Abstract
While the ability to process fermented fruits and alcohols was once an adaptive trait that improved nutrition and quality of life, the availability and prevalence of high potency alcoholic drinks has contributed to alcohol abuse disorders in a vulnerable portion of the population. Although the neural reward systems take part in the initial response to alcohol, negative reinforcement and stress, which are normally adaptive responses, can intersect to promote continued alcohol use at all stages of the addiction cycle. Eventually a point is reached where these once adaptive responses become dysregulated resulting in uncontrolled intake that constitutes a clinically important condition termed alcohol use disorder (AUD). Current research is targeted at both the behavioral and molecular adaptations in AUDs in an effort to better develop novel approaches to intervention. In this review, historical context is provided demonstrating the societal burden of alcohol use and abuse disorders. The importance of gender in the mechanism of action of alcohol is discussed. Finally, the impact of alcohol on stress-related circuitry, uncovered by preclinical research, is outlined to provide insight into potential novel pharmacological approaches to the treatment of AUD.
Keywords: Alcohol use disorders, locus coeruleus, central nucleus of the amygdala, sex differences
Introduction: Alcohol in Antiquity
Alcohol consumption is deeply woven into the fabric of human history. While alcohol use in antiquity often conjures images of Dionysian debauchery on artefactual Greek hydriai, there is evidence to suggest that fermented fruits contributed to shaping human evolution (Carrigan et al., 2012). Paleogenetic evidence regarding the history of alcohol dehydrogenase (ADH) indicates that this enzyme, critical for the metabolism of alcohol, may have first developed in primates before the divergence of old and new world monkeys. The emergence of the ADH genes at this time point is hypothesized to have both dietary and behavioral consequences as fruits that had fallen from trees and initiated the process of fermentation would now be digestible rather than toxic, opening a previously inaccessible food niche (Brenner, 2013, Carrigan, Uryasev, 2012). Further, according to the Drunken Monkey Hypothesis of Dudley (2004), volatilized alcohols from fruit may have acted as olfactory signals for food localization in early primates. The primates would have associated the alcohol with nutritive reward (Dudley, 2004). Dudley proposed that these genetically rooted behaviors which were once advantageous may underlie adverse reward- associated behaviors in a modern context where there is open access to higher concentrations of alcohol (Dudley, 2004).
While animals from robins to elephants have been recorded as recognizing the mind altering effects of fermented fruits, the earliest evidence for fermented beverage production among modern humans comes from pre-historic China in approximately 7000 BC (McGovern et al., 2004), and can be found in Mesopotamia, Egypt, and Greece shortly after (McGovern, 2007). The use of fermented foods and beverages throughout history likely represents a convergence of critical qualities. First, with the advent of agricultural societies, the preservation of foods became increasingly important and the stability of fermented beverages became a desirable trait. Alcohol also became revered for its antiseptic properties and was often added to drinking water to decrease bacterial contamination (Dasgupta, 2011), arguably improving the quality of life. At a cultural level, ancient civilizations worshiped gods of wine, such as Osiris in Egypt and Dionysus in Greece, and alcohol has been associated with celebrations from antiquity into modern times.
A dichotomy exists, however, between simple alcohol consumption and intake to excess. In ancient Greece, alcohol consumption leading to a point of mental status alteration was denounced by the early physician Hippocrates, and in China an imperial edict from 1116 BC clarifies that moderation in alcohol consumption was not only important but prescribed from heaven (Dasgupta, 2011). Beyond the social implications, moderate alcohol consumption may also have a biological basis. If primates were frequently exposed to low levels of alcohol from fermenting fruit, Dudley theorized that evolution would ultimately favor adaptations that minimize detrimental effects while favoring the physiological benefits of consumption (Dudley, 2004). Indeed, it is known from human studies that those who consume moderate amounts of wine on a regular basis have reduced all-cause mortality compared to excessive drinkers or those who are abstinent (Abramson et al., 2001, Micallef et al., 2007). Modern times have, however, increased the prevalence and availability of high concentration alcohol, and evidence has been found for J-shaped consumption risk curves. For example, while one drink has been shown to promote antioxidant activity in plasma, three drinks increases pro-oxidant activity (Prickett et al., 2004). Thus, while alcohol consumption may have positive origins as a highly adaptive behavior, the cultural shifts in alcohol use that outpaced evolutionary changes may, in part, underlie contemporary patterns of alcohol usage and abuse.
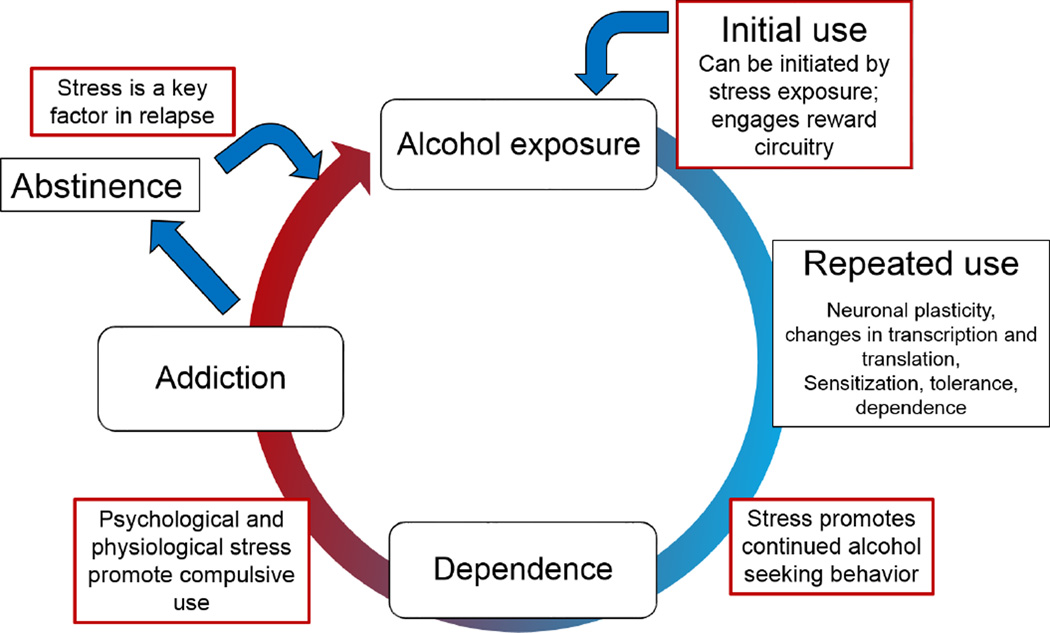
Addiction is often thought of as a cycle (Figure 1). Acute use of a drug may activate the brain reward systems (as can the context within which a drug is used), encouraging continued use. Various adaptations gradually transition the continued use of a substance into abuse and addiction, where the motivational state switches from reward to the circumvention of withdrawal (Koob and Le Moal, 2005). The physiological state of withdrawal represents the point at which dependence occurs. Much of the work on substance use and alcoholism has focused on the start and end points of this cycle – acute use and withdrawal– as they represent sudden perturbations to an organism, and critical states of the physiological adaptation to exposure. However, emerging evidence reveals that the gradual changes in neural plasticity associated with repeated use are more subtle and gaps exist in our knowledge regarding the temporal sequence of addiction as well as the adaptations occurring following chronic exposure (Cui et al., 2013).
Figure 1.
Stress is known to impact every aspect of the addiction cycle. Stress and reward intersect to promote initial use of a substance, and stress promotes continued usage often leading to compulsive alcohol seeking behaviors. Physiological adaptations following alcohol exposure lead to dependence and discontinuing usage precipitates withdrawal. Withdrawal acts as a stressor and negative reinforcer, perpetuating the addiction cycle. Patients and treatment providers both point to stress as a source of relapse and a barrier to abstinence, as stressors can promote resumption of addictive behaviors many years after remission.
It is well-established that stress impacts every aspect of the addiction cycle (Figure 1) (Alcoholism, 2012). Stressful situations often promote drug use as well as encourage continued usage. Withdrawal itself is a stressor, and patients and treatment providers both point to stress as a source of relapse and a barrier to abstinence (Keyes et al., 2011). This review will focus on the interplay between alcohol and stress circuitry with new insights into the mechanism by which alcohol alters stress response systems. Further, with emerging evidence regarding a gender bias in the negative sequelae following chronic exposure to abused substances, data will be reviewed highlighting potential therapeutic approaches for targeting AUDs across the sexes.
Prevalence, Current Definitions and Gender Differences
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) defines alcohol abuse as “continued drinking despite adverse effects on: health; family, work, or personal relationship; interpersonal problems; or alcohol-related legal problems”(Alcoholism, 2005). The most detrimental extreme of the alcohol use spectrum, alcohol dependence or alcoholism, advances this definition to include physical withdrawal symptoms in the absence of exposure, or “the need to drink substantially large amounts despite continued alcohol-related problems with cognitive, behavioral, and physiologic symptoms”(Alcoholism, 2005). Highlighting the evolving nature of our understanding of alcoholism as a disease, the most recent Diagnostic and Statistical Manual of Mental Disorders (DSM-5), updated by the American Psychiatric Association in May of 2013, combines alcohol abuse and alcohol dependence into one disorder: alcohol use disorder (AUD). There are 11 criteria for diagnosis, and individuals are classified as having mild, moderate, or severe AUD based on the number of criteria met (Alcoholism, 2013).
While the effects of alcohol use are often considered at an individual level, alcohol is a societal issue. Data from the Lancet and World Health Organization estimate that 3.8% of all deaths are attributable to alcohol, in addition to 4.6% of the global disease burden (Rehm et al., 2009). Results from the 2012 U.S. Department of Health and Human Services Administration’s National Survey on Drug Use and Health indicate that 52.1% of Americans 12 years old or older are self-reported current alcohol drinkers, with the highest rates of heavy alcohol use being reported by 21–25 year olds (Administration, 2013). A gender disparity exists among drinkers, with 56.5% of all men and 47.9% of all women self-reporting current alcohol use (at least one drink in the past month). Further, unlike some other drugs of abuse, alcohol use was seen to increase with education level – almost doubling between those without a high school education (36.6%) and college graduates (68.6%) - and to be increased among adults with full-time employment compared to the unemployed (64.8% versus 54.9%) (Administration, 2013).
There is an increasing body of evidence to support a greater impact of alcohol exposure on the female brain as compared to males (Hommer, 2004), a potential vulnerability that may be related to the observation that many psychiatric diseases exhibit a gender disparity with regards to prevalence and/or severity. For example, affective disorders and many anxiety disorders are nearly twice as prevalent in females compared to males (Fukushiro et al., 2012, Kessler et al., 1994). The NIAAA states that 5.3 million women in the United States use alcohol in a way that “threatens their health, safety and general well-being,” and that in addition, the problems that plague women drinkers are equal to, or greater than, those that affect men (Alcoholism, 2008). Compared to men, women experience a higher incidence of liver disease and cardiomyopathy (Fernandez-Sola et al., 1997, Thurman, 2000), enhanced motor and cognitive impairment following exposure to alcohol (Ceylan-Isik et al., 2010, Nixon et al., 2002), and have a 50 to 100 percent higher death rate due to accidents, suicides, and health problems (Alcoholism, 2008).
The effect of long-term ethanol use on changes in neuronal structures has also been detailed across genders. Results of investigations focusing on overall brain shrinkage, a common marker of brain damage, are conflicting (Alcoholism, 2003), with one group publishing evidence for enhanced total brain shrinkage in alcoholic women (Hommer, 2003), while other groups indicate more regionalized diversity (Pfefferbaum et al., 2001). Ultimately these differences may be attributable to methodological approaches, with the studies employing differing measurement techniques (Hommer, 2004). When examining regional morphological differences, multiple studies have indicated gender differences (Pfefferbaum, Rosenbloom, 2001), with recent work noting enhanced susceptibility to corpus callosum damage in men and an enhanced susceptibility to damage in the frontal and temporal lobes of women (Ruiz et al., 2013). Differences were noted in the neuronal response to abstinence as well, with women regaining white matter volume more quickly than men during the first year, and men regaining white matter volume more quickly than women after one year (Ruiz, Oscar-Berman, 2013).
Preclinical evidence for sex differences in the context of ethanol exposure is abundant, and has been seen in a variety of systems. Recent mouse model work examined the effect of chronic ethanol exposure on the immune system with a particular focus on the brain. Greater increases in inflammatory mediators and cytokines were seen in female compared to male mice, suggesting an enhanced susceptibility to the neurotoxic effects of ethanol (Alfonso-Loeches et al., 2013). Another study examined the interplay between ethanol and another common drug, caffeine. Non-toxic doses of caffeine given in conjunction with alcohol withdrawal were shown to cause cytotoxicity in the hippocampus in a sex dependent manner, with females showing enhanced damage in the CA1 region of the hippocampus and dentate gyrus (Butler et al., 2009). Thus, in addition to the damaging effects of ethanol itself, it may potentiate the impact of other common substances in a sexually dichotomous manner.
Alcohol Actions on Stress-integrative Neuronal Circuitry
Previous theories regarding the mechanism of action of alcohol in the brain were centered on lipid theory, which posited that alcohol acted via perturbations of membrane lipids (Spanagel, 2009). More recent studies have since revealed that the primary sites of action for alcohol are membrane receptors and ion channels (Lovinger et al., 1989). As with other addictive substances, ethanol initially activates the classical dopaminergic reward pathways (Fortuna et al., 1991, Spanagel, 2009). With continued use and physiological dependence, ethanol use switches from positive reinforcement to negative reinforcement, wherein continued use becomes a mechanism to avoid the negative state of withdrawal (Koob and Le Moal, 2005). Like benzodiazepine anxiolytics, ethanol is associated with the modulation of gamma-aminobutyric acid (GABA) inhibitory neurotransmission. Often GABA is co-localized with a variety of neuropeptides, as can be seen in the central nucleus of the amygdala (CeA) where GABA-ergic neurons contain corticotropin releasing factor (CRF), a key peptide in the stress response. As knowledge regarding ethanol’s actions continues to evolve, studies indicate that it affects, at some level, the majority of neurotransmitter and ion channel systems (Sharrett-Field et al., 2013).
Due to its effects on the stress axis and the evolving understanding of how stress impacts each phase of ethanol use, combined with the increased findings of sex differences in the stress systems, the effects of chronic ethanol use on the neural stress systems are an important area of investigation. Early attempts at defining stress were largely based around the concept of homeostasis, which posited that the response to stress was a reaction intended to return the organism to a pre-defined set point (McEwen and Stellar, 1993). This definition has evolved over the last 25 years, and the understanding of stress and the stress response has been shaped by the concepts of allostasis and allostatic load. Proposed by Sterling and Eyre in 1998 (P. Sterling, 1998), allostasis describes the physiological changes that take place and the range in which an organism is able to respond to a stress challenge. This concept was further advanced by McEwen and Stellar who proposed the concept of allostatic load (McEwen and Stellar, 1993), which encompasses the temporal aspects of allostasis, and accounts for the creeping increase in the toll of chronic stressors over time.
In humans, the experience of stress follows exposure to environmental situations in which adaptive responses are perceived to be inadequate (Cohen et al., 2007). Stressors can be varied and nuanced; physical as well experiential. The experience of stress has been shown to produce molecular as well as cellular changes, which can have pervasive clinical sequelae, such as sleep disturbance, cardiovascular disease, and psychiatric conditions including abuse of substances. McEwen and Stellar (1993) state that alcohol is specifically noted for its dual actions on the stress systems. On one hand, its use as a mechanism of coping with stress is well established, particularly as a method of self-medicating in conditions such as post-traumatic stress disorder (PTSD) (Simpson et al., 2014); while on the other, continued usage is known to cause increased stress-related symptoms (Sinha, 2008).
The primary neural pathway in the stress response is the hypothalamic-pituitary-adrenal (HPA) axis. Canonically, the paraventricular nucleus (PVN) of the hypothalamus releases CRF into the hypophysial portal vessels, which then signals the anterior pituitary to release adrenocorticotropic hormone (ACTH) systemically. ACTH targets the adrenal cortex where it stimulates the synthesis and secretion of glucocorticoids (cortisol or corticosterone), which are also released systemically, and the glucocorticoids modulate physiological changes thorough the body and brain (Smith and Vale, 2006). The circulating glucocorticoids also provide a negative feedback onto the axis, predominantly in the PVN and hippocampus (Jacobson and Sapolsky, 1991, Sawchenko, 1987).
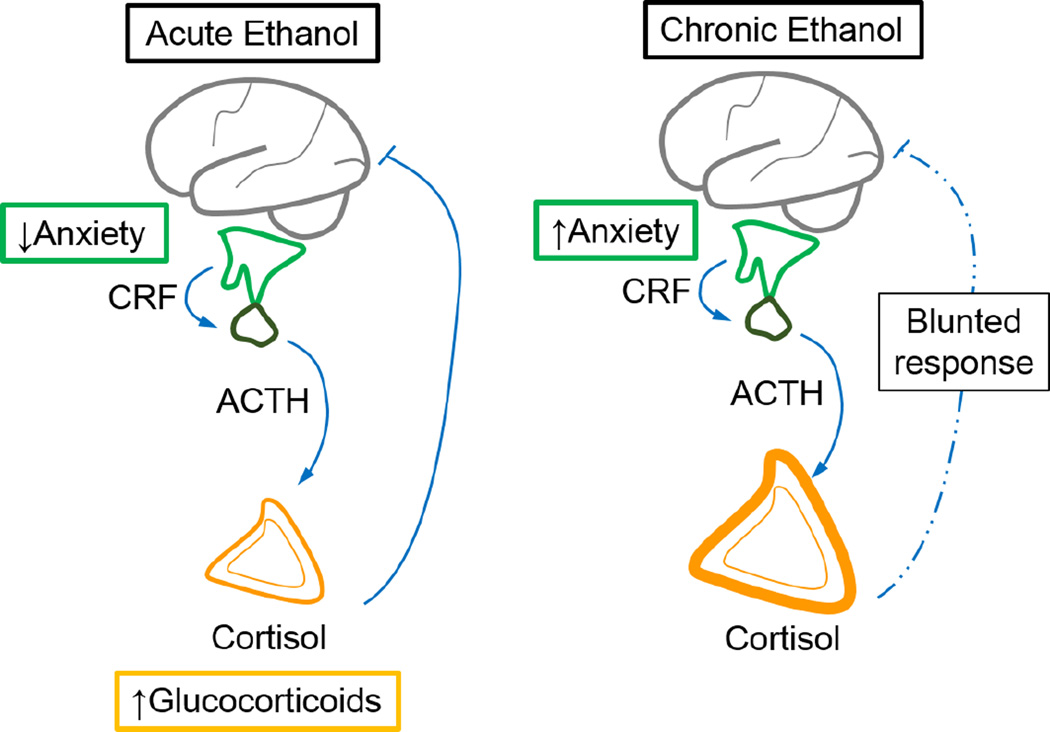
Alcohol use on both a short and long time scale has been shown to disrupt the HPA axis (Figure 2). Acute alcohol activates the HPA axis which results in elevated glucocorticoid levels (Richardson et al., 2008), and at the same time decreases anxiety (File and Seth, 2003, Varlinskaya and Spear, 2006). In contrast, prolonged ethanol exposure is seen as anxiogenic, and is known to cause HPA axis dysregulation associated with adrenal hypertrophy, and blunted corticosterone responses (King et al., 2006). Dysregulation of the HPA axis has been shown on a long-term scale after the cessation of chronic ethanol use, with drastic impairment giving way to subtle alterations. A study by Adinoff et al. (1990) examining the ACTH and cortisol responses to CRF challenge noted an attenuated ACTH response to CRF administration at one and three weeks after cessation of drinking in human AUD subjects. At 6 months after cessation of ethanol the ACTH and cortisol levels became normalized, but in AUD individuals the normal negative feedback response of the pituitary to glucocorticoids was blunted (Adinoff et al., 1990).
Figure 2.
Action of alcohol exposure on the hypothalamic-pituitary-adrenal (HPA) axis. (Left) Acutely, alcohol decreases anxiety and promotes systemic release of glucocorticoids. (Right) Chronic alcohol exposure elicits increased anxiety, causes hypertrophy of the adrenal glands and blunting of the corticosterone feedback response.
Evidence has been presented for HPA axis dysregulation as a cause, effect, or both, of alcohol use. Studies in youth suggest that HPA axis dysfunction at baseline may increase the risk for future AUDs, particularly when combined with stressors (Schepis et al., 2011, Sher, 2007). Alcohol use is posited as a mechanism of coping with HPA axis dysregulation, which may be a result of stress, traumatic events, or affective disorders (Schepis, Rao, 2011). Studies of AUD patients found that those with attenuated cortisol responses were more likely to relapse, although this attenuation was not seen universally (Clapp et al., 2008, Obara et al., 2009). Other studies have shown that patients with AUDs who also had attenuated stress responses were more likely to relapse (Clapp, Bhave, 2008, Obara, Bell, 2009), and a blunting of the stress response was observed during abstinence (Errico et al., 2002, Junghanns et al., 2003, Junghanns et al., 2005). Consistent with these observations, preclinical studies have shown that stress has been implicated in the neuronal dysregulation associated with alcohol withdrawal, and promoting sustained alcohol intake, and reinstatement of alcohol seeking behavior (Breese et al., 2005, Le et al., 2000, Martin-Fardon et al., 2010).
Alterations of baseline HPA axis function have also been observed in patients with a family history of AUD, leading to theories about the genetic components of this disease. When examining circadian ACTH and cortisol cycles, individuals with a family history of AUDs had lower baseline levels of ACTH whereas cortisol levels were normal, suggesting dysfunction (Gianoulakis et al., 2005). There is also evidence for an alteration in perception of stress among patients with a family history of AUD. These patients had lower self-reported anxiety measures when exposed to alcohol and a stressor, compared to controls who had lower self-reported anxiety when exposed to alcohol alone (Dai et al., 2007). Thus, it can be hypothesized that alcohol use in these patients has a potentiated anxiolytic effect initially, a trait correlated to increased vulnerability to addiction in humans (McClung et al., 2004).
While several factors are involved in the HPA axis modulation after ethanol, CRF dysregulation appears to play a pivotal role (Rivier and Lee, 1996). The CRF system is becoming increasingly linked to the dysregulation associated with alcohol use, and interventions targeting the CRF system are particularly effective in modulating HPA axis alterations. For example, ethanol-induced elevations in ACTH can be suppressed by blocking CRF (Rivier et al., 1984). Stressors have been shown to increase voluntary ethanol consumption in rodent models. When examining genetically modified animal models lacking the CRF receptor (CRFr), or after the administration of CRFr antagonists, this increased consumption behavior is delayed (Lowery et al., 2008). In a similar study where the effect of repeated stressors was examined on ethanol consumption, repeated stressors had less of an effect on consumption in the knockout mice compared to wild types, further supporting a role for CRFrs in stress-induced consumption of ethanol (Pastor et al., 2011).
Dysregulation of Stress Responses by Alcohol
There is growing preclinical evidence supporting differences in behavioral responses to chronic and acute ethanol exposure. While acute ethanol exposure is anxiolytic (Figure 2), continued exposure switches this effect from an anxiolytic to anxiogenic phenotype (File and Seth, 2003, Richardson, Lee, 2008, Varlinskaya and Spear, 2006). A large body of evidence exists to support this physiological stressor hypothesis, as chronic use and relapse vulnerability has been shown to involve dysregulation of the HPA axis (Errico, King, 2002, Junghanns, Backhaus, 2003, Junghanns, Tietz, 2005, Sher, 2007, Stephens and Wand, 2012). Evidence also exists for differential ethanol effects on the female and male stress systems. Studies in rats show that there is an increased stimulation of the HPA axis in females compared to males (Rivier, 1996), and in humans alcoholic women exhibited more signs of depression than alcoholic men (Skaff et al., 1999), perhaps owing to an increase in baseline neuronal stress circuitry activity.
When examining behavior, Kulkarni et al. tested the responses of mice in an elevated zero maze (EZM) after ethanol exposure. In this paradigm, exploratory behaviors are well correlated with anxiolytic administration, whereas anxiogenic drugs decrease these behaviors (Shepherd et al., 1994). Kulkarni et al observed that after an acute administration of ethanol, there was an increase in exploratory behaviors compared to controls, indicating anxiolysis (Kulkarni et al., 2007). Acevedo et al. (2014) also noted increased anxiolytic effects of acute ethanol in rats in the EZM at multiple ethanol doses (Acevedo et al., 2014). In contrast, Fukushiro et al. described an increase in locomotion following administration of acute ethanol compared with a decrease in exploratory behaviors after chronic administration, indicating a switch to anxiogenesis (Fukushiro, Josino, 2012). In agreement with the work by Fukushiro et al. and expanding upon it, a recent study by our lab examined behaviors in the EZM in both sexes after chronic ethanol exposure. Here we showed a similar behavioral phenotype across genders, with ethanol treated subjects of both sexes displaying increased anxiety-like behaviors. The treated subjects spent more time in the closed sections of the maze, and in addition, engaged in fewer exploratory behaviors such as standing and head dips compared to their control counterparts. These findings provide further evidence for an anxiogenic switch after chronic ethanol exposure (Retson et al., 2014b). While gaps exist in the understanding of the nature of these adaptations, changes can be seen at molecular and epigenetic levels (Koob and Le Moal, 2001, Leshner and Koob, 1999, Ponomarev, 2013), as well as in neural activity patterns between acute and chronic use (Vilpoux et al., 2009).
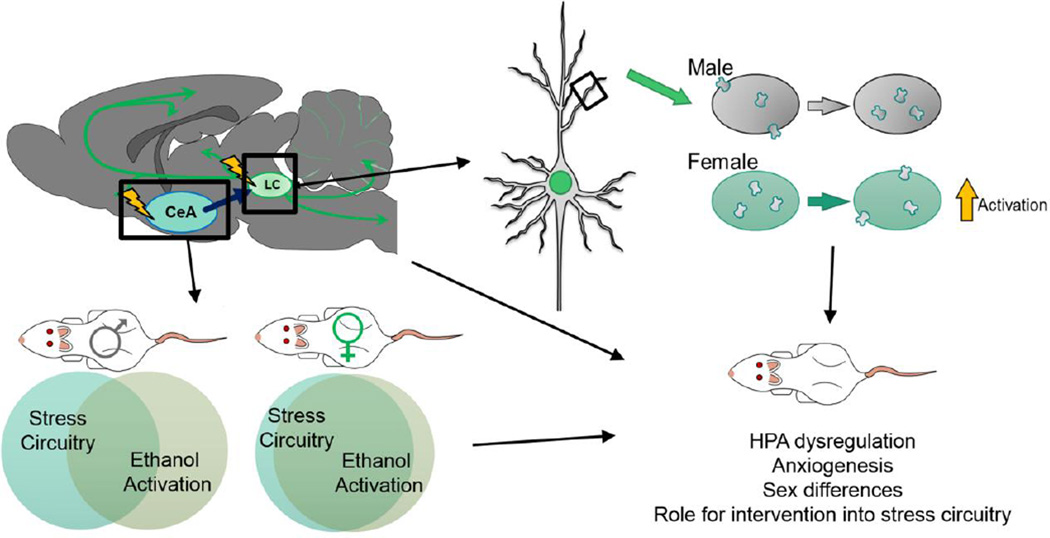
Alterations in neuronal activity in key stress-sensitive areas such as the locus coeruleus (LC) and central nucleus of the amygdala (CeA) can also be seen after acute and chronic ethanol administration (Thiele et al., 1997, Vilpoux, Warnault, 2009). For example, in males, the CeA shows increased neuronal markers of activity after acute ethanol administration which were potentiated after chronic ethanol administration (Vilpoux, Warnault, 2009). The LC also showed increased activity after acute ethanol, which was not seen after chronic treatment. These changes are thought to be the result of neuronal adaptations to chronic ethanol in male animals (Vilpoux, Warnault, 2009). Several recent studies from our group examined the neuronal activity in these regions and observed changes in activity patterns following repeated ethanol use that varied in a sex dependent manner (Figure 3) (Retson et al., 2014a, Retson, Reyes, 2014b). In the CeA, we examined a marker of recent neuronal activity, c-Fos, and a marker of long-term neuronal activity, ΔFosB, in order to identify differences in neuronal activation patterns across the sexes (Retson, Hoek, 2014a). Following exposure to chronic ethanol, there was an increased amount of ΔFosB immunoreactivity (IR) in male versus female subjects. When compared with c-Fos (marker of recent neuronal activation), male ethanol-treated subjects had similar c-Fos IR levels compared to controls, whereas female ethanol-treated subjects exhibited robust activation when compared to both control and male counterparts. In males, a significant increase in ΔFosB (marker of long-term activity) contrasted with c-Fos levels that were similar between ethanol and control groups, suggesting that males habituate to the neuronal activation induced by ethanol exposure. The pattern seen in females, with ethanol-treated subjects showing an increase in both c-Fos and ΔFosB seems to indicate that the female subjects do not habituate to chronic ethanol exposure in the same way, and this may be a mechanism underlying sex differences emerging in the alcohol addiction field (Retson, Hoek, 2014a). Similar findings were observed in the LC, where significant increases in c-Fos were observed in the female ethanol-treated subjects compared to males, again illustrating a lack of habituation to chronic ethanol exposure in females. This finding is consistent with the sexually dichotomous nature of the LC, and may indicate a dysregulation of the LC-NE system in females exposed to chronic ethanol that may pre-dispose them to increased vulnerability to continued stressors.
Figure 3.
(Top Left) The activity patterns of the CeA and LC are dysregulated after chronic ethanol exposure, with males showing a neuronal habituation to chronic use, and females showing a higher rate of activation at baseline. (Bottom Left) The stress activation response of the CeA is potentiated after chronic ethanol exposure in both sexes. In females, neuronal activity levels after a stressor are similar to those after chronic ethanol exposure, while males show differential activation patterns in ethanol-treated compared to control. (Top Right) The LC shows a sexually dimporphic response to chronic ethanol exposure with CRFr distribution patterns mimicking a stress response. Habituation is observed in males, while females do not show such neuronal adaptations.
To assess the impact of chronic ethanol exposure on stress circuitry, a recent study from our group examined neuronal activity after a robust stressor (a forced swim test). Here, similar distributions of neurons were activated in the CeA after stress and chronic ethanol treatment in female animals, which indicates that at baseline, chronic ethanol is activating the same neuronal populations as stressors. However, in males, distributions of activated neurons differed between stress and chronic ethanol exposure, which may point to ethanol engaging both the stress systems and other, yet undefined CeA circuitry concurrently. When considering the LC-NE system, previous work from our group exposed male and female animals to a stressor and measured the change in CRFr localization on a sub-cellular level (Bangasser et al., 2010). The alteration in CRFr sub-cellular localization in female animals exposed to a stressor is similar to the localization pattern recently revealed in ethanol treated females. In contrast, the difference in localization reported in males exposed to a stressor is almost twice that seen in males exposed to ethanol (Bangasser, Curtis, 2010, Retson, Reyes, 2014b). This would indicate that while ethanol is altering the CRFr system in both sexes, and altering it in the direction that would be expected by a stressor, chronic ethanol exposure is not as robust a stressor as swim stress in males. In females however, it is altering the CRFr system in a manner similar to that seen after a robust stressor. This may explain why the use of CRFr and stress related pharmacologics is particularly targeted at women (Clinicaltrials.gov, 2013, 2014). While these interventions would be expected to be effective in both genders, they might be particularly effective in females if this system is dysregulated even before discontinuation of ethanol use. Taken together, convergent lines of evidence suggest a lack of neuronal adaptation in females that renders this group more vulnerable to future stressors (Retson, Reyes, 2014b).
In human studies, stress alters the neuronal response to stimuli in a nuanced manner, and this response can vary between genders. For example, when exposed to a stressor and asked to consider the emotional connotation of faces there is a sexually dimorphic response of the fusiform face area, where the response of men is diminished and other emotional interpretation areas of the brain become less coordinated. In women, however, the connections between emotional regions are increased, illustrating that stress has a direct impact on emotional perception and that this response differs between genders (Mather, Lighthall et al. 2010).
Current treatments of AUD, and practical applications of preclinical work
While large strides have been made in the understanding of the biological basis for AUD, treatment options remain a continued focus of investigation. The goal for any treatment paradigm is to assist a patient in achieving stable abstinence, free from relapse after discontinuation of alcohol use. Often, pharmacological therapies are offered concurrently with cognitive behavioral interventions (CBI). CBIs can take a variety of forms, with the majority centered on the facilitation of skills necessary to cope with high risk life situations, managing negative mood and irrational thoughts, and preventing relapse (2011). Numerous studies have found that CBI is an effective intervention (Longabaugh R, 1999). Patients receiving concomitant CBI having an increased quality of life, decreases in depression (Laaksonen et al., 2013), and a higher index of percentage of days abstinent (Anton et al., 2006).
One of the earliest pharmacologic interventions was disulfram (Antabuse®). Disulfram’s mechanism of action is to inhibit acetaldehyde dehydrogenase, an enzyme critical in the breakdown of alcohol. This results in a severe and unpleasant reaction to alcohol use including flushing, nausea, and anxiety (Koppaka et al., 2012). The unpleasantness of this reaction was meant to discourage continued drinking, and while effective at preventing relapse if used correctly, rates of non-compliance are notoriously high (Fuller et al., 1986). As alcohol works at least in part through the classical reward pathways, naltrexone, an opioid antagonist, is also used in various treatment paradigms to prevent alcohol interaction with the endogenous opioid receptors (Franck and Jayaram-Lindstrom, 2013, O'Brien et al., 1996). Approved by the FDA for this use in 1994, studies indicate that its use decreases the rewarding effects of alcohol, and it has been shown to be as effective in increasing abstinence rates. Two of the more recent additions to the pharmacological treatment options include drugs that work on the GABA receptor system. Baclofen and topiramate suppress the cortico-mesolimbic dopamine system, and have shown efficacy at promoting abstinence and decreasing cravings in the case of baclofen (Muzyk et al., 2012), and reducing the number of heavy drinking days in the case of topiramate (Franck and Jayaram-Lindstrom, 2013).
A proposed pharmacologic target aimed at decreasing the burden of stress on abstinence is antagonism of CRFrs. CRFrs have been known from pre-clinical work to play a role in the reinstatement of alcohol seeking behaviors after a stressor (Le, Harding, 2000), and studies have shown dysregulation of the CRFr system after both stress and chronic ethanol use in rats (Bangasser, Curtis, 2010, Retson, Reyes, 2014b, Reyes et al., 2008, Waselus et al., 2009). Sexually dichotomous findings of alterations in the CRFr system after chronic ethanol exposure in an animal model may indicate that CRFr antagonists will be more effective in women where there is a stronger dysregulation of the CRFr system. Indeed, several CRFr antagonists are currently being examined as a clinical intervention for the dysphoria involved in ethanol abstinence (Clinicaltrials.gov, 2013, 2014). Of particular interest are the ongoing trials for the CRFr1 antagonists verucerfont and pexacerfont (Franck and Jayaram-Lindstrom, 2013). Pexacerfont is being tested by the National Institutes of Health Clinical Center on anxious alcoholics of both genders to decrease anxiety-related alcohol craving (Clinicaltrials.gov, 2014). Verucerfont is a similar compound developed by GlaxoSmithKline that is being tested in anxious, recently abstinent, alcoholic women. Initial testing indicated efficacy at reducing cravings in this population after stressful stimuli (Clinicaltrials.gov, 2013), and the composition of the study population highlights the increasing considerations being made in addiction research for gender differences.
Previous work showing neuronal activity adaptations in male but not female animals after chronic ethanol exposure suggest that it may be possible to identify gender-specific therapeutic targets for AUDs. Chronic activation of the neuronal stress systems, a proposed sequelae of chronic exposure to alcohol, is associated with downstream consequences of generalized autonomic dysregulation and affective disorders (Van Bockstaele et al., 1996). Studies in rodent models of ethanol exposure show an increased stimulation of the HPA axis in females compared to males (Rivier, 1996), and in human studies of AUD patients women exhibited more signs of depression than men (Skaff, Finney, 1999). If AUDs are associated with an increase in neuronal activation in stress responsive brain regions in women it may shed light on one mechanism by which AUDs and affective disorders show high rates of comorbidity.
A system that may represent a translatable target is the LC-norepinephrine (LC-NE) system. Increased LC activity is seen in females in a preclinical animal model of chronic alcohol use, which may indicate hyperactivity of the NE system and may predict an enhanced efficacy of serotonin norepinephrine reuptake inhibitors (SNRIs) (Retson, Reyes, 2014b). Stress and depression act on similar circuitry (Itoi and Sugimoto, 2010, McEwen et al., 2012), and the SNRIs are already well studied with findings showing that women diagnosed with depression are more responsive to SNRI therapy (Khan et al., 2005). Experiments aimed at testing the efficacy of SNRIs in AUD therapy may be of interest, as this drug class would specifically address the NE stress system that is shown to be dysregulated in preclinical models.
Conclusions
Current research at numerous sites is aimed at identifying molecular and circuitry targets for AUD treatments. Alcohol addiction in humans is a multi-faceted process that evolves from acute use to physiological dependence over time. Preclinical and clinical work highlight the importance of individualized treatment strategies for maximal patient benefit, with particular considerations for gender differences. The findings here from preclinical studies using rodent ethanol addiction models highlights dysregulation of neuronal activity patterns in key stress integrative loci, alterations at a molecular level in stress-responsive receptor systems, a complex phenotype of increased anxiety-like coping behaviors, and differences between males and females in each of these factors.
A long-term prospective study by Moos and Moos confirmed that among individuals seeking abstinence after AUDs, those who sought treatment were more successful than those who did not (Moos and Moos, 2006). Meta-analysis of AUD treatment outcomes indicated that among patients who did not receive treatment, abstinence rates were around 21% (Moos and Moos, 2006, Moyer and Finney, 2002), which is approximately half the rate of those who received treatment (where abstinence rates were closer to 40%) (Moos and Moos, 2006, Weisner et al., 2003). The impact of stress system dysregulation on the inability to maintain abstinence is a well-established observation. In an article for the National Institute on Drug Abuse, NIDA director Dr. G.R. Hanson pointed out that “patients and treatment providers alike point to stress as the most common cause of relapse” (Hanson, 2002). Thus, interventions that target the stress-response system with individualized therapies are poised to have a positive impact on treatment outcomes.
Supplementary Material
Acknowledgements
This project was supported by National Institute on Alcohol Abuse and Alcoholism grant AA021637 awarded to T. Retson, and National Institute on Drug Abuse grant DA009082 awarded to E. Van Bockstaele. The authors would also like to thank Diane Stringer, N.R., Leela Doge, M.A. Rubel, M. Van Tassel, J.A. Yost, Matthew Joseph and Andrew Lybek-Martori for their ongoing discourse and support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramson JL, Williams SA, Krumholz HM, Vaccarino V. Moderate alcohol consumption and risk of heart failure among older persons. Jama. 2001;285:1971–1977. doi: 10.1001/jama.285.15.1971. [DOI] [PubMed] [Google Scholar]
- Acevedo MB, Nizhnikov ME, Molina JC, Pautassi RM. Relationship between ethanol-induced activity and anxiolysis in the open field, elevated plus maze, light-dark box, and ethanol intake in adolescent rats. Behav Brain Res. 2014;265:203–215. doi: 10.1016/j.bbr.2014.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Martin PR, Bone GH, Eckardt MJ, Roehrich L, George DT, et al. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Archives of general psychiatry. 1990;47:325–330. doi: 10.1001/archpsyc.1990.01810160025004. [DOI] [PubMed] [Google Scholar]
- Administration SAaMHS. Admnistration SAaMHS. Rockville, MD: 2013. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- Alcoholism NIoAAa. Alcohol Research & Health. National Institutes of Health, Department of Health and Human Services; 2003; 2004. Oct, Alcoholic Brain Damage. [Google Scholar]
- Alcoholism NIoAAa. Epidemiology of Alcohol Problems in the United States. In: Disorders NSWEftPaToAU, editor. NIAAA publications. Module 1 ed. NIAAA; 2005. [Google Scholar]
- Alcoholism NIoAAa. Alcohol a Women's Health Issue. NIAAANIHGOV; 2008. [Google Scholar]
- Alcoholism NIoAAa. Alcohol Alert: The link between stress and alcohol. Alcohol Research: Current Reviews. 2012;34 [Google Scholar]
- Alcoholism NIoAAa. NIAAANIHGOV; 2013. Alcohol Use Disorder: A Comparison Between DSM–IV and DSM–5. [Google Scholar]
- Alfonso-Loeches S, Pascual M, Guerri C. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology. 2013;311:27–34. doi: 10.1016/j.tox.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Jama. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:877, 96–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a "kindling"/stress hypothesis. Psychopharmacology (Berl) 2005;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. AAAS - Paleogenetics and the History of Alcohol in Primates AAAS. 2013 [Google Scholar]
- Butler TR, Smith KJ, Berry JN, Sharrett-Field LJ, Prendergast MA. Sex differences in caffeine neurotoxicity following chronic ethanol exposure and withdrawal. Alcohol Alcohol. 2009;44:567–574. doi: 10.1093/alcalc/agp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan MA, Uryasev O, Davis RP, Zhai L, Hurley TD, Benner SA. The natural history of class I primate alcohol dehydrogenases includes gene duplication, gene loss, and gene conversion. PLoS One. 2012;7:e41175. doi: 10.1371/journal.pone.0041175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceylan-Isik AF, McBride SM, Ren J. Sex difference in alcoholism: Who is at a greater risk for development of alcoholic complication? Life Sciences. 2010;87:133–138. doi: 10.1016/j.lfs.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp P, Bhave SV, Hoffman PL. How Adaptation of the Brain to Alcohol Leads to Dependence: A Pharmacological Perspective. Alcohol Res Health. 2008;31:310–339. [PMC free article] [PubMed] [Google Scholar]
- Clinicaltrials.gov. Alcoholism NIoAAa, editor. Effects of Corticotropin-Releasing Hormone Receptor 1 (CRH1) Antagonism on Stress-Induced Craving in Alcoholic Women With High Anxiety. 2013
- Clinicaltrials.gov. Corticotropin-Releasing Hormone Receptor 1 (CRH1) Antagonism in Anxious Alcoholics. In: Heilig MA, editor. NCT01227980. Bethesda: National Institute on Alcohol Abuse and Alcoholism (NIAAA); 2014. [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. Jama. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Cui C, Noronha A, Morikawa H, Alvarez VA, Stuber GD, Szumlinski KK, et al. New insights on neurobiological mechanisms underlying alcohol addiction. Neuropharmacology. 2013;67:223–232. doi: 10.1016/j.neuropharm.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Santella S, Gianoulakis C. Response of the HPA-axis to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Psychoneuroendocrinology. 2007;32:293–305. doi: 10.1016/j.psyneuen.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Dasgupta A. The Science of Drinking: How Alcohol Affects Your Body and Mind. Rowman & Littlefield Publishers; 2011. [Google Scholar]
- Dudley R. Ethanol, fruit ripening, and the historical origins of human alcoholism in primate frugivory. Integr Comp Biol. 2004;44:315–323. doi: 10.1093/icb/44.4.315. [DOI] [PubMed] [Google Scholar]
- Errico AL, King AC, Lovallo WR, Parsons OA. Cortisol dysregulation and cognitive impairment in abstinent male alcoholics. Alcohol Clin Exp Res. 2002;26:1198–1204. doi: 10.1097/01.ALC.0000025885.23192.FF. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sola J, Estruch R, Nicolas JM, Pare JC, Sacanella E, Antunez E, et al. Comparison of alcoholic cardiomyopathy in women versus men. Am J Cardiol. 1997;80:481–485. doi: 10.1016/s0002-9149(97)00399-8. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Fortuna S, Pintor A, Michalek H. Adaptive processes of the central and autonomic cholinergic neurotransmitter system: age-related differences. Life Sciences. 1991;48:831–842. doi: 10.1016/0024-3205(91)90099-w. [DOI] [PubMed] [Google Scholar]
- Franck J, Jayaram-Lindstrom N. Pharmacotherapy for alcohol dependence: status of current treatments. Curr Opin Neurobiol. 2013;23:692–699. doi: 10.1016/j.conb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Fukushiro DF, Josino FS, Saito LP, Berro LF, Morgado F, Frussa-Filho R. Acute and chronic ethanol differentially modify the emotional significance of a novel environment: implications for addiction. Int J Neuropsychopharmacol. 2012;15:1109–1120. doi: 10.1017/S1461145711001283. [DOI] [PubMed] [Google Scholar]
- Fuller RK, Branchey L, Brightwell DR, Derman RM, Emrick CD, Iber FL, et al. Disulfiram treatment of alcoholism. A Veterans Administration cooperative study. Jama. 1986;256:1449–1455. [PubMed] [Google Scholar]
- Gianoulakis C, Dai X, Thavundayil J, Brown T. Levels and circadian rhythmicity of plasma ACTH, cortisol, and beta-endorphin as a function of family history of alcoholism. Psychopharmacology (Berl) 2005;181:437–444. doi: 10.1007/s00213-005-0129-x. [DOI] [PubMed] [Google Scholar]
- Hanson GR. New Insights Into Relapse. NIDA Notes. 2002:17. [Google Scholar]
- Health NCCfM. NICE Clinical Guidelines. Leicester (UK): British Psychological Society & The Royal College of Psychiatrists; 2011. Alcohol-Use Disorders: Diagnosis, Assessment and Management of Harmful Drinking and Alcohol Dependence. [PubMed] [Google Scholar]
- Hommer DW. Male and female sensitivity to alcohol-induced brain damage. Alcohol Res Health. 2003;27:181–185. [PMC free article] [PubMed] [Google Scholar]
- Hommer DW. Male and Female Sensitivity to Alcohol–Induced Brain Damage. NIAAAgov. 2004 [PMC free article] [PubMed]
- Itoi K, Sugimoto N. The brainstem noradrenergic systems in stress, anxiety and depression. J Neuroendocrinol. 2010;22:355–361. doi: 10.1111/j.1365-2826.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, et al. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 2003;38:189–193. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Tietz U, Dibbelt L, Kuether M, Jurth R, Ehrenthal D, et al. Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol. 2005;40:80–85. doi: 10.1093/alcalc/agh107. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, Hasin DS. Stressful life experiences, alcohol consumption, and alcohol use disorders: the epidemiologic evidence for four main types of stressors. Psychopharmacology (Berl) 2011;218:1–17. doi: 10.1007/s00213-011-2236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Brodhead AE, Schwartz KA, Kolts RL, Brown WA. Sex differences in antidepressant response in recent antidepressant clinical trials. J Clin Psychopharmacol. 2005;25:318–324. doi: 10.1097/01.jcp.0000168879.03169.ce. [DOI] [PubMed] [Google Scholar]
- King A, Munisamy G, de Wit H, Lin S. Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol. 2006;59:203–209. doi: 10.1016/j.ijpsycho.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koppaka V, Thompson DC, Chen Y, Ellermann M, Nicolaou KC, Juvonen RO, et al. Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol Rev. 2012;64:520–539. doi: 10.1124/pr.111.005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SK, Singh K, Bishnoi M. Elevated zero maze: a paradigm to evaluate antianxiety effects of drugs. Methods Find Exp Clin Pharmacol. 2007;29:343–348. doi: 10.1358/mf.2007.29.5.1117557. [DOI] [PubMed] [Google Scholar]
- Laaksonen E, Vuoristo-Myllys S, Koski-Jannes A, Alho H. Combining medical treatment and CBT in treating alcohol-dependent patients: effects on life quality and general well-being. Alcohol Alcohol. 2013;48:687–693. doi: 10.1093/alcalc/agt053. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Koob GF. Drugs of abuse and the brain. Proc Assoc Am Physicians. 1999;111:99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- Longabaugh RMJ. Cognitive-Behavioral Coping-Skills Therapy for Alcohol Dependence. Alcohol Research and Health. 1999;23:78–85. [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lowery EG, Sparrow AM, Breese GR, Knapp DJ, Thiele TE. The CRF-1 receptor antagonist, CP-154,526, attenuates stress-induced increases in ethanol consumption by BALB/cJ mice. Alcohol Clin Exp Res. 2008;32:240–248. doi: 10.1111/j.1530-0277.2007.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res. 2010;1314:145–161. doi: 10.1016/j.brainres.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McGovern PE. Ancient Wine: The Search for the Origins of Viniculture. Princeton University Press; 2007. [Google Scholar]
- McGovern PE, Zhang J, Tang J, Zhang Z, Hall GR, Moreau RA, et al. Fermented beverages of pre- and proto-historic China. Proc Natl Acad Sci U S A. 2004;101:17593–17598. doi: 10.1073/pnas.0407921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef M, Lexis L, Lewandowski P. Red wine consumption increases antioxidant status and decreases oxidative stress in the circulation of both young and old humans. Nutr J. 2007;6:27. doi: 10.1186/1475-2891-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101:212–222. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer A, Finney JW. Outcomes for untreated individuals involved in randomized trials of alcohol treatment. J Subst Abuse Treat. 2002;23:247–252. doi: 10.1016/s0740-5472(02)00264-7. [DOI] [PubMed] [Google Scholar]
- Muzyk AJ, Rivelli SK, Gagliardi JP. Defining the role of baclofen for the treatment of alcohol dependence: a systematic review of the evidence. CNS Drugs. 2012;26:69–78. doi: 10.2165/11597320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Tivis R, Ceballos N, Varner JL, Rohrbaugh J. Neurophysiological efficiency in male and female alcoholics. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:919–927. doi: 10.1016/s0278-5846(02)00206-3. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Volpicelli LA, Volpicelli JR. Naltrexone in the treatment of alcoholism: a clinical review. Alcohol. 1996;13:35–39. doi: 10.1016/0741-8329(95)02038-1. [DOI] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, et al. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009;33:1924–1934. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- P. Sterling JE. Allostasis: a new paradigm to explain arousal pathology. In: J. Fisher JR, editor. Handbook of Life Stress, Cognition, and Health. New York: John Wiley and Sons, Inc; 1998. pp. 629–649. [Google Scholar]
- Pastor R, Reed C, Burkhart-Kasch S, Li N, Sharpe AL, Coste SC, et al. Ethanol concentration-dependent effects and the role of stress on ethanol drinking in corticotropin-releasing factor type 1 and double type 1 and 2 receptor knockout mice. Psychopharmacology (Berl) 2011;218:169–177. doi: 10.1007/s00213-011-2284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan E. Sex differences in the effects of alcohol on brain structure. Am J Psychiatry. 2001;158:188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Ponomarev I. Epigenetic control of gene expression in the alcoholic brain. Alcohol Res. 2013;35:69–76. doi: 10.35946/arcr.v35.1.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickett CD, Lister E, Collins M, Trevithick-Sutton CC, Hirst M, Vinson JA, et al. Alcohol: Friend or Foe? Alcoholic Beverage Hormesis for Cataract and Atherosclerosis is Related to Plasma Antioxidant Activity. Nonlinearity Biol Toxicol Med. 2004;2:353–370. doi: 10.1080/15401420490900272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Retson TA, Hoek JB, Sterling RC, Van Bockstaele EJ. Amygdalar neuronal plasticity and the interactions of alcohol, sex, and stress. Brain Struct Funct. 2014a doi: 10.1007/s00429-014-0851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retson TA, Reyes BA, Van Bockstaele EJ. Chronic alcohol exposure differentially affects activation of female locus coeruleus neurons and the subcellular distribution of corticotropin releasing factor receptors. Progress in neuro-psychopharmacology & biological psychiatry. 2014b;56C:66–74. doi: 10.1016/j.pnpbp.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. Alcohol stimulates ACTH secretion in the rat: mechanisms of action and interactions with other stimuli. Alcohol Clin Exp Res. 1996;20:240–254. doi: 10.1111/j.1530-0277.1996.tb01636.x. [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984;229:127–131. [PubMed] [Google Scholar]
- Rivier C, Lee S. Acute alcohol administration stimulates the activity of hypothalamic neurons that express corticotropin-releasing factor and vasopressin. Brain Res. 1996;726:1–10. [PubMed] [Google Scholar]
- Ruiz SM, Oscar-Berman M, Sawyer KS, Valmas MM, Urban T, Harris GJ. Drinking history associations with regional white matter volumes in alcoholic men and women. Alcohol Clin Exp Res. 2013;37:110–122. doi: 10.1111/j.1530-0277.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE. Evidence for a local site of action for glucocorticoids in inhibiting CRF and vasopressin expression in the paraventricular nucleus. Brain Res. 1987;403:213–223. doi: 10.1016/0006-8993(87)90058-8. [DOI] [PubMed] [Google Scholar]
- Schepis TS, Rao U, Yadav H, Adinoff B. The limbic-hypothalamic-pituitary-adrenal axis and the development of alcohol use disorders in youth. Alcohol Clin Exp Res. 2011;35:595–605. doi: 10.1111/j.1530-0277.2010.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrett-Field L, Butler TR, Reynolds AR, Berry JN, Prendergast MA. Sex differences in neuroadaptation to alcohol and withdrawal neurotoxicity. Pflugers Arch. 2013;465:643–654. doi: 10.1007/s00424-013-1266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated "zero-maze" as an animal model of anxiety. Psychopharmacology (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- Sher L. The role of the hypothalamic-pituitary-adrenal axis dysfunction in the pathophysiology of alcohol misuse and suicidal behavior in adolescents. Int J Adolesc Med Health. 2007;19:3–9. doi: 10.1515/ijamh.2007.19.1.3. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Stappenbeck CA, Luterek JA, Lehavot K, Kaysen DL. Drinking motives moderate daily relationships between PTSD symptoms and alcohol use. J Abnorm Psychol. 2014;123:237–247. doi: 10.1037/a0035193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaff MM, Finney JW, Moos RH. Gender differences in problem drinking and depression: different "vulnerabilities?". Am J Community Psychol. 1999;27:25–54. doi: 10.1023/A:1022813727823. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34:468–483. doi: 10.35946/arcr.v34.4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Thiele TE, van Dijk G, Bernstein IL. Ethanol-induced c-Fos expression in rat lines selected for low and high alcohol consumption. Brain Res. 1997;756:278–282. doi: 10.1016/s0006-8993(97)00228-x. [DOI] [PubMed] [Google Scholar]
- Thurman RG. Sex-related liver injury due to alcohol involves activation of Kupffer cells by endotoxin. Can J Gastroenterol. 2000;14(Suppl D):129D–135D. doi: 10.1155/2000/735262. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol. 1996;364:523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Vilpoux C, Warnault V, Pierrefiche O, Daoust M, Naassila M. Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcohol Clin Exp Res. 2009;33:945–969. doi: 10.1111/j.1530-0277.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry. 2009;66:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisner C, Matzger H, Kaskutas LA. How important is treatment? One-year outcomes of treated and untreated alcohol-dependent individuals. Addiction. 2003;98:901–911. doi: 10.1046/j.1360-0443.2003.00438.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.