.
Plot the graph on a log-log scale and calculate the insulin concentration in the unknown samples using a cubic spline regression analysis.
Paired glucose and insulin readings can be used to assess the insulin resistance index using the Homeostatic Model Assessment (HOMA-IR). The formula for calculating HOMA-IR = (Glucose X Insulin)/405 (glucose in mg/dL and insulin in mU/L). If glucose is expressed in molar units (mmol/L), then the formula for calculating HOMA-IR= (Glucose X Insulin)/22.5 (insulin in mU/L).
3.6 Islet isolation
Islets play an important role in the pathophysiology of metabolic dysfunction during obesity and diabetes. Islet isolation is an important technique to generate islets for ex vivo studies, such as addressing endocrine function, the viability of cells, or in vivo transplantation. The method is based on collagenase infusion into the common bile duct of the euthanized animal; enzymatic degradation of the pancreatic architecture, separation of islets from other tissues and culturing viable islets. Although the procedure appears simple, it can be very challenging to obtain a pure and viable islet preparation.
Euthanize the mouse and surgically expose the abdomen (see Note 13).
Carefully locate the entry of bile duct into the duodenum and block the common bile duct opening by clamping it. To isolate islets, slowly inject the collagenase solution into the common bile duct opening to maximize solution entry into the pancreas. Since the opening into the small intestine is blocked, this will force the collagenase solution to enter the pancreas and it will expand like a balloon (see Note 14).
Perfusion of the pancreas from the inside through the common bile duct in this manner provides efficient access to collagenase compared to other methods such as dissection of pancreas, cutting small pieces and incubating in the collagenase solution (see Note 15).
The type of Collagenase used for digesting the pancreas affects the yield of islets. We routinely use collagenase fraction V from Sigma, which provides good yield as well as good viability.
Carefully remove the pancreas and incubate in collagenase solution in a 50 mL conical tube for 8–11 min. It is important to incubate for the optimum time as longer incubation in collagenase solution will increase the yield of smaller islets and may also decrease the viability of islets.
Apply the digested slurry on a discontinuous gradient of Histopaque (sodium diatrizoate; 1.109, 1.096, 1.070, and 0.570 g/mL) and isolate the islets from the interface of the 1.070/1.096 and 1.096/1.109 g/mL layers.
Further clean the fractions obtained from the Histopaque interfaces by individually handpicking the islets from a petri plate under a dissecting microscope. Pool islets of similar size. For experiments, islets are mixed from each group in similar numbers (typically ~10).
Islets can be cultured in DMEM with 11 mM glucose and 10% FBS overnight to let them recover from the isolation procedure.
To measure insulin secretion, first wash the islets and incubate in DMEM with 3 mM glucose for 90 min and subsequently incubate in DMEM with 3 mM or 16 mM glucose (+/− treatments) for 60 min.
Recover the medium and measure insulin using the Ultrasensitive Mouse Insulin Elisa kit (as in 3.5) (see Note 16).
Acknowledgments
The authors have been supported by NIH grants CA116662, CA127731 and CA163890 (ERP) and the UNM Cancer Center (CA118100).
Footnotes
The adipocytes isolated from the adipose tissue ex vivo should be used for experiments as soon as possible as they may start producing increased pro-inflammatory cytokines following collagenase treatment and that may induce experimental artifacts.
After adipogenesis, fat in the adipocytes can cause them to come off the plate so assays should be performed as soon as possible after differentiation and maturation. Data should be normalized to the protein or DNA content of the well.
In glucose uptake assays, starvation times and conditions can play a key role. Unlike other cells, adipocytes enter senescence when starved overnight in low glucose medium. Thus, we starve cells for three hours in KRH buffer directly and bypass the low glucose medium step.
Insulin responses in adipocytes peak within 30 min so it is advisable to plan the treatment time such that the reaction is stopped at 30 min after the insulin stimulation to achieve a good positive control.
In glucose uptake experiments, the final lysate should be left in the scintillation fluid overnight to disperse evenly before placing in the scintillation counter.
Since mice display significant variation, care should be taken to perform experiments with adequate numbers of animals to achieve statistical significance. A minimum of 6–8 animals should be used for each of the in vivo experiments. If large variations are observed, outlier analysis using statistical software such as Graphpad Prism can be performed.
Reference values for metabolite and hormone levels in a number of mouse strains are available at the Jackson Laboratories website (http://www.jacksonlaboratory.com).
A very important prerequisite for the mice that will undergo GTT/ITT is that they should be kept as calm as possible (no extraneous noises, smells, personnel, etc.). Also, trained personnel should handle the animals. Care should be taken to ensure that mice are not stressed between readings. Only a limited number of mice should be planned for this experiment that can be handled effectively given the constraints of the assay time points.
For GTT assay, we sometimes also take a 7.5 min glucose measurement post- glucose challenge. This reading is a good measure of the first phase of the pancreatic response. However, if employing a 7.5 min time point, care should be taken to use a small group of animals as handling larger numbers of animals in such a short time span can lead to missing time points.
For GTT or ITT experiments, determining the area under the curve (AUC) may provide statistically significant differences between groups, where effects at individual time points may not. In GTT, glucose levels first peak, then recover over time. Early time points reveal the dynamics of the first phase of insulin response from the pancreas and later time points provide information about the combined effect of insulin secretion from pancreas and insulin responsiveness in peripheral tissues. This combined effect can be further delineated by analyzing the ITT graph, which provides information regarding insulin responsiveness.
In the ITT AUC graph, two phases are evident: the first when glucose levels decrease in response to insulin, and the second when the glucose levels return to normal levels in response to glucagon and other liver enzymes. Plotting these two phases separately can provide information regarding responsiveness of these two contributing pathways.
Insulin sensitivity may vary between the strains or sex of mice used for the study. The insulin dose recommended above may be too high and may lead to hypoglycemia or even death in certain cases. Thus, performing a small pilot experiment to determine the appropriate dose of insulin for the animals under study is helpful. A hypoglycemic mouse approaching death can be “rescued” by injecting glucose, avoiding the loss of an animal from an experimental cohort, and allowing further studies at later times.
For islet isolation, the procedure should be performed as soon as possible after euthanasia as the pancreas is prone to autolysis.
To obtain a pure and viable islet preparation, it is important to perfuse the pancreas with collagenase solution through the common bile duct. This procedure is challenging and should be performed by trained and experienced personnel.
During the islet isolation procedure, the incubation time of the perfused pancreas in the collagenase solution is critical. Too much or too little time will lead to an over-digested or under-digested slurry that will eventually affect the quantity and quality of the islets. It is advisable to standardize the incubation time with each new lot of Collagenase V and also the animal model.
The number of islets in the insulin secretion assays should typically be ~10–12. If small islets are obtained (e.g., in insulin-resistant obese mice where new islets form resulting in hyperinsulinemia), then the number of islets can be increased accordingly.
Disclosures
E.R.P. is an inventor on US patents assigned to the University of New Mexico for GPER-selective ligands and imaging agents.
References
- 1.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 2.Sherwin R, Jastreboff AM. Year in diabetes 2012: The diabetes tsunami. J Clin Endocrinol Metab. 2012;97:4293–4301. doi: 10.1210/jc.2012-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol. 2012;8:228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 4.Faulds MH, Zhao C, Dahlman-Wright K, Gustafsson JA. The diversity of sex steroid action: regulation of metabolism by estrogen signaling. J Endocrinol. 2012;212:3–12. doi: 10.1530/JOE-11-0044. [DOI] [PubMed] [Google Scholar]
- 5.Barton M. Cholesterol and atherosclerosis: modulation by oestrogen. Curr Opin Lipidol. 2013;24:214–220. doi: 10.1097/MOL.0b013e3283613a94. [DOI] [PubMed] [Google Scholar]
- 6.Meyer MR, Clegg DJ, Prossnitz ER, Barton M. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol (Oxf) 2011;203:259–269. doi: 10.1111/j.1748-1716.2010.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nedungadi TP, Clegg DJ. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J Cardiovasc Transl Res. 2009;2:321–327. doi: 10.1007/s12265-009-9101-1. [DOI] [PubMed] [Google Scholar]
- 8.Clegg DJ. Minireview: the year in review of estrogen regulation of metabolism. Mol Endocrinol. 2012;26:1957–1960. doi: 10.1210/me.2012-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional beta-cell mass in diabetes. Nat Rev Endocrinol. 2012;8:342–351. doi: 10.1038/nrendo.2011.242. [DOI] [PubMed] [Google Scholar]
- 11.Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metab. 2011;14:289–299. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB- EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 13.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 14.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 15.Prossnitz ER, Arterburn JB. International Union of Basic and Clinical Pharmacology: G protein-coupled estrogen receptor (GPER) and its pharmacologic modulators. Pharm Rev. 2015 doi: 10.1124/pr.114.009712. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barton M, Prossnitz ER. Emerging roles of GPER in diabetes and atherosclerosis. Trends Endocrinol Metab. 2015 doi: 10.1016/j.tem.2015.02.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prossnitz ER, Barton M. Estrogen biology: New Insights into GPER function and clinical opportunities. Mol Cell Endocrinol. 2014;389:71–83. doi: 10.1016/j.mce.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dennis MK, Field AS, Burai R, et al. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J Steroid Biochem Mol Biol. 2011;127:358–366. doi: 10.1016/j.jsbmb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis MK, Burai R, Ramesh C, et al. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bologa CG, Revankar CM, Young SM, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 22.Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ, Prossnitz ER. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology. 2013;154:4136–4145. doi: 10.1210/en.2013-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma G, Prossnitz ER. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic beta-cells. Endocrinology. 2011;152:3030–3039. doi: 10.1210/en.2011-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martensson UE, Salehi SA, Windahl S, et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 25.Romacho T, Elsen M, Rohrborn D, Eckel J. Adipose tissue and its role in organ crosstalk. Acta Physiol (Oxf) 2014;210:733–753. doi: 10.1111/apha.12246. [DOI] [PubMed] [Google Scholar]
- 26.McGown C, Birerdinc A, Younossi ZM. Adipose tissue as an endocrine organ. Clin Liver Dis. 2014;18:41–58. doi: 10.1016/j.cld.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Exley MA, Hand L, O’Shea D, Lynch L. Interplay between the immune system and adipose tissue in obesity. J Endocrinol. 2014;223:R41–48. doi: 10.1530/JOE-13-0516. [DOI] [PubMed] [Google Scholar]
- 28.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 29.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]