Abstract
Background and Objectives
Social anxiety is characterized by biased attentional processing of social information. However, heterogeneity of extant findings suggests that it may be informative to elucidate individual difference factors that modulate the processing of emotional information. The current study examined whether individual differences in components of attentional control (AC – shifting and focusing) moderated the link between social anxiety and attentional engagement and disengagement biases for threat-relevant cues.
Methods
Seventy-five undergraduate students completed well-established measures of social anxiety symptoms, AC, and attentional bias for social threat information (modified probe detection task).
Results
Moderation analyses revealed that at low levels of AC-shifting, increased social anxiety was associated with slower disengagement from threat-relevant compared to neutral social cues. In contrast, at high levels of AC-shifting, social anxiety was associated with faster disengagement from threat-relevant compared to neutral stimuli. Individual differences in AC-focusing did not moderate the social anxiety-attentional bias link.
Limitations
Causal inferences cannot be made given the cross-sectional study design. The sample comprised individuals displaying a range of self-reported social anxiety symptoms; thus, generalizability to clinical samples remains to be established. The measurement of AC relied on subjective participant report.
Conclusions
The current findings underscore the importance of AC processes in understanding the nature of attentional bias mechanisms in anxiety.
Keywords: Attentional bias, attentional control, disengagement, engagement, individual differences, social anxiety
1. Introduction
The tendency to preferentially attend to threat-relevant social information is hypothesized to play an important role in the onset and maintenance of social anxiety disorder (SAD; Clark, 2001; Clark & Wells, 1995; Hofmann, 2007; Rapee & Heimberg, 1997). Although research generally supports this proposal (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoom, 2007; Cisler & Koster, 2010), the corpus of extant studies points to variability in both the nature and magnitude of attentional biases that characterize individuals with elevated social anxiety symptoms (Chen, Ehlers, Clark, & Mansell, 2002; Gotlib et al., 2004; Mansell, Clark, Ehlers, & Chen, 1999; Ononaiye, Turpin, & Reidy, 2007; Pineles & Mineka, 2005; Yuen, 1994). To the extent that attentional processes are important in understanding the etiology and/or persistence of SAD, it may be informative to elucidate individual difference variables that account for differential patterns of attentional responding to threat cues. Thus, the goal of the present study was to examine whether individual differences in components of attentional control, i.e., the capacity to use attentional resources to modulate processing of emotional stimuli (Derryberry & Reed, 2002), account in part for differential patterns of attentional biases observed across individuals with elevated social anxiety symptoms.
A commonly used paradigm to assess attentional bias for emotional information is the modified probe detection task (MacLeod, Mathews, & Tata, 1986; for a review see Bar-Haim et al. 2007). In this task, response latencies to identify a visual probe replacing one of two simultaneously presented stimuli are used to measure prioritization of attentional allocation for emotional compared to neutral stimuli. Although prior studies tend to support a link between social anxiety and preferential attentional allocation toward social threat relative to neutral information (Asmundson & Stein, 1994; Mogg & Bradley, 2002; Mogg, Philippot, & Bradley, 2004; Pishyar, Harris, & Menzies, 2004; see Bar-Haim et al., 2007 for a meta-analysis), there is also evidence to suggest that socially anxious individuals display an attentional bias away from threat-relevant social information (Chen et al., 2002; Mansell et al., 1999; Yuen, 1994), or do not display biased attentional responding to social threat versus neutral stimuli when compared to non-anxious control participants (Gotlib et al., 2004; Ononaiye et al., 2007; Pineles & Mineka, 2005). Together, these findings suggest that individual differences may contribute to varying patterns of attentional processing across individuals who experience elevated social anxiety symptoms.
Aside from examining the general association between social anxiety and attentional biases for threat, researchers have attempted to disentangle subcomponents of attentional mechanisms using variants of the probe detection task, namely enhanced engagement with threat-related stimuli (i.e., an attentional shift toward threat-related stimuli), or impaired disengagement from threat-related stimuli (i.e., difficulties shifting attention away from threat-related stimuli; Grafton, Watkins, & MacLeod, 2012; Grafton & MacLeod, 2014). Klumpp and Amir (2009) found that socially anxious individuals displayed increased engagement with threat-relevant faces in comparison to individuals without social anxiety. In contrast, other studies using similar methodology found that increased trait anxiety was associated with difficulty disengaging from threat-related information, but not engagement for threat-related stimuli (e.g., Koster, Crombez, Verschuere, & De Houwer, 2004; Salemink, Van den Hout, & Kindt, 2007; see also Amir, Elias, Klumpp, & Przeworski, 2003 for similar findings using a spatial cueing task in a socially anxious sample). More recent refinements of the probe detection task designed to better disambiguate attentional engagement and disengagement mechanisms (Clarke, MacLeod, & Guastella, 2013) revealed that anxiety was associated with both increased attentional engagement with negative images as well as increased impairment with disengaging attention from negative images (Rudaizky, Basanovic, & MacLeod, 2014).
To summarize, previous studies suggest that (1) social anxiety is associated with distinct patterns of attentional processing in the context of threat-relevant social information in comparison to non-anxious individuals, including biases either toward or away from threat-relevant information; (2) these patterns of attentional processing may reflect enhanced engagement with and/or difficulties disengaging attention from threat-relevant information, or both; and (3) even within socially anxious samples, individuals vary considerably in the nature and degree of biased attentional processing. What might account for individual variability in patterns of attentional processing associated with social anxiety? As a step toward addressing this question, and to further understand the nature of extant findings and clarify the role of attentional processes in social anxiety, we drew on attentional control theory (Eysenck, Derakshan, Santos, & Calvo, 2007) as a model for understanding and making predictions about individual variation in attentional bias patterns associated with social anxiety.
Attentional control (AC) is defined as the ability to effortfully regulate attention to override automatic emotional responses (Derryberry & Reed, 2002). Corbetta and Shulman (2002) found evidence for stimulus-driven (i.e., a bottom-up process driven by salient information) and goal-directed (i.e., a top-down process directed by knowledge and current goals) attentional systems. AC theory posits that anxiety disturbs the equilibrium between these two systems, such that the stimulus-driven system is more influential on attentional processing than the goal-directed system (Eysenck et al., 2007). By this account, a stimulus-driven attentional system characterized by hyper-responsiveness to emotionally salient stimuli paired with decreased regulation by the goal-driven system may lead to biased processing of salient, threat-relevant stimuli for anxious individuals.
Researchers have found that AC plays an important role in the relationship between anxiety and the processing of emotional information. In an influential study, Derryberry and Reed (2002) found that attentional bias for threat-related stimuli exhibited by individuals with elevated trait anxiety was moderated by AC. Individuals with higher AC were better at disengaging from threat in comparison to individuals with lower AC. Most relevant to the current study, past research has examined the role of AC in the relationship between anxiety and attentional bias to threat using probe detection paradigms. For example, Bardeen and Orcutt (2011) found that self-reported AC moderated the relationship between attentional bias for threat and posttraumatic stress symptoms (PTSS) such that individuals with low AC and high PTSS were more likely to attend to threat relative to neutral stimuli at a shorter (i.e., 150 ms) stimulus presentation durations. Similarly, other studies have also shown AC as a moderator of the relationship between anxiety and attentional bias for threat-related stimuli (Hou et al., 2014; Schoorl, Putman, Van Der Werff, & Van Der Does, 2014). These findings converge with a growing literature across numerous paradigms and measures suggesting that AC plays a role in the relationship between anxiety and the processing of emotional information (Reinholdt-Dunne, Mogg, & Bradley, 2009).
Despite growing evidence supporting the role of AC in modulating anxiety-related attentional processes, several questions remain unanswered. First, although AC has been shown to modulate affective and behavioral responses in relation to social anxiety (Jones, Fazio, & Vasey, 2012; Morrison & Heimberg, 2013), no studies to our knowledge, have examined the influence of AC on the relationship between social anxiety and attentional processes. Addressing this issue may explain, in part, variability in extant studies investigating the relationship between social anxiety and attentional biases. Second, AC has not been examined in relation to subcomponents of attentional processes linked to anxiety, namely attentional engagement and disengagement from threat-relevant stimuli. Thus, it remains to be established whether AC modulates specific attentional mechanisms (e.g., disengagement) or exerts more generic control over attentional processing. Third, AC itself is a multifaceted construct, and prior research supports empirically distinct dimensions underlying AC. Most relevant to the current study, factor analytic studies of the Attention Control Scale (ACS; Derryberry & Reed, 2002), a well-established self-rated measure of AC, revealed two dimensions underlying AC, namely shifting and focusing (Judah, Grant, Mills, & Lechner, 2014; Olafsson et al., 2011). The shifting dimension measures the ability to flexibly distribute attentional processes across multiple tasks that compete for cognitive processing resources (e.g., "It is easy for me to read or write while I’m also talking on the phone"), whereas the focusing dimension measures the ability to maintain attentional resources on task-relevant demands (e.g., "My concentration is good even if there is music in the room around me"). Examining subcomponents of both AC and threat-related attentional biases may provide a more precise understanding of information processing mechanisms that characterize social anxiety.
The goal of the present study was to examine whether dimensions of AC moderate the relationship between social anxiety symptoms and subcomponents of attentional engagement and disengagement for threat-relevant information. A cross-section of individuals endorsing a range of social anxiety symptoms completed the ACS to measure shifting and focusing dimensions of AC as well as a modified probe detection paradigm designed to measure engagement and disengagement components of attentional allocation for social-threat stimuli (negative faces). Drawing on AC theory (Eysenck et al., 2007) and prior findings (Derryberry & Reed, 2002), we predicted that individual differences in the capacity to shift attentional allocation would moderate the relationship between level of social anxiety and attentional disengagement from threat-relevant information, such that individuals endorsing elevated social anxiety symptoms and low levels of AC shifting would display greater difficulty disengaging from threat cues relative to high anxious participants with higher AC shifting scores. AC shifting, however, was expected to be less sensitive in moderating the relationship between social anxiety and attentional engagement threat biases given that attentional engagement (cf. disengagement) biases are hypothesized to reflect more bottom-up (stimulus-driven) processes compared to top-down cognitive control mechanisms (Corbetta & Shulman, 2002). The thematic content of the ACS-focusing scale, namely the capacity to maintain attentional allocation on a specific task or target stimuli, does not clearly map onto engagement and disengagement mechanisms as measured by probe detection tasks. Thus, we did not make predictions about its relationship with our measure of attentional engagement and disengagement, and consider such analyses exploratory.
2. Material and Methods
2.1. Participants
Participants were 75 individuals (34 men, 40 women)1 drawn from a pool of undergraduate students at a large university (mean age = 20.66, SD = 4.43; mean years of education = 14.05, SD = 1.25). These individuals responded to an advertisement for “individuals with difficulty giving speeches”. We expected that although this recruitment strategy would yield a sample of individuals scoring higher than non-anxious samples on mean levels of social anxiety, it would also allow for a wide range of social anxiety symptoms. See Table 1. Participants were offered course credit for their participation.
Table 1.
Means, standard deviations, and range of scores for the primary measures.
| Measure | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| LSAS-SR | 44.58 | 26.19 | 0 | 128 |
| ACS-Shifting | 25.99 | 4.17 | 16 | 38 |
| ACS-Focusing | 21.59 | 4.60 | 11 | 31 |
| Disengagement Bias | −3.77 | 106.45 | −207.91 | 310.55 |
| Engagement Bias | 3.04 | 117.03 | −309.77 | 295.04 |
Note. LSAS-SR = Liebowitz Social Anxiety Scale – Self-report; ACS = Attentional Control Scale.
2.2. Measures
2.2.1. Social Anxiety
The Liebowitz Social Anxiety Scale–Self-report version (Liebowitz, 1987) was used to assess level of social anxiety. The LSAS-SR consists of 24 social situations (e.g., public speaking, going to parties, meeting strangers) and asks the individual to rate their level of Fear and Avoidance for each situation on a 4-point scale (where 0 is ‘none/never’ and 3 is ‘severe/usually’). Items are summed to create a total score reflecting level of social anxiety symptoms (current sample Cronbach’s α = .96). The LSAS-SR displays strong psychometric properties that converge with the interviewer-administered LSAS (Fresco et al., 2001).
2.2.2. Depression
The Beck Depression Inventory – II (BDI-II; Beck, Steer, & Brown, 1996) is a 21-item self-report inventory that assesses severity of depression during the past two weeks. The BDI–II demonstrates excellent psychometric properties (e.g., Beck et al., 1996; Dozois, Dobson, & Ahnberg, 1998; current sample Cronbach’s α = .89) and was used to examine whether co-occurring symptoms of depression accounted for the predicted outcomes.
2.2.3. Attentional Control
The Attentional Control Scale (ACS; Derryberry & Reed, 2002) is a 20-item self-report questionnaire used to measure individual differences in attentional regulation and asks the individual to rate how they feel about situations related to concentrating and attentional flexibility on a 4-point scale (where 1 is ‘almost never’ and 4 is ‘always’). This questionnaire has shown to be a valid measure of attentional regulation (Judah et al., 2014; Olafsson et al. 2011) and to have good internal consistency (α = .88; Derryberry & Reed, 2002). Following prior factor analytic research (Olafsson et al., 2011), we used the two subscales of the ACS, attentional shifting (10 items) and attentional focusing (9 items). In the current study, Cronbach's alphas were .67 and .78 for the shifting and focusing scales, respectively.
2.2.4. Attention Bias Assessment Task
To measure attentional allocation for social threat information, participants completed a modified probe detection task (MacLeod et al., 1986). The stimuli comprised a standardized set of four male and four female faces portraying either a disgust and neutral expression or a happy2 and neutral expression (Matsumoto & Ekman, 1989)3. Disgust faces have been shown to be threat-relevant stimuli for socially anxious individuals (Amir, Najmi, Bomyea, & Burns, 2010). Each face measured 640 x 480 pixels with a resolution of 72,000 pixels per inch. Faces were positioned 3.0 cm from the top of the screen and separated by 1.5 cm between the bottom of the top image and the top of the bottom image. Both faces were centered horizontally and 17.5 cm from the left edge of the screen. Faces were 3.75 cm tall x 5 cm wide.
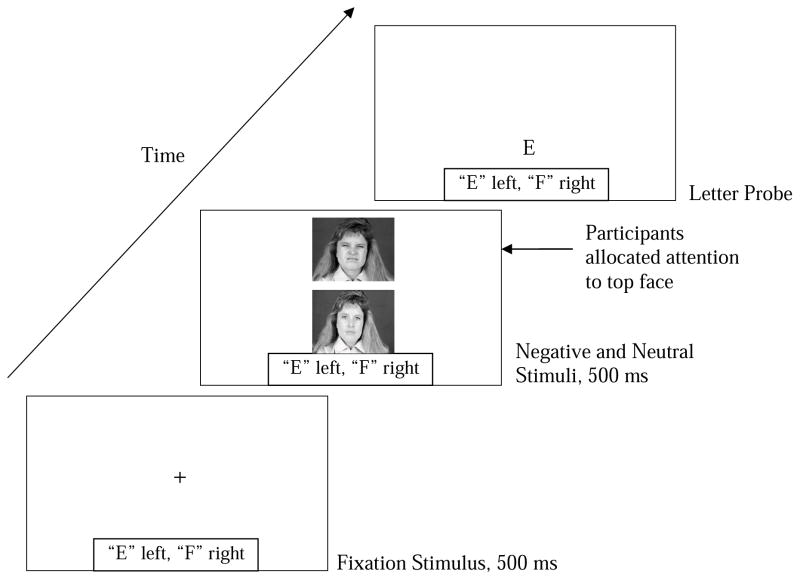
Each attentional bias assessment trial began with a centered fixation cross presented on the computer screen for 500ms. Next, this cross was replaced by a face-pair presented in the center of the screen for 500ms, one face above the other. Consistent with prior probe detection studies (e.g., Asmundson & Stein, 1994; Klumpp & Amir, 2009; MacLeod et al., 1986) and to control for individual differences in initial orienting to one of two stimuli (i.e., top vs. bottom), we asked participants to attend to the top picture at the start of every trial, thereby anchoring their attention to the top locus (Rudaizky et al., 2014). On trials in which a disgust face was present, it appeared in the top position 50% of the time. The faces then disappeared and a probe (i.e., the letter “E” or “F”) appeared immediately in the location of one of the two faces. The probe was either distal or proximal to the original locus of the participant’s attention. Participants were instructed to indicate whether the letter was an E or an F by pressing the corresponding mouse button. The importance of both speed and accuracy was emphasized to participants. The letter probe remained on the screen until participants responded. Response latencies to identify the probe were recorded from the onset of the letter probe to the button press. Trials were separated by 500ms intervals of a blank screen, and subsequent trials began with the presentation of a fixation cross. See Figure 1.
Figure 1.
This is an example of a trial in which participants must disengage from the negative stimulus to attend to the letter probe. Initial attention was anchored to the top locus by instructing participants to look at the top picture.
There were two critical trials of interest: (1) Trials in which both the disgust face and probe were presented distal to (i.e., bottom locus) the participant's original locus of attention. Such trials permitted an assessment of the speed with which participants' attention shifted to engage with threat-relevant information relative to maintaining attention in the vicinity of neutral information; (2) Trials in which the disgust face was presented in the same locus as the participant's initial focus of attention (i.e., top) and the probe was presented distal to the participant's original locus of attention (i.e., bottom). Such trials permitted an assessment of the speed with which participants' attention shifted from the location occupied by the disgust face to a distal location occupied by the neutral face. See Figure 1.
The assessment consisted of 96 trials: 64 trials included one neutral face and one emotional face (e.g., disgust) and 32 trials included only neutral faces. Trials were presented in a new random order to each participant. Participants were seated approximately 30cm from the computer screen. The computer program was written in Delphi (Embarcadero, Inc.) for this experiment.
2.3. Procedure
Upon arriving to the laboratory, participants provided informed written consent and completed the self-report questionnaires (i.e., demographics questionnaire, LSAS-SR, BDI-II, and ACS). Next, participants completed the probe detection task to assess attentional bias for social information. Participants subsequently completed a number of other tasks intended to address a different research question reported elsewhere.
3. Results
3.1. Preliminary Analyses and Data Preparation
3.1.1. Computation of Attentional Engagement and Disengagement Indices
Prior to the main analyses, one participant was removed from analysis due to low trial accuracy (44.75% of trials incorrect). Response latency [reaction time (RT)] data from the attention bias assessment were prepared in keeping with recommendations from Ratcliff (1993). First, trials with incorrect responses were removed (3.35%). Response latencies less than 200ms or greater than 3000ms were eliminated from analysis of the assessment task (0.20% of trials with correct responses). Response latencies ±3.0 SD from each participant’s mean response latency were also eliminated from analysis of the assessment task, respectively (1.56% of remaining trials). See Table 2 for means and standard deviations of response latencies by trial type.
Table 2.
Means and standard deviations of response latencies (in milliseconds) by trial type.
| DNT | DNB | NDT | NDB | NNT | NNB | |
|---|---|---|---|---|---|---|
| Mean | 573.52 | 572.55 | 574.66 | 574.42 | 564.02 | 566.81 |
| SD | 126.87 | 134.31 | 134.91 | 135.24 | 120.43 | 111.43 |
Note. DNT = Disgust face top, neutral face bottom, probe replaces top face; DNB = Disgust face top, neutral face bottom, probe replaces bottom face; NDT = Neutral face top, disgust face bottom, probe replaces top face; NDB = Neutral face top, disgust face bottom, probe replaces bottom face; NNT = Neutral face top and bottom, probe replaces top face; NNB = Neutral face top and bottom, probe replaces bottom face.
Attentional engagement and disengagement bias scores were computed following recommendations underscoring the importance of anchoring the participant’s attention in a specified spatial location and presenting the target emotional stimuli in a location either proximal or distal to the initial focus of the participant’s attention (Clarke, MacLeod, & Guastella, 2013; see also Rudaizky et al., 2014).
Attentional engagement trials were trials in which both the target emotional face and probe were presented distal to (i.e., bottom locus) the participant's original locus of attention (i.e., top face). Thus, on disgust-neutral trials, the target emotional face (i.e., disgust face) was presented in the bottom location; and on neutral-neutral trials, the target emotional face (i.e., neutral face) was also presented in the bottom location. On engagement trials, the speed to respond to the probe appearing in the location of the disgust face on disgust-neutral trials, in comparison to the probe appearing in the locus of the target neutral face on neutral-neutral trials, will be relatively fast to the extent that attention shifts to engage with the disgust face. If attention is faster to engage with disgust faces compared to neutral faces, then this is indicative of an attentional engagement bias towards disgust (threat-relevant) faces.
The engagement bias index was calculated using trials in which the disgust face was presented distal to the participant's original locus of attention as follows: Engagement bias index: (Neutral face at top of screen and disgust face at bottom: RT for probe distal to disgust face minus RT for probe proximal to disgust face) minus (Neutral face top and bottom of screen: RT for probe on bottom minus RT for probe on top). Higher scores reflect selectively enhanced shifting of attention towards initially unattended threat faces relative to neutral faces.4
Attentional disengagement trials were trials in which the target emotional disgust face was presented in the same locus as the participant's initial locus of attention (i.e., top face) and the probe was presented distal to the participant's original locus of attention (i.e., bottom locus). Thus, on disgust-neutral trials, the target emotional face (i.e., disgust face) was presented in the top location; and on neutral-neutral trials, the target emotional face (i.e. neutral face) was also presented in the top location. On disengagement trials, the speed to respond to the probe appearing in the location of the neutral face on disgust-neutral trials, in comparison to the probe appearing in the location of the bottom neutral face on neutral-neutral trials, will be relatively slowed to the extent that attention has been held in the location of the initially presented disgust face. If attention is slower to disengage from disgust faces compared to neutral faces, then this is indicative of impairment with disengaging from disgust (threat-relevant) faces.
The disengagement bias index was calculated using trials in which the disgust face was presented in the same locus as the participant's initial focus of attention as follows: Disengagement bias index: (Disgust face at top of screen and neutral face at bottom: RT for probe distal to disgust face minus RT for probe proximal to disgust face) minus (Neutral face top and bottom of screen: RT for probe on bottom minus RT for probe on top). Higher scores reflect a greater tendency for attention to be held in the spatial location of initially attended threat faces relative to neutral faces. 4
The means, standard deviations, and ranges of the measures are presented in Table 1. Bivariate correlations between measures are presented in Table 3. Social anxiety symptoms were not significantly associated with attentional engagement or disengagement indices, r(71) = −.22 and .11, respectively, both p > . 05. Higher levels of social anxiety were associated with lower scores on both ACS focusing and shifting subscales, r(71) = −.37 and −.35, respectively, both p < .01.
Table 3.
Bivariate correlations between social anxiety, attentional control, engagement and disengagement bias indices for threat stimuli.
| Measure | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. LSAS-SR | 1.00 | ||||
| 2. ACS-Shifting | −.35** | 1.00 | |||
| 3. ACS-Focusing | −.37** | −.50** | 1.00 | ||
| 4. Disengagement Bias | .11 | −.24* | .07 | 1.00 | |
| 5. Engagement Bias | −.22 | .19 | .01 | −.63** | 1.00 |
Note. LSAS-SR = Liebowitz Social Anxiety Scale – Self-report; ACS = Attentional Control Scale.
p < .05.
p < .01.
3.2. Main Analyses
3.2.1. Overview of Regression Analyses
Hierarchical regression analyses were used to test the hypothesis that individual differences in AC would moderate the relationship between social anxiety symptoms and attentional processing of negative social information. Given the specificity of our hypotheses regarding the influence of subfacets of AC on subcomponents of attentional processing of threat, separate regression models were tested for attentional disengagement and engagement bias indices as well as AC shifting and focusing subscales. In each model, level of social anxiety (LSAS total score) and AC subscale scores (shifting or focusing) served as predictors. Attentional disengagement and engagement scores served as the dependent variables. Prior to the analyses, continuous predictor variables included in interaction terms were centered (Aiken & West, 1991). The two predictor variables, LSAS and ACS (shifting or focusing), were entered separately in steps one and two of the regression equation, respectively. The LSAS × ACS interaction term was entered in step three of the regression analysis. Significant interactions were probed by conducting a regions of significance analysis using the Johnson-Neyman technique (Johnson & Neyman, 1936). This approach identifies the specific values of the moderator variable (ACS) at which the relationship between social anxiety symptoms and attentional bias for negative social information become statistically significant at α = .05. This analysis was implemented using an SPSS macro developed by Hayes and Matthes (2009).
3.2.2. Attentional Control and Disengagement Bias
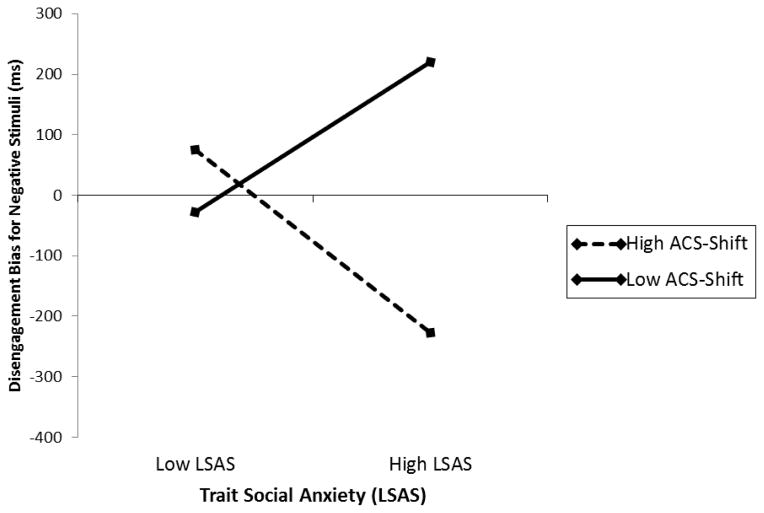
Table 4 presents the results of the hierarchical regression analyses predicting attentional disengagement bias scores. Our main hypothesis involved examining the relationship between individual differences in AC shifting abilities and disengagement from negative social information. Results of this analysis revealed a significant LSAS × ACS-shifting interaction, ΔR2 = .069, p = .023, which indicated that individual differences in AC shifting abilities moderated the relationship between level of social anxiety and attentional disengagement bias for negative social stimuli. 5 A regions of significance analysis identified 20.88 and 31.44 on the ACS-shifting measure as points of transition between a statistically significant and a statistically non-significant relationship between social anxiety and attentional disengagement scores. Specifically, this analysis revealed that for ACS-shifting scores less than 20.88 to the lowest value observed (16), level of social anxiety was positively associated with attentional disengagement scores. That is, higher levels of social anxiety symptoms were associated with significantly greater attentional disengagement bias scores for negative compared to neutral social cues. In contrast, for ACS-shifting scores greater than 31.44 to the largest observed value (38), level of social anxiety was negatively associated with attentional disengagement scores. That is, higher levels of social anxiety symptoms were associated with significantly faster attentional disengagement from negative compared to neutral social information. For ACS-shifting scores between 20.88 to 31.44, the relationship between social anxiety symptoms and attentional disengagement bias scores was not significant. These findings are illustrated in Figure 2. Note that the specific values obtained from the regions of significance analysis are estimates based on the current sample, and thus, should not be interpreted as an absolute threshold that is generalizable beyond the current sample.
Table 4.
Hierarchical Regression Analyses of Individual Differences in Social Anxiety and (a) Attentional Control – Shifting and (b) Attentional Control – Focusing Predicting Attentional Disengagement Bias Scores for Threat Stimuli.
| Moderator | (a) ACS-Shifting | (b) ACS-Focusing | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| B | SE B | β | ΔR2 | B | SE B | β | ΔR2 | |
| Step 1 | .01 | .01 | ||||||
| LSAS | .45 | .48 | .11 | .45 | .48 | .11 | ||
| Step 2 | .05 | .01 | ||||||
| LSAS | .13 | .50 | .031 | .64 | .52 | .16 | ||
| Moderator | -5.88 | 3.16 | −.23 | 2.93 | 2.94 | .13 | ||
| Step 3 | .07* | .001 | ||||||
| LSAS | −.33 | .53 | −.08 | .69 | .54 | .17 | ||
| Moderator | −6.41 | 3.07 | −.25* | 2.96 | 2.96 | .13 | ||
| LSAS × Moderator | −.26 | .11 | −.28* | .03 | .10 | .04 | ||
Note. ACS = Attentional Control Scale; LSAS = Liebowitz Social Anxiety Scale.
p < .05.
p < .01.
p < 001.
Figure 2.
Simple regression slopes of levels of attentional control – shifting (ACS – Shift) predicting disengagement bias for negative stimuli at levels of trait social anxiety (LSAS).
The regression analysis examining ACS-focusing as a moderator of the relationship between social anxiety and attentional disengagement scores revealed that the LSAS × ACS-focusing interaction was not significant, ΔR2 = .001, p = .77. These findings indicated that individual differences in AC focusing abilities did not influence the relationship between social anxiety symptoms and attentional disengagement scores. See Table 4.
3.2.3. Attentional Control and Engagement Bias
Table 5 presents the results of the hierarchical regression analyses predicting attentional engagement bias scores. In both regression models, the LSAS × ACS interaction was not significant, ΔR2 = .03, ΔR2 = .001, both p > .10, respectively. These findings indicated that individual differences in self-reported AC did not influence the relationship between social anxiety symptoms and attentional engagement for negative social information.
Table 5.
Hierarchical Regression Analyses of Individual Differences in Social Anxiety and (a) Attentional Control – Shifting and (b) Attentional Control – Focusing Predicting Attentional Engagement Bias Scores for Threat Stimuli.
| Moderator | (a) ACS-Shifting | (b) ACS-Focusing | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| B | SE B | β | ΔR2 | B | SE B | β | ΔR2 | |
| Step 1 | .05 | .05 | ||||||
| LSAS | −.97 | .52 | −.22 | −.97 | .52 | −.22 | ||
| Step 2 | .02 | .01 | ||||||
| LSAS | −.77 | .55 | −.17 | −1.11 | .56 | −.25 | ||
| Moderator | 3.65 | 3.47 | .13 | −2.08 | 3.19 | −.08 | ||
| Step 3 | .03 | .001 | ||||||
| LSAS | −.43 | .59 | −.10 | −1.16 | .59 | −.26 | ||
| Moderator | 4.04 | 3.44 | .14 | −2.11 | 3.21 | −.08 | ||
| LSAS × Moderator | .20 | .13 | .19 | −.03 | .11 | −.04 | ||
Note. ACS = Attentional Control Scale; LSAS = Liebowitz Social Anxiety Scale.
p < .05.
p < .01.
p < 001.
There were no significant main or interaction effects in predicting attentional engagement bias scores for negative stimuli.
4. Discussion
Dysregulation of attention is considered an important mechanism of social anxiety development and maintenance. The aim of the current study was to examine whether individual differences in subcomponents of attentional control (AC) moderated the relationship between level of social anxiety and attentional engagement and disengagement tendencies during processing of threat-relevant stimuli. The main finding was that individual variability in the self-rated capacity to flexibly shift attentional allocation moderated the association between social anxiety and attentional disengagement from, but not engagement with, social threat stimuli. This study adds to a growing literature underscoring the importance of AC processes in understanding the nature of anxiety-related attentional mechanisms (Bardeen & Orcutt, 2011; Derryberry & Reed, 2002; Hou et al., 2014; Reinholdt-Dunne et al., 2009; Schoorl et al., 2014). The current findings extend the extant literature, however, by pointing to the potential value of investigating subcomponents of both AC and attentional bias mechanisms in anxiety.
Social anxiety symptoms were not directly related to attentional engagement or disengagement threat bias indices. These findings are consistent with prior studies that did not find evidence of an association between social anxiety and attentional biases for threat (Gotlib et al., 2004; Ononaiye et al., 2007; Pineles & Mineka, 2005). Consistent with our prediction and prior studies (Bardeen & Orcutt, 2011; Derryberry & Reed, 2002; Hou et al., 2014; Reinholdt-Dunne et al., 2009; Schoorl et al., 2014), however, AC moderated the social anxiety-attentional bias relationship. Individual differences in the shifting (but not focusing) subscale of the ACS were associated with differential patterns of attentional disengagement for threat cues at high levels of social anxiety. A regions of significance analysis revealed two distinct patterns of attentional processing: (1) At low levels of AC-shifting, higher levels of social anxiety were associated with greater difficulties disengaging attention from social threat cues. These findings converge with prior cross-sectional studies demonstrating a link between social anxiety and attentional disengagement biases for social threat information (e.g., Amir et al., 2003; Buckner, Maner, & Schmidt, 2010). (2) In contrast, at high levels of AC-shifting, increasing levels of social anxiety were associated with faster attentional disengagement from threat-relevant cues. Taken together, this pattern of findings mirrors those reported in a recent study (Gorlin & Teachman, in press) in which general inhibitory control (a component of attentional control) measured using the color-word Stroop paradigm moderated the relationship between threat interference biases (measured using the emotional Stroop task) and indices of trait and state social anxiety (e.g., anxiety and negative cognitions during a laboratory speech task). Specifically, among participants with weaker inhibitory control, greater social threat interference was associated with higher anxiety, whereas among participants with stronger inhibitory control, lower social threat interference was associated with greater anxiety. All in all, the current findings suggest that individual differences in AC may be important in understanding both the nature and magnitude of attentional biases in individuals with elevated social anxiety symptoms, and may in part explain variability in extant findings regarding the link between social anxiety and attentional biases for threat.
The present results extend the extant literature by pointing to the potential importance of investigating subfacets of AC and threat-related biases in anxiety, and provide evidence to support the discriminant predictive validity of ACS dimensions reported in previous research (Judah et al. 2014; Olafsson et al. 2011). Prior studies have treated AC as a unitary construct. However, the current findings suggest that specific subfacets of AC, namely AC-shifting, account for the link between social anxiety and attentional disengagement biases. The AC-focusing subscale did not explain the pattern of attentional engagement or disengagement biases. Tasks that require sustained attentional focus (e.g., continuous performance task; Conners, 2002) may be more sensitive to revealing links between social anxiety, AC-focusing, and attentional processes (Kane et al., 2007; Stawarczyk, Majerus, Catale, & D'Argembeau, 2014). Future research is needed to address that issue.
How would impoverished AC contribute to difficulties disengaging attention from threat relative to benign stimuli in individuals with high levels of social anxiety? According to dual process models of cognition and emotion (Corbetta & Shulman, 2002; Eysenck et al. 2007), anxiety is associated with a hyper-responsiveness of the bottom-up stimulus driven attentional system that facilitates processing of salient, i.e., threat-relevant information (Derakshan & Eysenck, 2009; see Taylor & Amir, 2010 for a review). To the extent that negative social information is salient for individuals with heightened social anxiety given their fears of negative evaluation, such information is likely to preferentially demand attentional resources. However, under conditions in which threatening social information is task-irrelevant (e.g., the probe follows the non-threat stimulus), individuals with poor AC will have difficulties removing their attention from threat to efficiently respond to the task at hand. Consistent with this proposal, a recent study found that individuals with elevated social anxiety symptoms displayed difficulties disengaging their attention from threat stimuli, but only under conditions of high working memory load, ostensibly when attentional control resources were taxed (Judah, Grant, Lechner, & Mills, 2013). Thus, the current and prior data demonstrate the important role of AC in the expression of anxiety-related attentional biases. These findings are consistent with neurocognitive accounts of AC in which anxious individuals have been shown to display decreased activation of prefrontal brain regions (e.g., dorsolateral prefrontal cortex) thought to play a role in down-regulating amygdala activation when processing threat-relevant information (Bishop, Duncan, Brett, & Lawrence, 2004; Bishop, 2008, 2009).
The present findings also beg the question: Can AC be maladaptive in anxiety? One might argue that individuals in the current study with high levels of social anxiety and greater AC-shifting scores demonstrated attentional avoidance of threat-relevant cues; that is, enhanced atttentional disengagement from threat vs. neutral cues. Attentional avoidance has been proposed as a putative maintaining factor in anxiety. For example, vigilance-avoidance models of anxiety (Mogg & Bradley, 1998) suggest that attentional avoidance could result in repeated brief exposures to threat stimuli, which may impede emotional processing and thereby lead threat-relevant stimuli to retain their anxiety-provoking properties (e.g., Foa & Kozak, 1986; Foa, Huppert, & Cahill, 2006). By this account, AC may facilitate strategic attentional avoidance of cues initially appraised as threat relevant [(Heeren, De Raedt, Koster, & Philippot, 2013; Mogg & Bradley, 1998; see Price and Mohlman (2007) for a similar account regarding a potential maladaptive function of strong inhibitory control in anxiety. Note, however, that attentional avoidance of threat cues has also been shown to occur in individuals with poorer shifting ability, suggesting that avoidance may not always be a controlled or strategic response to threat (Booth, 2014)]. Considered together, social anxiety may be maintained by one of two attentional mechanisms: (1) poor AC that interferes with attentional disengagement from threat stimuli under conditions in which threat cues are task-irrelevant, or (2) high AC that facilitates strategic avoidance of threat-related cues and therefore prevents adequate emotional processing of that material (see also Gorlin & Teachman, in press). To the extent that these distinct mechanisms perpetuate social anxiety, they underscore the importance of investigating AC-informed treatment targets. Experimental studies designed to manipulate different subcomponents of AC are needed to test these hypotheses.
Given that the current study design was cross-sectional, causal inferences cannot be made. However, to the extent that AC regulates the expression of biased patterns of attentional responding implicated in the development and/or maintenance of anxiety, it may serve as an important target in prevention or treatment programs. Indeed, research supports the efficacy of numerous interventions that target attention processes in treatment (see Taylor & Amir, 2010 for a review). Moreover, modulating activity in brain regions implicated in AC may influence anxiety reactivity and attentional biases (Heeren et al., 2013). For example, experimentally manipulating attentional allocation away from threat-relevant stimuli in individuals with elevated social anxiety symptoms was found to enhance activation in prefrontal brain regions implicated in AC (i.e., ventromedial prefrontal cortex; vmPFC) as well as decrease activation in limbic regions (e.g., amygdala) implicated in biased attentional responding for salient (e.g., threat-relevant) stimuli (Taylor et al., 2014; see also Britton et al., in press). Moreover, greater increases in vmPFC activation following the attentional manipulation were associated with larger reductions in attentional disengagement scores for threat cues as well as attenuated anxiety reactivity to a subsequent laboratory stressor (Taylor et al.). All in all, the current findings warrant further investigation regarding the causal role of AC and attentional biases in anxiety, and suggest that it may be informative to understand how different subcomponents of AC mechanisms confer vulnerability to anxiety.
Future research could build upon the current study in several ways. First, the present sample comprised undergraduate students, and generalizability to community and clinical samples is needed. Moreover, a non-anxious control group is needed to establish a benchmark for the seemingly enhanced attentional disengagement from threat cues observed in participants with high levels of social anxiety and greater AC-shifting scores. Second, although AC was assessed using a validated instrument, it relied on subjective participant report. Moreover, consistent with prior studies (Olafsson et al., 2011), the internal consistency of the ACS-shifting subscale was low, which suggests that all of the items may not be measuring the same latent variable, in this case, attentional shifting. It is promising, however, that prior studies found relationships between the ACS-shifting scale and cognitive-experimental tasks designed to measure attentional shifting abilities (Judah et al., 2014), supporting its convergent validity. Nevertheless, additional work is needed to strengthen the psychometric properties of the ACS. Accordingly, future research is needed to assess AC using more objective measures (e.g., Attentional Network Task; Fan, McCandliss, Sommer, Raz, & Posner, 2002).
The number of trials per trial type used in the computation of attentional bias scores is lower compared to some prior probe detection studies. Although the optimal number of trials needed to maximize reliability of attentional bias indices has not been established, this question is an important one for future research. It is also important to note that although we instructed participants to fixate their attention on a predetermined spatial location during the presentation of stimuli in order to anchor their attention, it was not possible to verify whether participants' initial attentional allocation was in the vicinity of the top face as instructed. Extensions of this research could implement recently developed variants of the probe detection task in which participants' initial attentional allocation is verified by requiring correct identification of a cue probe at the end of each trial (Rudaizky et al., 2014).
5. Conclusion
This study suggests that individual differences in AC may be important in understanding both the nature and magnitude of attentional biases in social anxiety. The current results point to the potential value of investigating subcomponents of both AC and anxiety-related attentional bias mechanisms. Future experimental research is needed to examine the causal role of AC in anxiety development and maintenance in order to inform AC-targeted intervention approaches.
Highlights.
We assessed the link between social anxiety and threat engagement and disengagement.
We examined whether attentional control (AC) moderated those relationships.
Increased social anxiety related to slower threat disengagement at low levels of AC.
Increased social anxiety related to faster threat disengagement at high levels of AC.
AC may be an important process underlying variability in attentional bias in anxiety.
Acknowledgments
This research was supported by grants from the National Institute of Mental Health awarded to the first author (K99MH090243) and third author (R01MH087623). We would like to thank Laura Greathouse, John Plocharczyk, Daniel Fry, and Acacia Schmidt for their help with data collection and management, and Eleni Kapoulea for her editorial assistance.
Footnotes
Demographic information was missing for one participant. We reanalyzed the data in the sub-sample of participants who had complete demographic data. Results of these analyses did not differ from those reported for the entire sample.
Only disgust-neutral face pairs were analyzed for the current study because happy-neutral face pairs were not relevant to the study hypotheses.
Participants were randomly assigned to view one of two face sets (A or B) during the attention bias assessment task. We repeated the analyses entering face set as a covariate. Results did not differ according to face set, i.e., all main and interaction effects including face set were not significant (all p > .10). Thus, we report findings for both groups combined in the main text.
Upon examining frequency distributions of attentional bias indices, we detected one participant with attentional engagement and disengagement scores that were noticeably detached from the rest of the distribution. This participant also had standardized residual scores greater than 3 SDs from the predicted scores across all regression analyses predicting attentional bias indices. Accordingly, this person was removed from the analysis.
Depression is also associated with difficulties in attentional control (Olafsson et al., 2011) and frequently co-occurs with social anxiety (Schneier, Johnson, Hornig, Liebowitz, & Weissman, 1992). At the suggestion of an anonymous reviewer, we conducted a sensitivity analysis in which we modeled the main effect of depression (BDI-II scores), the interaction of depression with social anxiety (LSAS), and the three-way interaction of depression by social anxiety by ACS-shifting scores in the regression equation predicting attentional disengagement biases. Results revealed that neither the main nor interactive effects of depression accounted for significant variance in explaining attentional disengagement scores for threat stimuli, R2 = .060, p = .19. Moreover, ACS-shifting remained a robust moderator of the social anxiety-attentional disengagement bias relationship when accounting for the effects of depression in the regression model, B = −.24, t = −2.01, p = .049. Thus, the current pattern of findings cannot be accounted for by co-occurring symptoms of depression.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Newbury Park, Calif: Sage Publications; 1991. [Google Scholar]
- Amir N, Elias J, Klumpp H, Przeworski A. Attentional bias to threat in social phobia: facilitated processing of threat or difficulty disengaging attention from threat? Behaviour Research and Therapy. 2003;41(11):1325–1335. doi: 10.1016/S0005-7967(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Amir N, Najmi S, Bomyea J, Burns M. Disgust and anger in social anxiety. International Journal of Cognitive Therapy. 2010;3(1):3–10. doi: 10.1521/ijct.2010.3.1.3. [DOI] [Google Scholar]
- Asmundson GJG, Stein MB. Selective processing of social threat in patients with generalized social phobia: Evaluation using a dot-probe paradigm. Journal of Anxiety Disorders. 1994;8(2):107–117. doi: 10.1016/0887-6185(94)90009-4. [DOI] [Google Scholar]
- Bardeen JR, Orcutt HK. Attentional control as a moderator of the relationship between posttraumatic stress symptoms and attentional threat bias. Journal of Anxiety Disorders. 2011;25(8):1008–1018. doi: 10.1016/j.janxdis.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoom MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer A, Brown K. Beck Depression Inventory-II. San Antonio, TX: Harcourt Brace; 1996. [Google Scholar]
- Bishop SJ. Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences. 2008;1129:141–152. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12(1):92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7(2):184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Booth RW. Uncontrolled avoidance of threat: Vigilance-avoidance, executive control, inhibition, and shifting. Cognition & Emotion. 2014;28(8):1465–1473. doi: 10.1080/02699931.2014.882294. [DOI] [PubMed] [Google Scholar]
- Britton JC, Suway JG, Clementi MA, Fox NA, Pine DS, Bar-Haim Y. Neural changes with attention bias modification for anxiety: A randomized trial. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsu141. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Maner JK, Schmidt NB. Difficulty disengaging attention from social threat in social anxiety. Cognitive Therapy and Research. 2010;34(1):99–105. doi: 10.1007/s10608-008-9205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YP, Ehlers A, Clark DM, Mansell W. Patients with generalized social phobia direct their attention away from faces. Behaviour Research and Therapy. 2002;40(6):677–687. doi: 10.1016/S0005-7967(01)00086-9. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Koster EH. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30(2):203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DM. A cognitive perspective on social phobia. In: Crozier WR, Alden LE, editors. International handbook of social anxiety: concepts, research and interventions relating to the self and shyness. West Sussex, England: John Wiley & Sons Ltd; 2001. pp. 405–430. [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg R, Liebowitz M, Hope DA, Schneier FR, editors. Social phobia: Diagnosis, assessment, and treatment. New York: Guilford Press; 1995. pp. 69–93. [Google Scholar]
- Clarke PJ, MacLeod C, Guastella AJ. Assessing the role of spatial engagement and disengagement of attention in anxiety-linked attentional bias: a critique of current paradigms and suggestions for future research directions. Anxiety, Stress, and Coping. 2013;26(1):1–19. doi: 10.1080/10615806.2011.638054. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners’ continuous performance test. Vol. 2002 Toronto: Multi-Health System; 2002. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews: Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Eysenck MW. Anxiety, processing efficiency, and cognitive performance: new developments from attentional control theory. European Psychologist. 2009;14(2):168–176. doi: 10.1027/1016-9040.14.2.168. [DOI] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111(2):225–236. doi: 10.1037/0021-843X.111.2.225. [DOI] [PubMed] [Google Scholar]
- Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory - II. Psychological Assessment. 1998;10(2):83–89. doi: 10.1037/1040-3590.10.2.83. [DOI] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7(2):336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14(3):340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Foa EB, Huppert JD, Cahill SP. Emotional processing theory: An update. In: Rothbaum BO, editor. Pathological anxiety: Emotional processing in etiology and treatment. New York: Guilford Press; 2006. pp. 3–24. [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychological Bulletin. 1986;99(1):20–35. [PubMed] [Google Scholar]
- Fresco DM, Coles ME, Heimberg RG, Liebowitz MR, Hami S, Stein MB, Goetz D. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychological Medicine. 2001;31(6):1025–1035. doi: 10.1017/S003329170105405. [DOI] [PubMed] [Google Scholar]
- Gorlin EI, Teachman BA. Inhibitory control as a moderator of threat-related interference biases in social anxiety. Cognition & Emotion. doi: 10.1080/02699931.2014.931275. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Kasch KL, Traill S, Joormann J, Arnow BA, Johnson SL. Coherence and specificity of information-processing biases in depression and social phobia. Journal of abnormal psychology. 2004;113(3):386–398. doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- Grafton B, MacLeod C. Enhanced probing of attentional bias: The independence of anxiety-linked selectivity in attentional engagement with and disengagement from negative information. Cognition & Emotion. 2014;28(7):1287–1302. doi: 10.1080/02699931.2014.881326. [DOI] [PubMed] [Google Scholar]
- Grafton B, Watkins E, MacLeod C. The ups and downs of cognitive bias: Dissociating the attentional characteristics of positive and negative affectivity. Journal of Cognitive Psychology. 2012;24(1):33–53. doi: 10.1080/20445911.2011.578066. [DOI] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavior Research Methods. 2009;41(3):924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Heeren A, De Raedt R, Koster EH, Philippot P. The (neuro)cognitive mechanisms behind attention bias modification in anxiety: Proposals based on theoretical accounts of attentional bias. Frontiers in Human Neuroscience. 2013;7:119. doi: 10.3389/fnhum.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG. Cognitive factors that maintain social anxiety disorder: a comprehensive model and its treatment implications. Cognitive Behaviour Therapy. 2007;36(4):193–209. doi: 10.1080/16506070701421313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou R, Moss-Morris R, Risdale A, Lynch J, Jeevaratnam P, Bradley BP, Mogg K. Attention processes in chronic fatigue syndrome: Attentional bias for health-related threat and the role of attentional control. Behaviour Research and Therapy. 2014;52:9–16. doi: 10.1016/j.brat.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Johnson PO, Neyman J. Tests of certain linear hypotheses and their application to some educational problems. Statistical Research Memoirs. 1936;1:57–93. [Google Scholar]
- Jones CR, Fazio RH, Vasey MW. Attentional control buffers the effect of public speaking anxiety on performance. Social Psychological and Personality Science. 2012;3(5):556–561. doi: 10.1177/1948550611430166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judah MR, Grant DM, Lechner WV, Mills AC. Working memory load moderates late attentional bias in social anxiety. Cognition & Emotion. 2013;27(3):502–511. doi: 10.1080/02699931.2012.719490. [DOI] [PubMed] [Google Scholar]
- Judah MR, Grant DM, Mills AC, Lechner WV. Factor structure and validation of the attentional control scale. Cognition & Emotion. 2014;28(3):433–451. doi: 10.1080/02699931.2013.835254. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin-Germeys I, Kwapil TR. For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychological Science. 2007;18(7):614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Amir N. Examination of vigilance and disengagement of threat in social anxiety with a probe detection task. Anxiety Stress and Coping. 2009;22(3):283–296. doi: 10.1080/10615800802449602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Verschuere B, De Houwer J. Selective attention to threat in the dot probe paradigm: differentiating vigilance and difficulty to disengage. Behaviour Research and Therapy. 2004;42(10):1183–1192. doi: 10.1016/j.brat.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR. Social phobia. Modern Problems of Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95(1):15–20. doi: 10.1037/0021-843X.95.1.15. [DOI] [PubMed] [Google Scholar]
- Mansell W, Clark DM, Ehlers A, Chen YP. Social anxiety and attention away from emotional faces. Cognition & Emotion. 1999;13(6):673–690. doi: 10.1080/026999399379032. [DOI] [Google Scholar]
- Matsumoto D, Ekman P. American-Japanese cultural differences in intensity ratings of facial expressions of emotion. Motivation and Emotion. 1989;13(2):143–157. doi: 10.1007/Bf00992959. [DOI] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36(9):809–848. doi: 10.1016/S0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behaviour Research and Therapy. 2002;40(12):1403–1414. doi: 10.1016/S0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113(1):160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Morrison AS, Heimberg RG. Attentional control mediates the effect of social anxiety on positive affect. Journal of anxiety disorders. 2013;27(1):56–67. doi: 10.1016/j.janxdis.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsson RP, Smari J, Guethmundsdottir F, Olafsdottir G, Harethardottir HL, Einarsson SM. Self reported attentional control with the Attentional Control Scale: Factor structure and relationship with symptoms of anxiety and depression. Journal of Anxiety Disorders. 2011;25(6):777–782. doi: 10.1016/j.janxdis.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Ononaiye MSP, Turpin G, Reidy JG. Attentional bias in social anxiety: Manipulation of stimulus duration and social-evaluative anxiety. Cognitive Therapy and Research. 2007;31(6):727–740. doi: 10.1007/s10608-006-9096-8. [DOI] [Google Scholar]
- Pineles SL, Mineka S. Attentional biases to internal and external sources of potential threat in social anxiety. Journal of Abnormal Psychology. 2005;114(2):314–318. doi: 10.1037/0021-843X.114.2.314. [DOI] [PubMed] [Google Scholar]
- Pishyar R, Harris LM, Menzies RG. Attentional bias for words and faces in social anxiety. Anxiety, Stress & Coping. 2004;17(1):23–36. doi: 10.1080/10615800310001601458. [DOI] [Google Scholar]
- Price RB, Mohlman J. Inhibitory control and symptom severity in late life generalized anxiety disorder. Behaviour Research and Therapy. 2007;45(11):2628–2639. doi: 10.1016/j.brat.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behaviour Research and Therapy. 1997;35(8):741–756. doi: 10.1016/S0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. Methods for dealing with reaction-time outliers. Psychological Bulletin. 1993;114(3):510–532. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- Reinholdt-Dunne ML, Mogg K, Bradley BP. Effects of anxiety and attention control on processing pictorial and linguistic emotional information. Behaviour Research and Therapy. 2009;47(5):410–417. doi: 10.1016/j.brat.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Rudaizky D, Basanovic J, MacLeod C. Biased attentional engagement with, and disengagement from, negative information: Independent cognitive pathways to anxiety vulnerability? Cognition & Emotion. 2014;28(2):245–259. doi: 10.1080/02699931.2013.815154. [DOI] [PubMed] [Google Scholar]
- Salemink E, van den Hout MA, Kindt M. Selective attention and threat: Quick orienting versus slow disengagement and two versions of the dot probe task. Behaviour Research and Therapy. 2007;45(3):607–615. doi: 10.1016/j.brat.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Johnson J, Hornig CD, Liebowitz MR, Weissman MM. Social phobia. Comorbidity and morbidity in an epidemiologic sample. Archives of General Psychiatry. 1992;49(4):282–288. doi: 10.1001/archpsyc.1992.01820040034004. [DOI] [PubMed] [Google Scholar]
- Schoorl M, Putman P, Van Der Werff S, Van Der Does AJ. Attentional bias and attentional control in posttraumatic stress disorder. Journal of Anxiety Disorders. 2014;28(2) doi: 10.1016/j.janxdis.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Catale C, D'Argembeau A. Relationships between mind- wandering and attentional control abilities in young adults and adolescents. Acta Psychologica. 2014;148:25–36. doi: 10.1016/j.actpsy.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Amir N. Attention and emotion regulation. In: Kring AM, Sloan DM, editors. Emotion regulation and psychopathology: A transdiagnostic approach to etiology and treatment. New York: Guilford Press; 2010. pp. 380–404. [Google Scholar]
- Taylor CT, Aupperle RL, Flagan T, Simmons AN, Amir N, Stein MB, Paulus MP. Neural correlates of a computerized attention modification program in anxious subjects. Social Cognitive and Affective Neuroscience. 2014;9(9):1379–1387. doi: 10.1093/scan/nst128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen PK. Unpublished research project. Department of Experimental Psychology. University of Oxford; Oxford: 1994. Social anxiety and the allocation of attention: Evaluation using facial stimuli in dot-probe paradigm. [Google Scholar]