Abstract
While allogeneic hematopoietic stem cell transplantations have a curative potential, infections and graft-versus-host disease remain significant problems. The intestinal microbiota can influence responses to cancer chemotherapy and the role of the microbiota in affecting allogeneic hematopoietic stem cell transplantation outcomes is increasingly appreciated. The following paper discusses the most recent developments in this area.
Keywords: Allo-HSCT, bacteremia, diversity, intestinal microbiota, microbial dominance, metronidazole, mortality, survival
Introduction
The intestinal microbiota comprises microbial populations that colonize the human gastrointestinal tract and has been shown to play a crucial role in human health by mediating resistance to infection [1–3]. Studies of the microbiome of humans have revealed that healthy individuals harbor diverse microbial populations in the gut. The majority of bacterial taxa belong to the Firmicutes and Bacteroidetes phyla and bacteria belonging to the Actinobacteria, Proteobacteria, Verrucomicrobia, and Fusobacteria are also represented [1]. The composition of the intestinal microbiota differs from person to person, which can have implications in health and disease [4]. For example, the composition of the intestinal microbiota has been associated with malnutrition, obesity, and predisposition to rheumatoid arthritis [5–7]. Recently, two studies have indicated that the intestinal microbiota can influence the immune response to systemic cancer chemotherapy and disruption of the intestinal microbiome was associated with resistance to cancer therapy [8,9]. It has become clear that the intestinal microbiota not only mediates resistance to colonization by pathogens but also modulates immune function by promoting differentiation of different T-cell phenotypes. Given that infections and graft-versus-host disease are significant problems associated with allogeneic hematopoietic stem cell transplantation (allo-HSCT), the study of intestinal microbiota may provide clues to improve outcomes among allo-HSCT patients [10]. The following discussion provides a brief summary on the impact of intestinal microbiota in allo-HSCT outcomes.
Intestinal microbiota and bacteremia in allo-HSCT
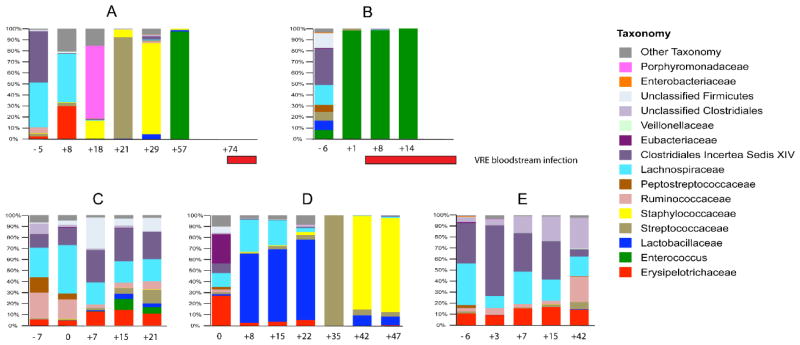
Although allo-HSCT represents a potentially curative treatment option for some patients with hematologic malignancies, infection and bacteremia are frequent complications [10]. A study of the fecal samples from five patients undergoing allo-HSCT showed that the composition of the intestinal microbiota can change dramatically following allo-HSCT (Figure 1) [11]. While all patients presented with a diverse intestinal microbiota before allo-HSCT, three of the patients showed dramatic fluctuations in the composition of the microbiota after allo-HSCT. In two of these patients, vancomycin-resistant Enterococcus (VRE) was shown to dominate the gastrointestinal tract before the onset of VRE bacteremia. However, the exact reason for this intestinal domination by VRE was not clear in this study.
Figure 1.
VRE dominates the intestinal microbiota in humans prior to invading the bloodstream [11].
Stool samples from 5 patients (A–E) were studied prior to and during the transplant period. Each bar on the graph depicts the microbiota of 1 stool sample. Days the samples were collected relative to the day of transplant are indicated along the x-axis. The red horizontal bars indicate the timing of vancomycin-resistant Enterococcus bloodstream infections in patients A and B.
Republished with permission of Journal of Clinical Investigation, from Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans, Ubeda C et al, volume 120, number 12, copyright 2010; permission conveyed through Copyright Clearance Center, Inc.
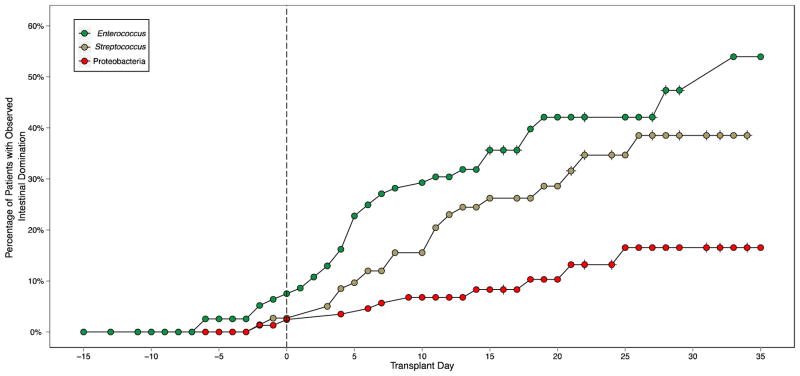
To identify correlative factors that explain the loss of diversity of the intestinal microbiota and the intestinal dominance by certain microorganisms following allo-HSCT, a larger longitudinal study with 94 allo-HSCT patients was performed [12]. Fecal samples were collected from these 94 patients pre-allo-HSCT and for up to 35 days post-allo-HSCT. These samples were characterized for intestinal microbiota by 454 pyrosequencing of the V1-V3 region of bacterial 16S ribosomal RNA genes. Intestinal domination was defined as the endpoint in which a particular bacterial taxon attains 30% or greater relative abundance and is more abundant than any other population member within a single fecal specimen. As observed in Figure 2, the risk of intestinal domination with Proteobacteria, Enterococcus, and Streptococcus increased over time following allo-HSCT [12]. Notably, Enterococcus domination was associated with administration of metronidazole, which strongly inhibits obligate anaerobes. Similarly, Streptococcus domination was associated with beta-lactam antibiotics such as cephalosporins, beta-lactam/beta-lactamase combinations, and carbapenems. On the other hand, administration of fluoroquinolones such as ciprofloxacin and levofloxacin reduced the risk of domination with Proteobacteria (Table 1) [12]. Consequently, while domination with Enterococcus increased the risk of developing VRE bacteremia, domination with Proteobacteria resulted in an increased likelihood of developing gram-negative bacteremia (Table 2) [12].
Figure 2.
Risk of intestinal domination of the microbiota increases over time following allo-HSCT [12].
This Kaplan-Meier plot shows intestinal domination by Enteroccocus (top), Streptococcus (middle), and Proteobacteria (bottom) at various times throughout the transplant observation period.
Republished by permission of Oxford University Press and the Infectious Diseases Society of America. Taur Y, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012;55:905–914.
Table 1.
Antibiotic risk factors for bacterial domination [12].
Republished by permission of Oxford University Press and the Infectious Diseases Society of America. Taur Y, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012;55:905–914.
| Clinical Predictor | Enterococcus Domination | Streptococcus Domination | Proteobacteria Domination | |||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Vancomycin | 2.10 (0.67 – 10.14) | 0.228 | 0.95 (0.33 – 3.75) | 0.931 | 5.08 (0.52 – 693.3) | 0.196 |
| Metronidazole | 3.40 (1.66 – 6.75) | 0.001 | 1.94 (0.81–4.31) | 0.130 | 1.73 (0.41 – 6.04) | 0.425 |
| Fluoroquinolones | 1.09 (0.49–2.25) | 0.824 | 1.19 (0.52 – 2.61) | 0.673 | 0.09 (0 – 0.75) | 0.020 |
| Beta-lactam | 1.19 (0.47 – 3.45) | 0.724 | 3.56 (0.83 – 33.20) | 0.094 | 0.64 (0.15 – 3.27) | 0.574 |
Table 2.
Domination as risk factors for subsequent bacteremia [12].
Republished by permission of Oxford University Press and the Infectious Diseases Society of America. Taur Y, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012;55:905–914.
| Domination by | VRE bacteremia | Gram negative bacteremia | ||
|---|---|---|---|---|
| Haz Ratio (95% CI) | P-value | Haz Ratio (95% CI) | P-value | |
| Enterococcus | 9.47 (2.46 – 46.0) | 0.001 | 1.53 (0.28 – 5.97) | 0.583 |
| Streptococcus | 0.22 (0.00 – 1.77) | 0.188 | 0.92 (0.10 – 4.17) | 0.925 |
| Proteobacteria | 0.76 (0.01 – 6.20) | 0.842 | 6.20 (1.15 – 23.37) | 0.036 |
Diversity of intestinal microbiota in allo-HSCT
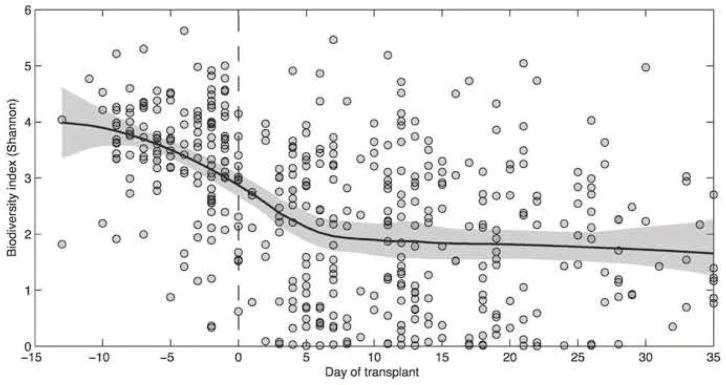
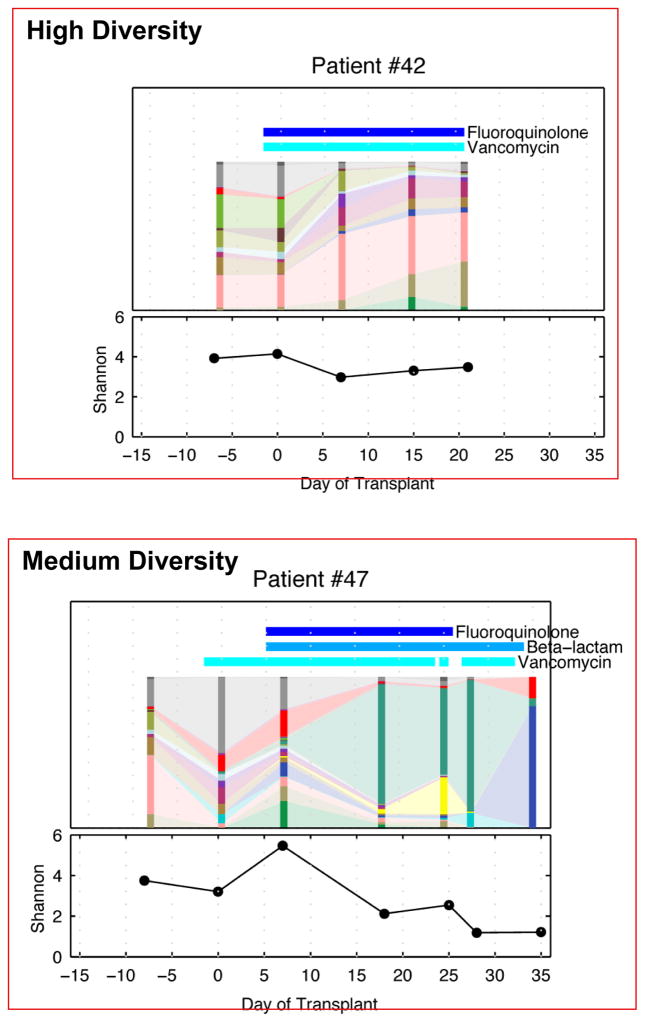
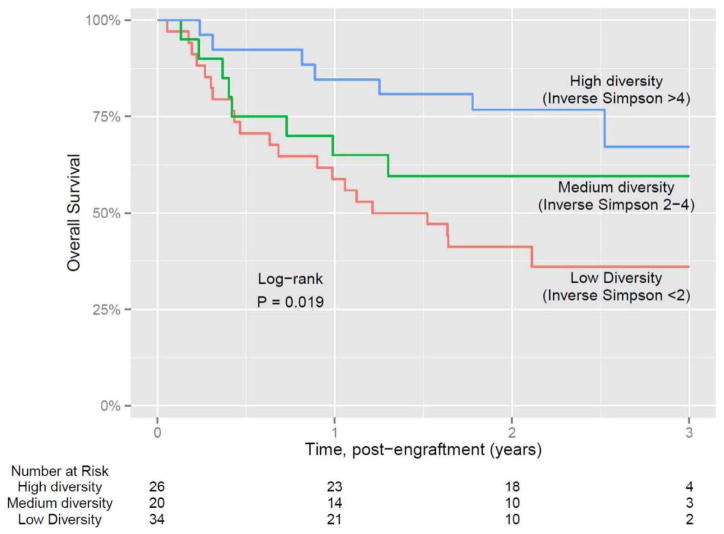
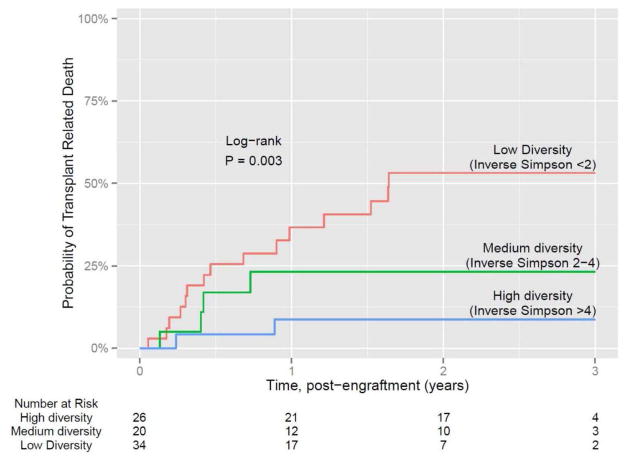
Another important observation made in this study was the decrease in the diversity of the intestinal microbiota of the patients undergoing allo-HSCT. As shown in Figure 3, most of the patients demonstrate considerable diversity in the intestinal microbiota before undergoing allo-HSCT. However, as the patients underwent allo-HSCT and following administration of conditioning regimens, there was a decrease in the diversity of the intestinal microbiota. Certain patient samples continued to maintain diversity and hence the next step was to determine whether there was a correlation between the loss of diversity and some specific clinical parameters [12]. As suggested by Figure 4, diversity loss correlated with the administration of antibiotics during allo-HSCT [12]. High intestinal microbiota diversity was observed among patients receiving fewer antibiotics such as fluoroquinolone and vancomycin, which lack broad anaerobic coverage. Intermediate intestinal microbiota diversity loss was associated with the addition of some beta-lactam antibiotics and the greatest loss of intestinal microbiota diversity was correlated with antibiotics that specifically target anaerobic microorganisms (Figure 4) [12]. Classification of allo-HSCT patients into low, medium, and high microbiota diversity groups at the time of stem cell engraftment demonstrated that diversity correlates with survival (Figure 5) [13]. Univariate and multivariate analysis further corroborated that low diversity of the intestinal microbiota was an independent risk factor for mortality. Specific examination of transplant-related death confirmed that low diversity of the intestinal microbiota was associated with increased mortality (52%) as opposed to 8% probability of transplant-related death among patients with high intestinal microbiota diversity at the time of stem cell engraftment (Figure 6) [13].
Figure 3.
Intestinal microbiota diversity decreases following allo-HSCT [12].
A microbiota diversity trend, solid black line, was calculated for each fecal specimen of each patient during the transplant period using the Shannon diversity index. The gray area indicates the 95% confidence interval. Intestinal microbiota diversity decreased after allo-HSCT (Day 0).
Republished by permission of Oxford University Press and the Infectious Diseases Society of America. Taur Y, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012;55:905–914.
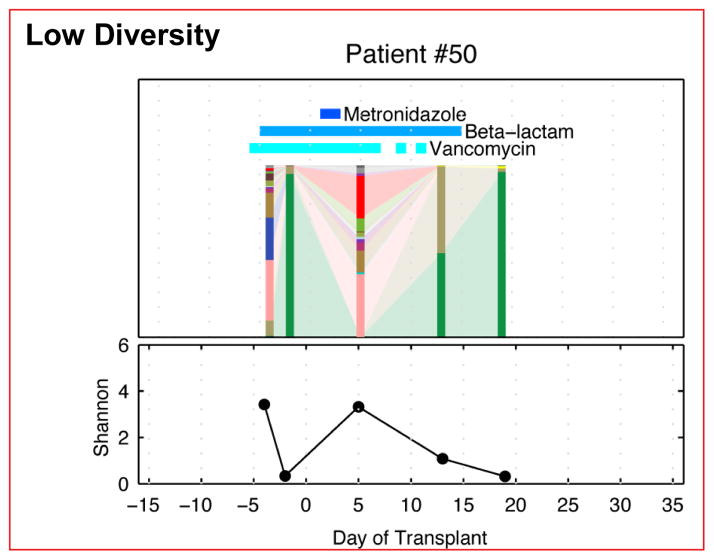
Figure 4.
Loss of microbiota diversity following allo-HSCT is variable [12].
The microbiota diversity of 3 patients following allo-HSCT is depicted here. The vertical bars represent the microbial composition of a single fecal sample, and the horizontal bars indicate antibiotics administered concurrently. As can be seen, the diversity is variable from patient to patient.
Republished by permission of Oxford University Press and the Infectious Diseases Society of America. Taur Y, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012;55:905–914.
Figure 5.
After transplant engraftment: Intestinal microbial diversity correlates with survival [13].
This research was originally published in Blood. Taur Y, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014;124:1174–1182. © the American Society of Hematology.
Figure 6.
Transplant-related mortality is markedly reduced in patients with a diverse microbiota following engraftment [13].
This research was originally published in Blood. Taur Y, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014;124:1174–1182. © the American Society of Hematology.
Summary
The intestinal microbiota stimulates immune development and defense against microbial pathogens. The diversity of the intestinal microbiota at the time of stem cell engraftment improves allo-HSCT outcomes. Decreased diversity and intestinal domination by Enterococci correlates with the spectrum of antibiotics administered to patients and leads to reduced survival. Ongoing studies are investigating whether reconstitution of intestinal microbiota using fecal microbial transplantation following allo-HSCT may provide an approach to optimize clinical outcomes and survival (clinicaltrials.gov identifier NCT02269150).
Abbreviations
- allo-HSCT
allogeneic HSCT
- HSCT
hematopoietic stem cell transplantation
- VRE
vancomycin-resistant Enterococcus
Footnotes
Conflict of interest
Eric G. Pamer: No relevant financial relationships with any commercial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother. 1994;38:409–414. doi: 10.1128/aac.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hentges DJ, Freter R. In vivo and in vitro antagonism of intestinal bacteria against Shigella flexneri. I. Correlation between various tests. J Infect Dis. 1962;110:30–37. doi: 10.1093/infdis/110.1.30. [DOI] [PubMed] [Google Scholar]
- 4.The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339:548–554. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 7.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 11.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–4341. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55:905–914. doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:1174–1182. doi: 10.1182/blood-2014-02-554725. [DOI] [PMC free article] [PubMed] [Google Scholar]