SUMMARY
Despite fundamental advances in the research on respiratory syncytial virus (RSV) since its initial identification almost 60 years ago, recurring failures in developing vaccines and pharmacologic strategies effective in controlling the infection have allowed RSV to become a leading cause of global infant morbidity and mortality. Indeed, the burden of this infection on families and health care organizations worldwide continues to escalate and its financial costs are growing. Furthermore, strong epidemiologic evidence indicates that early-life lower respiratory tract infections caused by RSV lead to the development of recurrent wheezing and childhood asthma. While some progress has been made in the identification of reliable biomarkers for RSV bronchiolitis, a “one size fits all” biomarker capable of accurately and consistently predicting disease severity and post-acute outcomes has yet to be discovered. Therefore, it is of great importance on a global scale to identify useful biomarkers for this infection that will allow pediatricians to cost-effectively predict the clinical course of the disease, as well as monitor the efficacy of new therapeutic strategies.
Keywords: Asthma, Brain-derived growth factor (BDNF), Bronchiolitis, Lung development, Nerve growth factor (NGF)
INTRODUCTION
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infections (LRTI) in infants and pre-school children. As virtually all children are infected with this virus at least once by 2 years of age, current estimates suggest RSV infection is responsible for greater than 60% of all acute LRTI in young children and more than 80% of all acute LRTI in infants under one year old worldwide [1, 2]. RSV LRTI are typically hallmarked by the development of bronchiolitis, defined as inflammation of the bronchiolar airways leading to obstruction [3]. Accordingly, bronchiolitis is the principal cause of hospitalization in infants under one year of age in the U.S., accounting for 16.4% of total hospitalizations in this age group [1], while more than one third of children will have developed bronchiolitis at some point prior to becoming two years old [4]. The clinical severity of bronchiolitis ranges from mild forms manageable on an outpatient basis to severe cases that require mechanical ventilation or extracorporeal membrane oxygenation (ECMO) in intensive care units (ICU) [2]. Highlighting the potential threat posed by bronchiolitis, a recent retrospective study found the ICU admission rate for all children under age 2 with bronchiolitis to be 22%, with 89% of the ICU admission cases testing positive for RSV infection and 47% ultimately requiring mechanical ventilation [5].
Early-life RSV infection has also been shown to lead to chronic airway dysfunction, with an increased risk of subsequent wheezing episodes that lasts for several years after the acute infection [6-9]. This characteristic of the virus has been the basis of a longstanding debate as to whether RSV infection is simply a trigger that uncovers an intrinsic predisposition, or rather a causative agent contributing to the inception of childhood asthma [10, 11]. In addition to a plethora of studies focusing on the role of innate and adaptive immune system, other studies have looked into the effects of RSV on airway innervation and reactivity both during and after infection and have shown that specific neurotrophic proteins and their cognate receptors are upregulated by this infection leading to persistent airway hyperreactivity [12-14]. In addition, the establishment of a lytic RSV infection cycle has been shown to involve a series of pro-survival mechanisms - including host immune system evasion and prevention of apoptosis by infected host cells – mediated through modulation of nerve growth factor (NGF) activity [12, 15-17].
Even with intensive research focused on RSV since its discovery in 1956 [18, 19], pediatric clinicians currently lack an effective vaccine capable of preventing the infection and must instead rely on supportive therapies aimed at managing complications of bronchiolitis (e.g., nasal suctioning, hydration, supplemental oxygen) [2, 20]. Once the infection is established, clinicians will empirically assess the trajectory of a patient’s symptoms in an attempt to predict its evolution, and will tailor their management strategy accordingly [21]. Over time, clinical observation has led to the identification of specific risk factors for severe RSV bronchiolitis: premature birth (<32 weeks gestational age), male sex, lack of breastfeeding, chronic lung disease, hemodynamically-significant congenital heart disease, and severe immunodeficiency amongst others [22-28]. However, numerous studies have also demonstrated that a majority of children hospitalized with RSV bronchiolitis lack these risk factors and were healthy prior to their clinical event [5, 29, 30]. As a result, efforts aimed at identifying novel biological markers measurable in the host during the course of RSV bronchiolitis have multiplied in hopes of developing a clinically effective method to predict disease severity outcomes. To this end, some advances in the development of biomarkers of RSV bronchiolitis have been made over the last several years and the primary purpose of this review is to state the need and discuss potential solutions for such biomarkers in more detail.
CHARACTERISTICS OF RSV INFECTION
The highly contagious nature of RSV stems from its ability to survive outside of a host up to 6 hours on hard surfaces and on the skin for 20 minutes [31]. This prolonged viability permits the inoculation of mucous membranes in the eyes or nose and allows the virus to spread quickly from the hands of adult hosts to infants and young children [32, 33]. Upon inoculation, RSV first infects the nasopharyngeal epithelium of the upper respiratory tract and subsequently spreads towards its more competent replication site in the bronchiolar epithelium within 1-3 days with peak infectivity achieved around 5-days post-inoculation [15, 34]. Host innate and adaptive immune responses are continuously triggered during this spread to the lower airways, but fail to effectively clear RSV in young children and immunocompromised individuals [23, 35, 36].
The inherent ability of RSV to manipulate host innate, adaptive, and memory immune responses is a contributing factor to an inability to develop a fully effective vaccine against the virus [17]. From initial failings in vaccine development leading to increased mortality in 1966 [37, 38] to more recent RSV vaccine approaches that initially showed promise [20], there still remains a void of safe and effective prophylaxis, other than the passive protection provided by the humanized monoclonal antibody palivizumab (Synagis®; marketed by MedImmune, LLC) [39-43]. As the prohibitive costs of this intervention limit its use to a minority of high-risk infants, the need for research aimed at identifying reliable biomarkers of RSV bronchiolitis in parallel with safe and effective therapy remain strong [44].
IMPACT OF RSV INFECTION ON PEDIATRIC HEALTH
In the U.S. population under one year of age, RSV-associated LRTI account for approximately 126,000 hospitalizations (25.2 per 1,000) [45] with estimated direct costs ranging from $394 million to $1.1 billion annually [46, 47]. When examining the global burden of RSV infection on developing nations, a systematic review and analysis of available literature estimated that nearly 33.8 million new cases of RSV-associated LRTI occur worldwide in children under 5 years of age leading to approximately 3.4 million hospitalizations annually [48]. These figures also fail to take into account the rates of hospitalization and mortality that RSV infection imparts on the elderly, a population now recognized as extremely susceptible to the virus and for which the associated health care costs are surely tremendous [49-53]. When taken together on a global scale, high RSV-associated LRTI hospitalization rates coupled with the increasing price of healthcare potentially cost the world hundreds of billions of dollars annually and put significant strain on infected individuals, health care providers, and health care systems alike.
In the U.S., mortality associated with RSV infection is uncommon in the 21st century due to effective hygiene and supportive care, with the approximately 40 deaths per year mostly occurring in infants with complex chronic conditions or in those with life-threatening conditions preexisting the infection [54]. In contrast, the global impact of RSV infection on infants and young children is staggering: worldwide, RSV-associated LRTI are the second leading cause of pathogenic mortality in infants less than one year of age [48]. Varied estimates place the global mortality rate somewhere between 200,000 and 1 million deaths annually in children under 5 years of age [2, 48]. Therefore, the need for effective strategies aimed at reducing RSV bronchiolitis morbidity and mortality rates has never been greater with the development and implementation of these tactics holding the potential to save hundreds of thousands of lives around the globe each year.
RSV INFECTION AND ASTHMA DEVELOPMENT
In immunocompetent children, RSV is fully cleared from the lower airways within 8 days after initial infection though its ability to cause persistent airway dysfunction is well documented [31, 55]. Multiple prospective epidemiological studies have associated these respiratory sequelae, chiefly recurrent episodes of wheezing and asthma development within the first decade of life, with early-life RSV LRTI [9-11, 56]. Mechanistic studies have demonstrated the involvement of both neurological networks [14, 57-60] and immune cells [61-63] within infected host tissues as being directly involved in post-infection airway obstruction. Numerous studies have also underscored the important effects that NGF and other neurotrophins have on airway tissues such as smooth muscle and fibroblasts during both acute bronchiolitis and chronic asthma [64-66]. The interplay between neural and immune networks has been described in numerous publications [2, 55, 61] and has led to continued and vigorous debate within the field of pediatric asthma as to which avenue has a greater impact on chronic sequelae development: nerves and nerve-derived proteins or immune effector cells and the cytokines associated with their responses.
NEUROTROPHINS AS BIOMARKERS OF RSV INFECTION
Neurotrophins are a family of proteins responsible for neuronal differentiation and survival that are continuously present in all vertebrates from the early embryonic stage forward throughout life [67, 68]. The neurotrophins family includes four highly conserved proteins with similar structure and function: NGF, brain-derived neurotrophic factor (BDNF), NT3, and NT4. While all of the classical NTs can bind to the low-affinity p75NTR receptor, each also preferentially binds specific high-affinity receptors of the tropomyosin-related tyrosine kinase (Trk) family, being TrkA (NGF), TrkB (BDNF and NT4) and TrkC (NT3) [69-72]. All of the classical NTs and their cognate receptors are widely expressed within numerous structural, neuronal, and auxiliary cells of the lungs [73, 74] where they have been found to contribute to a number of lung pathologies (e.g. bronchopulmonary dysplasia and asthma) either with their direct effect on neuronal structure and function or by interacting with multiple components of the immune system [15, 73, 75-83].
Elevated expression of NGF within the lower airways has been shown in animal models of early-life RSV infection (Table 1) [14, 57, 84]. Other studies focused on BDNF expression during neonatal lung pathologies associated with childhood asthma development also found high levels of BDNF and/or its cognate receptor TrkB [85, 86]. In a more recent animal study on vertical transmission during pregnancy, RSV was shown to cross the placental barrier where it could directly infect the lung buds of developing fetuses leading to increased airway expression of NGF and acetylcholine that persists through development and predisposes to airway hyperreactivity upon early-life RSV re-challenge [12]. The increased airway reactivity following vertical RSV transmission was found to be critically dependent on the NGF-TrkA axis, as blockade of either using anti-NGF antibodies or the Trk-dependent signal transduction inhibitor K252a returned post-natal response to methacholine challenge to normal range.
Table #1.
Neurotrophins as Biomarkers of Airway Disease
| Neurotrophin | Cognate Receptor | Changes with Airway Disease |
|---|---|---|
| Nerve Growth Factor (NGF) |
TrkA p75NTR |
|
| Brain-derived Neurotrophic Factor (BDNF) |
TrkB p75NTR |
|
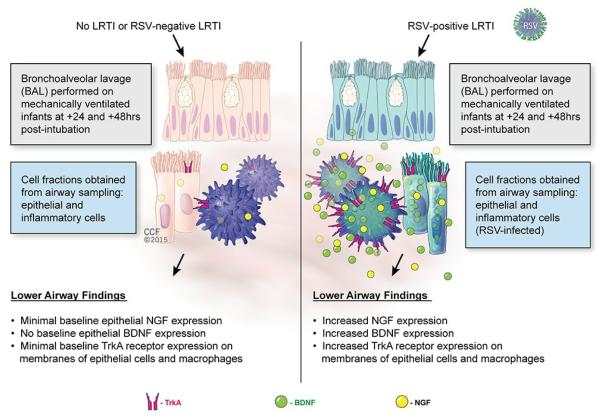
The relevance of this animal data for human disease was confirmed by a clinical study of mechanically ventilated infants who underwent bronchoalveolar lavage (BAL), concluding that patients with acute RSV infections had significantly elevated levels of NGF and BDNF proteins in the cellular fraction of their BAL compared to mechanically ventilated non-infected infants and infants with respiratory failure caused by adenoviral or parainfluenza infections (Figure 1) [13]. At 48 hours post-intubation, NGF in the RSV-positive LRTI group was 7-fold higher compared with the RSV-negative LRTI group (p <0.001) and 5-fold higher compared with the group without LRTI (p <0.001). At the same time points, absolute BDNF concentrations were one order of magnitude larger than NGF concentrations, and were 12-fold higher in the RSV-positive LRTI group compared with the RSV-negative LRTI group (p <0.05) and 5-fold higher compared with the group without LRTI (p <0.01).
Figure 1. Neurotrophins as biomarkers of RSV bronchiolitis.
Patients with acute RSV bronchiolitis have significantly higher concentrations of NGF and BDNF proteins in the cellular fractions sampled from their airways compared to non-infected infants or infants with acute bronchiolitis caused by adenoviral or parainfluenza infections whose airways express minimal baseline levels of both neurotrophins. Furthermore, epithelial cells and macrophages from the airways of RSV-infected infants have strong surface expression of the high-affinity NGF receptor TrkA, whereas in the absence of RSV infection this receptor is virtually absent.
Cytologic analysis of the BAL showed no significant differences in absolute and differential leukocyte counts between RSV-positive and RSV-negative infants with LRTI, suggesting that the increased synthesis of neurotrophic factors derives from RSV-infected structural airway cells rather than bone marrow-derived inflammatory cells recruited from the circulation to fight the infection. However, strong TrkA immunofluorescence was detected both in desquamated epithelial cells and macrophages recovered from RSV-infected airways, whereas the NGF receptor was virtually undetectable in cells recovered from RSV-negative infants, either non-infected or infected with non RSV viruses. Of importance, these preparations were examined by a pathologist blind to the illness groups, who detected immunofluorescence staining in all specimens from RSV-positive patients, whereas all specimens from RSV-negative patients were all read as negative. Collectively, these data suggest that NGF-TrkA axis expression levels in the lower airways could be used as a sensitive and specific biomarker of acute RSV infection. Although it cannot be ruled out whether other pathogens have similar neurotrophic effects, the finding that RSV-negative LRTI was not associated with changes in neurotrophic pathways suggests that this effect may be unique to RSV, or at least shared by specific pathogens only.
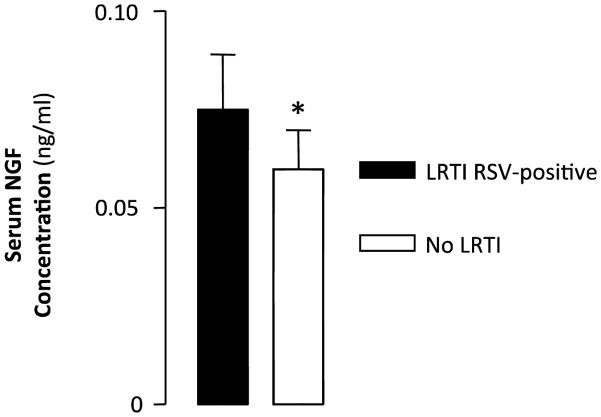
In contrast, no change was detected in serum samples and BAL supernatants collected during the first 48 hours after intubation in the same patients, suggesting that these specimens reflect only indirectly the actual concentrations of neurotrophic factors present in the cells and tissues where they are produced, secreted, and involved in biological functions. However, in a subgroup of RSV-infected infants requiring mechanical ventilation for more than 48 hours, significantly higher NGF concentrations were measured in serum samples obtained at 72 hours (Figure 2), suggesting that the transfer of locally secreted neurotrophins to the systemic circulation is gradual, and becomes measurable only later in the course of the infection and/or only in infants with more prolonged and severe RSV LRTI. This interpretation would also explain why higher NGF levels were found in the blood [87] and BAL fluid [88] of patients with chronic asthma, as in our study serum neurotrophin levels may have been low because the sampling was done early in the course of the illness.
Figure 2. Blood NGF in RSV bronchiolitis.
Bar graph shows NGF concentrations in the blood drawn at 72 hours post-intubation from 6 infants with RSV-positive LRTI and 6 RSV-negative post-surgical infants without LRTI. Blood samples drawn from the same patients at earlier time points showed no statistical significance. *P < 0.05.
Therefore, although blood samples are much more accessible than tracheal or bronchoalveolar lavage fluid, systemic concentrations of neurotrophic protein levels tend to increase less and later in the course of RSV disease compared to local airway expression, making their sensitivity and usefulness more problematic. Elevated levels of NGF were also found in human airways after allergen challenge [89] reinforcing the idea that this protein plays an important role in the pathophysiology of airway inflammation and hyperreactivity. Furthermore, strong airway expression of neurotrophic factors and receptors has been shown in active sarcoidosis [90] and in lung cancer [91], suggesting that neurotrophins not only are essential for neuro-immune integration, but are also controlling growth and differentiation of lung cells.
A relatively recent and surprising twist in this story has revealed that the lungs may not be the only source of RSV-induced NGF (Table 2). Indeed, akin to animal studies displaying RSV infection penetrating extrapulmonary sites [12], a wealth of clinical evidence in humans exists which supports the notion that this infection is not limited to its primary target in the lungs. To date, RSV RNA has been detected in peripheral blood mononuclear cells (PBMCs), cerebrospinal fluid (CSF), myocardium, primary nerve cells, the middle ear, the liver, and bone marrow [92-100]. Once seeded in these extrapulmonary sites RSV infection can cause a number of important secondary pathologies, such as sino-atrial blockade [101] or increased liver enzymes and hepatitis development [99], though the exact mechanisms behind these occurrences are not known. Similar to RSV, the recent finding of latent Mycobacterium tuberculosis infection within human bone marrow stem cells [102] supports the idea that the bone marrow may serve as a sanctuary for latent RSV infection from which persistent post-bronchiolitis airway dysfunction, failures in development of immunity to the virus, and possibly even later reactivation arise [103].
Table #2.
Extrapulmonary RSV Infection
| Extrapulmonary Site of RSV Detection | Biological Effects |
|---|---|
| Central Nervous System |
|
| Peripheral Nervous System | May modulate neurotransmitter synthesis/release |
| Bone Marrow Stroma |
|
| Peripheral Blood Mononuclear Cells |
|
| Myocardium | Sino-atrial blockade |
| Liver |
|
| Middle Ear | Otitis media |
Taken together, these studies have demonstrated that RSV can invade multiple extrapulmonary targets where one of its primary survival mechanisms within infected host cells would involve increased expression of the anti-apoptotic protein NGF. The specific pattern of RSV-induced NGF overexpression in infected tissues warrants an expansion of research into the potential of NGF as a useful biomarker to assess RSV bronchiolitis severity in humans. Going one step further, if NGF overexpression during RSV infection promotes virus survival in the short-term while also leading to long-term development of post-bronchiolitis airway dysfunction, then drug design strategies successfully inhibiting this pathway in humans could reshape our approaches towards treating RSV bronchiolitis and airway dysfunction.
CYTOKINES AS BIOMARKERS OF RSV INFECTION
There is no question that host immune response and subsequent lower respiratory tract inflammation during the progression of RSV infection play a key role in determining the severity of disease witnessed in patients [37, 38]. There is a continuous presence of lymphocytes and phagocytes both resident in the airway tissues and circulating within the peripheral bronchial and pulmonary vasculature, which serve as sentries to respond swiftly to any pathogenic challenge reaching the lower airways [104]. During RSV infection, these immune sentinels respond through cytokine signaling to activate the downstream cascade that leads to infiltration of the bronchiolar airways by neutrophils, eosinophils macrophages, B-lymphocytes, CD4+ and CD8+ T cells [104-106]. Both animal and human studies of RSV infection have suggested that a T helper 2 (Th2)-polarized immune response may be generated in certain conditions, leading to the activation of cytotoxic CD8+ T cells [62, 63] as well as the synthesis of interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13) [107-111].
While CD8+ T cell presence is detectable in the lower airways during RSV infection, the functional cytotoxicity of these cells may be compromised due to the infiltration of neutrophils and eosinophils resulting from local Th2 cytokine expression [112, 113] as well as by the virus itself which is inherently capable of blocking the activation, proliferation, and function of CD8+ T cells [104, 114-119]. The result of this multifactorial inhibition of CD8+ T cells, accompanied by parallel reductions in overall Th1 immune response functionality, is a substantial reduction in viral clearance and increased severity of disease. The host immune response to RSV and the accompanying inflammation that is produced within the lower airways due to immune effector cells infiltration are much more important that the direct cytopathic effects of the virus in determining the symptoms of RSV disease. As a corollary, RSV infection in immunocompromised hosts usually presents with lower levels of wheeze and higher probability for invasive pneumonia, whereas immunocompetent hosts tend to display greater levels of airway obstruction and wheezing but no progression to the pulmonary parenchyma [94, 120].
In immunocompetent subjects, the host immune system is ultimately able to clear the virus from lung tissues and mitigate the course of disease severity. However, the immunologic memory developed during an acute infection with RSV is incomplete and does not prevent reinfection of the host even within the same epidemic season, though it does appear to reduce the overall severity of recurrent RSV infections [121-124]. What level of protection to RSV infection is conferred correlates well with the serum level of neutralizing antibodies, a finding confirmed by multiple clinical studies of human infants [125-128] and further reinforced by the finding that decreased serum anti-RSV immunoglobulin titers are associated with increased incidence of severe disease [123]. These findings explain why prophylaxis with the humanized monoclonal antibody palivizumab (Synagis®) is a safe and effective tool for clinicians to protect those early childhood populations carrying the most significant risk of developing severe RSV bronchiolitis [20, 42, 43].
Human blood plasma and serum are clinical sample sources possessing numerous qualities that make them of significant importance to clinicians. They are easily attainable in most situations and circumstances and across age groups, procurement methods are of generally low cost, they are well-preserved long term, many commercial kits are available for testing of a plethora of proteins, and the data gathered from these tests represent systemic levels which are more indicative of overall body-wide affects. Therefore, clinical blood plasma and serum testing are powerful methods for biomarker monitoring of RSV bronchiolitis in an effort to predict disease severity outcomes, and several host cytokines display differential blood plasma and serum levels during RSV infection (Table 3). Levels of interleukin-8 (IL-8), interferon-gamma (IFN-γ) and the chemokine (C-C motif) ligand 5 (CCL-5) have been shown to be predictors of mechanical ventilator requirement during RSV infection and bronchiolitis [129, 130]. Low levels of plasma TNF-α and IL-6 were correlated with increased infant hospitalization [131, 132], with peripheral blood mononuclear cell proliferation also being associated with increased infant ICU admission during RSV bronchiolitis [133] and CD4+ T cell count predictive of mechanical ventilator requirement [129]. While the use of tracheal aspirate fluid (TAF) as a diagnostic tool during RSV bronchiolitis course is only possible in specific clinical situations, it has been shown to harbor potential biomarkers of severity with one study finding increased IL-6 and IL-17 levels in the TAF of infants with severe RSV bronchiolitis [134].
Table #3.
Immunological Biomarkers of RSV Infection
| Cytokine | Effect of RSV Infection | Immune Cell | Effect of RSV Infection |
|---|---|---|---|
| IL-A | Elevated with Th2 polarization by RSV LRTI | Dendritic Cells | Increased in bronchiolar airways |
| IL-5 | Elevated with Th2 polarization by RSV LRTI | Eosinophils | Infiltration of bronchiolar airways |
| IL-13 | Elevated withTh2 polarization by RSV LRTI | Neutrophils | Infiltration of bronchiolar airways |
| TNF-α | Reduced plasma level correlated with hospitalization | Macrophages | Infiltration of bronchiolar airways |
| IFN-γ |
|
CD4+ T cells |
|
| IL-8 |
|
CDS* T Cells |
|
| IL-6 |
|
Peripheral Blood
Mononuclear Cells |
Proliferation associated with increased infant ICU admissions |
|
CCL5
(RANTES) |
|
||
| IL-17 | Elevated in TAP during severe RSV LRTI |
LEUKOTRIENES AS BIOMARKERS OF RSV INFECTION
The acute inflammatory response of airways infected by RSV in early life involves the release of cysteinyl leukotrienes and activation of the cysLT1 receptor, as manifested by the anti-inflammatory effect of the receptor antagonist montelukast in animal models [135] and in human studies [136]. Following the early phase of the viral respiratory infection, leukotriene production and release rapidly return to baseline levels, but can be reactivated when airborne irritants (e.g., tobacco smoke) stimulate nociceptive nerve fibers connected to the numerous mast cells still present in the lung tissues. Indeed, LTC4 is elevated in the nasopharyngeal secretions of children during acute RSV infection and correlates with clinical severity, with more appreciable LTC4 elevation in children displaying higher degrees of lower respiratory tract involvement [137, 138]. Accordingly, the terminal urine metabolite of cysteinyl leukotrienes (LTE4) is elevated in the urine of young infants with RSV bronchiolitis as compared to controls without respiratory infection [139], and a study in intubated patients with severe RSV bronchiolitis found that increased LTE4 in endotracheal aspirates correlates well with urinary LTE4 levels [140]. An important practical implication of these findings is that the increased excretion of LTE4 during acute bronchiolitis could not only be used to assess severity, but also to assist in the decision of starting therapy with leukotriene modifiers that have been shown to have therapeutic value in this setting, and maybe monitor evolution of the disease and response to therapy (Table 4).
Table #4.
Other Potential Biomarkers of RSV Bronchiolitis
| Potential Blomarker | Findings During RSV Bronchiolitis |
|---|---|
| Cysteinyl Leukotrienes |
|
| Lactate Dehydrogenase (LDH) | Concentration within nasal washings predicts admissions from ER with 88% accuracy |
| Blood-borne Biomarkers |
|
| Patient-Specific Gene Expression Profiling |
|
LTE4 is the end-product of the 5-LO pathway in activated mast cells, eosinophils, and monocytes [141]. LTA4, the primary 5-LO metabolite, is converted to LTC4 and sequentially to LTD4 and LTE4 in the host cell. This metabolism is rapid and complete, in that LTC4 is virtually undetectable in plasma. Because of its short half-life (approximately 7 minutes), LTE4 is likewise difficult to detect in plasma as a consequence of the low rate of production and rapid elimination [142]. Normal human urine contains low but detectable amounts of LTE4, ranging from 10 to 60 pg/ml [143]. Asthmatic patients with an acute episode of bronchoconstriction may have elevations of urinary LTE4 to several hundred pg/ml, although their baseline concentration is not consistently abnormal. LTE4 titration in urinary samples also avoids risks of ex vivo formation of metabolites during or after sampling, which may be a major problem when measuring arachidonic acid metabolism in other biological specimens.
The overproduction of cysteinyl leukotrienes reflected by urinary LTE4 is more prominent in the younger patients infected with RSV [139]. Also, an intrinsic predisposition to develop atopy or airway hyperreactivity seems to amplify the effect of the virus, as higher LTE4 concentrations were found in infants with a medical history of eczema or dry cough and/or family history of asthma. In particular, eczema and dry cough had the strongest association with urinary LTE4 levels in multivariate analysis. Age or atopy per se did not affect leukotriene synthesis in the absence of infection. Another important advantage of LTE4 monitoring in the setting of RSV infections is that this biomarker is not affected by extrinsic environmental factors, such as pre- and/or post-natal exposure to tobacco smoke [139] or environmental pollution [144], as such factors do not affect leukotriene synthesis, either alone or in combination with the viral infection. New prospective, controlled studies are needed to establish whether urinary LTE4 is more sensitive or specific than historical data alone in identifying young infants who will develop recurrent wheezing or asthma after RSV bronchiolitis, or in identifying potential responders to anti-leukotriene therapy for this infection.
ADDITIONAL BIOMARKERS SHOWING PROMISE
The lack of any single biomarker of RSV bronchiolitis that displays adequate predictive value and can be applied to all clinical scenarios is a recurring problem and has led many clinicians and researchers to pursue alternative strategies (Table 4). A predictive model designed to identify which bronchiolitis patients within an emergency department are most likely to be admitted used concentration measurements of lactate dehydrogenase in nasal washings, which was found to have a positive predictive value of 88% [145]. Increased liver transaminase and alanine aminotransferase have been observed in children with RSV bronchiolitis [146], while decreased blood sodium levels accompanied by elevated antidiuretic hormone (ADH) levels have been described in patients specifically having RSV LRTI compared to RSV infections of the upper respiratory tract [147-149].
In addition, RSV titers have shown some limited promise as predictors of bronchiolitis severity, whether hailing from the blood [150, 151] or from nasal swabs [152-156]. More specific to active RSV infection, viral RNA has been detected in human PBMCs from infants and children with RSV LRTI [93-95], and RSV RNA levels within PBMCs were correlated with disease severity in a murine model [94]. More recently, the advent of next-generation whole genome sequencing coupled with the decreased cost and increased ease of performing these powerful assays has driven some investigators to conclude that identifying unique genetic traits specific to each patient affected by RSV bronchiolitis is as equally important as targeting the virus itself. This has led to one study of specific host gene expression patterns within the nasopharyngeal region during RSV infection progression [157], whereas others have sought to assess RSV disease severity in infants by generating whole blood gene expression profiles [158].
In the latter multi-center observational study, researchers analyzed whole blood RNA sequencing profiles from children under 2 years of age positively identified through clinical testing as being infected with either RSV, human rhinovirus (hRV), or influenza A. Their model distinguished infants with RSV LRTI versus LRTI from hRV or influenza A with 95% accuracy. More importantly as this study relates to RSV, patients with RSV LRTI displayed unique transcriptional profiles compared to healthy controls hallmarked by significant overexpression of gene modules related to inflammation and innate immunity (including monocyte, neutrophil, and innate immune response genes) and decreased expression of gene modules related to adaptive immunity (including B cell and cytotoxic T/natural killer cell genes).
During the acute phase, patients with RSV LRTI displayed the highest levels of neutrophil-related gene expression paralleled by significant suppression of B cell, T cell, and lymphoid lineage response gene expression levels, which were either strikingly milder or even absent in patients with hRV or influenza A LRTI. Sub-classifying RSV LRTI by disease severity and tracking out to 1-month post-hospitalization, it was found that most host immune response gene patterns, such as those related to T cell and lymphoid lineage responsiveness, had ablated whereas the levels of B cell gene expression remained persistently suppressed during follow-up visits. This last finding provides further evidence as to the ability of RSV to modulate host immune responses with effects that specifically target anti-RSV immunoglobulin producing B cells and persist well after the period of symptomology and viral shedding/infectivity has subsided.
FUTURE RESEARCH DIRECTIONS
- Continue to research the full scope of NGF activity during acute RSV bronchiolitis, as well as during the post-bronchiolitis phase in which persistent airway dysfunction occurs.
- Continue to delineate the pathways and processes by which extrapulmonary sites become directly infected with RSV, what roles virus-mediated NGF expression may play in these sites, and what the immediate and long-term consequences to the health of the host are as a result of extrapulmonary RSV infections.
- Continued research using next generation whole genome sequencing to profile and pattern host immune responses at the gene level during RSV bronchiolitis will most likely yield significant advances in our understanding of how the virus modulates the ability of our immune system during the acute phase of infection and possibly for the weeks, months, and years that follow.
- Coupling this trend in host immune response gene research with studies of bone marrow and other extrapulmonary site RSV infections may yet elucidate the exact mechanism(s) behind the long-term suppression of host immune B-cell and memory responses to the virus.
- By understanding how host immunity to RSV is being evaded, we may be able to identify novel therapeutic targets for intervention both prior to and during RSV infection, which could ultimately lead to the development of new anti-viral pharmacologic strategies as well as a safe and effective vaccine.
EDUCATIONAL AIMS.
The reader will come to:
- Understand that RSV infection of the lower airways is the primary cause of acute bronchiolitis in infants and children worldwide.
- Learn the evidence linking early-life RSV lower respiratory tract infections to the development of childhood asthma.
- Recognize the multifaceted role of nerve growth factor (NGF) and other neurotrophins in the pathophysiology of RSV bronchiolitis.
- Know that a limited number of host biomarkers have been identified recently as predictive of RSV bronchiolitis severity outcomes.
ACKNOWLEDGMENTS
This article is dedicated to the memory of Dr. Caroline Breese-Hall, source of knowledge and inspiration for any scholar interested in RSV. We also thank the National Heart, Lung and Blood Institute of the National Institute of Health for the generous support of the research on the role of neurotrophins and leukotrienes in RSV disease. We are very grateful to the many faculty members, fellows, and technical and administrative staffers, without whom our research would not have been possible.
REFERENCES
- 1.Shay DK, et al. Bronchiolitis-associated hospitalizations among US children, 1980-1996. Jama-Journal of the American Medical Association. 1999;282(15):1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 2.Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr Pulmonol. 2011;46(4):324–47. doi: 10.1002/ppul.21377. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics Subcommittee on, D. and B. Management of Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–93. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 4.Zorc JJ, Hall CB. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010;125(2):342–9. doi: 10.1542/peds.2009-2092. [DOI] [PubMed] [Google Scholar]
- 5.Sala KA, et al. Factors associated with disease severity in children with bronchiolitis. J Asthma. 2014:1–5. doi: 10.3109/02770903.2014.956893. [DOI] [PubMed] [Google Scholar]
- 6.Sims DG, et al. Study of 8-year-old children with a history of respiratory syncytial virus bronchiolitis in infancy. Br Med J. 1978;1(6104):11–14. doi: 10.1136/bmj.1.6104.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mok JY, Simpson H. Outcome for acute bronchitis, bronchiolitis, and pneumonia in infancy. Arch Dis Child. 1984;59(4):306–9. doi: 10.1136/adc.59.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray M, et al. Respiratory status and allergy after bronchiolitis. Arch Dis Child. 1992;67(4):482–7. doi: 10.1136/adc.67.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noble V, et al. Respiratory status and allergy nine to 10 years after acute bronchiolitis. Arch Dis Child. 1997;76(4):315–9. doi: 10.1136/adc.76.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein RT, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354(9178):541–5. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 11.Sigurs N, et al. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161(5):1501–7. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 12.Piedimonte G, Walton C, Samsell L. Vertical transmission of respiratory syncytial virus modulates pre- and postnatal innervation and reactivity of rat airways. PLoS One. 2013;8(4):e61309. doi: 10.1371/journal.pone.0061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tortorolo L, et al. Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am J Respir Crit Care Med. 2005;172(2):233–7. doi: 10.1164/rccm.200412-1693OC. [DOI] [PubMed] [Google Scholar]
- 14.Hu C, et al. Nerve growth factor and nerve growth factor receptors in respiratory syncytial virus-infected lungs. Am J Physiol Lung Cell Mol Physiol. 2002;283(2):L494–502. doi: 10.1152/ajplung.00414.2001. [DOI] [PubMed] [Google Scholar]
- 15.Othumpangat S, et al. NGF is an essential survival factor for bronchial epithelial cells during respiratory syncytial virus infection. PLoS One. 2009;4(7):e6444. doi: 10.1371/journal.pone.0006444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Othumpangat S, Walton C, Piedimonte G. MicroRNA-221 modulates RSV replication in human bronchial epithelium by targeting NGF expression. PLoS One. 2012;7(1):e30030. doi: 10.1371/journal.pone.0030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espinoza JA, et al. Modulation of host adaptive immunity by hRSV proteins. Virulence. 2014;5(7) doi: 10.4161/viru.32225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chanock R, Finberg L. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). II. Epidemiologic aspects of infection in infants and young children. American Journal of Hygiene. 1957;66(3):291–300. doi: 10.1093/oxfordjournals.aje.a119902. [DOI] [PubMed] [Google Scholar]
- 19.Chanock R, Roizman B, Myers R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. American Journal of Hygiene. 1957;66(3):281–90. doi: 10.1093/oxfordjournals.aje.a119901. [DOI] [PubMed] [Google Scholar]
- 20.Murata Y. Respiratory syncytial virus vaccine development. Clin Lab Med. 2009;29(4):725–39. doi: 10.1016/j.cll.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo G, et al. A systematic review of predictive modeling for bronchiolitis. Int J Med Inform. 2014;83(10):691–714. doi: 10.1016/j.ijmedinf.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Milner ME, de la Monte SM, Hutchins GM. Fatal respiratory syncytial virus infection in severe combined immunodeficiency syndrome. Am J Dis Child. 1985;139(11):1111–4. doi: 10.1001/archpedi.1985.02140130049028. [DOI] [PubMed] [Google Scholar]
- 23.Hall CB, et al. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315(2):77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 24.Navas L, et al. Improved outcome of respiratory syncytial virus infection in a high-risk hospitalized population of Canadian children. Pediatric Investigators Collaborative Network on Infections in Canada. J Pediatr. 1992;121(3):348–54. doi: 10.1016/s0022-3476(05)90000-0. [DOI] [PubMed] [Google Scholar]
- 25.King JC, Jr., et al. Respiratory syncytial virus illnesses in human immunodeficiency virus- and noninfected children. Pediatr Infect Dis J. 1993;12(9):733–9. doi: 10.1097/00006454-199309000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Wang EE, et al. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study of admission and management variation in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr. 1996;129(3):390–5. doi: 10.1016/s0022-3476(96)70071-9. [DOI] [PubMed] [Google Scholar]
- 27.Boyce TG, et al. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000;137(6):865–70. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 28.Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis. 2004;189(2):233–8. doi: 10.1086/380907. [DOI] [PubMed] [Google Scholar]
- 29.Hall CB, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brooks AM, et al. Predicting deterioration in previously healthy infants hospitalized with respiratory syncytial virus infection. Pediatrics. 1999;104:463–7. doi: 10.1542/peds.104.3.463. 3 Pt 1. [DOI] [PubMed] [Google Scholar]
- 31.Hall CB, Douglas RG, Jr., Geiman JM. Possible transmission by fomites of respiratory syncytial virus. J Infect Dis. 1980;141(1):98–102. doi: 10.1093/infdis/141.1.98. [DOI] [PubMed] [Google Scholar]
- 32.Hall CB, Douglas RG., Jr. Modes of transmission of respiratory syncytial virus. J Pediatr. 1981;99(1):100–3. doi: 10.1016/s0022-3476(81)80969-9. [DOI] [PubMed] [Google Scholar]
- 33.Hall CB, et al. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun. 1981;33(3):779–83. doi: 10.1128/iai.33.3.779-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917–28. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 35.Englund JA, et al. Respiratory syncytial virus infection in immunocompromised adults. Ann Intern Med. 1988;109(3):203–8. doi: 10.7326/0003-4819-109-3-203. [DOI] [PubMed] [Google Scholar]
- 36.Chandwani S, et al. Respiratory syncytial virus infection in human immunodeficiency virus-infected children. J Pediatr. 1990;117:251–4. doi: 10.1016/s0022-3476(05)80539-6. 2 Pt 1. [DOI] [PubMed] [Google Scholar]
- 37.Kim HW, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–34. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 38.Chin J, et al. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89(4):449–63. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 39.Carbonell-Estrany X, et al. Clinical relevance of prevention of respiratory syncytial virus lower respiratory tract infection in preterm infants born between 33 and 35 weeks gestational age. Eur J Clin Microbiol Infect Dis. 2008;27(10):891–9. doi: 10.1007/s10096-008-0520-8. [DOI] [PubMed] [Google Scholar]
- 40.Hussman JM, et al. A review of cost-effectiveness of palivizumab for respiratory syncytial virus. Expert Rev Pharmacoecon Outcomes Res. 2012;12(5):553–67. doi: 10.1586/erp.12.45. [DOI] [PubMed] [Google Scholar]
- 41.Hussman JM, Lanctot KL, Paes B. The cost effectiveness of palivizumab in congenital heart disease: a review of the current evidence. J Med Econ. 2013;16(1):115–24. doi: 10.3111/13696998.2012.734886. [DOI] [PubMed] [Google Scholar]
- 42.Resch B, Resch E, Muller W. Should respiratory care in preterm infants include prophylaxis against respiratory syncytial virus infection? The case in favour. Paediatric Respiratory Reviews. 2013;14(2):130–136. doi: 10.1016/j.prrv.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Isaacs D. Should respiratory care in preterm infants include prophylaxis against respiratory syncytial virus? The case against. Paediatric Respiratory Reviews. 2013;14(2):128–129. doi: 10.1016/j.prrv.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Frogel MP, et al. A systematic review of compliance with palivizumab administration for RSV immunoprophylaxis. J Manag Care Pharm. 2010;16(1):46–58. doi: 10.18553/jmcp.2010.16.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J. 2002;21(7):629–32. doi: 10.1097/00006454-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Paramore LC, et al. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics. 2004;22(5):275–84. doi: 10.2165/00019053-200422050-00001. [DOI] [PubMed] [Google Scholar]
- 47.Smart KA, Lanctot KL, Paes BA. The cost effectiveness of palivizumab: a systematic review of the evidence. J Med Econ. 2010;13(3):453–63. doi: 10.3111/13696998.2010.499749. [DOI] [PubMed] [Google Scholar]
- 48.Nair H, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson WW, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 50.Falsey AR, et al. Acute respiratory tract infection in daycare centers for older persons. J Am Geriatr Soc. 1995;43(1):30–6. doi: 10.1111/j.1532-5415.1995.tb06238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Falsey AR, et al. Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J Infect Dis. 1995;172(2):389–94. doi: 10.1093/infdis/172.2.389. [DOI] [PubMed] [Google Scholar]
- 52.Sorvillo FJ, et al. An outbreak of respiratory syncytial virus pneumonia in a nursing home for the elderly. J Infect. 1984;9(3):252–6. doi: 10.1016/s0163-4453(84)90530-9. [DOI] [PubMed] [Google Scholar]
- 53.Nicholson KG, et al. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ. 1997;315(7115):1060–4. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Byington CL, et al. Respiratory syncytial virus–associated mortality in hospitalized infants and young children. Pediatrics. 2015;135:e24–e31. doi: 10.1542/peds.2014-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piedimonte G. Contribution of neuroimmune mechanisms to airway inflammation and remodeling during and after respiratory syncytial virus infection. Pediatr Infect Dis J. 2003;22(2 Suppl):S66–74. doi: 10.1097/01.inf.0000053888.67311.1d. discussion S74-5. [DOI] [PubMed] [Google Scholar]
- 56.Openshaw PJM, Dean GS, Culley FJ. Links between respiratory syncytial virus bronchiolitis and childhood asthma: clinical and research approaches. Pediatric Infectious Disease Journal. 2003;22(2):S58–S65. doi: 10.1097/01.inf.0000053887.26571.eb. [DOI] [PubMed] [Google Scholar]
- 57.Piedimonte G, Hegele RG, Auais A. Persistent airway inflammation after resolution of respiratory syncytial virus infection in rats. Pediatr Res. 2004;55(4):657–65. doi: 10.1203/01.PDR.0000112244.72924.26. [DOI] [PubMed] [Google Scholar]
- 58.Auais A, et al. Immunomodulatory effects of sensory nerves during respiratory syncytial virus infection in rats. Am J Physiol Lung Cell Mol Physiol. 2003;285(1):L105–13. doi: 10.1152/ajplung.00004.2003. [DOI] [PubMed] [Google Scholar]
- 59.Wedde-Beer K, et al. Leukotrienes mediate neurogenic inflammation in lungs of young rats infected with respiratory syncytial virus. Am J Physiol Lung Cell Mol Physiol. 2002;282(5):L1143–50. doi: 10.1152/ajplung.00323.2001. [DOI] [PubMed] [Google Scholar]
- 60.King KA, et al. Exaggerated neurogenic inflammation and substance P receptor upregulation in RSV-infected weanling rats. Am J Respir Cell Mol Biol. 2001;24(2):101–7. doi: 10.1165/ajrcmb.24.2.4264. [DOI] [PubMed] [Google Scholar]
- 61.Leon A, et al. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994;91(9):3739–43. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aung S, Rutigliano JA, Graham BS. Alternative mechanisms of respiratory syncytial virus clearance in perforin knockout mice lead to enhanced disease. J Virol. 2001;75(20):9918–24. doi: 10.1128/JVI.75.20.9918-9924.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Legg JP, et al. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2003;168(6):633–9. doi: 10.1164/rccm.200210-1148OC. [DOI] [PubMed] [Google Scholar]
- 64.Renz H, Kerzel S, Nockher WA. The role of neurotrophins in bronchial asthma: contribution of the pan-neurotrophin receptor p75. Prog Brain Res. 2004;146:325–33. doi: 10.1016/s0079-6123(03)46020-2. [DOI] [PubMed] [Google Scholar]
- 65.Braun A, et al. Neurotrophins: a link between airway inflammation and airway smooth muscle contractility in asthma? Int Arch Allergy Immunol. 1999;118(2-4):163–5. doi: 10.1159/000024056. [DOI] [PubMed] [Google Scholar]
- 66.Micera A, et al. Nerve growth factor displays stimulatory effects on human skin and lung fibroblasts, demonstrating a direct role for this factor in tissue repair. Proc Natl Acad Sci U S A. 2001;98(11):6162–7. doi: 10.1073/pnas.101130898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 68.Cohen S, Levi-Montalcini R, Hamburger V. A Nerve Growth-Stimulating Factor Isolated from Sarcom as 37 and 180. Proc Natl Acad Sci U S A. 1954;40(10):1014–8. doi: 10.1073/pnas.40.10.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110(2):167–73. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- 70.Zampieri N, Chao MV. Mechanisms of neurotrophin receptor signalling. Biochem Soc Trans. 2006;34:607–11. doi: 10.1042/BST0340607. Pt 4. [DOI] [PubMed] [Google Scholar]
- 71.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545–64. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets. 2008;7(1):46–62. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]
- 73.Ricci A, et al. Neurotrophin and neurotrophin receptor protein expression in the human lung. Am J Respir Cell Mol Biol. 2004;30(1):12–9. doi: 10.1165/rcmb.2002-0110OC. [DOI] [PubMed] [Google Scholar]
- 74.Ricci A, et al. Increased pulmonary neurotrophin protein expression in idiopathic interstitial pneumonias. Sarcoidosis Vasc Diffuse Lung Dis. 2007;24(1):13–23. [PubMed] [Google Scholar]
- 75.Rochlitzer S, Nassenstein C, Braun A. The contribution of neurotrophins to the pathogenesis of allergic asthma. Biochem Soc Trans. 2006;34:594–9. doi: 10.1042/BST0340594. Pt 4. [DOI] [PubMed] [Google Scholar]
- 76.Hahn C, et al. Airway epithelial cells produce neurotrophins and promote the survival of eosinophils during allergic airway inflammation. J Allergy Clin Immunol. 2006;117(4):787–94. doi: 10.1016/j.jaci.2005.12.1339. [DOI] [PubMed] [Google Scholar]
- 77.Nockher WA, Renz H. Neurotrophins and asthma: novel insight into neuroimmune interaction. J Allergy Clin Immunol. 2006;117(1):67–71. doi: 10.1016/j.jaci.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 78.Nockher WA, Renz H. Neurotrophins in allergic diseases: from neuronal growth factors to intercellular signaling molecules. J Allergy Clin Immunol. 2006;117(3):583–9. doi: 10.1016/j.jaci.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 79.Kobayashi H, et al. Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood. 2002;99(6):2214–20. doi: 10.1182/blood.v99.6.2214. [DOI] [PubMed] [Google Scholar]
- 80.Noga O, et al. The production, storage and release of the neurotrophins nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3 by human peripheral eosinophils in allergics and non-allergics. Clin Exp Allergy. 2003;33(5):649–54. doi: 10.1046/j.1365-2222.2003.01586.x. [DOI] [PubMed] [Google Scholar]
- 81.Santambrogio L, et al. Nerve growth factor production by lymphocytes. J Immunol. 1994;153(10):4488–95. [PubMed] [Google Scholar]
- 82.Noga O, et al. Selective induction of nerve growth factor and brain-derived neurotrophic factor by LPS and allergen in dendritic cells. Clin Exp Allergy. 2008;38(3):473–9. doi: 10.1111/j.1365-2222.2007.02907.x. [DOI] [PubMed] [Google Scholar]
- 83.Raap U, et al. Modulation of neurotrophin and neurotrophin receptor expression in nasal mucosa after nasal allergen provocation in allergic rhinitis. Allergy. 2008;63(4):468–75. doi: 10.1111/j.1398-9995.2008.01626.x. [DOI] [PubMed] [Google Scholar]
- 84.Mohtasham L, Auais A, Piedimonte G. Nerve growth factor mediates steroid-resistant inflammation in respiratory syncytial virus infection. Pediatr Pulmonol. 2007;42(6):496–504. doi: 10.1002/ppul.20607. [DOI] [PubMed] [Google Scholar]
- 85.Yao Q, et al. Hyperoxia enhances brain-derived neurotrophic factor and tyrosine kinase B receptor expression in peribronchial smooth muscle of neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2005;289(2):L307–14. doi: 10.1152/ajplung.00030.2005. [DOI] [PubMed] [Google Scholar]
- 86.Yao Q, et al. Neonatal lung and airway injury: a role for neurotrophins. Semin Perinatol. 2006;30(3):156–62. doi: 10.1053/j.semperi.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 87.Bonini S, et al. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc Natl Acad Sci USA. 1996;93:10955–10960. doi: 10.1073/pnas.93.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olgart Hoglund C, et al. Nerve growth factor levels and localisation in human asthmatic bronchi. Eur Respir J. 2002;20:1110–1116. doi: 10.1183/09031936.02.00205402. [DOI] [PubMed] [Google Scholar]
- 89.Virchow JC, et al. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am J Respir Crit Care Med. 1998;158(6):2002–5. doi: 10.1164/ajrccm.158.6.9803023. [DOI] [PubMed] [Google Scholar]
- 90.Dagnell C, et al. Increased levels of nerve growth factor in the airways of patients with sarcoidosis. J Intern Med. 2008;264(5):463–471. doi: 10.1111/j.1365-2796.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- 91.Ricci A, et al. Neurotrophins and neurotrophin receptors in human lung cancer. Am J Respir Cell Mol Biol. 2001;25(4):439–446. doi: 10.1165/ajrcmb.25.4.4470. [DOI] [PubMed] [Google Scholar]
- 92.Rezaee F, et al. Respiratory syncytial virus infection in human bone marrow stromal cells. Am J Respir Cell Mol Biol. 2011;45(2):277–86. doi: 10.1165/rcmb.2010-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rohwedder A, et al. Detection of respiratory syncytial virus RNA in blood of neonates by polymerase chain reaction. J Med Virol. 1998;54(4):320–7. doi: 10.1002/(sici)1096-9071(199804)54:4<320::aid-jmv13>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 94.Torres JP, et al. Respiratory syncytial virus (RSV) RNA loads in peripheral blood correlates with disease severity in mice. Respir Res. 2010;11:125. doi: 10.1186/1465-9921-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yui I, et al. Detection of human respiratory syncytial virus sequences in peripheral blood mononuclear cells. J Med Virol. 2003;70(3):481–9. doi: 10.1002/jmv.10421. [DOI] [PubMed] [Google Scholar]
- 96.Menchise A. Myocarditis in the setting of RSV bronchiolitis. Fetal Pediatr Pathol. 2011;30(1):64–8. doi: 10.3109/15513815.2010.505632. [DOI] [PubMed] [Google Scholar]
- 97.Li XQ, et al. Respiratory syncytial virus (RSV) infects neuronal cells and processes that innervate the lung by a process involving RSV G protein. J Virol. 2006;80(1):537–40. doi: 10.1128/JVI.80.1.537-540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Graham BS, Johnson TR, Peebles RS. Immune-mediated disease pathogenesis in respiratory syncytial virus infection. Immunopharmacology. 2000;48(3):237–47. doi: 10.1016/s0162-3109(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 99.Kirin BK, Topic RZ, Dodig S. Hepatitis during respiratory syncytial virus infection--a case report. Biochem Med (Zagreb) 2013;23(1):112–6. doi: 10.11613/BM.2013.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sweetman LL, et al. Neurologic complications associated with respiratory syncytial virus. Pediatr Neurol. 2005;32(5):307–10. doi: 10.1016/j.pediatrneurol.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 101.Esposito S, et al. Altered cardiac rhythm in infants with bronchiolitis and respiratory syncytial virus infection. BMC Infect Dis. 2010;10:305. doi: 10.1186/1471-2334-10-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Das B, et al. CD271(+) bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci Transl Med. 2013;5(170):170ra13. doi: 10.1126/scitranslmed.3004912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Piedimonte G. Respiratory syncytial virus and asthma: speed-dating or long-term relationship? Curr Opin Pediatr. 2013;25(3):344–9. doi: 10.1097/MOP.0b013e328360bd2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bueno SM, et al. Host immunity during RSV pathogenesis. Int Immunopharmacol. 2008;8(10):1320–9. doi: 10.1016/j.intimp.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 105.Lay MK, et al. Advances in understanding respiratory syncytial virus infection in airway epithelial cells and consequential effects on the immune response. Microbes Infect. 2013;15(3):230–42. doi: 10.1016/j.micinf.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 106.Avendano LF, Palomino MA, Larranaga C. Surveillance for respiratory syncytial virus in infants hospitalized for acute lower respiratory infection in Chile (1989 to 2000) J Clin Microbiol. 2003;41(10):4879–82. doi: 10.1128/JCM.41.10.4879-4882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Varga SM, et al. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity. 2001;15(4):637–46. doi: 10.1016/s1074-7613(01)00209-6. [DOI] [PubMed] [Google Scholar]
- 108.Wan YY, Flavell RA. How Diverse-CD4 Effector T Cells and their Functions. Journal of Molecular Cell Biology. 2009;1(1):20–36. doi: 10.1093/jmcb/mjp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Becker Y. Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy--a review. Virus Genes. 2006;33(2):235–52. doi: 10.1007/s11262-006-0064-x. [DOI] [PubMed] [Google Scholar]
- 110.Lee HC, et al. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol. 2012;130(5):1187–1196 e5. doi: 10.1016/j.jaci.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gut W, et al. RSV respiratory infection in children under 5 y.o.--dynamics of the immune response Th1/Th2 and IgE. Przegl Epidemiol. 2013;67(1):17–22. 105-9. [PubMed] [Google Scholar]
- 112.Olson MR, Hartwig SM, Varga SM. The number of respiratory syncytial virus (RSV)-specific memory CD8 T cells in the lung is critical for their ability to inhibit RSV vaccine-enhanced pulmonary eosinophilia. J Immunol. 2008;181(11):7958–68. doi: 10.4049/jimmunol.181.11.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Munir S, et al. Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response. PLoS Pathog. 2011;7(4):e1001336. doi: 10.1371/journal.ppat.1001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gonzalez PA, et al. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc Natl Acad Sci U S A. 2008;105(39):14999–5004. doi: 10.1073/pnas.0802555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gonzalez PA, et al. Impairment of T cell immunity by the respiratory syncytial virus: targeting virulence mechanisms for therapy and prophylaxis. Curr Med Chem. 2009;16(34):4609–25. doi: 10.2174/092986709789760724. [DOI] [PubMed] [Google Scholar]
- 116.Singleton R, et al. Inability to evoke a long-lasting protective immune response to respiratory syncytial virus infection in mice correlates with ineffective nasal antibody responses. J Virol. 2003;77(21):11303–11. doi: 10.1128/JVI.77.21.11303-11311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vallbracht S, Unsold H, Ehl S. Functional impairment of cytotoxic T cells in the lung airways following respiratory virus infections. Eur J Immunol. 2006;36(6):1434–42. doi: 10.1002/eji.200535642. [DOI] [PubMed] [Google Scholar]
- 118.Bueno SM, et al. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc Natl Acad Sci U S A. 2008;105(52):20822–7. doi: 10.1073/pnas.0806244105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gonzalez PA, et al. Understanding respiratory syncytial virus infection to improve treatment and immunity. Curr Mol Med. 2013;13(7):1122–39. doi: 10.2174/1566524011313070007. [DOI] [PubMed] [Google Scholar]
- 120.Whimbey E, et al. Respiratory syncytial virus pneumonia in hospitalized adult patients with leukemia. Clin Infect Dis. 1995;21(2):376–9. doi: 10.1093/clinids/21.2.376. [DOI] [PubMed] [Google Scholar]
- 121.Henderson FW, et al. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300(10):530–4. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 122.Hall CB, et al. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163(4):693–8. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 123.Glezen WP, et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–6. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 124.Hall CB, Long CE, Schnabel KC. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis. 2001;33(6):792–6. doi: 10.1086/322657. [DOI] [PubMed] [Google Scholar]
- 125.Glezen WP, et al. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98(5):708–15. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 126.Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. The PREVENT Study Group. Pediatrics. 1997;99(1):93–9. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 127.Prevention of respiratory syncytial virus infections: indications for the use of palivizumab and update on the use of RSV-IGIV. American Academy of Pediatrics Committee on Infectious Diseases and Committee of Fetus and Newborn. Pediatrics. 1998;102(5):1211–6. doi: 10.1542/peds.102.5.1211. [DOI] [PubMed] [Google Scholar]
- 128.Stensballe LG, et al. Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J Allergy Clin Immunol. 2009;123(2):398–403. doi: 10.1016/j.jaci.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 129.Brand HK, et al. CD4+ T-cell counts and interleukin-8 and CCL-5 plasma concentrations discriminate disease severity in children with RSV infection. Pediatr Res. 2013;73(2):187–93. doi: 10.1038/pr.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bont L, et al. Local interferon-gamma levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. J Infect Dis. 2001;184(3):355–8. doi: 10.1086/322035. [DOI] [PubMed] [Google Scholar]
- 131.Kott KS, et al. Effect of secondhand cigarette smoke, RSV bronchiolitis and parental asthma on urinary cysteinyl LTE4. Pediatr Pulmonol. 2008;43(8):760–6. doi: 10.1002/ppul.20853. [DOI] [PubMed] [Google Scholar]
- 132.Mella C, et al. Innate immune dysfunction is associated with enhanced disease severity in infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis. 2013;207(4):564–73. doi: 10.1093/infdis/jis721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mandelberg A, et al. Lipopolysaccharide hyporesponsiveness as a risk factor for intensive care unit hospitalization in infants with respiratory syncitial virus bronchiolitis. Clin Exp Immunol. 2006;144(1):48–52. doi: 10.1111/j.1365-2249.2006.03030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Littel-van den Hurk SV, Watkiss ER. Pathogenesis of respiratory syncytial virus. Current Opinion in Virology. 2012;2(3):300–305. doi: 10.1016/j.coviro.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 135.Wedde-Beer K, et al. Leukotrienes mediate neurogenic inflammation in lungs of young rats infected with respiratory syncytial virus. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1143–L1150. doi: 10.1152/ajplung.00323.2001. [DOI] [PubMed] [Google Scholar]
- 136.Bisgaard H. A randomized trial of montelukast in respiratory syncytial virus postbronchiolitis. Am J Respir Crit Care Med. 2003;167:379–383. doi: 10.1164/rccm.200207-747OC. [DOI] [PubMed] [Google Scholar]
- 137.Volovitz B, et al. The release of leukotrienes in the respiratory tract during infection with respiratory syncytial virus: role in obstructive airway disease. Pediatr Res. 1988;24(4):504–7. doi: 10.1203/00006450-198810000-00018. [DOI] [PubMed] [Google Scholar]
- 138.van Schaik SM, et al. Increased production of IFN-gamma and cysteinyl leukotrienes in virus-induced wheezing. J Allergy Clin Immunol. 1999;103(4):630–6. doi: 10.1016/s0091-6749(99)70235-6. [DOI] [PubMed] [Google Scholar]
- 139.Piedimonte G, et al. Leukotriene synthesis during respiratory syncytial virus bronchiolitis: influence of age and atopy. Pediatr Pulmonol. 2005;40(4):285–91. doi: 10.1002/ppul.20285. [DOI] [PubMed] [Google Scholar]
- 140.Sznajer Y, et al. Airway eicosanoids in acute severe respiratory syncytial virus bronchiolitis. J Pediatr. 2004;145:115–118. doi: 10.1016/j.jpeds.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 141.Maclouf J, et al. Entry rate and metabolism of leukotriene C4 into vascular compartment in healthy subjects. Am J Physiol. 1992;263:H244–H249. doi: 10.1152/ajpheart.1992.263.1.H244. [DOI] [PubMed] [Google Scholar]
- 142.Huber M, et al. Metabolism of cysteinyl leukotrienes in monkey and man. Eur J Biochem. 1990;194:309–315. doi: 10.1111/j.1432-1033.1990.tb19458.x. [DOI] [PubMed] [Google Scholar]
- 143.Kumlin M, et al. Validation and application of a new simple strategy for measurements of urinary leukotriene E4 in humans. Clin Exp Allergy. 1995;25:467–479. doi: 10.1111/j.1365-2222.1995.tb01079.x. [DOI] [PubMed] [Google Scholar]
- 144.Renzetti G, et al. Less air pollution leads to rapid reduction of airway inflammation and improved airway function in asthmatic children. Pediatrics. 2009;123(3):1051–1058. doi: 10.1542/peds.2008-1153. [DOI] [PubMed] [Google Scholar]
- 145.Laham FR, et al. LDH concentration in nasal-wash fluid as a biochemical predictor of bronchiolitis severity. Pediatrics. 2010;125(2):e225–33. doi: 10.1542/peds.2009-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Eisenhut M, Thorburn K. Hepatitis associated with severe respiratory syncytial virus-positive lower respiratory tract infection. Scand J Infect Dis. 2002;34(3):235. doi: 10.1080/00365540110077191. [DOI] [PubMed] [Google Scholar]
- 147.Eisenhut M. Extrapulmonary manifestations of severe RSV bronchiolitis. Lancet. 2006;368(9540):988. doi: 10.1016/S0140-6736(06)69409-9. [DOI] [PubMed] [Google Scholar]
- 148.Eisenhut M. Extrapulmonary manifestations of severe respiratory syncytial virus infection--a systematic review. Crit Care. 2006;10(4):R107. doi: 10.1186/cc4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hanna S, et al. Incidence of hyponatraemia and hyponatraemic seizures in severe respiratory syncytial virus bronchiolitis. Acta Paediatr. 2003;92(4):430–4. doi: 10.1111/j.1651-2227.2003.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 150.Houben ML, et al. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J Med Virol. 2010;82(7):1266–71. doi: 10.1002/jmv.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Borchers AT, et al. Respiratory syncytial virus--a comprehensive review. Clin Rev Allergy Immunol. 2013;45(3):331–79. doi: 10.1007/s12016-013-8368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.DeVincenzo JP. Factors predicting childhood respiratory syncytial virus severity: what they indicate about pathogenesis. Pediatr Infect Dis J. 2005;24(11 Suppl):S177–83. doi: 10.1097/01.inf.0000187274.48387.42. discussion S182. [DOI] [PubMed] [Google Scholar]
- 153.DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis. 2005;191(11):1861–8. doi: 10.1086/430008. [DOI] [PubMed] [Google Scholar]
- 154.Perkins SM, et al. Comparison of a real-time reverse transcriptase PCR assay and a culture technique for quantitative assessment of viral load in children naturally infected with respiratory syncytial virus. J Clin Microbiol. 2005;43(5):2356–62. doi: 10.1128/JCM.43.5.2356-2362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hon KL, et al. Respiratory syncytial virus morbidity, premorbid factors, seasonality, and implications for prophylaxis. J Crit Care. 2012;27(5):464–8. doi: 10.1016/j.jcrc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 156.El Kholy AA, et al. Morbidity and outcome of severe respiratory syncytial virus infection. Pediatr Int. 2013;55(3):283–8. doi: 10.1111/ped.12051. [DOI] [PubMed] [Google Scholar]
- 157.van den Kieboom CH, et al. Nasopharyngeal gene expression, a novel approach to study the course of respiratory syncytial virus infection. Eur Respir J. 2014 doi: 10.1183/09031936.00085614. [DOI] [PubMed] [Google Scholar]
- 158.Mejias A, et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med. 2013;10(11):e1001549. doi: 10.1371/journal.pmed.1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]