Abstract
The renal endothelin system contributes to sex differences in blood pressure with males demonstrating greater endothelin type-A receptor-mediated responses relative to females. Intrauterine growth restriction programs hypertension and enhanced renal sensitivity to acute angiotensin II in male growth-restricted rats. Endothelin is reported to work synergistically with angiotensin II. Thus, this study tested the hypothesis that endothelin augments the blood pressure response to acute angiotensin II in male growth-restricted rats. Systemic and renal hemodynamics were determined in response to acute angiotensin II (100 nanogram/kilogram/minute for 30 minutes) with and without the endothelin type-A receptor antagonist, ABT 627(10 nanogram/kilogram/minute for 30 minutes), in rats pretreated with enalapril (250 milligram/Liter for one week) to normalize the endogenous renin angiotensin system. Endothelin type-A receptor blockade reduced angiotensin II-mediated increases in blood pressure in male control and male growth-restricted rats. Endothelin type-A receptor blockade also abolished hyper-responsiveness to acute angiotensin II in male growth-restricted rats. Yet, blood pressure remained significantly elevated above baseline following endothelin type-A receptor blockade suggesting that factors in addition to endothelin contribute to the basic angiotensin II-induced pressor response in male rats. We also determined sex-specific effects of endothelin on acute angiotensin II-mediated hemodynamic responses. Endothelin type-A receptor blockade did not reduce acute angiotensin II-mediated increases in blood pressure in female control or growth-restricted rats, intact or ovariectomized. Thus, these data suggest that endothelin type-A receptor blockade contributes to hypersensitivity to acute angiotensin II in male growth-restricted rats and further supports the sex-specific effect of endothelin on blood pressure.
Keywords: sex differences, intrauterine growth-restriction, angiotensin II, endothelin, blood pressure
Offspring of pregnancies complicated by placental ischemia, hypertension and preeclampsia exhibit a greater risk for the development of increased blood pressure (BP) (1, 2) and cardiovascular (CV) disease in later life (1, 3). A mechanical reduction in uteroplacental perfusion in the rat results in placental ischemia leading to hypertension in the mother and intrauterine growth restriction (IUGR) in the offspring (4). Male growth-restricted offspring exhibit a marked increase in BP that initiates in early life and persists in adulthood (5). Male growth-restricted offspring also exhibit an enhanced sensitivity to acute angiotensin II (Ang II) (6) further implicating that fetal exposure to pregnancies complicated by placental ischemia programs increased CV risk in the offspring. The mechanisms that contribute to the etiology of increased BP that has its origins in fetal life are multifactorial and have not been fully elucidated. However, programmed increases in BP (7) and the enhanced BP response to acute Ang II (6) programmed by fetal exposure to placental ischemia in male growth-restricted offspring are testosterone dependent implicating a role for sex steroids. Androgens can potentiate renal responsiveness to Ang II via the Rho kinase signaling pathway (8). Previously, we reported that the enhanced BP response to acute Ang II in the male growth-restricted rats is independent of the Rho kinase pathway (9). Thus, one goal of this study was to investigate another potential mechanism that mediates hypersensitivity to acute Ang II in male growth-restricted rats.
Numerous experimental and human studies suggest that the endothelin (ET) system contributes to the development and progression of hypertension through its effects on vascular and renal function (10, 11). ET is a powerful vasoconstrictor, which exerts its effects on vascular smooth muscle via its endothelin type-A (ETA) and endothelin-type B (ETB) receptors (11). Hypertension induced by chronic Ang II infusion in the male rat can be blocked by an ETA receptor antagonist (12) and ET is reported to enhance the pressor response to Ang II (13) implicating a synergistic effect of ET and Ang II on BP. Numerous studies also indicate that the ET system contributes to sex differences in BP with ETA receptor-dependent increases in BP more prevalent in males relative to females (14). Although male growth-restricted rats are hypertensive in early adulthood, female growth-restricted rats are normotensive (5). Ovariectomy induces hypertension in female growth-restricted rats in young adulthood (15) and also enhances the BP response to acute Ang II (16). Thus, the aims of this study were to test the hypothesis that ET contributes to the enhanced BP response to acute Ang II in male growth-restricted rats and to determine if the effect of ET on acute Ang II-mediated pressor responses in growth-restricted offspring is sex-specific.
MATERIALS AND METHODS
Detailed Materials and Methods are available in the online-only Data Supplement.
RESULTS
Birth weight, body weight, and kidney weight
Birth weight was significantly reduced in growth-restricted rats compared to same-sex control counterparts (Table 1). Males were heavier than females, but body weight did not differ relative to same-sex counterparts at 16 weeks of age (Table 1); kidney weight (Table 1) and kidney to body weight (data not shown) did not differ in same sex groups. Kidney weight was greater in males relative to females and ovariectomy had no significant effect on kidney weight among groups; however, body weight was increased in ovariectomized female rats (Table 1).
Table 1.
Birth weight, body weight, and kidney weight in male and female, intact and ovariectomized, control and growth-restricted (IUGR) rats at 16 weeks of age.
| Experimental group | Birth weight (g) | Body weight (g) | Kidney weight (g) |
|---|---|---|---|
| Males | |||
| Control | 6.7±0.2 | 409±13 | 2.7±0.2 |
| IUGR | 5.5±0.1* | 432±19 | 2.7±0.1 |
| Females | |||
| Control Intact | 6.1±0.1 | 256±8 | 1.6±0.2 |
| IUGR Intact | 5.1±0.1* | 254±6 | 1.5±0.1 |
| Control Ovariectomized | 6.0±0.1 | 315±9 † | 1.6±0.1 |
| IUGR Ovariectomized | 5.0±0.1* | 318±9† | 1.6±0.1 |
P<0.05 vs. same-sex counterpart;
P<0.05 vs. intact counterpart. Values represent mean±SEM
The effect of an endothelin type A receptor antagonist on mean arterial pressure
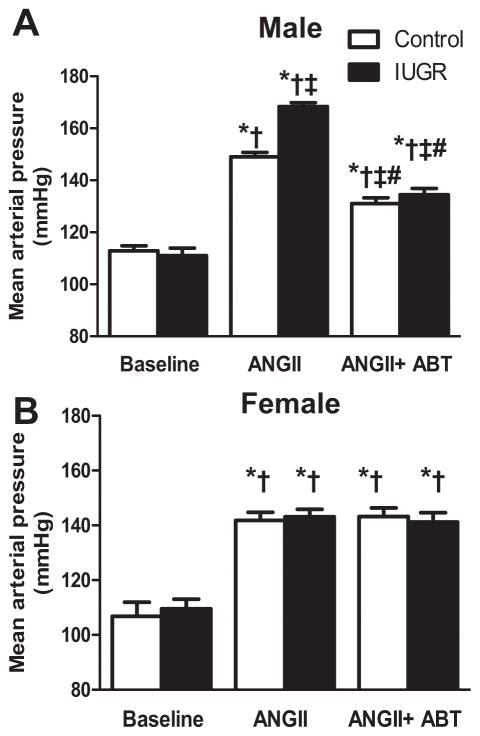
BP was increased to a greater degree by acute infusion of ANG II in male growth-restricted relative to male control (P<0.05) (Figure 1a). However, female growth-restricted did not demonstrate a similar response (Figure 1b). Blockade of the ETA receptor significantly reduced the BP response to acute Ang II in male rats (Figure 1a) but BP remained significantly elevated relative to baseline (P<0.05) (Figure 1a). However, blockade of the ETA receptor abolished the differential response to acute Ang II in male growth-restricted rats relative to male control. Blockade of the ETA receptor with ABT-627 had no effect on BP in intact Ang II-treated female control or growth-restricted rats (P<0.05 vs. baseline female control or IUGR) (Figure 1b). Blockade of the ETA receptor with ABT-627 also did not significantly alter BP in ovariectomized female control or growth-restricted rats (Figure S1).
Figure 1. Mean arterial Pressure (MAP).
a) Male control and growth-restricted (IUGR) rats. b) Female control and growth-restricted rats. MAP was measured at 16 weeks of age in chronically instrumented, conscious animals pretreated with the angiotensin convertor enzyme inhibitor, enalapril (250mg/L for 1 week). MAP was measured at baseline during an acute infusion of ANG II (100 ng/kg/min) for 30 min, and during a 30 minute infusion of ANG II plus the ETA receptor antagonist, ABT-627 (10 ng/kg/min for 30min). Values were allowed to return to baseline between acute treatments. *P<0.05 versus baseline control. †P<0.05 versus baseline IUGR. ‡ P< 0.05 vs. ANG II control. # P<0.05 vs. Ang II IUGR.. Data values represent mean±SEM
The effect of an endothelin type A receptor antagonist on renal hemodynamics
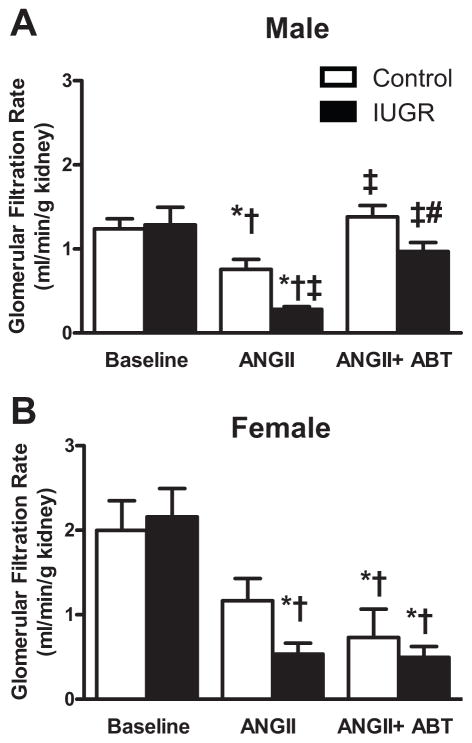
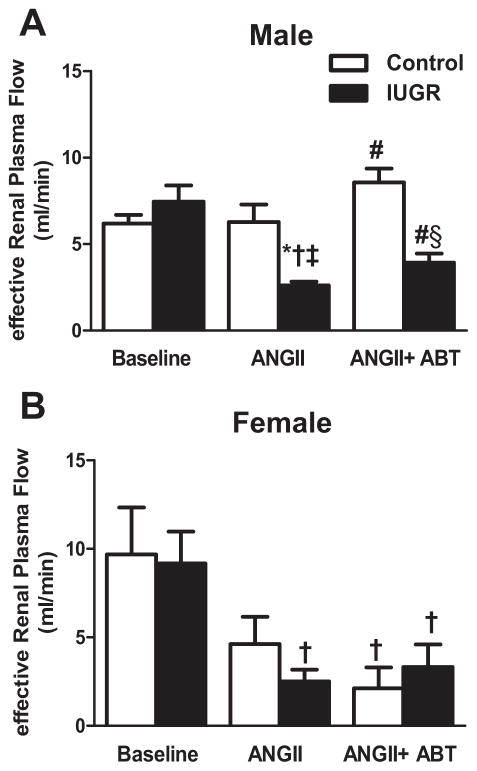
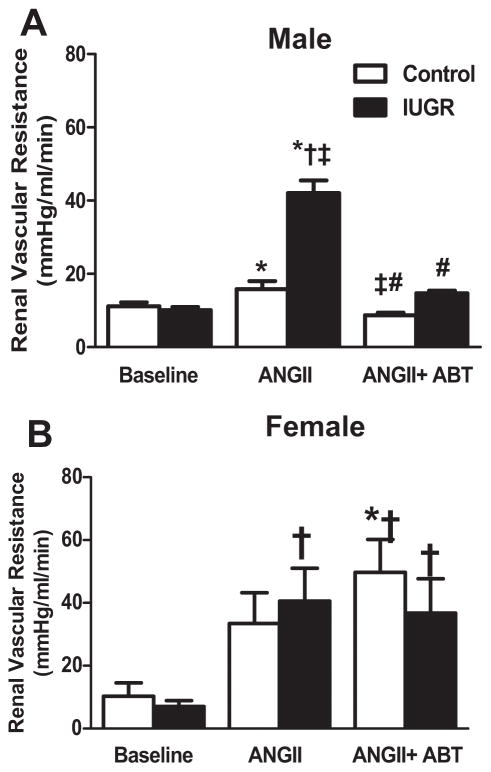
Acute Ang II induced a significant decrease in GFR in male control and male growth-restricted rats that was greater in male growth-restricted compared to male control (P<0.05) (Figure 2a). Blockade of the ETA receptor with ABT-627 abolished the decline in GFR induced with acute Ang II in male control and growth-restricted rats restoring values back to baseline (Figure 2a). ETA receptor blockade was unable to restore GFR in intact (Figure 2b) or ovariectomized (Figure S1) female control or growth-restricted offspring (P<0.05 vs. baseline control). Inhibition of the ETA receptor attenuated the significant decline in eRPF that occurred in response to acute ANG II in male growth-restricted offspring (P<0.05) (Figure 3a); yet, eRPF remained significantly lower in ABT-627 treated male growth-restricted relative to ABT-627 treated male control (Figure 3a). Ang II induced a decrease in eRPF that was not abolished by ETA receptor blockade in intact female growth-restricted rats (P<0.05 IUGR baseline versus vs. IUGR Ang II and IUGR Ang II + ABT) (Figure 3b) and did not significantly alter eRPF in ovariectomized rats versus their baseline treated counterpart (Figure S1). Normalizing GFR or eRPF to body weight or kidney weight did not alter outcomes (data not shown). The marked increase in RVR in male growth-restricted rats relative to male control was abolished by ETA receptor blockade (P<0.05) (Figure 4a) and normalized RVR in male growth-restricted rats relative to male baseline, control or growth-restricted. However, RVR remained significantly elevated in ABT-627-treated intact female growth-restricted rats relative to baseline intact female control and growth-restricted (Figure 4b); ETA receptor blockade had no effect on RVR in Ang II-treated female ovariectomized rats (Figure S1). Filtration Fraction did not differ between groups under baseline conditions, before or after acute infusion of Ang II, or in conjunction with ABT-627 (data not shown).
Figure 2. Glomerular Filtration Rate (GFR).
a) Male control and growth-restricted (IUGR) rats. b) Female control and growth-restricted rats. GFR was measured at 16 weeks of age in chronically instrumented, conscious animals pretreated with the angiotensin convertor enzyme inhibitor, enalapril (250mg/L for 1 week). Renal function was measured at baseline during an acute infusion of ANG II (100 ng/kg/min) for 30 min, and during a 30 minute infusion of ANG II plus the ETA receptor antagonist, ABT-627 (10 ng/kg/min for 30 min). Values were allowed to return to baseline between acute treatments. *P<0.05 versus baseline control. †P<0.05 versus baseline IUGR. ‡ P< 0.05 vs. ANG II control. # P<0.05 vs. Ang II IUGR. Data values represent mean±SEM.
Figure 3. Effective Renal Plasma Flow (eRPF).
a) Male control and growth-restricted (IUGR) rats. b) Female control and growth-restricted rats. eRPF was measured at 16 weeks of age in chronically instrumented, conscious animals pretreated with the angiotensin convertor enzyme inhibitor, enalapril (250mg/L for 1 week). Renal function was measured at baseline during an acute infusion of ANG II (100 ng/kg/min) for 30 min, and during a 30 minute infusion of ANG II plus the ETA receptor antagonist, ABT-627 (10 ng/kg/min for 30min). Values were allowed to return to baseline between acute treatments. *P<0.05 versus baseline control. †P<0.05 versus baseline IUGR. ‡ P< 0.05 vs. ANG II control. # P<0.05 vs. Ang II IUGR. § P< 0.05 vs. ANG+ABT II control. Data values represent mean±SEM.
Figure 4. Renal Vascular Resistance (RVR).
a) Male control and growth-restricted (IUGR) rats. b) Female intact control and growth-restricted rats. RVR was measured at 16 weeks of age in chronically instrumented, conscious animals pretreated with the angiotensin convertor enzyme inhibitor, enalapril (250mg/L for 1 week). Renal function was measured at baseline during an acute infusion of ANG II (100 ng/kg/min) for 30 min, and during a 30 minute infusion of ANG II plus the ETA receptor antagonist, ABT-627 (10 ng/kg/min for 30min). Values were allowed to return to baseline between acute treatments. *P<0.05 versus baseline control. †P<0.05 versus baseline IUGR. ‡ P< 0.05 vs. ANG II control. # P<0.05 vs. Ang II IUGR. Data values represent mean±SEM.
Renal expression of the endothelin system
Whole kidney pre-pro-endothelin (ppET) mRNA expression and 24-hour urinary excretion of endothelin did not differ in male or female growth-restricted relative to same-sex control counterpart (Figure S2). Protein expression of the ETA and the ETB receptor were significantly elevated in male growth-restricted offspring within the cortex and medulla compared to male control (Figure S3). In females ETA receptor protein expression did not differ but medullary ETB receptor protein expression was significantly decreased in female growth-restricted relative to female control (Figure S4).
Discussion
The main findings from this study demonstrate a role for the ET system in the sexual dimorphic developmental programming of BP control in growth-restricted rats exposed to placental ischemia during fetal life. Male growth-restricted rats exhibited a significantly greater increase in BP in response to acute Ang II compared to male control. The increased BP response to acute Ang II was significantly reduced by blockade of the ETA receptor in male control and growth-restricted rats. Blockade of the ETA receptor also abolished the exaggerated BP response to Ang II in male growth-restricted rats suggesting hypersensitivity to acute Ang II is mediated by the ETA receptor. However, ETA receptor blockade during acute infusion of Ang II failed to restore BP entirely back to baseline in male control and growth-restricted rats indicating that other factors in addition to ET may contribute to the basal acute Ang II-mediated BP response in male rats. ETA receptor blockade had no significant effect on acute Ang II-induced increases in BP in female control or growth-restricted rats regardless of ovarian status. Therefore, administration of an ETA receptor antagonist at a dose that significantly reduced Ang II-mediated increases in BP in male rats had no effect in female control or growth-restricted rats, intact or ovariectomized. These findings strongly suggest that the sexual dimorphism observed in the regulation of BP in adult rats is partially mediated by the ET System.
Investigation into the contribution of the ET system to the developmental programming of BP control is very limited. Male offspring exposed to early life stress (ELS) are normotensive under basal conditions but exhibit a hyperresponsiveness to chronic Ang II that is androgen dependent in male ELS rats (17). Male ELS rats also exhibit an exaggerated BP response to acute air jet stress (AJS) (18). The exaggerated response to AJS is abolished in male ELS rats homozygous for ETB receptor deficiency (18) suggesting a role for the ET system in the etiology of increased CV risk in ELS rats. Whether the ET system contributes to the enhanced BP response to chronic Ang II in male or female ELS rats has not been examined. Male offspring exposed to hypoxia during fetal life develop IUGR and a significant increase in BP with age that is not observed in their female IUGR counterparts (19). Blockade of the ETA/B receptor attenuates the increase in BP in male IUGR rats programmed by fetal exposure to hypoxia but has no effect on baseline BP in female rats, control or IUGR (19). Thus, these studies suggest that the ET system contributes to the developmental programming of BP in male rats and indicates a sex-specific effect of ET on BP in the model of IUGR induced via prenatal hypoxia.
Numerous models of developmental programming exhibit a sex difference in BP control with females protected relative to their male counterparts (15, 17, 19, 20). We previously reported that female growth-restricted rats are normotensive during young adulthood (15); whereas male growth-restricted rats exhibit a significant increase in BP that is testosterone dependent (7). Hyperresponsiveness to acute Ang II is also testosterone-dependent in male growth-restricted rats (6) whereas BP is increased and hypersensitivity to acute Ang II is exacerbated by ovariectomy in female growth-restricted rats in young adulthood (15, 16). Many experimental models that mimic essential hypertension also exhibit sex differences in the importance of the ET system in BP regulation. Females are protected against the development of Ang II-induced hypertension (21) and exhibit a delay in the development of hypertension induced via deoxycorticosterone acetate (DOCA)-salt that is also attenuated relative to their male DOCA-salt counterparts (22). The renal ET system contributes to sex differences in the regulation of BP in these experimental models with males exhibiting greater ETA-mediated responses whereas the ETB receptor is protective against increased BP in the female (23). ETA receptor blockade reduces BP in male but not female salt-loaded stroke-prone spontaneously hypertensive rats (SHR), an experimental model of more severe hypertension (24). However, ETA receptor blockade has no effect on BP in female SHRs in young adulthood but attenuates the hypertension that develops in post-cycling SHR, a model of postmenopausal hypertension (25). ET levels increase with age (26) and circulating ET-1 levels are positively associated with increased testosterone levels in postmenopausal women (27). Circulating ET-1 levels are also positively associated with increased testosterone in women with polycystic ovary syndrome (PCOS) (28). Testosterone up-regulates ET-1 mRNA and induces an increase in secretion of ET-1 from endothelial cells (29). Thus, elevated testosterone levels that occur in menopause or women with PCOS could contribute to increase in circulating levels of ET in female-specific conditions that are associated with increased CV risk and involve a role for the ET system in their etiology of elevated BP. Elevations in circulating levels of testosterone may also contribute to increased activation of the ET system in male growth-restricted rats. Hypertension and enhanced sensitivity to acute Ang II in male growth-restricted rats are testosterone dependent and are also associated with a significant increase in circulating testosterone (6, 7). However, estradiol also mediates an influence on ET production. In vitro estradiol reduces the increase in ET-1 production stimulated by Ang II (29). Whether estradiol exerts similar actions in vivo is not clear. Nonetheless, these studies indicate that modulation of the ET system by sex steroids may contribute to sex differences in Ang II sensitivity in growth-restricted offspring.
The mechanism that mediates ET-induced amplification of acute Ang II-mediated systemic and renal hemodynamic responses is unknown. Riggleman et al. demonstrated that ET acting via its ETA receptor contributes to the acute pressor response to acute Ang II (30). ET also contributes to the enhanced pressor response to acute Ang II in the SHR relative to WKY rats (31) implicating that ET amplifies the actions of acute Ang II. Ang II receptor density and ligand affinity are increased in the SHR (32) suggesting that differences in the binding and distribution of the Ang II receptors may be a contributory factor in the hyperresponsiveness to acute Ang II observed in the SHR. Renal AT1 receptor expression and glomerular 125I-Ang II binding are increased in male offspring exposed to maternal protein restriction (33). A greater reduction in GFR following acute Ang II is noted in male offspring exposed to a maternal low protein diet relative to control (34) suggesting that differences in Ang II receptor expression and binding may be a contributor factor in the developmental programming of impaired renal function. However, renal AT1 receptor mRNA expression and density are not elevated in male growth-restricted rats programmed by exposure to placental ischemia (35). Oriji and Keiser demonstrated that Ang II stimulation of rat aortic rings results in the rapid release of ET mediated via protein kinase C (36). Thus, the enhanced actions of ET on Ang II-mediated responses could also involve the rapid release of ET from the vasculature.
Renal ppET mRNA expression and urinary excretion of ET-1 were not significantly different in female or male growth-restricted rats relative to their control counterpart. Urinary ET-1 is reported not to differ in male versus female rats (37). Whether expression of the ET system was altered in males relative to females was not directly compared in this study. Nevertheless, renal protein expression of the ETA receptor was up-regulated in the renal cortex and medulla of male but not female growth-restricted rats relative to same-sex control. The ET system including type A and type B receptors is highly expressed in the collecting duct with males rats reported to exhibit greater ETA receptor expression relative to females (38). Activation of the ETA receptor is also enhanced in male rats relative to females (38) suggesting differences in signaling of the ET receptor may contribute to sex differences in IUGR-induced hypertension. ET is tightly linked to the nitric oxide (NO) system within the medulla and renal ET can differentially regulate nitric oxide synthase (NOS) activity within the kidney (39). Sex steroids can also modulate NO availability (40) and renal levels of endothelial NOS are higher in female rats relative to males (41). Yet, male rats are more susceptible to NOS inhibition than females (41) indicating that a loss of NO may uncover the actions of a vasoconstrictor such as ET which is more prominent in male versus female rats. Whether NOS activity is increased in a compensatory manner in female growth-restricted rats or whether enhanced activation of the ETA receptor mediates enhanced sensitivity to acute Ang II in male growth-restricted rats is not known. Thus, the mechanisms that contribute to enhanced renal sensitivity to acute Ang II-mediated responses in growth-restricted rats are not known. Additional studies are required to fully elucidate the mechanisms that mediate sex differences in the BP response to acute Ang II following IUGR.
Clinical Perspectives
Hypertension is more common in men than women prior to menopause (42). Birth weight is inversely associated with BP in both men and women (43) and low birth weight men exhibit a greater CV risk in young adulthood relative to their female low birth weight counterparts (44). The mechanisms that contribute to sex difference in BP control are multifactorial and within the low birth weight population, not clearly elucidated. This study supports an important role for the ET system in the etiology of increased CV risk that develops in male growth-restricted rats and also implicates the ET system as a contributor to sex differences in the developmental programming of BP.
Supplementary Material
NOVELTY AND SIGNFICANCE.
What is new?
Our study demonstrates that the ETA receptor contributes to the pressor response to acute Ang II in male rats and further reveals that the ETA receptor mediates hypersensitivity to acute Ang II in male growth-restricted rats.
Our study also shows that blockade of the ETA receptor at a dose that significantly reduces Ang II-mediated increases in BP in male rats has no effect on Ang II-mediated increases in BP in female rats regardless of their birth weight or ovarian hormone status.
What is Relevant?
Currently birth weight is not a consideration in the management of BP within the general population. Demonstration that the ETA receptor contributes to the developmental programming of high BP in male animals and that the effect of ETA receptor blockade on BP is sex-specific should encourage the development of endothelin antagonists for the treatment of high BP with the caveat that their use may be limited by sex yet, their viability as an antihypertensive agent may be enhanced in low birth weight men.
Summary
Further studies are necessary to discern the impact of sex and birth weight on effectiveness of an antihypertensive regimen in low birth weight individuals.
Acknowledgments
SOURCES OF FUNDING
Dr. Alexander is supported by the American Heart Grant GRNT19900004 and NIH grants HL074927 and HL51971. Dr. Intapad is supported by funding from the AHA, 12POST1198002, and the NIH P20GM104357. Dr. Ojeda is supported by the Discovery Grant from Pediatric Research Fund-UMMC.
Footnotes
DISCLOSURES
None.
References
- 1.Davis EF, Lewandowski AJ, Aye C, Williamson W, Boardman H, Huang RC, Mori TA, Newnham J, Beilin LJ, Leeson P. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: insights from a 20-year prospective follow-up birth cohort. BMJ Open. 2015;5:e008136. doi: 10.1136/bmjopen-2015-008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraser A, Nelson SM, Macdonald-Wallis C, Sattar N, Lawlor DA. Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension. 2013;62:614–620. doi: 10.1161/HYPERTENSIONAHA.113.01513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrera-Garcia G, Contag S. Maternal preeclampsia and risk for cardiovascular disease in offspring. Curr Hypertens Rep. 2014;16:1–10. doi: 10.1007/s11906-014-0475-3. [DOI] [PubMed] [Google Scholar]
- 4.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 5.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41:457–462. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 6.Ojeda NB, Royals TP, Black JT, Dasinger JH, Johnson JM, Alexander BT. Enhanced sensitivity to acute angiotensin II is testosterone dependent in adult male growth-restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1421–R1427. doi: 10.1152/ajpregu.00096.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2007;292:R758–R763. doi: 10.1152/ajpregu.00311.2006. [DOI] [PubMed] [Google Scholar]
- 8.Song J, Kost CK, Jr, Martin DS. Androgens potentiate renal vascular responses to angiotensin II via amplification of the Rho kinase signaling pathway. Cardiovasc Res. 2006;72:456–463. doi: 10.1016/j.cardiores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Ojeda NB, Royals TP, Alexander BT. Sex differences in the enhanced responsiveness to acute angiotensin II in growth-restricted rats: role of fasudil, a Rho kinase inhibitor. Am J Physiol Renal Physiol. 2013;304:F900–F907. doi: 10.1152/ajprenal.00687.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohan DE. Endothelin, hypertension and chronic kidney disease: new insights. Curr Opin Nephrol Hypertens. 2010;19:134–139. doi: 10.1097/MNH.0b013e328335f91f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speed JS, Pollock DM. Endothelin, kidney disease, and hypertension. Hypertension. 2013;61:1142–1145. doi: 10.1161/HYPERTENSIONAHA.113.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander BT, Cockrell KL, Rinewalt AN, Herrington JN, Granger JP. Enhanced renal expression of preproendothelin mRNA during chronic angiotensin II hypertension. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1388–R1382. doi: 10.1152/ajpregu.2001.280.5.R1388. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida K, Yasujima M, Kohzuki M, Kanazawa M, Yoshinaga K, Abe K. Endothelin-1 augments pressor response to angiotensin II infusion in rats. Hypertension. 1992;20:292–297. doi: 10.1161/01.hyp.20.3.292. [DOI] [PubMed] [Google Scholar]
- 14.Kittikulsuth W, Sullivan JC, Pollock DM. ET-1 actions in the kidney: evidence for sex differences. Br J Pharmacol. 2013;168:318–326. doi: 10.1111/j.1476-5381.2012.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50:679–685. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojeda NB, Intapad S, Royals TP, Black JT, Dasinger JH, Tull FL, Alexander BT. Hypersensitivity to acute ANG II in female growth-restricted offspring is exacerbated by ovariectomy. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1199–R1205. doi: 10.1152/ajpregu.00219.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loria AS, Yamamoto T, Pollock DM, Pollock JS. Early life stress induces renal dysfunction in adult male rats but not female rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R121–R129. doi: 10.1152/ajpregu.00364.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loria AS, D’Angelo G, Pollock DM, Pollock JS. Early life stress downregulates endothelin receptor expression and enhances acute stress-mediated blood pressure responses in adult rats. Hypertension. 2010;55:494–499. doi: 10.1152/ajpregu.00333.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourque SL, Gragasin FS, Quon AL, Mansour Y, Morton JS, Davidge ST. Prenatal hypoxia causes long-term alterations in vascular endothelin-1 function in aged male, but not female, offspring. Hypertension. 2013;62:753–758. doi: 10.1161/HYPERTENSIONAHA.113.01516. [DOI] [PubMed] [Google Scholar]
- 20.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1131–R1166. doi: 10.1152/ajpregu.00037.2003. [DOI] [PubMed] [Google Scholar]
- 21.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H2177–H2184. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 22.Crofton JT, Share L. Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension. 1997;29:494–499. doi: 10.1161/01.hyp.29.1.494. [DOI] [PubMed] [Google Scholar]
- 23.Kittikulsuth W, Looney SW, Pollock DM. Endothelin ET(B) receptors contribute to sex differences in blood pressure elevation in angiotensin II hypertensive rats on a high-salt diet. Clin Exp Pharmacol Physiol. 2013;40:362–370. doi: 10.1111/1440-1681.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Touyz RM, Turgeon A, Schiffrin EL. Endothelin-A-receptor blockade improves renal function and doubles the lifespan of stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2000;36:S300–S304. doi: 10.1097/00005344-200036051-00088. [DOI] [PubMed] [Google Scholar]
- 25.Yanes LL, Romero DG, Cucchiarelli VE, Fortepiani LA, Gomez-Sanchez CE, Santacruz F, Reckelhoff JR. Role of endothelin in mediating postmenopausal hypertension in a rat model. Am J Physiol Regul Integr Comp Physiol. 2005;288:R229–R233. doi: 10.1152/ajpregu.00697.2003. [DOI] [PubMed] [Google Scholar]
- 26.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–H432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maturana MA, Breda V, Lhullier F, Spritzer PM. Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. Metabolism. 2008;57:961–965. doi: 10.1016/j.metabol.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Christakou C, Economou F, Livadas S, Piperi C, Adamopoulos C, Marinakis E, Jdiamanti-Kandarakis E. Strong and positive association of endothelin-1 with AGEs in PCOS: a causal relationship or a bystander? Hormones (Athens) 2012;10:292–297. doi: 10.14310/horm.2002.1320. [DOI] [PubMed] [Google Scholar]
- 29.Pearson LJ, Yandle TG, Nicholls MG, Evans JJ. Regulation of endothelin-1 release from human endothelial cells by sex steroids and angiotensin-II. Peptides. 2008;29(6):1057–1061. doi: 10.1016/j.peptides.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Riggleman A, Harvey J, Baylis C. Endothelin mediates some of the renal actions of acutely administered angiotensin II. Hypertension. 2001;38:105–109. doi: 10.1161/01.hyp.38.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balakrishnan SM, Wang HD, Gopalakrishnan V, Wilson TW, McNeill JR. Effect of an endothelin antagonist on hemodynamic responses to angiotensin II. Hypertension. 1996;28:806–809. doi: 10.1161/01.hyp.28.5.806. [DOI] [PubMed] [Google Scholar]
- 32.Blunkenburg B, van Amelsvoort T, Rogg H, Wood JM. Receptor-mediated effects of angiotensin II on growth of vascular smooth muscle from spontaneously hypertensive rats. Hypertension. 1992;20:746–754. doi: 10.1161/01.hyp.20.6.746. [DOI] [PubMed] [Google Scholar]
- 33.Sahajpal V, Ashton N. Increased glomerular angiotensin II binding in rats exposed to a maternal low protein diet in utero. J Physiol. 2005;563:193–201. doi: 10.1113/jphysiol.2004.078642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahajpal V, Ashton N. Renal function and angiotensin AT1 receptor expression in young rats following intrauterine exposure to a maternal low-protein diet. Clin Sci (Lond) 2003;104:607–614. doi: 10.1042/CS20020355. [DOI] [PubMed] [Google Scholar]
- 35.Grigore D, Ojeda NB, Robertson EB, Dawson AS, Huffman CA, Bourassa EA, Speth RC, Brosnihan KB, Alexander BT. Placental insufficiency results in temporal alterations in the renin angiotensin system in male hypertensive growth restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2007;293:R804–R811. doi: 10.1152/ajpregu.00725.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oriji GK, Keiser HR. Protein kinase C mediates angiotensin II-induced contractions and the release of endothelin and prostacyclin in rat aortic rings. Prostaglandins Leukot Essent Fatty Acids. 1997;57:135–141. doi: 10.1016/s0952-3278(97)90003-x. [DOI] [PubMed] [Google Scholar]
- 37.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Gender differences in ET and NOS systems in ETB-receptor-deficient rats: effect of a high salt diet. Hypertension. 41:657–662. doi: 10.1161/01.HYP.0000048193.85814.78. [DOI] [PubMed] [Google Scholar]
- 38.Jin C, Speed JS, Hyndman KA, O’Connor PM, Pollock DM. Sex differences in ET-1 receptor expression and Ca2+ signaling in the IMCD. Hypertension. 2013;305:1099–1104. doi: 10.1152/ajprenal.00400.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan JC, Goodchild TT, Cai A, Pollock DM, Pllock JS. Endothelin(A) (ET(A)) and ET(B) receptor-mediated regulation of nitric oxide synthase 1 (NOS1) and NOS3 isoforms in the renal inner medulla. Acta Physiol (Oxf) 2007;191:329–336. doi: 10.1111/j.1748-1716.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 40.Hodgin JB, Knowles JW, Kim H-S, Smithies O, Maeda N. Interactions between endothelial nitric oxide synthase and sex hormones in vascular protection in mice. J Clin Investig. 2002;109:541–548. doi: 10.1172/JCI14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reckelhoff JF, Hennington BS, Moore AG, Blanchard EJ, Cameron J. Gender differences in the renal nitric oxide (NO) system: dissociation between expression of endothelial NO synthase and renal hemodynamic response to NO synthase inhibition. Am J Hypertens. 1998;11:97–104. doi: 10.1016/s0895-7061(97)00360-9. [DOI] [PubMed] [Google Scholar]
- 42.Hage FG, Mansur SJ, Xing D, Oparil S. Hypertension in women. Kidney Int Suppl. 2013;3:352–356. doi: 10.1038/kisup.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vos LE, Oren A, Bots ML, Gorissen WH, Grobbee DE, Uiterwaal CS. Birth size and coronary heart disease risk score in young adulthood. The Atherosclerosis Risk in Young Adults (ARYA) study. Eur J Epidemiol. 2006;21:33–38. doi: 10.1007/s10654-005-4658-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.