Summary
The bacterial pathogen Listeria monocytogenes induces internalization into mammalian cells and uses actin-based motility to spread within tissues. Listeria accomplishes this intracellular life cycle by exploiting or antagonizing several host GTPases. Internalization into human cells is mediated by the bacterial surface proteins InlA or InlB. These two modes of uptake each require a host actin polymerization pathway comprised of the GTPase Rac1, nucleation promotion factors, and the Arp2/3 complex. In addition to Rac1, InlB-mediated internalization involves inhibition of the GTPase Arf6 and participation of Dynamin and septin family GTPases. After uptake, Listeria is encased in host phagosomes. The bacterial protein GAPDH inactivates the human GTPase Rab5, thereby delaying phagosomal acquisition of antimicrobial properties. After bacterial-induced destruction of the phagosome, cytosolic Listeria uses the surface protein ActA to stimulate actin-based motility. The GTPase Dynamin 2 reduces the density of microtubules that would otherwise limit bacterial movement. Cell-to-cell spread results when motile Listeria remodel the host plasma membrane into protrusions that are engulfed by neighboring cells. The human GTPase Cdc42, its activator Tuba, and its effector N-WASP form a complex with the potential to restrict Listeria protrusions. Bacteria overcome this restriction through two microbial factors that inhibit Cdc42-GTP or Tuba/N-WASP interaction.
Introduction
Listeria monocytogenes is a Gram-positive bacterium that causes food-borne illnesses resulting in gastroenteritis, meningitis, or abortion (Posfay-Barbe and Wald, 2009). Critical for virulence is the ability of Listeria to actively induce its uptake into human cells, replicate within the host cytoplasm, and disseminate between cells while remaining in a cytoplasmic niche (Vazquez-Boland et al., 2001).
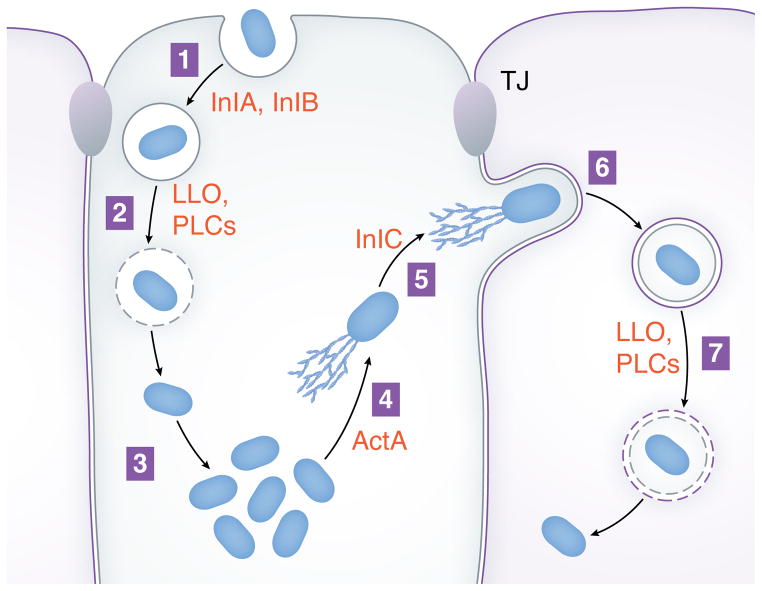
Details of the intracellular life cycle of Listeria are presented in Figure 1. Bacteria are internalized into human cells through plasma membrane remodeling mediated, in part, by the host actin cytoskeleton (step 1). Ingestion by professional phagocytes such as macrophages or neutrophils is promoted by antibody or complement and is likely independent of microbial factors (Vazquez-Boland et al., 2001). By contrast, internalization (‘entry’) into non-phagocytic cells, such as intestinal epithelial cells, involves interaction of specific bacterial surface proteins called InlA or InlB with their cognate host receptors (Ireton, 2007; Pizarro-Cerda et al., 2012). After internalization into human cells, bacteria are present in membranous structures termed ‘phagosomes’. In cell types that lack potent antimicrobial activity, a substantial proportion (~50%) of Listeria escape phagosomes from 30 min to 2 h post-infection (Vazquez-Boland et al., 2001). Phagosome lysis is promoted by a secreted pore-forming enzyme called Listeriolysin O (LLO) and the phospholipases PlcA and PlcB (step 2). Cytosolic pathogens replicate (step 3) and use a bacterial surface protein called ActA to stimulate the polymerization of host actin monomers into filaments (Gouin et al., 2005; Campellone and Welch, 2010). Filamentous (F) –actin becomes organized into tail- like structures that propel Listeria through the cytosol (step 4). Motile pathogens ultimately contact and remodel the host plasma membrane into thin membrane projections termed ‘protrusions’ (Ireton, 2013) (step 5). Efficient protrusion formation in polarized epithelial cells requires the secreted Listeria protein InlC (Rajabian et al., 2009). Bacteria-containing protrusions are internalized by neighboring human cells, resulting in microbes in double-membranous vacuoles (step 6). These vacuoles are destroyed by LLO and phospholipases, liberating bacteria into the cytosol of the newly infected cell (Vazquez-Boland et al., 2001).
Figure 1. Intracellular life cycle of Listeria.
Steps in the infection cycle are (1) internalization of bacteria into host cells, (2) escape from phagosomes, (3) replication in the cytosol, (4) actin-based motility, (5) formation of protrusions, (6) engulfment of protrusions, and (7) dissolution of the double membranous vacuole. The process of cell-to-cell spread comprises steps 4–7. Bacterial factors that promote various steps in the life cycle are in red lettering. ‘LLO’ denotes Listeriolysin O, and ‘PLCs’ indicates the phospholipases PlcA and PlcB. ‘TJ’ represents tight junctions.
The intracellular life cycle of Listeria involves pathogen-directed manipulation of the host actin cytoskeleton (steps 1 and 4) and remodeling of the plasma membrane (steps 1, 5, and 6). In eukaryotic cells, cytoskeletal assembly, vesicular trafficking, and membrane structure are controlled, in part, by GTPases of the Rho, Rab, Arf, and Dynamin families (Jaffe and Hall, 2005; Hutagalung and Novick, 2011; Menon and Schafer, 2013; Stalder and Antonny, 2013). Importantly, Listeria exploits or interferes with the functions of several of these GTPases in order to promote internalization, survival in the phagosome, actin-based motility, or protrusion formation (Bierne et al., 2005; Bosse et al., 2007; Sousa et al., 2007; Alvarez-Dominguez et al., 2008; Henmi et al., 2010; Rigano et al., 2014). The septin family of GTPases assembles into filaments that form cytoskeletal-like structures (Mostowy and Cossart, 2012). Interestingly, recent results indicate an involvement of septins in entry of Listeria (Mostowy et al., 2009a; Mostowy et al., 2009b). In this microreview, we first describe the regulation and biological functions of select Rho, Rab, Arf, Dynamin, and septin family GTPases. We then discuss several examples in which Listeria subverts or impairs host GTPase function to efficiently infect human cells.
GTPase regulation and function
Ras superfamily GTPases
The mammalian Ras superfamily of small GTPases contains more than 150 members and is organized into five branches: Ras, Rho, Rab, Ran, and Arf (Wennerberg et al., 2005). The activities of these GTPases are controlled by binding to GTP or GDP. When complexed with GTP, the GTPase protein is active and interacts with downstream effectors. When bound to GDP, the GTPase is inactive. The rate-limiting step in activation is disassociation of GDP, the first stage of nucleotide exchange. A class of regulatory proteins called guanine nucleotide exchange factors (GEFs) activate small GTPases by stimulating GDP dissociation (Schmidt and Hall, 2002). Intrinsic GTP hydrolysis activity is typically low in small GTPases (Wennerberg et al., 2005). A second class of regulatory proteins termed ‘GTPase Activating Proteins’ (GAPs)’ accelerate GTP hydrolysis, thereby terminating signaling (Tcherkezian and Lamarche-Vane, 2007). The cellular localization and/or activity of many GEFs or GAPs is controlled by environmental stimuli, such as cell-cell adhesion or engagement of integrin or growth factor receptors (Schmidt and Hall, 2002; Tcherkezian and Lamarche-Vane, 2007).
The diverse biological functions of Ras superfamily GTPases are described in several recent reviews (Jaffe and Hall, 2005; Wennerberg et al., 2005; Hutagalung and Novick, 2011; Ratheesh et al., 2013; Stalder and Antonny, 2013). Here we discuss only GTPases known to control Listeria infection: members of the Rho, Rab, and Arf branches.
Rho family GTPases include more than 20 proteins, the best characterized of which are RhoA, Rac1, and Cdc42 (Jaffe and Hall, 2005). Major roles of these GTPases include regulation of gene expression and the actin or microtubule cytoskeletons. Of particular relevance for Listeria infection is the extensively studied functions of Rac1, Cdc42, and RhoA in actin polymerization. Cdc42 and Rac1 each stimulate F-actin assembly through the Arp2/3 complex, an evolutionarily conserved, multi-component machinery that promotes the formation of branched actin filaments (Jaffe and Hall, 2005; Campellone and Welch, 2010). In order to nucleate the assembly of actin filaments, the Arp2/3 complex must be activated by one of several ‘nucleation promotion factors’ (NPFs). These NPFs are themselves regulated by various stimuli, including activated Cdc42 or Rac1. Cdc42-GTP relieves autoinhibition in the NPF N-WASP, whereas Rac1-GTP activates NPFs of the WAVE family. The end result of N-WASP or WAVE activation is the assembly of branched actin filaments via the Arp2/3 complex. Rac1 also controls the NPF cortactin, although in this case regulation occurs at the level of NPF localization (Weed et al., 1998). Finally, apart from effects on the Arp2/3 complex, some Rho family GTPases promote the formation of linear actin filaments by activating members of the mammalian Diaphanous-related formin (mDia) family (Campellone and Welch, 2010). Later in this review, we describe studies that demonstrated requirements for Rac1 or Cdc42 GTPases, NPFs, and the Arp2/3 complex in internalization of Listeria into host cells. We also discuss work indicating that Cdc42 and its effector N-WASP act together to restrict protrusion formation during cell-to-cell spread of Listeria.
Rab family GTPases control intracellular trafficking of membranes (Hutagalung and Novick, 2011). Over 60 mammalian Rab proteins exist, and many of these different proteins localize to distinct membrane compartments, including the plasma membrane, early endosomes, late endosomes, recycling endosomes, the endoplasmic reticulum (ER), the Golgi apparatus, or secretory vesicles (Hutagalung and Novick, 2011). The major function of Rab GTPases is to mediate the tethering and subsequent fusion of membrane compartments (Hutagalung and Novick, 2011). For example, one of the most extensively studied mammalian Rab proteins, Rab5, promotes fusion of early endosomes. Such fusion is needed for maturation of early endosomes to lysosomes. Rab5 also controls the maturation of phagosomes to phagolysosomes, which have potent antimicrobial properties (Flannagan et al., 2009). In this review, we will discuss how Listeria interferes with Rab5 function to impair phagosome maturation.
Like Rab proteins, Arf GTPases regulate membrane traffic at various cellular locales, including the Golgi, ER, or plasma membrane (Stalder and Antonny, 2013). The GTPase Arf6 is predominantly at the plasma membrane, where it promotes internalization and/or recycling of select membrane receptors and homeostasis of cholesterol (Naslavsky et al., 2004; Schweitzer et al., 2011). Later in the review, we describe the role of host Arf6 in entry of Listeria.
Dynamin GTPases
There are three Dynamin GTPase isoforms encoded by distinct genes (Gonzalez-Jamett et al., 2013). Dynamin 1 is neuronal specific, Dynamin 2 is ubiquitously expressed, and Dynamin 3 is expressed in the brain, testis, and lung. Dynamin 1 and 2 are the most extensively studied isoforms. These proteins promote clathrin- or caveolin-dependent endocytosis by stimulating scission at the tubular necks of plasma membrane invaginations (Gonzalez-Jamett et al., 2013). Membrane scission is dependent on GTP hydrolysis by Dynamin proteins. Apart from their roles in endocytosis, recent results indicate that Dynamins promote exocytic events (Gonzalez-Jamett et al., 2013). A third function of Dynamin proteins is to control the F-actin and microtubule cytoskeletons (Gonzalez-Jamett et al., 2013). Dynamin GTPases bind directly to actin filaments and promote filament elongation through the removal of the capping protein gelsolin. Dynamins also regulate the actin cytoskeleton through interaction with the NPF cortactin. Dynamin 2 affects microtubule function by promoting chromosome cohesion, cytokinesis, and microtubule instability. In this review, the role of Dynamin 2 in internalization and actin-based motility of Listeria will be discussed.
Septins
Human cells express 13 different septin GTPases, which are organized into four distinct groups based on amino acid similarity (Mostowy and Cossart, 2012; Oh and Bi, 2010). Septins form hetero-oligomeric complexes capable of assembling into higher ordered structures, including filaments, filament bundles, and rings. Overall, the role of GTP binding and hydrolysis in septin function is not well understood. However, the ability of GTP to induce conformational changes in domains involved in oligomerization suggests a role for nucleotide binding in filament assembly and/or stability (Sirajuddin et al., 2009). Importantly, septins interact with phosphoinositides and the actin and microtubule cytoskeletons (Mostowy and Cossart, 2012). Collectively, these findings indicate that septins form cytoskeletal-like elements that connect to ‘classic’ cytoskeletons and the plasma membrane.
A major function of septins is to control the subcellular localization of proteins in diverse processes including cytokinesis in budding yeast, tail formation in sperm, and ciliogenesis in animal cells (Mostowy and Cossart, 2012; Oh and Bi, 2010). Depending on the process, septins direct protein localization either by acting as scaffolds for recruitment or by forming barriers that restrict diffusion in the plasma membrane. Later in this review, we discuss the role of septin rings in entry of Listeria into human cells.
Role of host GTPases in Listeria entry
Listeria induces its internalization into human cells through two major pathways (Fig. 2A) (Ireton, 2007; Pizarro-Cerda et al., 2012). One of these routes is mediated by binding of the bacterial surface protein InlA (internalin) to the calcium-dependent cell-cell adhesion molecule E-cadherin. The other entry pathway is directed by interaction of the bacterial protein InlB with a host receptor tyrosine kinase called Met. Work with animal models indicates a critical role of InlA/E-cadherin interaction in infection of the intestinal epithelium (Disson and Lecuit, 2013). InlB promotes infection of hepatocytes in the liver (Ireton, 2007), and may also play a minor role in colonization of the intestine (Pentecost et al., 2010). Interestingly, both InlA and InlB contribute to infection of trophoblast tissue in the placenta (Disson and Lecuit, 2013).
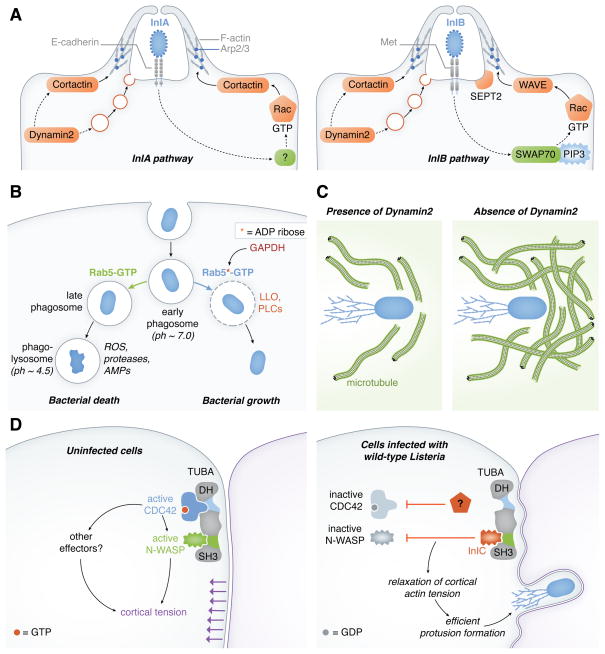
Figure 2. Subversion or inhibition of host GTPases by Listeria.
(A). GTPases involved in InlA- or InlB-mediated bacterial entry. Human GTPases and their effectors known to participate in internalization of Listeria are colored in orange. Solid arrows indicate known effects on bacterial entry, whereas dashed lines denote possible effects. InlA-dependent entry involves the GTPase Rac1 and the NPF cortactin, which promote actin polymerization through the Arp2/3 complex. ‘?’ indicates that the GEF involved in Rac1 activation is unknown. InlB-mediated entry is promoted by Rac1 and its NPFs WAVE and cortactin. The GEF SWAP70 is required for Listeria entry, and possibly activates Rac1 during this process. The septin GTPase SEPT2 is recruited to sites of Listeria entry and is required for bacterial uptake. The GTPase Dynamin 2 is needed for both InlA- and InlB- mediated internalization. Possible modes of action of Dynamin 2 include recruitment of cortactin, stimulation of focal exocytosis, or scission of the developing phagosome (not shown). For the sake of simplicity, some host GTPases that regulate Listeria entry, such as Cdc42, Arf6 and SEPT11, are not depicted in this diagram. ‘PIP3’ indicates phosphatidylinositol 3,4,5-tris phosphate. (B). Control of phagosomal survival by Rab5 GTPase. Rab5-GTP promotes the maturation of early phagosomes to phagolysosomes, contributing to killing of internalized bacteria (left pathway). By producing the protein GAPDH, Listeria diminishes Rab5-GTP levels and allows phagosomal survival until escape by LLOs and PLCs occurs (right pathway). GAPDH ADP-ribosylates Rab5, thereby inhibiting nucleotide exchange and maintaining Rab5 in a GDP bound state. ‘ROS’ indicates reactive oxygen species, and ‘AMPs’ denotes antimicrobial peptides. (C). Regulation of bacterial motility by Dynamin 2. The density of microtubules increases in cells depleted for Dynamin 2, resulting in impaired actin-based motility of Listeria. (D). Model of the mechanism of relief of host inhibition of Listeria spread by InlC and other bacterial factors. The images in each panel represent XY views (cross sections) at the level of the tight junction. Left panel: Without InlC or other bacterial proteins, cortical tension at the plasma membrane is generated by [1] The Tuba SH3 domain binding to N-WASP to recruit N-WASP to the plasma membrane and/or activate N-WASP, and [2] Tuba-mediated stimulation of Cdc42 to activate N-WASP and/or other effectors (?). Right panel: In cells infected with wild-type Listeria, the bacterial protein InlC blocks N-WASP binding to Tuba SH3 domain. In addition, an unknown bacterial factor ‘?’ reduces Cdc42 activity. The combined effects of InlC and the unidentified microbial factor reduce cortical tension and allow efficient protrusion formation by Listeria.
Rho family GTPases
Several studies have addressed the function of Rho GTPases in InlA- or InlB-dependent entry of Listeria into cultured human cells (Bierne et al., 2001; Bierne et al., 2005; Bosse et al., 2007; Sousa et al., 2007). Overall the results support the idea that these GTPases promote actin polymerization during bacterial uptake (Fig. 2A). F-actin assembly is essential for both InlA- and InlB-mediated internalization, and is thought to provide a protrusive force that drives the host plasma membrane around adherent bacteria (Ireton, 2007). InlA-mediated entry was found to require the Rho family GTPase Rac1, but not Cdc42 (Sousa et al., 2007). Dominant negative inhibition of the Arp2/3 complex impaired bacterial entry, suggesting an important role for Arp2/3-dependent F-actin assembly. Supporting this idea, Rac1 and Arp3 were recruited to adherent bacteria. Importantly, Arp3 recruitment occurred a few minutes prior to F-actin assembly associated with bacterial uptake. While the NPFs WAVE and N-WASP were dispensable for InlA-mediated entry, cortactin activity was needed for this process. Rac1 is known to promote recruitment of cortactin to the plasma membrane (Weed et al., 1998), suggesting that the GTPase might exert a similar function during Listeria entry.
Like InlA-dependent entry, the InlB internalization pathway requires host Rac1 and Arp2/3 (Bierne et al., 2001; Bierne et al., 2005; Bosse et al., 2007). WAVE proteins are also involved in InlB-mediated entry, suggesting that these proteins serve as NPFs downstream of Rac1 (Bierne et al., 2005; Bosse et al., 2007). While Rac1 is required for the InlB entry route in all cell lines tested, Cdc42 promotes bacterial uptake only in select cell lines, including HeLa cells and mouse embryonic fibroblasts (MEFs) (Bierne et al., 2001; Bierne et al., 2005; Bosse et al., 2007). Adding to this complexity, the precise way that Cdc42 controls entry varies with cell type. In HeLa cells, a requirement for N-WASP in Listeria internalization suggests that this NPF probably acts downstream of Cdc42 (Bierne et al., 2005). By contrast, N-WASP is dispensable for InlB-mediated entry into MEFs (Bosse et al., 2007). The role of Cdc42 in these cells is instead to activate Rac1 through a mechanism that has not been characterized.
An important question is how is Rac1 or Cdc42 activated downstream of E-cadherin or the Met receptor to promote bacterial entry? Several Rac1 and Cdc42 GEFs are stimulated upon binding to phosphatidylinositol 3,4,5-trisphosphate (PIP3), a lipid product of type IA PI 3 kinase (Schmidt and Hall, 2002). A recent RNA interference (RNAi)-based screen identified 9 components of the host PI 3-kinase signaling pathway that promote InlB-mediated entry of Listeria (Jiwani et al., 2012). One these human proteins, SWAP70, is a known GEF for Rac1. Several other Rac1 GEFs including DOCK180, SOS1, and Vav2 were dispensable for entry. These findings suggest that SWAP70 might control Rac1 activation during InlB-mediated entry. This idea needs to be tested through measurement of Rac1 activity in SWAP70-depleted cells infected with Listeria. Thus far Rac1 GEFs involved in InlA-dependent entry of Listeria have yet to be identified.
Interestingly, the above work with Rho family GTPases suggests that internalization of Listeria might occur through subversion of the same host signaling pathways that mediate the normal biological functions of E-cadherin or Met. Similarly to InlA-dependent entry, the formation and maintenance of adherens junctions requires Rac1, cortactin, and the Arp2/3 complex (Bershadsky, 2004; Ratheesh et al., 2013). Cell migration stimulated by the Met ligand hepatocyte growth factor (HGF) resembles InlB-dependent entry in the involvement of Rac1, Cdc42, N-WASP, and WAVE proteins (Kawamura et al., 2004; Ridley et al., 1995; Royal et al., 2000). It seems likely that InlA and InlB stimulate microbial uptake (rather than cell adhesion or motility) because these proteins are linked to the bacterial surface, allowing engagement of a small subset of E-cadherin or Met receptors. Such local engagement causes focused signal transduction and actin polymerization resulting in microbial engulfment (Bierne et al., 2001; Bierne et al., 2005; Veiga and Cossart, 2005; Sousa et al., 2007; Veiga et al., 2007; Bonazzi et al., 2008, Gavicherla et al., 2010). Conversely, homophilic interaction of E-cadherin molecules on adjacent cells or HGF stimulation of the Met receptor occurs over a broad surface area and likely results in more diffuse signaling events. Consistent with this view, latex beads (1 micrometer in diameter) coated with the E-cadherin extracellular domain are internalized by human cells expressing E-cadherin (Bonazzi et al., 2008). Conversely, ectopic expression of InlA on the surface of human cells results in the formation of junctions with neighboring E-cadherin –expressing cells (Bonazzi et al., 2012). Finally, soluble InlB protein stimulates epithelial cell motility (Ireton, 2007).
Arf GTPases
Recent results indicate that the Arf family protein Arf6 controls InlB-mediated internalization of Listeria into host cells (Gavicherla et al., 2010). Arf6 activity is antagonized by several GAPs, including the protein ARAP2 (Yoon et al., 2006). Importantly, experiments involving RNAi and dominant negative alleles indicate a critical role for host ARAP2 in entry of Listeria (Gavicherla et al., 2010). ARAP2 is also essential for accumulation of F-actin during uptake of InlB-coated latex beads, indicating that this human protein promotes F-actin remodeling that drives particle engulfment.
ARAP2 has several functional domains, including a pleckstrin homology (PH) domain that binds PIP3 and a GAP domain active on Arf6 (Yoon et al., 2006). Interaction of PIP3 with the PH domain stimulates GAP activity, indicating that ARAP2 is regulated by type IA PI 3-kinase. Since Listeria induces PIP3 accumulation during InlB-dependent entry (Ireton, 2007), it is plausible that bacteria stimulate ARAP2 activity. However, this idea remains to demonstrated. Importantly, experiments with allele mutated in the GAP domain indicate a requirement for GAP activity in Listeria entry (Gavicherla et al., 2010). Strikingly, the inhibition in entry caused by the GAP defective ARAP2 allele is suppressed by RNAi-mediated depletion of Arf6. In addition, overexpression of a constitutively activated allele of Arf6 blocks internalization of Listeria. These latter findings indicate that ARAP2 promotes entry through antagonism of Arf6-GTP. How unrestrained accumulation of Arf6-GTP in the absence of ARAP2 restricts InlB-mediated entry is not understood. Based on findings that constitutively activated Arf6 induces intracellular accumulation of cholesterol (Naslavsky et al., 2004), it was proposed that ARAP2 might be needed for plasma membrane localization of this lipid, which is known to promote Listeria entry (Ireton, 2007; Pizarro-Cerda et al., 2012). This idea needs to be tested.
Dynamin
Dynamin 2 is needed for both InlA- and InlB-mediated entry of Listeria (Veiga and Cossart, 2005; Veiga et al., 2007). How this GTPase controls bacterial uptake is not understood. Possible mechanisms include scission of the neck of the phagosome (akin to the role of Dynamins in endocytosis), control of the actin cytoskeleton through interaction with cortactin, or exocytosis (Fig. 2A) (Gonzalez-Jamett et al., 2013). Interestingly, Dynamin 2 promotes the internalization of antibody-coated particles in macrophages by stimulating focal exocytosis- i.e. the localized delivery of membrane to the phagocytic cup (Di et al., 2003). Focal exocytosis is thought to replenish plasma membrane that would otherwise be lost due to particle internalization. It would be interesting to investigate if Dynamin 2 has a similar exocytic function during entry of Listeria.
Septins
Recent studies have demonstrated an important role for select septins in InlB-mediated internalization of Listeria. Importantly, three septin proteins, SEPT2, SEPT9, and SEPT11, are recruited to bacteria undergoing InlB-mediated uptake into human cells (Mostowy et al., 2009b) (Fig. 2A). These septins accumulate immediately adjacent to areas of intense F-actin labeling, creating the appearance of septin ‘collars’ that underlie actin-rich extensions of the plasma membrane. Interestingly, treatment of host cells with cytochalasin D, an inhibitor of actin polymerization, blocked septin enrichment near adherent bacteria. These latter findings indicate that F-actin is needed for septin recruitment, and are consistent with a previous in vitro study showing that F-actin bundles can alter the higher ordered structure of septins (Mostowy et al., 2012). Importantly, experiments involving RNAi-mediated depletion indicate the SETP2 is needed for efficient InlB-mediated bacterial entry (Mostowy et al., 2009b) Scanning electron microscopy analysis showed that SEPT2 is required for remodeling of the host cell surface that normally mediates engulfment of InlB-coated particles. Finally, SEPT2 was found to promote signal transduction downstream of the Met receptor, since RNAi-mediated depletion of the septin impaired activation of type IA PI 3-kinase, as assessed by a FRET-based assay. In contrast to the situation observed with SEPT2, SEPT11 is not required for entry but instead negatively controls Listeria uptake (Mostowy et al., 2009a).
How are septins recruited to invading Listeria? In budding yeast, the Rho GTPase Cdc42 directs the assembly and maturation of septin rings that promote cytokinesis between the mother cell and bud (Oh and Bi, 2010). It would be interesting to determine the role of Cdc42 or other Rho GTPases in SEPT2 accumulation during InlB-mediated entry. Another important question is how do septins control bacterial uptake? Atomic Force Microscopy (AFM) analysis of human cells supports the idea that SEPT2 enhances binding of InlB to the Met receptor (Mostowy et al., 2011). Conversely, SEPT11 attenuates binding. In addition, depletion of SEPT2 reduces the effective viscosity and elasticity of cells. Collectively, the AFM findings were interpreted as meaning that SEPT2 might anchor the Met receptor and plasma membrane to the cortical actin cytoskeleton. If so, then it is possible that SEPT2 and actin restrict Met to particular membrane domains, resulting in enhanced interaction with InlB and subsequent downstream signaling. In this context, it would be interesting to examine the effect of SEPT2 depletion on clustering of the Met receptor normally induced during bacterial entry (Bierne et al., 2001). Given the established roles of septins in localization of cytoplasmic proteins through scaffolding or barrier functions (Mostowy and Cossart, 2012; Oh and Bi, 2010), it is also important to determine if SEPT2 affects recruitment of various signaling or endocytic proteins known to mediate entry downstream of Met (Ireton, 2007; Pizarro-Cerda et al., 2012).
Bacterial interference with Rab5-mediated phagosome maturation
The human GTPase Rab5 promotes the maturation of phagosomes in macrophages by stimulating fusion with early endosomes (Flannagan et al., 2009). The resulting phagolysosomes kill bacteria through several mechanisms, including acidic pH, production of reactive oxygen species (ROS), proteolytic activity, and antimicrobial peptides. Interestingly, Listeria retards phagosome maturation by producing a factor that inactivates host Rab5 (Prada-Delgado et al., 2001; Prada-Delgado et al., 2005; Alvarez-Dominguez et al., 2008). This strategy is thought to augment bacterial survival by providing sufficient time for phagosomal escape mediated by bacterial LLO and phospholipases (Fig. 2B).
Treatment of macrophages with IFN-γ leads to an enhanced killing of Listeria that correlates with phagosomal recruitment of Rab5 and acquisition of lysosomal markers (Prada-Delgado et al., 2001). Experiments involving antisense or dominant negative alleles indicate that Rab5 is needed for efficient ROS-mediated killing of phagosomal Listeria (Prada-Delgado et al., 2001; Prada-Delgado et al., 2005). Importantly, Listeria secretes a protein that inhibits nucleotide exchange on Rab5, resulting in decreased Rab5-GTP levels (Prada-Delgado et al., 2005). This bacterial protein was identified as glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which has NAD-dependent ADP ribosylation activity specific for Rab5 (Alvarez-Dominguez et al., 2008). The resulting covalently modified Rab5 is unable to interact with its GEF Vps9, and is therefore locked in an inactive GDP-bound state. An interesting unresolved question is how does bacterial GADPH, which is produced in the phagosome, access Rab5 bound to the cytoplasmic face of the cytoplasmic membrane? Interestingly, fractionation experiments indicate that GAPDH is present in the phagosomal membrane (Alvarez-Dominguez et al., 2008). How membrane insertion occurs is not known, but may involve the amphipathic amino-terminal domain of GAPDH. This same domain has ADP ribosylation activity, suggesting that GAPDH in the phagosome membrane might interact with host Rab5 in the cytoplasm. Collectively, the findings on GAPDH and Rab5 highlight a novel mechanism used by Listeria to enhance intracellular survival through delayed phagosome maturation.
Host GTPases involved in cell-to-cell spread
Modulation of actin-based motility by Dynamin 2
The ability of Listeria to spread to surrounding host cells requires the bacterial surface protein ActA (Gouin et al., 2005; Campellone and Welch, 2010). ActA localizes asymmetrically at one bacterial pole and stimulates the assembly of F-actin comet tails that drive microbial motility. Remarkably, ActA directly activates the human Arp2/3 complex, and is considered as a structural and functional mimic of the eukaryotic NPF N-WASP (Gouin et al., 2005; Campellone and Welch, 2010).
Although ActA does not require host factors to activate Arp2/3, recent findings reveal that host Dynamin 2 can indirectly modulate actin-based motility of Listeria. Dynamin 2 is present in Listeria comet tails (Lee and De Camilli, 2002) and RNAi-mediated depletion of this host protein reduces the speed of bacterial motility (Henmi et al., 2011). Interestingly, the mechanism by which Dynamin 2 impairs motility involves microtubules. Dynamin 2 depletion induces an increase in acetylated microtubules, which subsequently form a dense network in the cytoplasm (Henmi et al., 2011). Treatment of human cells with drugs that stabilize or depolymerize microtubules creates microtubule-free spaces in the cytoplasm. Importantly, these drug treatments also restore normal actin-based motility in cells depleted for Dynamin 2. These findings suggest that microtubules have the potential to act as a barrier to Listeria motility, and that Dynamin 2 helps remove this barrier (Fig. 2C).
Role of Cdc42 in Listeria protrusion formation
ActA-mediated motility ultimately results in the formation of membrane protrusions that mediate cell-to-cell spread of bacteria (Ireton, 2013). In polarized epithelial cells, Listeria produces at least two factors that act after ActA to enhance protrusion formation (Rajabian et al., 2009; Rigano et al., 2014). These microbial factors target a host protein complex comprised of Cdc42 GTPase, a Cdc42-specific GEF called Tuba, and the NPF N-WASP (Fig. 2D).
Tuba, Cdc42, and N-WASP form a complex at tight junctions of epithelial cells, where they control junctional structure (Otani et al., 2006). Tuba is a large scaffolding protein with a GEF domain that activates Cdc42, a Bar domain that interacts with lipids, and a carboxyl-terminal Src Homology 3 (SH3) domain that binds N-WASP (Salazar et al., 2003; Otani et al., 2006). The GEF and SH3 domains likely cooperate to activate N-WASP through production of Cdc42-GTP and engagement of proline-rich sequences in N-WASP, respectively (Suetsugu and Gautreau, 2012). The Tuba/Cdc42/N-WASP complex is thought to generate cortical tension at tight junctions, since inhibition of these proteins causes normally linear junctions to slacken (Otani et al., 2006; Rajabian et al., 2009).
Importantly, the Tuba/Cdc42/N-WASP complex has the potential to restrain protrusion formation by Listeria. The role of this host complex was revealed in studies with a Listeria mutant deleted for the inlC gene. The inlC deletion (ΔinlC) mutant is partly defective in protrusion formation (Rajabian et al., 2009). This defect is suppressed by RNAi-mediated depletion of Tuba or N-WASP or by dominant negative inhibition of Cdc42 (Rajabian et al., 2009; Rigano et al., 2014). By contrast, inhibition of Tuba, N-WASP, or Cdc42 does not affect protrusion formation by wild-type Listeria. These findings indicate that the Tuba/Cdc42/N-WASP complex antagonizes spread of bacteria lacking InlC. By contrast, wild-type Listeria relieves this antagonism and spreads efficiently.
There are at least two mechanisms by which wild-type Listeria counteracts the restriction of spread exerted by the Tuba/Cdc42/N-WASP complex (Fig. 2D). The first mode involves binding of InlC to the same SH3 domain in Tuba that normally interacts with N-WASP (Polle et al., 2014; Rajabian et al., 2009). InlC and N-WASP bind to partly overlapping sites in the Tuba SH3 domain (Polle et al., 2014). The higher affinity of InlC for this domain results in the displacement of N-WASP. The second mode of inhibition of the Tuba/Cdc42/N-WASP complex involves an unidentified Listeria factor that decreases the cellular levels of Cdc42-GTP (Rigano et al., 2014). The mechanism of antagonism of Cdc42 is not known. Importantly, the ability of Listeria to downregulate host Cdc42 is critical for bacterial spread, since a constitutively activated allele of Cdc42 blocks protrusion formation. How do the combined activities of InlC and the unidentified bacterial factor promote spread? Since Cdc42-GTP and SH3 domain engagement additively stimulate N-WASP (Suetsugu and Gautreau, 2012), it is possible that the two Listeria factors each affect protrusion formation through inhibition of N-WASP. Alternatively, Listeria inhibition of Cdc42-GTP may affect bacterial spread through both N-WASP and additional human effectors.
How does the Tuba/Cdc42/N-WASP complex restrict spread of ΔinlC mutant Listeria? Inhibition of protrusions is thought to involve the ability of the Tuba/Cdc42/N-WASP complex to generate cortical tension at tight junctions (Otani et al., 2006; Rajabian et al., 2009). This tension provides an inward force at the plasma membrane that likely counteracts the outward force made by motile bacteria. In support of a role for cortical tension, wild-type Listeria induces slackening of tight junctions in polarized epithelial cells (Polle et al., 2014; Rajabian et al., 2009; Rigano et al., 2014). By contrast, ΔinlC mutant bacteria fail to alter cell junctions. A constitutively activated allele of Cdc42 prevents the slackening of junctions normally caused by wild-type Listeria, demonstrating that bacterial inhibition of Cdc42 is needed for relief of cortical tension (Rigano et al., 2014). The collective results on Listeria spread and junctional structure indicate that the Tuba/Cdc42/N-WASP complex presents a barrier to bacterial spread that is overcome by microbial factors targeting Cdc42 and the Tuba SH3 domain (Fig. 2D).
Future Perspectives
The ability of Listeria to exploit or disrupt host GTPase function is important for bacterial entry, intracellular survival, actin-based motility, and protrusion formation. It seems likely that future work will uncover new roles for GTPases in other steps of the Listeria intracellular life cycle. For example, the process by which Listeria-containing protrusions are engulfed by neighboring human cells (Fig. 1; step 6) is not well understood. Since Rac1 and Cdc42 are often involved in phagocytic events (Flanagan et al., 2009) it is plausible that internalization of protrusions involves one or more of these Rho GTPases. Protrusion engulfment might also require Dynamin 2, which could direct scission of the invaginating plasma membrane in the cell receiving the protrusion (Ireton, 2013). In future investigations, it will be useful to perform genetic screens that comprehensively investigate the roles of host GTPases in Listeria infection. Such large-scale investigations may uncover novel functions for some of the less extensively studied GTPases in bacterial pathogenesis. In future work, it also will be useful to include analyses of host proteins that cooperate with GTPases to induce membrane remodeling. Prime examples are proteins with BAR domains (Suetsugu and Gautreau, 2012). BAR domains have convex or concave structures that sense and/or induce membrane curvature. Importantly, several BAR domain proteins act together with Rac or Cdc42 GTPases and their respective NPFs to induce invaginations or protrusions of the plasma membrane. Surprisingly, the functions of BAR domain proteins in membrane remodeling during Listeria entry, protrusion formation, and protrusion engulfment have yet to be addressed. Future work will lead to a better understanding of the molecular mechanisms of Listeria infection and also may provide new insights on mammalian GTPase function.
Acknowledgments
We apologize to those whose work could not be cited because of space constraints. Research in K.I.’s laboratory is supported by grants from the University of Otago, the Marsden Fund of the Royal Society of New Zealand (10-UOO-015 and 13-UOO-085), the Lottery Health Fund of New Zealand, and the National Institutes of Health (R01AI085072).
References
- Alvarez-Dominguez C, Madrazo-Toca F, Fernandez-Prieto L, Vanderkerckhove J, Pareja E, Tobes R, Gomez-Lopez MT, Del Cerro-Vadillo E, Fresno M, fLC, Carrasco-Marin E. Characterization of a Listeria monocytogenes protein interfering with Rab5a. Traffic. 2008;9:325–337. doi: 10.1111/j.1600-0854.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- Bershadsky A. Magic touch: how does cell-cells adhesion trigger actin assembly? Trends Cell Biol. 2004;14:589–593. doi: 10.1016/j.tcb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Bierne H, Gouin E, Roux P, Caroni P, Yin HL, Cossart P. A role for cofilin and LIM kinase in Listeria-induced phagocytosis. Journal of Cell Biology. 2001;155:101–112. doi: 10.1083/jcb.200104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierne H, Miki H, Innocenti M, Scita G, Gertler FB, Takenawa T, Cossart P. WASP-related proteins, Abi and Ena/VASP are required for Listeria invasion induced by the Met receptor. J Cell Sci. 2005;118:1537–1547. doi: 10.1242/jcs.02285. [DOI] [PubMed] [Google Scholar]
- Bonazzi M, Kühbacher A, Toledo-Arana A, Mallet A, Vasudevan L, Pizarro-Cerda J, Brodsky FM, Cossart P. A common clathrin-mediated machinery coordinates cell-cell adhesion and bacterial internalization. Traffic. 2012;13:1653–1666. doi: 10.1111/tra.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonazzi M, Veiga E, Pizarro-Cerda J, Cossart P. Successive post-translational modifications of E-cadherin are required for InlA-mediated internalization of Listeria monocytogenes. Cell Microbiol. 2008;10:2208–2222. doi: 10.1111/j.1462-5822.2008.01200.x. [DOI] [PubMed] [Google Scholar]
- Bosse T, Ehinger J, Czuchra A, Benesch S, Steffen A, Wu X, Schloen K, Niemann HH, Scita G, Stradal TEB, et al. Cdc42 and phosphoinositide 3-kinase drive Rac-mediated actin polymerization downstream of c-Met in distinct and common pathways. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–251. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di A, Nelson DJ, Bindokas V, Brown ME, Libunao F, Palfrey HC. Dynamin regulates focal exocytosis in phagocytosing macrophages. Mol Biol Cell. 2003;14:2016–2028. doi: 10.1091/mbc.E02-09-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disson O, Lecuit M. In vitro and in vivo models to study human listeriosis: mind the gap. Microbes Infect. 2013;15:971–980. doi: 10.1016/j.micinf.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Flannagan RS, Cosio B, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- Gavicherla B, Ritchey L, Gianfelice A, Kolokoltsov AA, Davey RA, Ireton K. Critical role for the host GTPase-activating protein ARAP2 in InlB-mediated entry of Listeria monocytogenes. Infec Immun. 2010;78:4532–4541. doi: 10.1128/IAI.00802-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Jamett AM, Momboisse F, Haro-Acuna V, Bevilacqua JA, Caviedes P, Cardenas AM. Dynamin-2 function and dysfunction along the secretory pathway. Front Endocrinol. 2013;4:126. doi: 10.3389/fendo.2013.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin E, Welch MD, Cossart P. Actin-based motility of intracellular pathogens. Curr Opin Microbiol. 2005;8:35–45. doi: 10.1016/j.mib.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Henmi Y, Tanabe K, Takei K. Disruption of microtubule network rescues aberrant acti comets in dynamin 2-depleted cells. PLoS ONE. 2011;6:e28603. doi: 10.1371/journal.pone.0028603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K. Entry of the bacterial pathogen Listeria monocytogenes into mammalian cells. Cell Microbiol. 2007;9:1365–1375. doi: 10.1111/j.1462-5822.2007.00933.x. [DOI] [PubMed] [Google Scholar]
- Ireton K. Molecular mechanisms of cell-cell spread of intracellular bacterial pathogens. Open Biol. 2013;3:130079. doi: 10.1098/rsob.130079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jiwani S, Wang Y, Dowd GC, Gianfelice A, Pichestapong P, Gavicherla B, Vanbennekom N, Ireton K. Identification of components of the type IA phosphoinositide 3-kinase pathway that promote internalization of Listeria monocytogenes. Infec Immun. 2012;80:1252–1266. doi: 10.1128/IAI.06082-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Takano K, Suetsugu S, Kurisu S, Yamazaki D, Miki H, Takenawa T, Endo T. N-WASP and WAVE2 acting downstream of phosphatidylinositol 3-kinase are required for myogenic cell migration induced by hepatocyte growth factor. J Biol Chem. 2004;279:54862–54871. doi: 10.1074/jbc.M408057200. [DOI] [PubMed] [Google Scholar]
- Lee E, De Camilli P. Dynamin at actin tails. Proc Natl Acad Sci USA. 2002;99:161–166. doi: 10.1073/pnas.012607799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S, Cossart P. Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol. 2012;13:183–194. doi: 10.1038/nrm3284. [DOI] [PubMed] [Google Scholar]
- Mostowy S, Danckaert A, Tham TN, Machu C, Guadagnini S, Pizarro-Cerda J, Cossart P. Septin 11 restricts InlB-mediated invasion by Listeria. J Biol Chem. 2009a;284:11613–11621. doi: 10.1074/jbc.M900231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S, Janel S, Forestier C, Roduit C, Kasas S, Pizarro-Cerda J, Cossart P, Lafont F. A role for septins in the interaction between Listeria monocytogenes invasion protein InlB and the Met receptor. Biophys J. 2011;100:1949–1959. doi: 10.1016/j.bpj.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S, Tham TN, Danckaert A, Guadagnini S, Boisson-Dupuis S, Pizarro-Cerda J, Cossart P. Septins regulate bacterial entry into host cells. PLoS ONE. 2009b;4:e4196. doi: 10.1371/journal.pone.0004196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Bi E. Septin structure and function in yeast and beyond. Trends Cell Biol. 2010;21:141–148. doi: 10.1016/j.tcb.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani T, Ichii T, Aono S, Takeichi M. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J Cell Biol. 2006;175:135–146. doi: 10.1083/jcb.200605012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentecost M, Kumaran J, Ghosh P, Amieva MR. Listeria monocytogenes Internalin B activates junctional endocytosis to accelerate intestinal invasion. PLoS Pathog. 2010;6:e1000900. doi: 10.1371/journal.ppat.1000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Cerda J, Kühbacher A, Cossart P. Entry of Listeria monocytogenes in mammalian epithelial cells: an updated view. Cold Spring Harb Perspect Med. 2012;2:a010009. doi: 10.1101/cshperspect.a010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle L, Rigano LA, Julian R, Ireton K, Schubert WD. Structural details of human Tuba recruitment by InlC of Listeria monocytogenes elucidate bacterial cell-cell spreading. Structure. 2014;22:304–314. doi: 10.1016/j.str.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Posfay-Barbe KM, Wald ER. Listeriosis. Semin Fetal Neonatal Med. 2009;14:228–233. doi: 10.1016/j.siny.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Prada-Delgado A, Carrasco-Marin E, Bokoch GM, Alvarez-Dominguez C. Interferon-γ listericidal action is mediated by novel Rab5a functions at the phagosomal environment. J Biol Chem. 2001;276:19059–19065. doi: 10.1074/jbc.M101639200. [DOI] [PubMed] [Google Scholar]
- Prada-Delgado A, Carrasco-Marin E, Pena-Macarro C, Del Cerro-Vadillo E, Fresno-Escudero M, Leyva-Cobian F, Alvarez-Dominguez C. Inhibition of Rab5a exchange activity is a key step for Listeria monocytogenes survival. Traffic. 2005 doi: 10.1111/j.1600-0854.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- Rajabian T, Gavicherla B, Heisig M, Muller-Altrock S, Goebel W, Gray-Owen SD, Ireton K. The bacterial virulence factor InlC perturbs apical cell junctions and promotes cell-to-cell spread of Listeria. Nat Cell Biol. 2009;11:1212–1218. doi: 10.1038/ncb1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratheesh A, Priya R, Yap AS. Coordinating Rho and Rac: The regulation of Rho GTPase signaling and cadherin junctions. Prog Mol Biol Transl Sci. 2013;116:49–68. doi: 10.1016/B978-0-12-394311-8.00003-0. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigano LA, Dowd GC, Wang Y, Ireton K. Listeria monocytogenes antagonizes the human GTPase Cdc42 to promote bacterial spread. Cell Microbiol. 2014 doi: 10.1111/cmi.12260. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal I, Lamarche-Vane N, Lamorte L, Kaibuchi K, Park M. Activation of Cdc42, Rac, PAK, and Rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol Biol Cell. 2000;11:1709–1725. doi: 10.1091/mbc.11.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar MA, Kwiatkowski AV, Pellegrini L, Cestra G, Butler MH, Rossman KL, Serna DM, Sondek J, Gertler FB, De Camilli P. Tuba, a novel protein containing Bin/Amphiphysin/Rvs and Dbl homology domains, links dynamin to regulation of the actin cytoskeleton. J Biol Chem. 2003;278:49031–49043. doi: 10.1074/jbc.M308104200. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- Schweitzer JK, Sedgwick AE, D’Souza-Schorey C. Arf6-mediated endocytic recycling impacts cell movement, cell division, and lipid homeostasis. Sem Cell Dev Biol. 2011;22:39–47. doi: 10.1016/j.semcdb.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirajuddin M, Farkasovsky M, Zent E, Wittinghofer A. GTP-induced conformational changes in septins and implications for function. Proc Natl Acad Sci USA. 2009;106:16592–16597. doi: 10.1073/pnas.0902858106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa S, Cabanes D, Bougneres L, Lecuit M, Sansonetti P, Tran-Van-Nhieu G, cossart P. Src, cortactin and Arp2/3 complex are required for E-Cadherin-mediated internalization of Listeria into cells. Cell Microbiol. 2007;9:2629–2643. doi: 10.1111/j.1462-5822.2007.00984.x. [DOI] [PubMed] [Google Scholar]
- Stalder D, Antonny B. Arf GTPase regulation through cascade mechanisms and positive feedback loops. FEBS Lett. 2013;587:2028–2035. doi: 10.1016/j.febslet.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Suetsugu S, Gautreau A. Synergistic BAR-NPF interactions in actin-driven membrane remodeling. Trends Cell Biol. 2012:22. doi: 10.1016/j.tcb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol Cell. 2007;99:67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga E, Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol. 2005;7:894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- Veiga E, Guttman JA, Bonazzi M, Boucrot E, Toledo-Arana A, Lin Ae, Enninga J, Pizarro-Cerda J, Finlay BB, Kirchhausen T, Cossart P. Invasive and adherent bacterial pathogens co-opt hos clathrin for infection. Cell Host Microbe. 2007;2:340–351. doi: 10.1016/j.chom.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed SA, Du Y, Parsons JT. Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J Cell Sci. 1998;111:2433–2443. doi: 10.1242/jcs.111.16.2433. [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- Yoon HY, Miura K, Cuthbert EJ, Davis KK, Ahvazi B, Casanova JE, Randazzo PA. ARAP2 effects on the actin cytoskeleton are dependent on Arf6-specific GTPase-activating-protein activity and binding to RhoA-GTP. Journal of Cell Science. 2006;119:4650–4666. doi: 10.1242/jcs.03237. [DOI] [PubMed] [Google Scholar]