Abstract
Although coronary artery calcium (CAC) is an established marker of coronary atherosclerosis, whether it also reflects the physiological significance is unknown. This study aims to evaluate if CAC could indicate physiological ischemia in intermediate stenosis defined by an invasive fractional flow reserve (FFR). CAC score (CACS) derived from either whole coronary arteries or individual arteries was measured by computed tomography among patients with intermediate de novo lesions (percent diameter stenosis from 30% to less than 70%). All stenoses were evaluated by invasive FFR; lesions with an FFR ≤ 0.80 were considered significant. We enrolled 119 patients with 143 lesions. Of these, 42 lesions (29.4%) demonstrated significant ischemia by FFR measurement. FFR values had modest but significant correlations with CACS in individual arteries with intermediate stenosis (r = − 0.290; p < 0.001). A receiver operating characteristic curve analysis showed that CACS of individual arteries with intermediate stenosis had 71.4% sensitivity and 67.3% specificity as a predictor of significant ischemia at a cut off value of 145.9. Multivariable analysis showed that percent diameter stenosis and CACS in individual arteries with intermediate stenosis were independent predictors for significant ischemia. By net reclassification improvement analysis, CACS in individual arteries with intermediate stenosis provided incremental prediction for significant ischemia over minimum lumen diameter, percent diameter stenosis, and lesion length. CACS measured in each artery, but not the total CACS, provides additional information as to whether an angiographically intermediate stenosis within the artery is significant enough to cause myocardial ischemia.
Keywords: coronary artery disease, intermediate stenosis, fractional flow reserve, CTCA, coronary calcification
Coronary artery calcium (CAC) is an established surrogate marker of coronary atherosclerotic burden.1 The amount of CAC assessed as the CAC score (CACS) by computed tomography coronary angiography (CTCA) has been shown to predict future cardiovascular events in numerous studies.2 3 4 Moreover, CAC may also suggest coronary artery dysfunction; for example, previous studies using magnetic resonance imaging, single-photon emission computed tomography (SPECT), or positron emission tomography (PET) imaging have showed an inverse relationship between CAC levels and myocardial perfusion.5 6 7
Fractional flow reserve (FFR) is an invasive, lesion-specific index that is used to assess the potential of a coronary stenosis to induce myocardial ischemia.8 The measurement of FFR has been shown to improve clinical outcomes by allowing significant stenosis with the need for revascularization to be identified and selected.9 The development of FFR has prompted a major shift in the assessment of coronary stenosis severity in cardiac catheterization.
To date, few studies have evaluated whether CAC levels were associated with FFR. Therefore, this study aims to evaluate the relationship between the magnitude of CAC and functional ischemia in patients with intermediate coronary stenosis undergoing both 320-multidetector row CTCA and conventional coronary angiogram (CCA) using invasive FFR measurements.
Methods
Patients and Setting
The retrospective observational study was conducted between August 2009 and July 2013 in Miyagi East Department of Interventional Cardiology. We identified all patients undergoing CTCA with stable chest complaints and/or suspected coronary artery disease (CAD). Coronary stenosis was classified as significant if it had over 50% diameter stenosis (DS) on the longitudinal images,10 and patients with significant lesions on CTCA were further evaluated by CCA. When intermediate DS was detected (30–69%) by visual assessment (i.e., CCA interpretation), we further performed invasive FFR measurement at the discretion of the interventional cardiologist. All patients receiving invasive FFR measurement were included. We excluded patients with a history of coronary revascularization (coronary stent implantation or coronary artery bypass graft surgery) and those in whom it was not possible to assess CACS in whole coronary arteries or because of motion artifacts leading to poor image quality. All study patients provided written informed consent.
Acquisition of Computed Tomography Coronary Angiography
All study patients were examined using a 320-multidetector row computed tomography scanner (Aquilion ONE, Toshiba Medical Systems, Otawara, Japan). Unless contraindicated, 100 mg of diltiazem was administered orally the night before examination if the patient's heart rate was more than 60 beats/min. If the target heart rate was not achieved, 2 to 4 mg of propranolol or 5 to 10 mg of landiolol was injected intravenously just before scanning.11 Sublingual nitroglycerin was administered before scanning if the systolic blood pressure was greater than 110 mm Hg. Patients underwent nonenhanced prospective electrocardiography-gated sequential scans to measure CACS. Calcium scanning was performed at 120 kV and 140 mA with a gantry rotation of 0.35 second, collimation of 0.5 mm, and scan range of 128 to 160 mm. Contrast media was injected through a 20 G trocar inserted into an antecubital vein.11
For the contrast-enhanced scan, 0.75 mL/kg of contrast agent containing iopromide 370 mgI/mL (Ultravist 370, Bayer-Schering Pharma, Berlin, Germany) was injected at 4 to 5.5 mL/s. If the heart rate was less than 66 beats/min at the time of imaging, prospective scan triggering was performed at 70 to 80% of one R–R interval with X-ray exposure times of 0.35 to 0.45 second. For patients with a heart rate more than 65 beats/min despite β-blockade, a two-heartbeat image acquisition protocol was applied with prospective scan triggering and a 40 to 80% exposure window.
Computed Tomography Coronary Angiography Image Analysis
After image acquisition, a three-dimensional workstation (Ziostation, Ziosoft, Tokyo, Japan) was used to determine the Agatston calcium score and to generate three-dimensional (volume rendering) and curved multiplanar reformat images. Calcium was considered present if there were at least three contiguous pixels with a density > 130 Hounsfield units. The overall and per-artery calcium burden was quantified according to the scoring algorithm proposed by Agatston et al.3
The presence and characteristics of coronary plaques in each segment was subsequently investigated. Coronary plaques were classified as noncalcified, mixed, or calcified. Coronary calcification was visually determined in the contrast-enhanced dataset. Noncalcified plaques were defined as a tissue structure > mm2 that could be clearly discriminated from the vessel lumen and surrounding tissue with a density below the contrast-enhanced blood pool. Mixed plaques were those that met this definition as well as those that had calcified areas of any extent. Calcified plaques were defined as a tissue with a predominantly high attenuation but without noncalcified plaque elements.12 These CTCA findings were interpreted by more than two experienced radiologists who were blinded to CCA and FFR findings.
Conventional Coronary Angiogram Protocol
All patients underwent CCA through the radial approach, using a 4 to 5F gauge diagnostic catheter. After intracoronary injection of 2 mg isosorbide dinitrate, right and left coronary angiograms were performed in multiple projections using standard techniques. All angiograms were analyzed offline by more than two cardiologists who were not involved in the patient's treatment. Quantitative coronary angiography (QCA) was performed using an edge-detection algorithm (QangioXA, Medis Medical Equipment, Leiden, The Netherlands), based on the angiographic projection with the most severe narrowing.13
Fractional Flow Reserve Measurement Protocol
FFR measurement was performed in cases of intermediate stenosis using a sensor-tipped 0.36 mm in guidewire (PressureWire Certus, St. Jude Medical Systems, Uppsala, Sweden; or ComboWire, Volcano Corporation, San Diego, CA). After positioning the pressure sensor in the distal vessel to be interrogated, maximal myocardial hyperemia was induced by continuous intravenous infusion of adenosine in a brachial vein at an infusion rate of 140 µg/kg body weight/min for at least 2 minutes. The FFR value was calculated as the ratio of the mean distal intracoronary pressure measured by the wire to the mean arterial pressure that was measured by the coronary catheter.8 FFR was considered diagnostic of ischemia at a threshold of ≤0.80.9
Statistical Analyses
All statistical analyses were performed using the JMP version 11 for Mac software (SAS Institute Inc., Cary, NC). For descriptive analysis, continuous variables were presented as the mean ± SD and categorical variables were presented as percentages. Categorical variables were compared with the chi-square statistic and continuous variables by the t-test. Pearson correlation coefficients were used to describe the relationship between corresponding FFR values on a per vessel basis and CACS or QCA findings. The determinants of FFR were assessed by univariable and multivariable linear logistic regression analyses. A p-value less than 0.05 was used to define statistical significance. To examine discrimination, receiver operating characteristic curves (ROCs) and area under ROC (AUC) were constructed to compare different coronary CTCA parameters in the corresponding logistic models using the method described by DeLong et al.14 Net reclassification improvement (NRI) was derived from logistic regression models based on QCA findings such as minimum lumen diameter (MLD), %DS, and lesion length, with and without the CACS.15 Here, we utilized the logarithmic transformation of CACS (log [CACS + 1]). To calculate NRI, we compared the rescaled lesion-specific predicted values from models with and without log (CACS + 1) using traditional QCA findings.
Results
Patients
The study flow schema is summarized in Fig. 1. CTCA was performed on 5,897 patients with stable chest complaints and/or suspected CAD during the study period in our institution. In total, 1,710 patients had significant lesions on CTCA, and 172 patients had intermediate %DS (30–69%) on visual assessment of CCA and underwent invasive FFR measurement. We excluded 43 patients with a history of stent implantation, 3 patients with a history of coronary artery bypass graft surgery, and 7 because it was not possible to assess CACS in whole coronary arteries or because of motion artifacts leading to poor image quality.
Fig. 1.
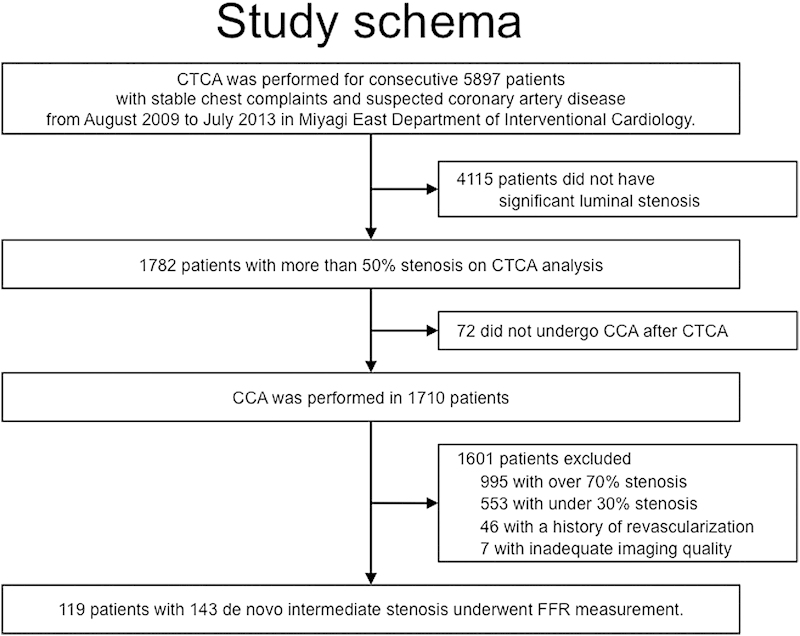
Patient diagram. Flow chart of the patients who underwent CTCA, CCA, and invasive FFR measurement included in the analysis. CCA, conventional coronary angiogram; CTCA, computed tomography coronary angiography; FFR, fractional flow reserve.
Patient and Lesion Characteristics
We retrospectively analyzed 119 consecutive patients (mean age 70.4 ± 8.8 years, 61.3% male) that had 143 de novo intermediate coronary stenoses between them and who underwent CTCA, invasive CCA, and invasive FFR measurement. The patient and lesion characteristics are summarized in Tables 1 and 2.
Table 1. Baseline characteristics.
| Characteristics | All (n = 119) |
FFR ≤ 0.80 (n = 40) |
FFR > 0.80 (n = 79) |
p-Value |
|---|---|---|---|---|
| Male, n (%) | 74 (62.2) | 23 (57.5) | 51 (64.6) | 0.45 |
| Mean age (y) | 70.5 ± 8.9 | 72.4 ± 9.3 | 69.6 ± 9.3 | 0.11 |
| Body mass index (kg/m2) | 24.9 ± 3.5 | 25.5 ± 3.9 | 24.5 ± 3.2 | 0.18 |
| Hypertension, n (%) | 108 (90.8) | 36 (90.0) | 72 (91.1) | 0.84 |
| Dyslipidemia, n (%) | 75 (63.0) | 28 (70.0) | 47 (59.5) | 0.26 |
| Statin use, n (%) | 66 (55.5) | 26 (65.0) | 40 (50.6) | 0.13 |
| Diabetes, n (%) | 42 (35.3) | 18 (45.0) | 24 (30.4) | 0.12 |
| Oral antidiabetic drug use, n (%) | 29 (24.4) | 14 (35.0) | 15 (19.0) | 0.06 |
| Insulin use, n (%) | 8 (6.7) | 5 (12.5) | 3 (3.8) | 0.08 |
| Familial history, n (%) | 18 (15.1) | 6 (15.0) | 12 (15.2) | 0.98 |
| Current smoking, n (%) | 32 (26.9) | 5 (12.5) | 27 (34.2) | <0.01 |
| Prior myocardial infarction, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
| Typical presentation of effort angina, n (%) | 23 (19.5) | 13 (33.3) | 10 (12.7) | <0.01 |
| Atypical presentation of effort angina, n (%) | 48 (40.7) | 9 (23.1) | 39 (49.4) | <0.01 |
| Asymptomatic, n (%) | 47 (39.8) | 17 (43.6) | 30 (38.0) | 0.56 |
| LVEF (%) | 71.4 ± 8.9 | 70.7 ± 9.0 | 71.7 ± 8.9 | 0.54 |
| Systolic blood pressure (mm Hg) | 136.5 ± 19.1 | 137.4 ± 21.0 | 136.1 ± 18.2 | 0.74 |
| Diastolic blood pressure (mm Hg) | 78.7 ± 14.1 | 75.7 ± 16.0 | 80.2 ± 13.0 | 0.12 |
| Heart rate (bpm) | 78.8 ± 14.0 | 77.8 ± 15.2 | 79.2 ± 13.4 | 0.62 |
| Hemoglobin (g/dL) | 13.8 ± 1.5 | 13.3 ± 1.5 | 14.5 ± 1.5 | <0.01 |
| Total cholesterol (mg/dL) | 193.9 ± 38.7 | 197.8 ± 45.3 | 191.9 ± 35.0 | 0.43 |
| LDL cholesterol (mg/dL) | 106.3 ± 32.0 | 107.8 ± 38.1 | 105.6 ± 28.6 | 0.72 |
| HDL cholesterol (mg/dL) | 54.6 ± 16.8 | 52.7 ± 15.2 | 55.6 ± 17.6 | 0.38 |
| Triglyceride (mg/dL) | 135.4 ± 79.8 | 148.8 ± 104.1 | 128.6 ± 63.7 | 0.02 |
| Fasting blood glucose (mg/dL) | 128.6 ± 47.5 | 126.4 ± 51.3 | 130.2 ± 45.2 | 0.77 |
| HbA1c (%) | 6.3 ± 1.0 | 6.5 ± 0.9 | 6.1 ± 1.0 | 0.13 |
| eGFR (mL/min/1.73 m2) | 59.0 ± 12.6 | 55.9 ± 11.2 | 60.5 ± 13.1 | 0.06 |
| C-reactive protein (mg/dL) | 0.5 ± 1.4 | 0.9 ± 2.3 | 0.2 ± 0.2 | 0.07 |
| CACS of whole arteries | 711.8 ± 930.6 | 1,086.6 ± 1,257.0 | 522.0 ± 713.4 | <0.01 |
Abbreviations: bpm, beats/min; CACS, CAC score; eGFR, estimated glomerular filtration rate; FFR, fractional flow reserve; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction.
Note: Values area mean ± SD or n (%).
Table 2. Lesion characteristics assessed by CCA and CTCA as a function of FFR value.
| Characteristics | All (n = 143) |
FFR ≤ 0.80 (n = 42) |
FFR > 0.80 (n = 101) |
p-Value |
|---|---|---|---|---|
| Mean value of FFR | 0.85 ± 0.09 | 0.74 ± 0.05 | 0.90 ± 0.05 | <0.01 |
| Target vessel, n (%) | 0.09 | |||
| Left anterior descending artery | 87 (60.8) | 30 (71.4) | 57 (55.5) | |
| Left circumflex artery | 32 (22.4) | 5 (11.9) | 27 (26.7) | |
| Right coronary artery | 24 (16.8) | 6 (14.3) | 18 (17.8) | |
| Lesion location, n (%) | 0.03 | |||
| Proximal | 50 (39.0) | 18 (45.2) | 31 (30.7) | |
| Mid | 59 (41.2) | 17 (40.5) | 42 (41.6) | |
| Distal | 24 (22.8) | 6 (14.3) | 18 (17.8) | |
| Other location | 10 (7.0) | 0 (0.0) | 10 (9.9) | |
| ACC/AHA classification, n (%) | <0.01 | |||
| Type A/B1 | 78 (54.5) | 13 (31.0) | 65 (64.4) | |
| Type B2/C | 65 (45.5) | 29 (69.0) | 36 (35.6) | |
| CACS in individual arteries with intermediate stenosis | 238.6 ± 300.4 | 428.5 ± 394.3 | 159.6 ± 207.2 | <0.01 |
| Classification of plaque, n (%) | <0.01 | |||
| Calcified plaque | 39 (27.3) | 20 (47.6) | 19 (18.8) | |
| Noncalcified plaque | 51 (35.7) | 6 (14.3) | 45 (44.6) | |
| Mixed plaque | 53 (37.0) | 16 (38.1) | 37 (36.6) | |
| QCA findings | ||||
| Reference vessel diameter (mm) | 2.57 ± 0.49 | 2.52 ± 0.54 | 2.59 ± 0.47 | 0.47 |
| Minimum lumen diameter (mm) | 1.33 ± 0.35 | 1.21 ± 0.36 | 1.38 ± 0.34 | 0.01 |
| % diameter stenosis (%) | 48.22 ± 9.72 | 51.56 ± 9.75 | 46.84 ± 9.41 | <0.01 |
| Lesion length (mm) | 9.76 ± 4.76 | 10.96 ± 6.13 | 9.27 ± 3.99 | 0.05 |
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; CACS, coronary artery calcium score; CCA, conventional coronary angiogram; CTCA, computed tomography coronary angiography; FFR, fractional flow reserve; QCA, quantitative coronary angiography.
Invasive FFR was measured at an average of 17.2 ± 9.9 days after CTCA. FFR measurements were successfully performed in all-study patients without complication. We observed no relevant symptom changes or adverse clinical events in the interval between CTCA and FFR measurement. None of the study patients had a history of myocardial infarction or coronary revascularization. Among the 143 stenotic lesions, 42 significant ischemic lesions (29.4%) were identified by FFR with an average FFR value of 0.74 ± 0.05. Compared with nonischemic lesions, ischemic lesions had comparable vessel diameters and lesion lengths, but significantly smaller MLD (1.21 vs. 1.38 mm, p = 0.01) and larger %DS (51.6 vs. 46.8%, p < 0.01) by QCA (Table 2).
Coronary Artery Calcium and Plaque Characteristics
The median total CACS was 711.8 (range, 0.0–4,590.5). In patients with significant ischemia (n = 40), the median total CACS was 1,086.6 ± 1,257.0; in patients without significant CAD (n = 79) the CACS was 522.0 ± 713.4 (p < 0.01; Table 1). The median CACS was 428.5 ± 394.3 and 159.6 ± 207.2 in arteries with and without significant ischemic lesions, respectively (p < 0.01).
The composition of coronary plaques in arteries with intermediate stenotic lesions is summarized in Table 2. Highly ischemic lesions contained calcified plaques more frequently than nonischemic lesions (47.6 vs. 18.8%; p < 0.01).
Correlation between Fractional Flow Reserve Value and Quantitative Coronary Angiography or Computed Tomography Coronary Angiography Findings
The FFR value was modestly correlated with MLD (r = 0.232; p = 0.005) and %DS (r = −0.219; p = 0.009). Furthermore, modest but significant correlations were observed between FFR values and CACS in individual arteries with intermediate stenosis (r = −0.290; p < 0.001). In contrast, the FFR value was not correlated with lesion length (r = −0.171; p = 0.041) or reference vessel diameter (r = 0.092, p = 0.275; Fig. 2).
Fig. 2.
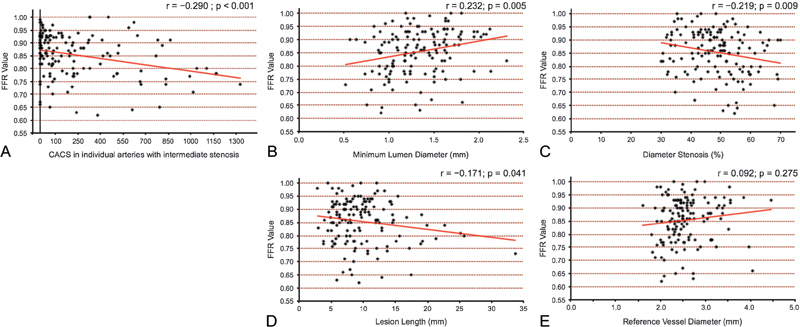
Scatterplot showing the correlation between FFR values and either CACSs in individual arteries with intermediate stenosis or traditional QCA findings. (A) A modest but significant correlation (r = − 0.290) was observed between FFR values and the CACS of an FFR-related vessel, whereas modest correlations were observed between FFR values and (B) minimum lumen diameter (r = 0.232), or (C) diameter stenosis (r = − 0.219). No correlation was observed between FFR values and (D) lesion length (r = − 0.171), or (E) reference vessel diameter (r = 0.092). CACS, coronary artery calcium score; FFR, fractional flow reserve; QCA, quantitative coronary angiogram.
Diagnosis of Ischemia in Intermediate Stenosis by CAC score and Quantitative Coronary Angiography
On univariable logistic regression analysis, lesion-specific ischemia was significantly related to total CACS, CACS in individual arteries with intermediate stenosis, calcified plaques, MLD, and %DS.
On multivariable logistic regression analysis, CACS in individual arteries with intermediate stenosis was an independent predictor for significant ischemia (odds ratio [OR] 1.004; 95% confidence interval [CI] 1.001–1.007; p = 0.013), as was %DS (OR 1.058; 95% CI 1.007–1.101; p = 0.024; Table 3).
Table 3. Conditional multivariate logistic regression analysis for determinant of impaired FFR value.
| Analysis | Univariate | Multivariate | ||
|---|---|---|---|---|
| p-Value | OR | 95% CI | p-Value | |
| CACS in whole vessels | 0.002 | 1.000 | 0.999–1.001 | 0.303 |
| CACS in individual arteries with intermediate stenosis | <0.001 | 1.004 | 1.001–1.007 | 0.005 |
| Calcified plaque | 0.001 | 1.412 | 0.499–3.844 | 0.507 |
| %DS measured by QCA | 0.008 | 1.054 | 1.007–1.105 | 0.023 |
Abbreviations: CACS, coronary artery calcium score; CI, confidence interval; DS, diameter stenosis; FFR, fractional flow reserve; OR, odds ratio; QCA, quantitative coronary angiogram.
AUC for lesion-specific ischemia was the highest for CACS in individual arteries with intermediate stenosis (OR 0.725; 95% CI 0.621–0.811; p < 0.001) than that for %DS (OR 0.650; 95%CI 0.540–0.746; p = 0.007), and MLD (OR 0.642; 95% CI 0.533–0.738; p = 0.009; Fig. 3). The optimal CTCA and QCA cut off values to identify significant stenosis were as follows: 145.9 for CACS in individual arteries with intermediate stenosis (71.4% sensitivity, 67.3% specificity, positive predictive value [PPV] 47.6%, negative predictive value [NPV] 85.0%, accuracy 68.5%), 1.05 mm for MLD (47.6% sensitivity, 81.2% specificity, PPV 51.3%, NPV 78.8%, accuracy 71.3%), and 52.0% for %DS (57.1% sensitivity, 77.2% specificity, PPV 51.1%, NPV 81.3%, accuracy 71.3%).
Fig. 3.
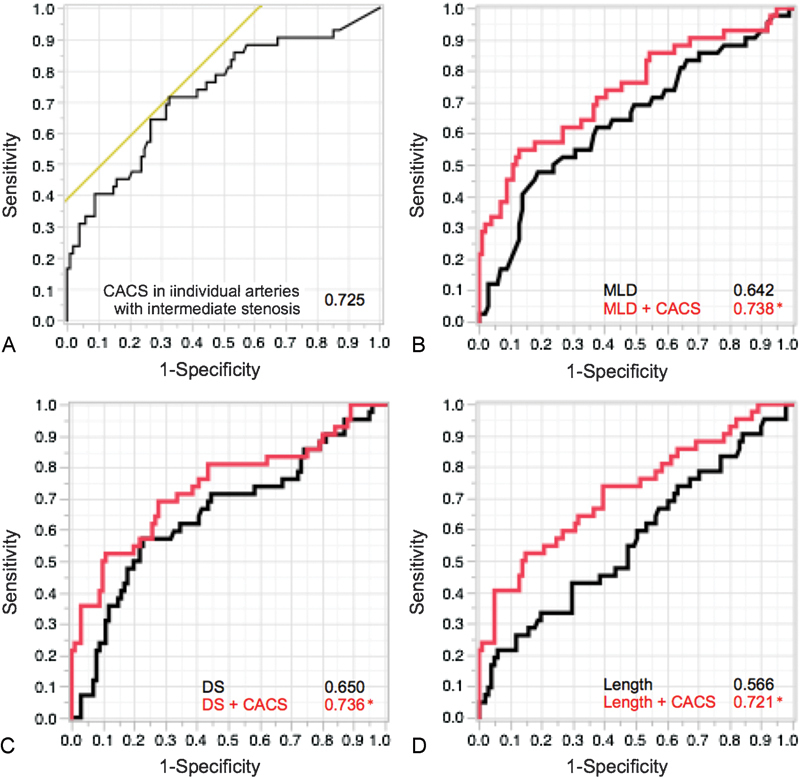
AUC for detection of significant ischemic lesions. (A) AUC was highest for the CACS of an FFR-related vessel at 0.725. The other AUC graphs illustrate that CACS provided incremental discrimination of ischemia over (B) MLD, (C) DS, or (D) lesion length of coronary artery disease severity considered alone. *p < 0.05. AUC, area under the receiver operating characteristic curve; CACS, coronary artery calcium score; DS, diameter stenosis; MLD, minimum lumen diameter.
On NRI analysis from adjusted logistic regression models, CACS in individual arteries with intermediate stenosis provided incremental prediction for significant ischemia over MLD alone (0.738 [95% CI 0.631–0.823] vs. 0.642 [95% CI 0.533–0.738], respectively, p = 0.032), %DS alone (0.734 [95% CI 0.627–0.823] vs. 0.650 [95% CI 0.540–0.746], respectively, p = 0.036), or lesion length alone (0.710 [95% CI 0.615–0.808] vs. 0.566 [95% CI 0.458–0.669], p = 0.003). Figs. 3 4 5 show representative cases of intermediate stenosis in the midleft anterior descending arteries with and without advanced arterial calcification, respectively.
Fig. 4.
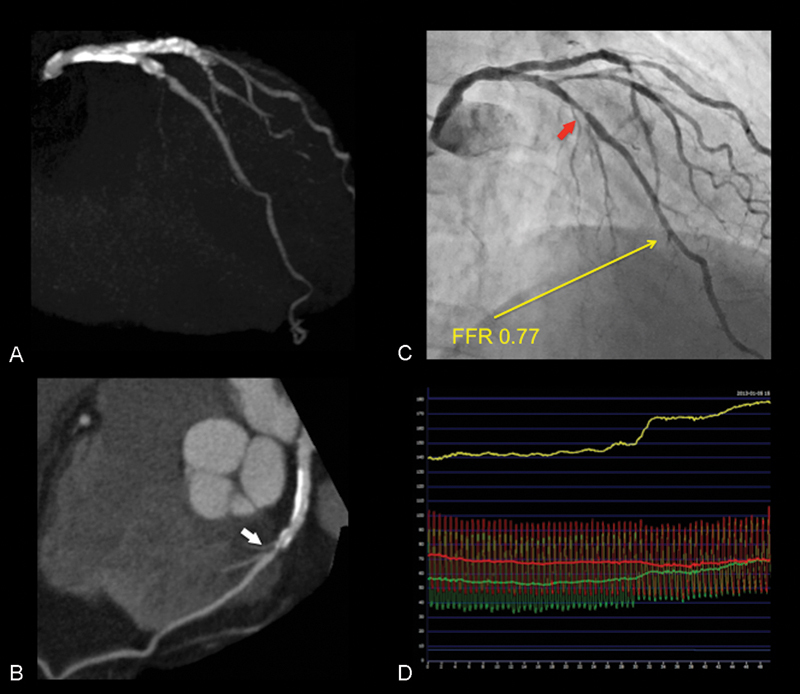
CACS, CCA, and FFR in vessels with intermediate stenosis and significant ischemia. (A) CACS of the LAD is 489.3, and that of whole coronary arteries is 1,448.0. (B) On CTCA, curved MPR revealed the presence of an atherosclerotic lesion (white arrow) with a calcified plaque in the mid-LAD. (C) Percentage DS is estimated to be 60.0% (red arrow). (D) FFR value in the LAD was 0.77. CACS, coronary artery calcium score; CCA, conventional coronary angiogram; CTCA, computed tomography coronary angiography; DS, diameter stenosis; FFR, fractional flow reserve; LAD, left anterior descending; MPR, multiplanar reconstruction.
Fig. 5.
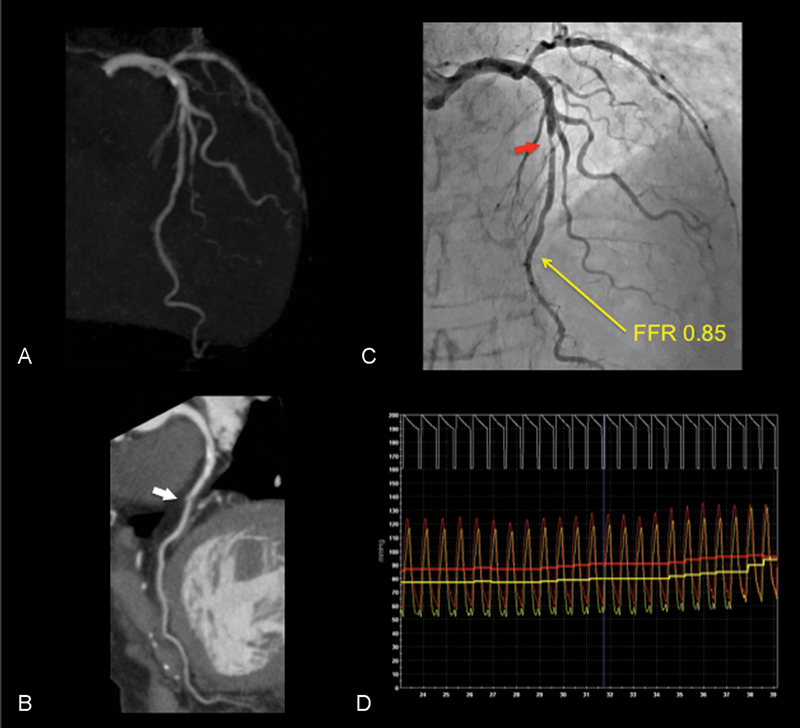
CACS, CCA, and FFR in intermediate stenosis with nonsignificant ischemia. (A) CACS of the LAD is 10.1 and that of whole coronary arteries is 23.9. (B) On CTCA, curved MPR revealed the presence of an atherosclerotic lesion (white arrow) with a noncalcified plaque in the mid-LAD. (C) Percentage DS is estimated to be 55.9% (red arrow). (D) FFR value in the LAD was 0.85. CACS, coronary artery calcium score; CCA, conventional coronary angiogram; CTCA, computed tomography coronary angiography; DS, diameter stenosis; FFR, fractional flow reserve; LAD, left anterior descending; MPR, multiplanar reconstruction.
Discussion
The main findings of this study are fourfold. First, quantitative CACS in individual arteries with intermediate ischemia can be used to detect physiologically significant ischemia as defined by invasive FFR, and is independent of traditional angiographic measures. Second, CACS in individual arteries with intermediate ischemia had a modest but significant correlation with the invasive FFR value (r = −0.290; p < 0.001). Third, the optimal cut off value of CACS in individual arteries with intermediate ischemia for significant ischemia is 145.9. Fourth, the combination of CACS in individual arteries with intermediate ischemia and QCA findings provided incremental discriminatory power for significant ischemia when compared with angiographic measures alone.
FFR is considered one of the most reliable methods for assessing the ischemic potential of coronary stenosis. This is evidenced by numerous clinical trials showing that the invasive assessment of coronary physiology improves clinical outcomes by allowing the selection of the most suitable patients for revascularization.8 9 The physiological effect of coronary stenosis is determined by many anatomical and clinical factors; therefore, some studies have revealed the relationship between the FFR value and anatomical parameters of coronary stenosis evaluated by coronary imaging techniques. Although the FFR value correlates with atherosclerosis, identified as lesion length16 or plaque burden,17 few studies have assessed the association of coronary artery calcification on invasive FFR. Because the volume of CAC is considered an excellent marker of overall atherosclerotic burden, a larger calcium burden might be associated with a higher likelihood of advanced atherosclerosis in coronary arteries.3 4 Previous investigations have suggested that calcium deposition only occurred when atherosclerosis was present, and that plaques with more advanced atherosclerotic characteristics tended to have a greater amount of calcium.12 Lori et al demonstrated that increased arterial calcium levels were associated with a higher atherosclerotic burden of noncalcified plaque in patients with low CACS after adjustment for traditional risk factors.18 In our results, we found that lower FFR values in calcified arteries might be associated with luminal stenosis due to advanced atherosclerosis, which could not be fully evaluated by angiography. To the best of our knowledge, this is the first result to show that, in angiographic intermediate stenosis, more extensive CAC indicated a higher likelihood of physiological ischemia. Moreover, it was a unique finding that the FFR value correlated with CACS in individual arteries with intermediate stenosis rather than the total CACS.
In the present study, AUC for lesion-specific ischemia based on either MLD, %DS, or lesion length alone was significantly improved when combined with CACS of individual arteries with intermediate stenosis. This improved diagnostic accuracy seems to be explained by the contribution of unmeasured atherosclerosis to the severity of arterial calcification. In addition, previous studies have reported that both diffuse atherosclerosis and single stenotic lesions in the coronary arteries also contribute to ischemia.19 By measuring coronary flow reserve, de Bruyne et al demonstrated that a diffuse atherosclerotic disease could cause a decline in the coronary flow despite the absence of obstructive CAD.19 Therefore, the severity of myocardial perfusion abnormalities is not only dependent on whether obstructive disease is present but also on the total atherosclerotic burden in a coronary artery. Our results suggest that advanced coronary artery calcification might reflect a diffuse atherosclerotic feature.
Nakazato et al evaluated the relationship between invasive FFR and aggregate plaque burden measured by CTCA from the vessel ostium to the distal stenosis-causing plaque. They reported that aggregate plaque volume provided a more accurate diagnosis of lesion-specific ischemia defined by invasive FFR than that by traditional angiographic measures of CAD lesion severity.17 Although the report showed a clear correlation between plaque burden and FFR value, a major limitation of CTCA is its inaccuracy in evaluating calcified vessels, even in the current era of improved resolution.20 Moreover, plaque measurements by CTCA are time-consuming and necessitate expert interpretation, whereas CACS can be assessed quantitatively, in almost all patients without stent implants, using simple software to apply the semiautomated scoring system. Although the predictive value of CACS is inferior to aggregate plaque volumes reported previously,17 CACS in individual arteries with intermediate stenosis appears to offer a feasible means of discriminating significant ischemia with improved diagnostic value when combined with traditional QCA findings.
To the best of our knowledge, this was the first study to evaluate the association between CACS on the invasive FFR value, and suggested that a heavy calcification has lower apparent FFR values for a given stenosis. The combined use of CACS and measures of luminal stenosis may offer a more precise approach in patients with both intermediate coronary stenosis and arterial calcification.
Several limitations of our study need to be considered when interpreting the results. First, this study was a single-center retrospective analysis conducted on a small sample. Furthermore, there is no information about other anatomical or physiological examinations, such as intravascular ultrasound, SPECT, or PET imaging. Finally, although our results suggest that the quantitative measurement of CAC has an adjunctive role in identifying physiologically significant ischemia in clinical practice, how coronary calcification affects invasive FFR cannot be determined from the present study design.
Conclusions
The severity of CAC in individual arteries rather than the total CACS provides additional information about whether angiographically intermediate stenosis within the artery is significant enough to cause myocardial ischemia.
Acknowledgments
The authors are very grateful for Yoshihiko Goto, Hidenori Miyamori, Hidekazu Takahashi, Hirotaka Hoshi, Yasuhiro Kinebuchi, Tatsuya Satoh, and Misato Ueno for their technical support in the analysis of CTCA and CCA findings.
Footnotes
Conflict of Interest No conflicts of interest or financial disclosures.
References
- 1.Rumberger J A, Schwartz R S, Simons D B, Sheedy P F III, Edwards W D, Fitzpatrick L A. Relation of coronary calcium determined by electron beam computed tomography and lumen narrowing determined by autopsy. Am J Cardiol. 1994;73(16):1169–1173. doi: 10.1016/0002-9149(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 2.Shaw L J, Raggi P, Berman D S, Callister T Q. Coronary artery calcium as a measure of biologic age. Atherosclerosis. 2006;188(1):112–119. doi: 10.1016/j.atherosclerosis.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Agatston A S, Janowitz W R, Hildner F J, Zusmer N R, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 4.Detrano R, Guerci A D, Carr J J. et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 5.Wang L Jerosch-Herold M Jacobs D R Jr Shahar E Detrano R Folsom A R; MESA Study Investigators. Coronary artery calcification and myocardial perfusion in asymptomatic adults: the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol 20064851018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramakrishna G, Miller T D, Breen J F, Araoz P A, Hodge D O, Gibbons R J. Relationship and prognostic value of coronary artery calcification by electron beam computed tomography to stress-induced ischemia by single photon emission computed tomography. Am Heart J. 2007;153(5):807–814. doi: 10.1016/j.ahj.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Curillova Z, Yaman B F, Dorbala S. et al. Quantitative relationship between coronary calcium content and coronary flow reserve as assessed by integrated PET/CT imaging. Eur J Nucl Med Mol Imaging. 2009;36(10):1603–1610. doi: 10.1007/s00259-009-1121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pijls N H, van Son J A, Kirkeeide R L, De Bruyne B, Gould K L. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87(4):1354–1367. doi: 10.1161/01.cir.87.4.1354. [DOI] [PubMed] [Google Scholar]
- 9.De Bruyne B, Pijls N H, Kalesan B. et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 10.Hou Z H, Lu B, Gao Y. et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5(10):990–999. doi: 10.1016/j.jcmg.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Shim S S, Kim Y, Lim S M. Improvement of image quality with beta-blocker premedication on ECG-gated 16-MDCT coronary angiography. AJR Am J Roentgenol. 2005;184(2):649–654. doi: 10.2214/ajr.184.2.01840649. [DOI] [PubMed] [Google Scholar]
- 12.Hadamitzky M, Achenbach S, Al-Mallah M. et al. Optimized prognostic score for coronary computed tomographic angiography: results from the CONFIRM registry (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry) J Am Coll Cardiol. 2013;62(5):468–476. doi: 10.1016/j.jacc.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 13.Reiber J H, Serruys P W, Kooijman C J. et al. Assessment of short-, medium-, and long-term variations in arterial dimensions from computer-assisted quantitation of coronary cineangiograms. Circulation. 1985;71(2):280–288. doi: 10.1161/01.cir.71.2.280. [DOI] [PubMed] [Google Scholar]
- 14.DeLong E R, DeLong D M, Clarke-Pearson D L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 15.Pencina M J D'Agostino R B Sr D'Agostino R B Jr Vasan R S Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond Stat Med 2008272157–172., discussion 207–212 [DOI] [PubMed] [Google Scholar]
- 16.Brosh D, Higano S T, Lennon R J, Holmes D R Jr, Lerman A. Effect of lesion length on fractional flow reserve in intermediate coronary lesions. Am Heart J. 2005;150(2):338–343. doi: 10.1016/j.ahj.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Nakazato R, Shalev A, Doh J H. et al. Aggregate plaque volume by coronary computed tomography angiography is superior and incremental to luminal narrowing for diagnosis of ischemic lesions of intermediate stenosis severity. J Am Coll Cardiol. 2013;62(5):460–467. doi: 10.1016/j.jacc.2013.04.062. [DOI] [PubMed] [Google Scholar]
- 18.Tam L M, Kim J, Blumenthal R S, Nasir K, Al-Mallah M H, Blaha M J. Absolute coronary artery calcium score is the best predictor of non-calcified plaque involvement in patients with low calcium scores (1–100) Atherosclerosis. 2013;230(1):76–79. doi: 10.1016/j.atherosclerosis.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 19.De Bruyne B, Hersbach F, Pijls N H. et al. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but “Normal” coronary angiography. Circulation. 2001;104(20):2401–2406. doi: 10.1161/hc4501.099316. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Levin D C, Halpern E J, Fischman D, Savage M, Walinsky P. Accuracy of MDCT in assessing the degree of stenosis caused by calcified coronary artery plaques. AJR Am J Roentgenol. 2008;191(6):1676–1683. doi: 10.2214/AJR.07.4026. [DOI] [PubMed] [Google Scholar]


