Abstract
Peripheral vascular disease (PVD) is a progressive atherosclerotic disease that leads to stenosis or occlusion of blood vessels supplying the lower extremities. Current diagnostic imaging techniques commonly focus on evaluation of anatomy or blood flow at the macrovascular level and do not permit assessment of the underlying pathophysiology associated with disease progression or treatment response. Molecular imaging with radionuclide-based approaches, such as PET and SPECT, can offer novel insight into PVD by providing non-invasive assessment of biological processes such as angiogenesis and atherosclerosis. This review discusses emerging radionuclide-based imaging approaches that have potential clinical applications in the evaluation of PVD progression and treatment.
Keywords: Peripheral vascular disease, molecular imaging, PET, SPECT, perfusion, angiogenesis, atherosclerosis
Introduction
Peripheral vascular disease (PVD) is a highly prevalent and progressive atherosclerotic disease of the lower extremities that is associated with claudication, non-healing ulcers, major amputations, and death.1 In addition to lower extremity complications, PVD is also associated with high rates of myocardial infarction and stroke.2 In a study of Medicare patients, annual costs for PVD-related treatment totaled 4.37 billion dollars.3
Due to the onset of atherosclerosis-induced tissue ischemia and necrosis, many PVD patients require lower extremity revascularization using endovascular procedures or surgical bypass.4 Non-surgical options have traditionally been limited to endovascular procedures that have resulted in high rates of stenosis, but recent developments in drug-eluting balloons5 and stents,6,7 which possess anti-proliferative agents, have increased the opportunities for novel therapeutics. In addition to endovascular procedures, systemic treatments such as antiplatelet and cholesterol lowering drugs, as well as renin-angiotensin system inhibitors, have been applied for the treatment of PVD patients who are not candidates for revascularization due to multiple diffuse stenosis or arterial calcification.8 Gene-based therapies using growth factors such as vascular endothelial growth factor (VEGF),9 fibroblast growth factor (FGF),10 hepatocyte growth factor (HGF),11 and hypoxia inducible factor 1 (HIF-1)12 have also gained attention in recent years. Additionally, cell-based therapies for PVD have been assessed using endothelial progenitor cells (EPCs),13 bone marrow mononuclear cells (BM-MNCs),14 and mesenchymal stem cells (MSCs)15 and demonstrated relative benefits. Overall, although early application of both gene- and cell-based therapies has produced some positive results, there are many inconsistencies that still remain with regard to proper dose size, frequency of therapeutics, and combination therapies incorporating multiple forms of growth factors or cells. Development of standardized end points to evaluate clinical outcomes should facilitate understanding of the clinical potential for these therapies. In particular, the use of non-invasive imaging techniques capable of evaluating physiological responses to novel therapeutic interventions could enhance the field and provide tools that complement standard clinical and anatomical indices which may lack the ability to fully assess underlying physiological changes responsible for positive clinical outcomes.
Standard Imaging Modalities for Evaluating PVD
Common clinical methods for detecting the response to medical treatment in the setting of PVD have been the ankle-brachial pressure index (ABI), duplex ultrasonography, and magnetic resonance (MR) or X-ray computed tomography (CT) angiographic parameters; however, each of these techniques have relative limitations for the evaluation of PVD.1 For example, ABI (i.e. the pressure differential between upper and lower extremity arteries) is only truly efficient for evaluating large vessel obstructions and has decreased sensitivity in the setting of microvascular disease.16 Additionally, ABI values can be over-exaggerated in the setting of medical calcification, further weakening their clinical utility.16 Duplex ultrasonography, although a relatively inexpensive, widely available, and fast imaging technique, only permits evaluation of blood flow in major vessels and is not useful for the estimation of collateral vessel flow.1 CT and MR angiography can characterize vessel morphology and allow for identification of significant stenosis or occlusion, thereby directing targeted revascularization procedures. However, both CT and MR angiography are limited by the lack of standard quantitative tools to assess the physiologic consequences associated with vessel stenosis and occlusion.17 Additional MR approaches exist for evaluation of lower extremity tissue perfusion and oxygenation, but commonly require exercise, pharmacological, or reactive hyperemia protocols to produce quantifiable changes in image signal intensity.18,19 Other techniques that have been utilized in more severe cases of PVD, such as critical limb ischemia (CLI), have included ankle and toe systolic pressures. However, ankle pressures, like ABIs, are subject to error in the setting of arterial calcification, and toe pressures are not always reliable for predicting amputation risk. Transcutaneous oxygen pressure (TcPO2) is considered to be a more reliable prognostic tool in the setting of CLI, as it provides functional information related to tissue perfusion and viability; however, TcPO2 also has limitations due to the ability to only measure superficial tissue viability.20
Lower extremity imaging using radionuclide-based approaches, such as positron emission tomography (PET) and single photon emission computed tomography (SPECT), may allow for not only the physiological assessment of PVD, but may permit evaluation of molecular events associated with disease progression or treatment response. PET and SPECT utilize targeted radionuclides capable of high-sensitivity detection of biological processes such as angiogenesis, atherosclerosis, and metabolism (Table 1).1 PET systems offer higher sensitivity than SPECT for targeted molecular imaging and also expose patients to lower amounts of ionizing radiation through the use of isotopes with relatively short half-lives. However, SPECT systems are more readily available, less expensive, and permit simultaneous imaging of multiple isotopes. Despite the high sensitivity of PET and SPECT for molecular imaging, both possess low spatial resolution when compared to CT and MR systems. Due to this limitation, hybrid imaging systems (PET/CT, SPECT/CT, PET/MR) have emerged that allow for pairing of high-sensitivity physiologic images with high-resolution anatomic images, permitting improved quantification of radionuclide uptake within anatomical regions of interest and allowing for attenuation correction and correction for partial volume effects.21 This review discusses recent trends in radionuclide imaging of PVD and highlights novel applications that may allow for serial evaluation of the progression and treatment of PVD.
Table 1.
Molecular Imaging Targets for Evaluation of PVD
| Physiologic Target | Radionuclide | Application |
|---|---|---|
| Perfusion/Blood Flow | 201Tl | Porcine,32 Clinical28–31,82 |
| 99mTc-sestamibi | Clinical34,35,37 | |
| 99mTc-tetrofosmin | Clinical1,38,39 | |
| 15O-water | Canine,43 Clinical40,42,83 | |
| C15O2 | Clinical84 | |
| 15O2 | Clinical83,84 | |
| 13N-ammonia | Mouse44 | |
| Angiogenesis | 99mTc-NC100692 | Mouse,47,52,85,86 |
| 111In-VEGF121 | Rabbit48 | |
| 125I-c(RGD(I)yV) | Mouse51 | |
| 76Br-nanoprobe | Mouse46 | |
| 68Ga-NOTA-RGD | Mouse50 | |
| 64Cu-DOTA-VEGF121 | Mouse49 | |
| 64Cu-DOTA-CANF-comb | Mouse53 | |
| 64Cu-NOTA-TRC105 | Mouse45,54 | |
| Atherosclerosis | 18F-FDG | Clinical55–58 |
| 18F-NaF | Clinical61–64 | |
| 11C-acetate | Clinical65 | |
| 18F-galacto-RGD | Mouse,68 Clinical87 | |
| 64Cu-DOTA-CANF | Rabbit67 | |
| 99mTc-PBMC | Clinical66 | |
| Metabolism | 18F-FDG | Clinical69 |
Radionuclide Imaging of Skeletal Muscle Perfusion and Blood Flow
Due to ongoing exposure to, and severity of skeletal muscle ischemia and hypoxia within the lower extremities, some of the earliest attempts at evaluating PVD with radionuclide approaches evaluated skeletal muscle blood flow and perfusion using intramuscular22–24 and intra-arterial25–27 injections of radionuclides. However, in the 1980s thallium-201 (201Tl) emerged as the predominant radionuclide for planar imaging in PVD patients due high first-pass extraction and the ability to inject 201Tl intravenously under conditions of rest28 or stress.29 Early studies established the clinical utility of 201Tl scintigraphy and SPECT for detecting perfusion abnormalities in PVD patients,28,30 while they also demonstrated high sensitivity for detecting impaired perfusion in the lower extremities of asymptomatic patients.31 Recent pre-clinical work by our research team has also demonstrated the feasibility of 201Tl SPECT imaging for tracking serial changes in lower extremity perfusion within specific muscle groups in association with ischemia-induced arteriogenesis and angiogenesis, further indicating the clinical utility of 201Tl.32
Despite the established value of 201Tl for evaluating PVD patients, technetium-99m (99mTc)-labeled tracers have recently emerged as the SPECT perfusion agents of clinical choice due to the shorter half-life, higher energy gamma rays, and minimal redistribution that characterizes 99mTc-labeled perfusion agents, thus reducing patient exposure to ionizing radiation while also improving image quality.33 Planar imaging studies using 99mTc-sestamibi (MIBI) have demonstrated significant reductions in proximal and distal lower extremity perfusion in asymptomatic patients who presented with early stages of atherosclerosis,34 and have also revealed impairments in rest and exercise stress perfusion when comparing PVD to control patients.35,36 Additionally, Miles et al.35 demonstrated that 99mTc-MIBI possessed a very high sensitivity (91%) and specificity (94%) for diagnosing PVD, and also demonstrated that 99mTc-MIBI uptake significantly correlated with angiographic and Doppler findings. Because of the limited redistribution associated with 99mTc-MIBI, there has been recent focus on 99mTc-MIBI lower extremity imaging in patients already undergoing myocardial perfusion imaging for the purposes of identifying potential lower extremity abnormalities in asymptomatic and symptomatic patients.37 Another 99mTc-labeled tracer, 99mTc-tetrofosmin, with no demonstrable redistribution has also emerged as a tool for assessing lower extremity perfusion, and has demonstrated potential clinical utility in evaluating the response to cell- and growth factor-based therapies in PVD patients.38,39 Our group has established tools for quantifying regional differences in 99mTc-tetrofosmin uptake in patients with unilateral PVD1 and have recently transitioned 99mTc-tetrofosmin SPECT imaging into PVD patients with CLI to evaluate serial changes in foot perfusion following revascularization procedures (Figure 1).
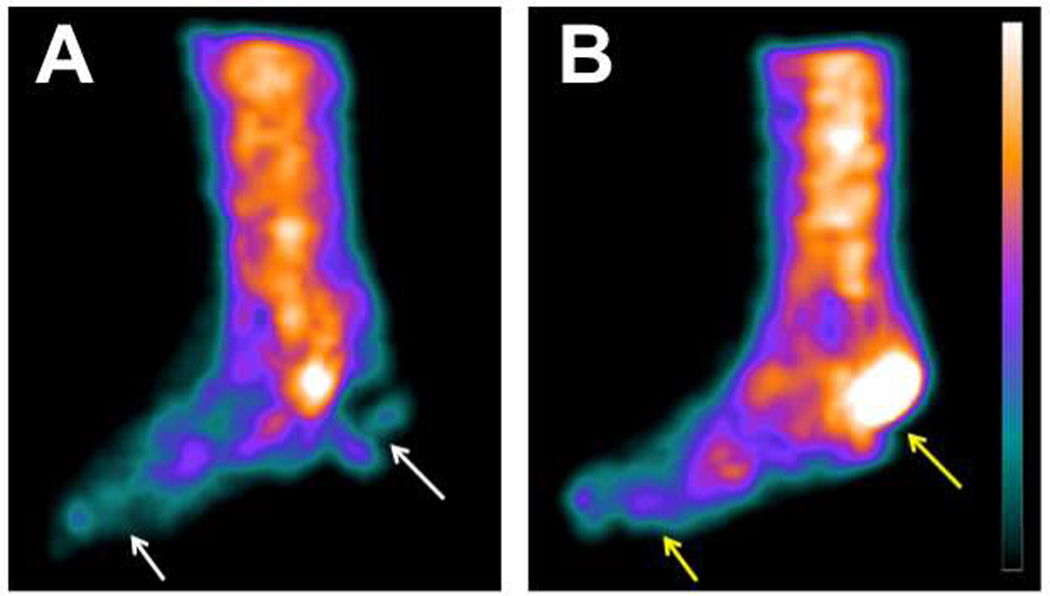
Figure 1.
Sagittal view of 99mTc-tetrofosmin SPECT imaging in a patient with non-healing heel ulcer before (A) and after (B) lower extremity revascularization and wound debridement demonstrates increased tracer uptake in the heel and distal foot. Pre-revascularization regions of ischemia are identified by white arrows and improvements in post-revascularization perfusion are denoted by yellow arrows.
In addition to radionuclides for SPECT imaging of lower extremity perfusion, PET tracers have also been utilized for evaluation of skeletal muscle blood flow. One potential benefit that PET may offer over SPECT in the evaluation of PVD is the ability to quantify absolute muscle blood flow, compared to the standard use of relative uptake values with SPECT. Additionally, PET tracers typically possess shorter half-lives, which allows for repeated measurements on a patient within the same day.40 However, one primary limitation of PET, when compared to SPECT imaging, is the requirement of exercise or a pharmacological stressor to induce quantifiable changes in muscle blood flow.41 An early study by Burchert et al.41 established the clinical utility of PET imaging for evaluation of muscle blood flow in PVD patients using vasodilator and exercise stress. Further work by Schmidt et al.40 demonstrated the ability of H2 15O PET imaging to detect significant impairments in calf muscle flow reserve in PVD patients compared to healthy subjects, and also found a close correlation between thermodilution- and PET-derived flow reserve values. In addition to the utility of PET imaging in the setting of PVD, H2 15O PET has also identified the anatomical level of muscle blood flow deficits in the limbs of patients with CLI, suggesting that radionuclide imaging may be a specific indicator of tissue viability and predictor of future amputation level of the lower extremity.42
Pre-clinical work with H2 15O PET imaging has demonstrated a close matching of PET-and microsphere-derived measurements of limb blood flow in healthy canines under baseline resting conditions and vasodilator stress.43 Additionally, PET-derived measurements of muscle blood flow have been found to significantly correlate with Doppler flow probe measurements across a variety of physiologic states (r2 = 0.92), further indicating the clinical potential of PET imaging for assessing lower extremity blood flow.43 In a mouse model of hind limb ischemia, PET imaging of nitrogen-13 (13N)-ammonia has been used to evaluate serial changes in tissue perfusion following iliac occlusion and has demonstrated that serial improvements in relative tissue perfusion of the ischemic hind limb closely correlated with the amount of tissue necrosis and fibrosis quantified histologically 30 days following occlusion.44
Application of SPECT and PET imaging for serial evaluation of muscle perfusion or blood flow in PVD patients has yet to reach full potential. Further development of fluorine-18 (18F)-labeled perfusion tracers for PET, which would possess longer half-lives than standard tracers (~110 min vs. < 5 min), may assist in expanding the application of PET for assessment of PVD through the combination of myocardial and lower extremity perfusion imaging. Additionally, ongoing development of high-sensitivity, high-resolution cadmium zinc telluride (CZT) SPECT systems should allow for absolute quantification of blood flow in the lower extremities, thereby improving the future potential of SPECT applications in the setting of PVD.
Radionuclide Imaging of Skeletal Muscle Angiogenesis
In addition to imaging of lower extremity perfusion and blood flow, radionuclide-based imaging of angiogenesis has also been established as an effective tool in the evaluation of limb ischemia in pre-clinical animal models.44–47 To date, molecular imaging of peripheral angiogenesis with PET and SPECT has relied on radionuclides targeting VEGF receptors and the αvβ3 integrin, which both play important roles in the angiogenic process. Early work by Lu et al.48 targeted VEGF receptors using indium-111 (111In)-labeled recombinant human VEGF121 and demonstrated increased tracer uptake in ischemic hind limbs that peaked 10 days following induction of limb ischemia. More recent work by Willmann et al.49 utilized in vivo micro PET imaging and gamma counting of copper-64 (64Cu)-VEGF121 for evaluating peripheral angiogenesis and revealed peak tracer uptake at 8 days post-femoral artery ligation. Additionally, 64Cu-VEGF121 demonstrated a significantly higher angiogenic response in mice exposed to an exercise-training regimen, suggesting that serial imaging of peripheral angiogenesis may have clinical utility for patients undergoing exercise therapy.
Along with targeting of VEGF receptors, other work has utilized gallium-68 (68Ga)-,50 iodine-125 (125I)-,51 and 99mTc-labeled52 RGD peptides (composed of L-arginine, glycine, and L-aspartic acid) for targeting of the αvβ3 integrin. The RGD peptide moiety allows for selective imaging of angiogenesis, as the αvβ3 integrin is expressed on proliferating endothelial cells and activated macrophages that are involved in the angiogenic process. Early work by Lee et al.51 assessed an 125I-labeled RGD peptide in mice and demonstrated serial changes in radionuclide uptake (via gamma counting) in ischemic limbs that corresponded with post-mortem immunohistochemistry evaluation of αvβ3 integrin expression. Serial in vivo Doppler imaging also revealed improvements in tissue perfusion within the ischemic limb post-surgery. Additional work by Hua et al.52 used planar imaging of another radiolabeled RGD tracer, 99mTc-NC100692 (Maraciclatide, GE Healthcare), to evaluate serial changes in angiogenesis and demonstrated increased radionuclide uptake at 3 and 7 days after induction of limb ischemia that was associated with serial improvements in microvascular density. Further immunofluorescent analysis revealed specificity and co-localization of NC100692 to endothelial cells. Our research team has expanded on this early work with RGD peptides to also validate SPECT/CT image analysis tools for serial regional assessment of angiogenesis in endothelial nitric oxide synthase (eNOS) deficient mice exposed to limb ischemia and has demonstrated impaired angiogenesis in the distal limbs of these mice when compared to wild type counterparts.47 Ongoing work in our laboratory is directed at applying 99mTc-NC100692 SPECT/CT imaging in large animal models of limb ischemia to assess serial changes in angiogenesis and has recently revealed the potential of this tracer for evaluating ischemic hind limb tissue in a porcine model 2 weeks post-femoral artery occlusion (Figure 2).
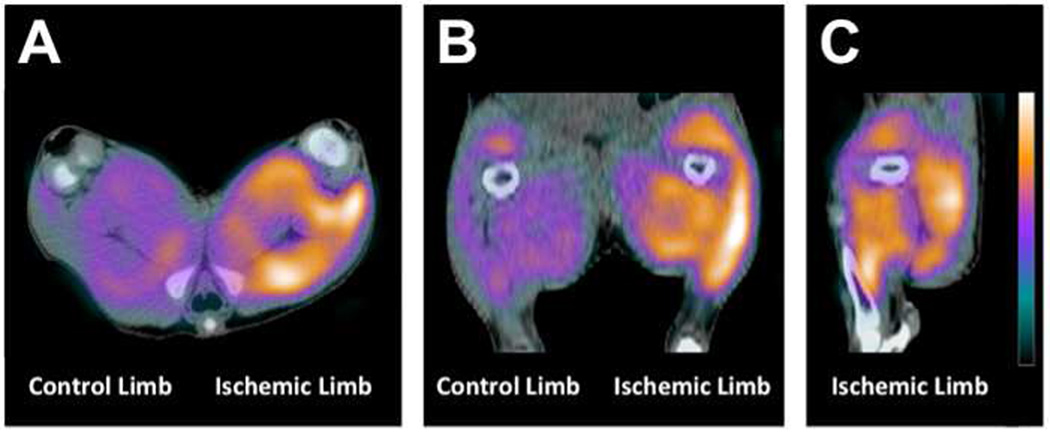
Figure 2.
Trasverse (A), coronal (B), and sagittal (C) views of angiogenesis targeted imaging in a pig model of unilateral hind limb ischemia using 99mTc-NC100692 SPECT/CT demonstrates marked tracer uptake in ischemic tissue 2 weeks following femoral artery occlusion.
Targeted nanoprobes have also been in recent development for non-invasive assessment of peripheral angiogenesis.46,53 Specifically, 64Cu-labeled C-type atrial natriuretic factor (CANF)-conjugated comblike nanoprobes have been developed and applied for PET imaging of angiogenesis in mice, demonstrating high sensitivity and specificity for angiogenesis specific vascular targets.53 Additionally, biodegradable dendritic nanoprobes labeled with either 125I or bromine-76 (76Br) have been developed for PET imaging of the αvβ3 integrin through the use of polyethylene oxide (PEO) chains fitted with RGD motifs and have revealed specific uptake in αvβ3-positive cells as well as in angiogenic tissue within the mouse hind limb.46
Along with the previously established αvβ3 and VEGF targets for non-invasive imaging of angiogenesis, 64Cu-NOTA-TRC1005, a CD105 antibody, has recently been developed and validated for evaluating serial changes in angiogenesis following surgically induced limb ischemia.45,54 In addition to 64Cu-NOTA-TRC1005 uptake demonstrating significantly increased uptake in the setting of hind limb ischemia, this tracer has also exhibited significantly higher uptake in mice exposed to a combination of limb ischemia and statin therapy, indicating further potential for clinical translation of angiogenesis targeted imaging in the assessment of medical treatment.45
Radionuclide Imaging of Atherosclerosis
Another area of radionuclide imaging research that has developed interest is molecular imaging of atherosclerosis. Atherosclerotic plaque progression and stability is regulated by multiple signaling events and cell interactions. Therefore, incorporation of non-invasive imaging tools may offer new insight into plaque evolution while also assisting with monitoring of disease progression and therapeutic responses. To date, the most popular plaque targets have been metabolism and inflammation using PET/CT imaging of 18F-fluurodeoxyglucose (FDG), which is a glucose analog that is metabolized by resident macrophages, thus providing a non-invasive dual marker of ongoing metabolic activity and inflammation within remodeling plaque.55–57 Early work by Yun et al.57 demonstrated a strong correlation (r = 0.99) between increasing age and increasing FDG uptake in peripheral arteries, and also found an increased prevalence of FDG uptake in peripheral vessels in patients with at least one atherogenic risk factor.58 Further work by Rudd et al.56 revealed that symptomatic, unstable plaques exhibited higher uptake of FDG when compared to asymptomatic lesions and demonstrated high reproducibility of 18F-FDG PET/CT imaging for evaluation of plaque burden within the carotid, iliac, and femoral arteries.55 Recent clinical studies have found significant reductions in 18F-FDG uptake within lower extremity vessels following the elevation of plasma high-density lipoprotein levels via atherogenic risk reduction,59 as well as reduced vascular FDG uptake following high-dose statin therapy.60 These results suggest that PET/CT imaging of 18F-FDG may provide a sensitive noninvasive tool for tracking atherosclerotic disease progression and therapeutic responses in PVD.
Another radionuclide approach that may allow for evaluation of atherosclerotic plaque progression is PET/CT imaging of 18F-sodium fluoride (NaF). 18F-NaF was originally approved by the Food and Drug Administration in 1972 as a bone imaging tracer due to the tendency of fluoride 18F to deposit within sites of calcium minerals. Recent work has demonstrated that PET/CT imaging of 18F-NaF may also have value as a non-invasive tool for evaluating active microcalcifications in atherosclerotic plaques (Figure 3).61–64 In initial atherosclerosis imaging work by Derlin et al.,61 18F-NaF uptake was highest in the femoral arteries when compared to other major peripheral vessels. Further PET/CT imaging studies have since demonstrated a strong correlation between 18F-NaF femoral64 and carotid63 artery uptake and atherogenic risk factors, as well as the ability to pair 18F-NaF with 18F-FDG for potential dual analysis of pathophysiologic stages of plaque formation and progression.62
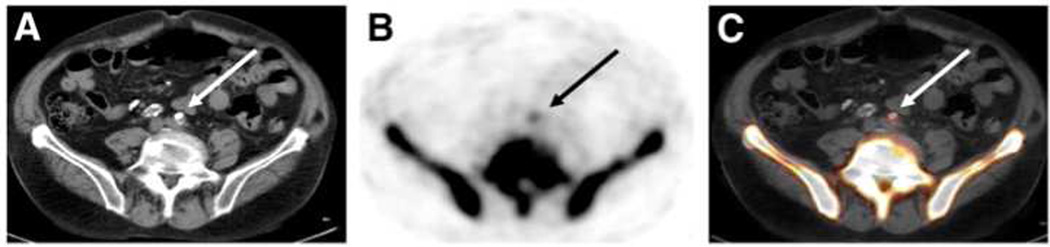
Figure 3.
Targeted imaging of atherosclerosis in the common iliac arteries using CT (A), 18F-NaF PET (B), and fused 18F-NaF PET/CT imaging (C). Fused imaging demonstrates 18F-NaF accumulation in atherosclerotic lesion of iliac artery that is co-localized with calcification. Arrows indicate region of calcified lesion.
From Derlin et al. Feasibility of 18F-Sodium Fluoride PET/CT for Imaging of Atherosclerotic Plaque. J Nucl Med 2010;51:862-5; with permission.
Additional radionuclide techniques have also demonstrated feasibility for targeted imaging of atherosclerosis. Derlin et al.65 have applied carbon-11 (11C)-acetate PET/CT imaging for evaluation of fatty acid synthesis within atherosclerotic plaque of patients and demonstrated increased tracer uptake that was co-localized with regions of arterial calcification. In addition to imaging of fatty acid synthesis, 99mTc-labeled peripheral blood mononuclear cells (PBMCs) have also been used for targeted SPECT/CT imaging of arterial wall inflammation in patients with advanced atherosclerosis and revealed a correlation between tracer uptake and disease severity.66 Pre-clinical imaging work in atherosclerosis-induced animal models has also demonstrated the potential for 64Cu-labeled natriuretic peptide in a rabbit model of hypercholesterolemia67 and 18F-galacto-RGD in evaluating the effect of dietary intervention in hypercholesterolemic mice.68 The active development of radionuclide-based approaches for evaluation of atherosclerosis should elucidate the various mechanisms regulating plaque progression and vulnerability to rupture, thereby improving management of patients with PVD at high-risk for cardiovascular events and allowing for assessment of therapeutic interventions.
Potential for Application of Novel Radionuclides
A variety of lesser-established radionuclide approaches may also have potential for evaluation of PVD and warrant further investigation and application. Specifically, Pande et al.69 have demonstrated impairment of skeletal muscle metabolism in PVD patients with claudication using 18F-FDG PET/CT imaging. 18F-FDG has also shown clinical potential for assessing local infection within fractured peripheral stents and guiding subsequent antibiotic therapy (Figure 4).70 Both of these studies represent novel applications of 18F-FDG imaging in the evaluation and management of PVD patients, and warrant future clinical investigations that may further expand on the current role of PET/CT imaging in PVD.
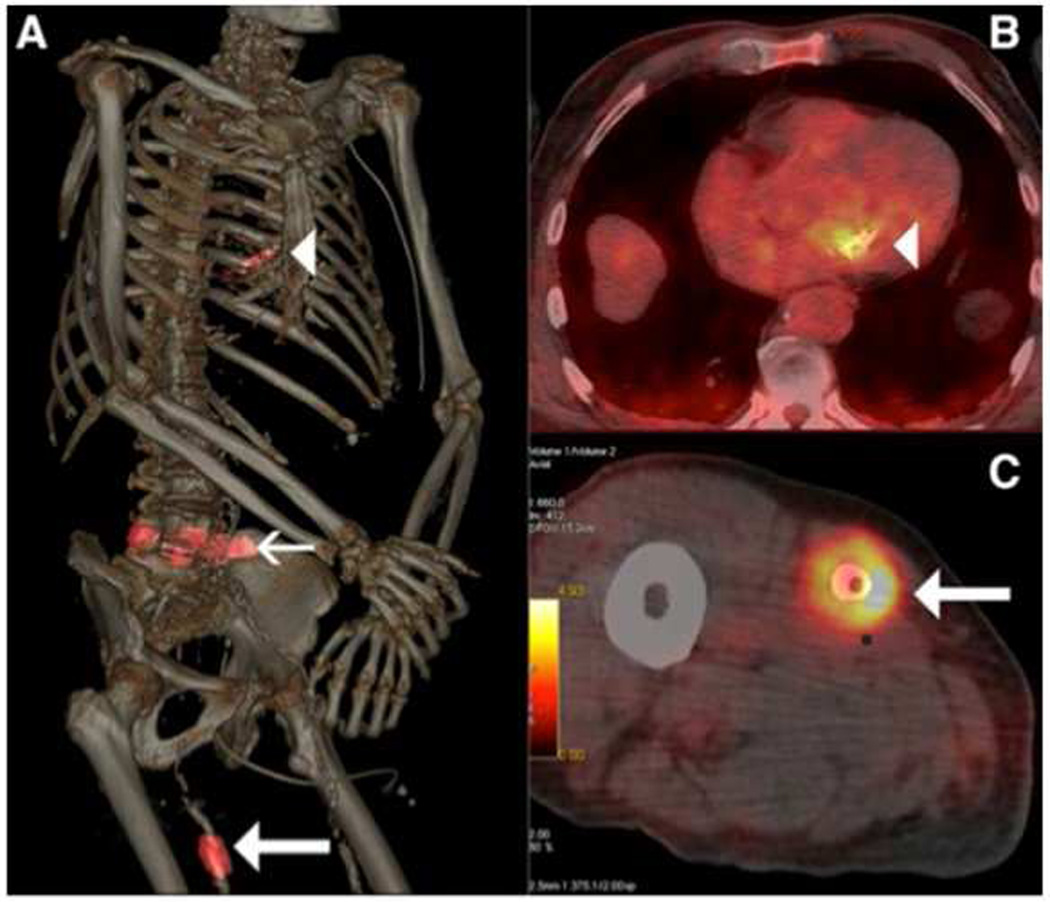
Figure 4.
A) Volume rendering of 18F-FDG PET/CT demonstrates focal uptake of tracer in regions of mitral valve (arrowhead), L4 to L5 discus (thin arrow), and right femoral artery stent (thick arrow) in a patient found to have mitral endocarditits, peridural abscess, and stent dislocation and fracture. Transverse images further reveal focal 18F-FDG uptake in the infected (B) mitral valve and (C) femoral artery stent.
From Berard X, Pinaquy J-B, Stecken L, et al. Use of 18F-fluorodeoxyglucose positron emission tomography-computed tomography and sonication for detection of infection after peripheral stent fracture. Circulation 2014;129:2437-9; with permission.
Other radiotracer-based imaging approaches have been developed, but have not yet been applied in the setting of PVD. For example, hypoxia targeted imaging has been previously developed and applied for assessment of myocardial ischemia, but application of hypoxia imaging for evaluation of PVD may represent an alternative use, providing an assessment of the balance between tissue blood flow and oxygen utilization.71 Another non-invasive probe for application in PVD might be the pH (low) insertion peptide (i.e. pHLIP), which has been applied for evaluating changing levels in tissue pH and acidosis in the setting of myocardial ischemia and demonstrated sensitivity for detecting tissue exposed to acidic pH levels.72 Additionally, an 18F-labeled PET tracer has been recently developed for assessment of reactive oxygen species and applied in the setting of myocardial inflammation, representing another novel non-invasive approach that may have potential for evaluating stages of atherosclerotic plaque progression or the angiogenic process.73
Ongoing development and application of cell- and gene-based therapies that are being used in clinical trials of patients with PVD should offer additional opportunities for high sensitivity radionuclide-based approaches for evaluation of PVD.8,74 The ability to noninvasively evaluate the biodistribution of transplanted cells should be achievable through the utilization of high-sensitivity PET and SPECT imaging of radiolabeled cells (~104 – 106 cells/voxel).75 Multiple clinical trials have already demonstrated the feasibility of tracking effective cell delivery in the myocardium of patients following myocardial infarction.76–79 However, to date, the relatively short half-lives of PET and SPECT radionuclides has limited the ability to track long-term cell fate in vivo. The development of reporter probes, such as the sodium iodide symporter (NIS), may overcome this limitation by allowing for non-invasive detection of viable NIS-transfected cells through retention of isotopes by NIS.80 Indeed, NIS has already demonstrated potential for non-invasive assessment of viable cells within infarcted porcine myocardium for 15 weeks following cell transplantation using 123I SPECT/CT imaging.81 Future use of radionuclide imaging for serial tracking of cell- and gene-based therapies is encouraging and should have an expanding role in the evaluation of these novel treatments of PVD.
Conclusions
Radionuclide-based approaches for evaluation of PVD continue to demonstrate potential in pre-clinical animal models as well as patient populations. Ongoing development of targeted PET and SPECT tracers should provide high sensitivity biomarkers for evaluation of a variety of pathophysiologic processes associated with PVD, while also offering non-invasive imaging tools that complement the existing anatomical and clinical indices. Continued progress in revascularization procedures and gene- and cell-based therapies for the management of PVD may further expand the role and potential applications for radionuclide-based imaging approaches in the serial evaluation of medical and/or surgical treatments.
Key Points.
Peripheral vascular disease is a prevalent atherosclerotic condition affecting the lower extremities that is associated with significant limb health complications and healthcare costs.
Current imaging techniques all have limitations with regard to non-invasive assessment of peripheral vascular disease pathophysiology and treatment responses.
Radionuclide-based imaging may provide novel opportunities for non-invasive assessment of peripheral vascular disease by allowing for evaluation of various physiologic indices, such as skeletal muscle perfusion, angiogenesis, atherosclerosis, and metabolism.
Acknowledgments
Funding Sources:
This work was supported in part by American Heart Association grant 14CRP20480404 to Dr. Stacy and NIH grant T32 HL098069 to Dr. Sinusas.
Disclosures:
Dr. Sinusas receives financial support and NC100692 from GE Healthcare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stacy MR, Zhou W, Sinusas AJ. Radiotracer imaging of peripheral vascular disease. J Nucl Med. 2013;54(12):2104–2110. doi: 10.2967/jnumed.112.115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Regensteiner JG, Hiatt WR, Coll JR, et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med (London, England) 2008;13(1):15–24. doi: 10.1177/1358863X07084911. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med. 2008;13(3):209–215. doi: 10.1177/1358863X08089277. [DOI] [PubMed] [Google Scholar]
- 4.Nasr B, Kaladji A, Vent PA, et al. State-of-the-art treatment of common femoral artery disease. J Cardiovasc Surg. 2015;56(2):309–316. [PubMed] [Google Scholar]
- 5.Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358(7):689–699. doi: 10.1056/NEJMoa0706356. [DOI] [PubMed] [Google Scholar]
- 6.Lammer J, Bosiers M, Zeller T, et al. First clinical trial of nitinol self-expanding everolimus-eluting stent implantation for peripheral arterial occlusive disease. J Vasc Surg. 2011;54(2):394–401. doi: 10.1016/j.jvs.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 7.Dake MD, Ansel GM, Jaff MR, et al. Sustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomized and single-arm clinical studies. J Am Coll Cardiol. 2013;61(24):2417–2427. doi: 10.1016/j.jacc.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Sanada F, Taniyama Y, Kanbara Y, et al. Gene therapy in peripheral artery disease. Expert Opin Biol Ther. 2015;15(3):381–390. doi: 10.1517/14712598.2015.1007039. [DOI] [PubMed] [Google Scholar]
- 9.Isner JM, Pieczek A, Schainfeld R, et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF 165 in patient with ischaemic limb. Lancet. 1996;348(9024):370–374. doi: 10.1016/s0140-6736(96)03361-2. [DOI] [PubMed] [Google Scholar]
- 10.Nikol S, Baumgartner I, Van Belle E, et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol Ther. 2008;16(5):972–978. doi: 10.1038/mt.2008.33. [DOI] [PubMed] [Google Scholar]
- 11.Makino H, Aoki M, Hashiya N, et al. Long-term follow-up evaluation of results from clinical trial using hepatocyte growth factor gene to treat severe peripheral arterial disease. Arter Thromb Vasc Biol. 2012;32(10):2503–2509. doi: 10.1161/ATVBAHA.111.244632. [DOI] [PubMed] [Google Scholar]
- 12.Rajagopalan S, Olin J, Deitcher S, et al. Use of a constitutively active hypoxia-inducible factor-1alpha transgene as a therapeutic strategy in no-option critical limb ischemia patients: phase I dose-escalation experience. Circulation. 2007;115(10):1234–1243. doi: 10.1161/CIRCULATIONAHA.106.607994. [DOI] [PubMed] [Google Scholar]
- 13.Kawamoto A, Katayama M, Handa N, et al. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells. 2009;27(11):2857–1864. doi: 10.1002/stem.207. [DOI] [PubMed] [Google Scholar]
- 14.Walter DH, Krankenberg H, Balzer JO, et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA) Circ Cardiovasc Interv. 2011;4(1):26–37. doi: 10.1161/CIRCINTERVENTIONS.110.958348. [DOI] [PubMed] [Google Scholar]
- 15.Lasala GP, Silva JA, Gardner PA, Minguell JJ. Combination stem cell therapy for the treatment of severe limb ischemia: safety and efficacy analysis. Angiology. 2010;61(6):551–556. doi: 10.1177/0003319710364213. [DOI] [PubMed] [Google Scholar]
- 16.Potier L, Abi Khalil C, Mohammedi K, Roussel R. Use and utility of ankle brachial index in patients with diabetes. Eur J Vasc Endovasc Surg. 2011;41(1):110–116. doi: 10.1016/j.ejvs.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Duran C, Bismuth J. Advanced imaging in limb salvage. Methodist Debakey Cardiovasc J. 2012;8(4):28–32. doi: 10.14797/mdcj-8-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollak AW, Meyer CH, Epstein FH, et al. Arterial spin labeling MR imaging reproducibly measures peak-exercise calf muscle perfusion: a study in patients with peripheral arterial disease and healthy volunteers. JACC Cardiovasc Imaging. 2012;5(12):1224–1230. doi: 10.1016/j.jcmg.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ledermann H-P, Schulte A-C, Heidecker H-G, et al. Blood oxygenation level-dependent magnetic resonance imaging of the skeletal muscle in patients with peripheral arterial occlusive disease. Circulation. 2006;113(25):2929–2935. doi: 10.1161/CIRCULATIONAHA.105.605717. [DOI] [PubMed] [Google Scholar]
- 20.Becker F, Robert-Ebadi H, Ricco JB, et al. Chapter I: Definitions, Epidemiology, Clinical Presentation and Prognosis. Eur J Vasc Endovasc Surg. 2011;42(52):S4–S12. doi: 10.1016/S1078-5884(11)60009-9. [DOI] [PubMed] [Google Scholar]
- 21.Stacy MR, Sinusas AJ. Emerging imaging modalities in regenerative medicine. Curr Pathobiol Rep. 2015;3(1):27–36. doi: 10.1007/s40139-015-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kety S. Measurement of regional circulation by the local clearance of radioactive sodium. Am Hear J. 1949;38:321–328. doi: 10.1016/0002-8703(49)90845-5. [DOI] [PubMed] [Google Scholar]
- 23.Lassen OA, Lindberg J, Munck O. Measurement of blood flow through skeletal muscle by intramuscular injection of Xenon-133. Lancet. 1964;1(7335):686–689. doi: 10.1016/s0140-6736(64)91518-1. [DOI] [PubMed] [Google Scholar]
- 24.Cutajar CL, Brown NJ, Marston A. Muscle blood-flow studies by the technetium (99m)Tc clearance technique in normal subjects and in patients with intermittent claudication. Br J Surg. 1971;58(7):532–537. doi: 10.1002/bjs.1800580717. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes BA, Greyson ND, Siegel ME, et al. The distribution of radioactive microspheres after intra-arterial injection in the legs of patients with peripheral vascular disease. Am J Roentgenol Radium Ther Nucl Med. 1973;118:820–826. doi: 10.2214/ajr.118.4.820. [DOI] [PubMed] [Google Scholar]
- 26.Coffmann JD, Mannick JA. A simple objective test for arteriosclerosis obliterations. N Engl J Med. 1965;273:1297–1301. doi: 10.1056/NEJM196512092732402. [DOI] [PubMed] [Google Scholar]
- 27.Sheda H, O’Hara I. Study in peripheral circulation using I-131 and macroaggregated serum albumin. J Exp Med. 1970;101:311–314. doi: 10.1620/tjem.101.311. [DOI] [PubMed] [Google Scholar]
- 28.Hamanaka D, Odori T, Maeda H, Ishii Y, Hayakawa K, Torizuka K. A quantitative assessment of scintigraphy of the legs using 201Tl. Eur J Nucl Med. 1984;9(1):12–16. doi: 10.1007/BF00254343. [DOI] [PubMed] [Google Scholar]
- 29.Siegel ME, Stewart CA. Thallium-201 peripheral perfusion scans : feasibility of single-dose, single-day, rest and stress study. AJR Am J Roentgenol. 1981;136(6):1179–1183. doi: 10.2214/ajr.136.6.1179. [DOI] [PubMed] [Google Scholar]
- 30.Oshima M, Akanabe H, Sakuma S, Yano T, Nishikimi N, Shionoya S. Quantification of leg muscle perfusion using thallium-201 single photon emission computed tomography. J Nucl Med. 1989;30(4):458–465. [PubMed] [Google Scholar]
- 31.Duet M, Virally M, Bailliart O, et al. Whole-body (201)Tl scintigraphy can detect exercise lower limb perfusion abnormalities in asymptomatic diabetic patients with normal Doppler pressure indices. Nucl Med Commun. 2001;22(9):949–954. doi: 10.1097/00006231-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Stacy MR, Yu DY, Maxfield MW, et al. Multimodality imaging approach for serial assessment of regional changes in lower extremity arteriogenesis and tissue perfusion in a porcine model of peripheral arterial disease. Circ Cardiovasc Imaging. 2014;7(1):92–99. doi: 10.1161/CIRCIMAGING.113.000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purushothaman K, Sinusas AJ. Technetium-99m-labeled myocardial perfusion agents: Are they better than thallium-201? Cardiol Rev. 2001;9(3):160–172. doi: 10.1097/00045415-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Kuśmierek J, Dąbrowski J, Bienkiewicz M, Szuminski R, Plachcinska A. Radionuclide assessment of lower limb perfusion using (99m)Tc-MIBI in early stages of atherosclerosis. Nucl Med Rev. 2006;9(1):18–23. [PubMed] [Google Scholar]
- 35.Miles KA, Barber RW, Wraight EP, Cooper M, Appleton DS. Leg muscle scintigraphy with (99)Tc-MIBI in the assessment of peripheral vascular (arterial) disease. Nucl Med Commun. 1992;13:593–603. [PubMed] [Google Scholar]
- 36.Sayman HB, Urgancioglu I. Muscle perfusion with technetium-MIBI in lower extremity peripheral arterial diseases. J Nucl Med. 1991;32(9):1700–1703. [PubMed] [Google Scholar]
- 37.Soyer H, Uslu I. A patient with peripheral arterial stenosis diagnosed with lower extremity perfusion scintigraphy. Clin Nucl Med. 2007;32:458–459. doi: 10.1097/RLU.0b013e318059b54a. [DOI] [PubMed] [Google Scholar]
- 38.Miyamoto M, Yasutake M, Takano H, et al. Therapeutic angiogenesis by autologous bone marrow cell implantation for refractory chronic peripheral arterial disease using assessment of neovascularization by 99mTc-tetrofosmin (TF) perfusion scintigraphy. Cell Transpl. 2004;13(4):429–437. doi: 10.3727/000000004783983837. [DOI] [PubMed] [Google Scholar]
- 39.Takagi G, Miyamoto M, Tara S, et al. Controlled-release basic fibroblast growth factor for peripheral artery disease: comparison with autologous bone marrow-derived stem cell transfer. Tissue Eng Part A. 2011;17:2787–2794. doi: 10.1089/ten.tea.2010.0525. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt MA, Chakrabarti A, Shamim-Uzzaman Q, Kaciroti N, Koeppe RA, Rajagopalan S. Calf flow reserve with H(2)(15)O PET as a quantifiable index of lower extremity flow. J Nucl Med. 2003;44(6):915–919. [PubMed] [Google Scholar]
- 41.Burchert W, Schellong S, van den Hoff J, Meyer G-J, Alexander K, Hundeshagen H. Oxygen-15-water PET assessment of muscular blood flow in peripheral vascular disease. J Nucl Med. 1996;37:93–98. [PubMed] [Google Scholar]
- 42.Scremin OU, Figoni SF, Norman K, et al. Preamputation evaluation of lower-limb skeletal muscle perfusion with (15)O H2O positron emission tomography. Am J Phys Med Rehabil. 2010;89(6):473–486. doi: 10.1097/PHM.0b013e3181d89b08. [DOI] [PubMed] [Google Scholar]
- 43.Fischman AJ, Hsu H, Carter Ea, et al. Regional measurement of canine skeletal muscle blood flow by positron emission tomography with H2(15)O. J Appl Physiol. 2002;92(4):1709–1716. doi: 10.1152/japplphysiol.00445.2001. [DOI] [PubMed] [Google Scholar]
- 44.Peñuelas I, Aranguren XL, Abizanda G, et al. (13)N-ammonia PET as a measurement of hindlimb perfusion in a mouse model of peripheral artery occlusive disease. J Nucl Med. 2007;48(7):1216–1223. doi: 10.2967/jnumed.106.039180. [DOI] [PubMed] [Google Scholar]
- 45.Orbay H, Hong H, Koch JM, et al. Pravastatin stimulates angiogenesis in a murine hindlimb ischemia model: a positron emission tomography imaging study with (64)Cu-NOTA-TRC105. Am J Transl Res. 2013;6(1):54–63. [PMC free article] [PubMed] [Google Scholar]
- 46.Almutairi A, Rossin R, Shokeen M, et al. Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis. Proc Natl Acad Sci U S A. 2009;106(3):685–690. doi: 10.1073/pnas.0811757106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dobrucki LW, Dione DP, Kalinowski L, et al. Serial noninvasive targeted imaging of peripheral angiogenesis: validation and application of a semiautomated quantitative approach. J Nucl Med. 2009;50(8):1356–1363. doi: 10.2967/jnumed.108.060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu E, Wagner WR, Schellenberger U, et al. Targeted in vivo labeling of receptors for vascular endothelial growth factor: approach to identification of ischemic tissue. Circulation. 2003;108(1):97–103. doi: 10.1161/01.CIR.0000079100.38176.83. [DOI] [PubMed] [Google Scholar]
- 49.Willmann JK, Chen K, Wang H, et al. Monitoring of the biological response to murine hindlimb ischemia with 64Cu-labeled vascular endothelial growth factor-121 positron emission tomography. Circulation. 2008;117(7):915–922. doi: 10.1161/CIRCULATIONAHA.107.733220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeong JM, Hong MK, Chang YS, et al. Preparation of a promising anigogenesis PET imaging agent: (68)Ga-labeled c(RGDyK)-isothiocyanatobenzyl1-1,4,7-triazacyclononane-1,4,7-triacetic acid and feasibility studies in mice. J Nucl Med. 2008;49:830–836. doi: 10.2967/jnumed.107.047423. [DOI] [PubMed] [Google Scholar]
- 51.Lee K, Jung K, Song S, et al. Radiolabeled RGD uptake and alpha(v) integrin expression is enhanced in ischemic murine hindlimbs. J Nucl Med. 2005;46:472–478. [PubMed] [Google Scholar]
- 52.Hua J, Dobrucki LW, Sadeghi MM, et al. Noninvasive imaging of angiogenesis with a (99m)Tc-labeled peptide targeted at alpha(v)beta(3) integrin after murine hindlimb ischemia. Circulation. 2005;111(24):3255–3260. doi: 10.1161/CIRCULATIONAHA.104.485029. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Pressly ED, Abendschein DR, et al. Targeting angiogenesis using a C-type atrial natriuretic factor-conjugated nanoprobe and PET. J Nucl Med. 2011;52:1956–1963. doi: 10.2967/jnumed.111.089581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orbay H, Zhang Y, Hong H, et al. Positron emission tomography imaging of angiogenesis in a murine hindlimb ischemia model with (64)Cu-labeled TRC105. Mol Pharm. 2013;10:2749–2756. doi: 10.1021/mp400191w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rudd JHF, Myers KS, Bansilal S, et al. Atherosclerosis inflammation imaging with 18FFDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49(6):871–878. doi: 10.2967/jnumed.107.050294. [DOI] [PubMed] [Google Scholar]
- 56.Rudd JHF, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708–2711. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 57.Yun M, Yeh D, Araujo LI, Jang S, Newberg A, Alavi A. F-18 FDG uptake in the large arteries. A new observation. Clin Nucl Med. 2001;26(4):314–319. doi: 10.1097/00003072-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Yun M, Jang S, Cucchiara A, Newberg AB, Alavi A. (18)F FDG uptake in the large arteries: a correlation study with the atherogenic risk factors. Semin Nucl Med. 2002;32:70–76. doi: 10.1053/snuc.2002.29279. [DOI] [PubMed] [Google Scholar]
- 59.Lee SJ, On YK, Lee EJ, Choi JY, Kim BT, Lee KH. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med. 2008;49:1277–1282. doi: 10.2967/jnumed.108.052233. [DOI] [PubMed] [Google Scholar]
- 60.Ishii H, Nishio M, Takahashi H, et al. Comparison of atorvastatin 5 and 20 mg/d for reducting F-18 fluorodeoxyglucose uptake in atherosclerotic plaques on positron emission tomography/computed tomography: A randomized, investigator-blinded, open-label, 6-month study in Japanese adults schedule. Clin Ther. 2010;32:2337–2347. doi: 10.1016/j.clinthera.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Derlin T, Richter U, Bannas P, et al. Feasibility of (18)F-sodium fluoride PET/CT for imaging of atherosclerosis plaque. J Nucl Med. 2010;51:862–865. doi: 10.2967/jnumed.110.076471. [DOI] [PubMed] [Google Scholar]
- 62.Derlin T, Toth Z, Papp L, et al. Correlation of inflammation assessed by (18)F-FDG PET, active mineral deposition assessed by (18)F-fluoride PET, vascular calcification in atherosclerotic plaque: A dual-tracer PET/CT study. J Nucl Med. 2011;52:1020–1027. doi: 10.2967/jnumed.111.087452. [DOI] [PubMed] [Google Scholar]
- 63.Derlin T, Wisotzki C, Richter U, et al. In vivo imaging of mineral deposition in carotid plaque using 18F-sodium fluoride PET/CT: Correlation with atherogenic risk factors. J Nucl Med. 2011;52:362–368. doi: 10.2967/jnumed.110.081208. [DOI] [PubMed] [Google Scholar]
- 64.Janssen T, Bannas P, Herrmann J, et al. Association of linear (18)F-sodium fluoride accumulation in femoral arteries as a measure of diffuse calcification with cardiovascular risk factors: A PET/CT study. J Nucl Cardiol. 2013;20:569–577. doi: 10.1007/s12350-013-9680-8. [DOI] [PubMed] [Google Scholar]
- 65.Derlin T, Habermann CR, Lengyel Z, et al. Feasibility of (11)C-acetate PET/CT for imaging of fatty acid synthesis in the atherosclerotic vessel wall. J Nucl Med. 2011;52:1848–1854. doi: 10.2967/jnumed.111.095869. [DOI] [PubMed] [Google Scholar]
- 66.Van der Valk FM, Kroon J, Potters WV, et al. In vivo imaging of enhanced leukocyte accumulation in atherosclerotic lesions in humans. J Am Coll Cardiol. 2014;64(10):1019–1029. doi: 10.1016/j.jacc.2014.06.1171. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Abendschein D, Woodard GE, et al. Molecular imaging of atherosclerotic plaque with (64)Cu-labeled natriuretic peptide and PET. J Nucl Med. 2010;51:85–91. doi: 10.2967/jnumed.109.066977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saraste A, Laitinen I, Weidl E, et al. Diet intervention reduces uptake of avB3 integrin-targeted PET tracer (18)F-galacto-RGD in mouse atherosclerotic plaques. J Nucl Cardiol. 2012;19:775–784. doi: 10.1007/s12350-012-9554-5. [DOI] [PubMed] [Google Scholar]
- 69.Pande RL, Park M-A, Perlstein TS, et al. Impaired skeletal muscle glucose uptake by [18F]Fluorodeoxyglucose-positron emission tomography in patients with peripheral artery disease and intermitten claudication. Arter Thromb Vasc Biol. 2011;31:190–196. doi: 10.1161/ATVBAHA.110.217687. [DOI] [PubMed] [Google Scholar]
- 70.Berard X, Pinaquy J-B, Stecken L, et al. Use of 18F-fluorodeoxyglucose positron emission tomography-computed tomography and sonication for detection of infection after peripheral stent fracture. Circulation. 2014;129:2437–2439. doi: 10.1161/CIRCULATIONAHA.114.009317. [DOI] [PubMed] [Google Scholar]
- 71.Sinusas AJ. The potential for myocardial imaging with hypoxia markers. Semin Nucl Med. 1999;29(4):330–338. doi: 10.1016/s0001-2998(99)80020-8. [DOI] [PubMed] [Google Scholar]
- 72.Sosunov EA, Anyukhovsky EP, Sosunov AA, et al. pH (low) insertion peptide (pHLIP) targets ischemic myocardium. Proc Natl Acad Sci U S A. 2013;110(1):82–86. doi: 10.1073/pnas.1220038110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chu W, Chepetan A, Zhou D, et al. Development of a PET radiotracer for non-invasive imaging of the reactive oxygen species, superoxide, in vivo. Org Biomol Chem. 2014;12(25):4421–4431. doi: 10.1039/c3ob42379d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moazzami K, Moazzami B, Roohi A, Nedjat S, Dolmatova E. Local intramuscular transplantation of autologous mononuclear cells for critical lower limb ischaemia. Cochrane Database Syst Rev. 2014;12:CD008347. doi: 10.1002/14651858.CD008347.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nguyen PK, Riegler J, Wu JC. Stem cell imaging: From bench to bedside. Cell Stem Cell. 2014;14:431–444. doi: 10.1016/j.stem.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med. 2006;47:1295–1301. [PubMed] [Google Scholar]
- 77.Schachinger V, Aicher A, Dobert N, et al. Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation. 2008;118(14):1425–1432. doi: 10.1161/CIRCULATIONAHA.108.777102. [DOI] [PubMed] [Google Scholar]
- 78.Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111(17):2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 79.Vrtovec B, Poglajen G, Lezaic L, et al. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013;128(Suppl 1):S42–S49. doi: 10.1161/CIRCULATIONAHA.112.000230. [DOI] [PubMed] [Google Scholar]
- 80.Dohan O, De la Vieja A, Paroder V, et al. The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24(1):48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- 81.Templin C, Zweigerdt R, Schwanke K, et al. Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iod. Circulation. 2012;126(4):430–439. doi: 10.1161/CIRCULATIONAHA.111.087684. [DOI] [PubMed] [Google Scholar]
- 82.Earnshaw JJ, Hardy JG, Hopkinson BR, Makin GS. Non-invasive investigation of lower limb revascularisation using resting thallium peripheral perfusion imaging. Eur J Nucl Med. 1986;12(9):443–446. doi: 10.1007/BF00254748. [DOI] [PubMed] [Google Scholar]
- 83.Depairon M, Depresseux J-C, Petermans J, Zicot M. Assessment of Flow and Oxygen Delivery to the Lower Extremity in Arterial Insufficiency: A PET-Scan Study Comparison with Other Methods. Angiology. 1991;42(10):788–795. doi: 10.1177/000331979104201003. [DOI] [PubMed] [Google Scholar]
- 84.Depairon M, Zicot M. The Quantitation of Blood Flow/Metabolism Coupling at Rest and After Exercise in Peripheral Arterial Insufficiency, Using PET and 15-0 Labeled Tracers. Angiology. 1996;47(10):991–999. doi: 10.1177/000331979604701008. [DOI] [PubMed] [Google Scholar]
- 85.Mehra VC, Jackson E, Zhang XM, et al. Ceramide-activated phosphatase mediates fatty acid-induced endothelial VEGF resistance and impaired angiogenesis. Am J Pathol. 2014;184(5):1562–1576. doi: 10.1016/j.ajpath.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hedhli N, Dobrucki LW, Kalinowski A, et al. Endothelial-derived neuregulin is an important mediator of ischaemia-induced angiogenesis and arteriogenesis. Cardiovasc Res. 2012;93(3):516–524. doi: 10.1093/cvr/cvr352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beer AJ, Pelisek J, Heider P, et al. PET/CT imaging of integrin alpha(v)beta(3) expression in human carotid atherosclerosis. JACC Cardiovasc Imaging. 2014;7(2):178–187. doi: 10.1016/j.jcmg.2013.12.003. [DOI] [PubMed] [Google Scholar]