Abstract
Objectives
We examined the utility of circulating total and IgG4+ plasmablasts as biomarkers of diagnosis and disease activity in IgG4-related disease (IgG4-RD).
Materials & Methods
We evaluated patients with active, untreated, biopsy-proven IgG4-RD affecting an array of organs. Flow cytometry was used to measure total plasmablast and IgG4+ plasmablast counts by gating peripheral blood for CD19lowCD38+CD20−CD27+cells and CD19lowCD38+CD20−CD27+IgG4+cells. Serum IgG4 concentrations were measured by nephelometry. We compared 37 IgG4-RD patients to 35 controls, including healthy individuals (n=14) and patients with other inflammatory diseases prior to treatment (n=21).
Results
TheIgG4-RD patients’ mean age was 59, and 68% were male. Fourteen patients (38%) had three or more organs involved. The IgG4-RD patients had substantially elevated total plasmablast counts (median: 4,698/mL; range: 610–79,524/mL) compared to both untreated disease controls (median: 592/mL; range: 19–4,294/mL;P<0.001) and healthy controls (median: 94/mL; range: 1–653/mL; P<0.001).
Thirteen IgG4-RD patients (36%) had normal serum IgG4 concentrations (mean: 60 mg/dL; range: 5–123 mg/dL; normal: <135 mg/dL). However, the median plasmablast count was not significantly lower in that subset with normal serum IgG4 concentrations compared to those with elevated serum IgG4: 3,784/mL versus 5,155/mL, respectively (P=0.242). Among the 12 rituximab (RTX)-treated patients, the median plasmablast level during disease flare was 6,356/mL (range: 1,123–41,589/mL), declining to 1,419/ml (range: 386/mL–4,150/mL) during remission (P<0.01).
Conclusions
Circulating plasmablasts are elevated in active IgG4-RD, even in patients with normal serum IgG4 concentrations. Plasmablast counts are a potentially useful biomarker for diagnosis, assessing response to treatment, and determining the time to re-treat patients.
Keywords: IgG4-Related Disease, IgG4, Plasmablast, Rituximab
IgG4-related disease (IgG4-RD) is an immune-mediated, fibro-inflammatory disease that leads to tissue damage, organ dysfunction and, if untreated, to organ failure[1]. The disease can affect almost any anatomic location, but the sites involved most commonly are the pancreas, salivary glands, orbital adnexa, lymph nodes, and retroperitoneum[2, 3]. IgG4-RD, typically diagnosed among individuals who are middle-aged, is characterized by a male predominance except with regard to organs of the head and neck (e.g., the salivary glands and orbits), where the gender distribution is approximately equal[3–5]. The epidemiology of IgG4-RD remains poorly understood because of its recognition only recently as a multi-organ disease. However, IgG4-RD accounts for many conditions once regarded as disparate, single-organ disorders[6].
The current gold standard for the diagnosis of IgG4-RD is the identification of characteristic histology and immunohistochemistry features through biopsy. These pathology features are consistent across the full range of organs affected by IgG4-RD[7–9]. However, histopathologic variation can occur according to the stage of the lesion; that is, longstanding disease may be predominately fibrotic and a cellular. Confirming the diagnosis of IgG4-RD in such cases can be difficult[10]. Moreover, IgG4-RD organ pathology and IgG4-RD mimickers, such as granulomatosis with polyangiitis (formerly Wegener’s), sarcoidosis, histiocytosis, and malignancies (e.g., lymphoma and adenocarcinoma of the pancreas), may share similar features including an IgG4-positive plasma cell infiltrate[11, 12]. Reliance upon serum IgG4 concentrations to diagnose IgG4-RD is similarly problematic because both the specificity and positive predictive value of serum IgG4 concentrations are poor[13].
B cell depletion appears to be an effective treatment strategy for IgG4-RD but the assessment of disease activity following treatment and appropriate timing of re-treatment remains challenging. In patients with a normal serum IgG4 concentration at baseline, for example, serial measurements are of limited utility. Even in patients with elevated baseline IgG4 concentrations, the decline of IgG4 following successful treatment is variable; measurements frequently remain elevated, albeit lower. A biomarker with high sensitivity and specificity for active IgG4-RD would be of tremendous value for confirming diagnostic suspicion, assessing response to treatment, and identifying impending relapse.
Both humoral immunity and cell-mediated immunity have been implicated in IgG4-RD pathophysiology[14–16]. The disease’s response to depletion of peripheral CD20+ naïve and memory B-cells with rituximab supports the hypothesis that B-cells play an important role in IgG4-RD pathogenesis, either directly or through the effects of B-cell depletion on T-cell behavior, as is theorized in other conditions[17–19].
Plasmablasts, derived from the B-cell lineage and characterized as CD19lowCD20−CD38+CD27+, comprise a stage intermediate between activated B-cells and plasma cells[20]. Plasmablasts are generally rare in the peripheral blood of healthy individuals[20, 21], but expansions are observed briefly during responses to infection or vaccination[22]. In contrast, in the setting of autoimmunity and persistent self-antigen(s), plasmablasts can circulate for prolonged periods[20, 23, 24].
Circulating plasmablasts have been described previously in inflammatory bowel disease, rheumatoid arthritis, systemic lupus erythematosus, and multiple myeloma[20, 21, 23–26]. In this study, we describe the clinical characteristics of patients with active, untreated IgG4-RD and elevated plasmablastlevels in blood, stratified according to serum IgG4 concentration. We also report the relationship between total and IgG4+ plasmablasts and disease activity, and describe serial assessments of plasmablasts following treatment.
MATERIALS & METHODS
Patients
This study was approved by the institutional review board and all subjects provided informed, written consent. From the database of the Massachusetts General Hospital Center for IgG4-Related Disease, we identified 37 sequentially-evaluated patients who had active, untreated disease. The IgG4-RD patients were compared to 14 healthy controls and to 21 disease controls with active, untreated inflammatory diseases or malignancies at the time their samples were drawn. The 21 disease control subjects included five patients with rheumatoid arthritis (RA), four with granulomatosis with polyangiitis (GPA; formerly Wegener’s), two with pancreatic cancer, one with diffuse large B cell lymphoma, three with sarcoidosis, and one each with livedoid vasculopathy, Sjögren’s syndrome, primary biliary cirrhosis, chronic pain, acute Lyme disease, and gout. Twelve of the patients with active, untreated IgG4-RD were treated with rituximab (RTX, 1000 mg in two doses separated by 15 days). Peripheral blood was analyzed by flow cytometry before and after this therapy.
Pathology
All 37 patients had clinical histories consistent with IgG4-RD and biopsies of involved organs met criteria for a definite diagnosis[7]. Hematoxylin and eosin-stained slides were reviewed to determine the histopathologic features. Immunohistochemistry for IgG4 and IgG was performed using antibodies to IgG4 (Zymed; 1:200 dilution) and IgG (Dako; 1: 3,000 dilution). For each case, the number of plasma cells staining for IgG4 was assessed in three non-overlapping high-power fields (HPF; magnification of ×400)[7]. The fields with the highest degree of IgG4 reactivity were counted. The number of IgG4+ plasma cells was then divided by the total number of IgG+ plasma cells in these fields to determine the IgG4+:IgG+ plasma cell ratio.
Disease Activity
The IgG4-RD Responder Index (RI) was used to identify patients with active disease, defined by an RI score ≥ to 3[27].In serial assessments, disease activity was categorized as “flare” or “not flare”[28]. Details regarding demographic features and reported standard laboratory tests reported were collected from the electronic medical record; one patient did not have data on serum IgG4 concentrations at baseline available. Patients with other immune-mediated conditions were determined to have active disease based on the assessment by their treating providers. None of the controls had received treatment for their condition prior to blood sampling.
Flow cytometry
Flow cytometry was used to measure the absolute plasmablast count per milliliter (mL) by gating peripheral blood for CD19lowCD20−CD38+CD27+and staining for IgG4+cells in a subset of patients. Flow cytometry was performed on all samples at the time of patients’ presentations with active, untreated disease. Patients who were treated with rituximab were re-evaluated by flow cytometry within three to six months. Serum IgG4 concentrations were measured by immunonephelometry using a Siemens BNII instrument and reagent sets obtained from either Siemens or The Binding Site. The prozone effect, known to occur with some nephelometry assays in the setting of high antigen excess, was avoided by diluting the samples sufficiently until consistent measurements were obtained from one serial dilution to the next[29].
Statistical Analysis
All statistical tests were performed using SPSS Version 21. Statistical differences were found by paired and unpaired Student t-tests and by nonparametric tests, including all assessments of plasmablast levels. Linear correlations were measured by Pearson’s correlation coefficient. ROC curves were generated based on the assumption that ascending rank of plasmablast counts would increase the likelihood of an IgG4-RD diagnosis. A P value of <0.05 was considered significant for all statistical testing.
RESULTS
Clinical characteristics and demographics
The clinical characteristics of the 37 IgG4-RD patients and the subset of 12 who were treated with RTX are described in Table 1. The mean age of the IgG4-RD group was 59 years (range: 33–82). Twenty-five (68%) of the patients were male. Fourteen (38%) had at least three organs involved (range: 3–6). The remaining 23 patients had active IgG4-RD in only one or two organs.
Table 1.
Clinical and Laboratory Characteristics of IgG4-RD Patients
| All IgG4-RD Patients (n=37) |
IgG4-RD Patients Treated with RTX (n=12) |
|
|---|---|---|
| Age (mean, years) | 59 (range: 33–82) | 60.4 (range: 44–77) |
| Gender (% male) | 68% | 82% |
| Race | 76% Caucasian | 64% Caucasian |
| Plasmablast Count (median, /mL) | 4,698 (range: 610–79,524) | 6,356 (range: 1,145–41,589) |
| Serum IgG4 (mean, nml <135 mg/dL) | 611.56 (range: 5.3–4,780) | 1,014 (range: 26–4,780) |
| C3 (mean, nml 86–184 mg/dL) | 94.3 | 90 |
| C4 (mean, nml 16–38 mg/dL) | 17.4 | 14.4 |
| ESR (mean, nml 0–20 mm/hr) | 37 | 39.4 |
| CRP (mean, nml <8 mg/L) | 16.6 | 15.4 |
| IgG4-RD Responder Index (mean) | 11 (range: 3–36) | 13.8 (range: 3–36) |
| Average # of Organs Involved | 2.6 (range: 1–6) | 2.8 (range: 1–6) |
| Number with Normal Serum IgG4 (%) | 13 (36%) | 3 (25%) |
The most commonly involved organs were the lymph nodes and submandibular glands (11 cases each). Lymphadenopathy was the sole disease manifestation in only one patient. Other frequent sites of involvement included the pancreas (8 cases), parotid gland (7 cases), and orbit (7 cases). In considering all 37 IgG4-RD patients, however, 18 different anatomic locations were involved in at least one patient. The average IgG4-RD RI score was 11 (range: 3–36). The mean ages of the disease controls and healthy controls were 53 years old (range: 25–81) and 43 years old (range: 23–60), respectively. Twenty-nine percent of the disease controls and 60% of the healthy controls were males.
Plasmablast counts
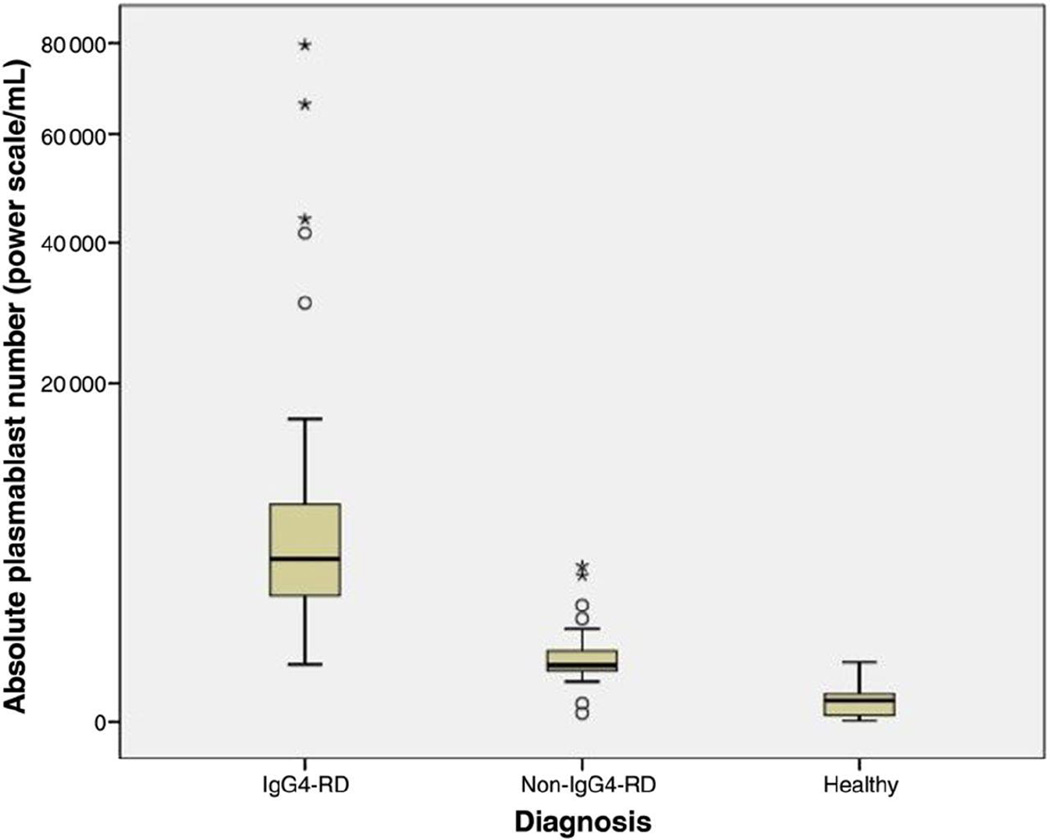
All IgG4-RD patients had expanded circulating plasmablast populations (median: 4,698/ml; range: 610 – 79,524/mL). Male and female IgG4-RD patients had similar plasmablast counts (male median: 4,093/mL, range: 610 – 79,524/mL; female median: 5,155/ml, range: 910–15,528/ml;P=0.67). The median plasmablast count among healthy controls was 94/mL (range: 1–653/mL; P<0.001 compared with IgG4-RD)(Figure 1). The median plasmablast count among patients with other immune-mediated conditions and malignancies was 592.0/mL (range: 19–4,294/mL, P<0.001 compared with IgG4-RD)(Figure 1). Several patients with other immune-mediated conditions and one healthy control had elevations in their plasmablast counts. These values were 644/mL (a GPA patient), 653/mL (healthy control), 752/mL (RA), 798/ml (sarcoidosis), 1,917/ml (primary biliary cirrhosis), 2,428 (GPA), 3,802/mL (Sjögren’s syndrome), and 4,294/ml (RA).
Figure 1.
Box plot of median plasmablast count (power scale /mL) by diagnosis. Open circles represent outliers and asterisks represent extreme outliers.
Serum IgG4 concentrations
Among IgG4-RD patients, thirteen (36%) had normal serum IgG4 concentrations (mean 59.5 mg/dL; range 5.3–123 mg/dL; normal < 135 mg/dL). Table 2, which compares the features of patients with elevated serum IgG4 concentrations to those of patients with normal serum IgG4 concentrations, is shown on the following page. Patients with elevated serum IgG4 concentrations (mean 924 mg/dL; range 138–4780 mg/dL; normal < 135 mg/dL) were more likely to have involvement of three or more organs (57% versus 15%, P<0.01), a greater mean number of organs involved (2.9 versus 1.7, P<0.01), and abnormal inflammatory markers (P<0.05 for C3, C4, ESR, CRP)(Table 2). Patients with a normal serum IgG4 concentrations had lower mean RI scores nearly 7 points lower compared to those with elevated serum IgG4 concentrations (mean 6.5 [range 3–14] versus 13.3 [range: 6–36]; P<0.01). The mean serum IgG4 concentration was higher among the male patients (mean 728 mg/dL versus 378 mg/dL for females, P=0.05).
Table 2.
IgG4-Related Disease Patients Stratified by Serum IgG4 Concentration
| Variable | Elevated Serum IgG4 (n=23) |
Normal Serum IgG4 (n=13) |
P-value |
|---|---|---|---|
| Gender (% male) | 65% | 69% | 0.81 |
| Age (Years) | 64 | 50 | 0.001 |
| Number of Organs (mean) | 2.9 | 1.7 | 0.003 |
| Percentage with ≥ 3 organs involved | 57% | 15% | <0.01 |
| Median PlasmablastCount (#/mL) | 3,981 | 3,659 | 0.82 |
| Serum IgG4 (mg/dL) | 923.6 | 59.5 | 0.001 |
| C3 (mg/dL) | 82 | 113 | 0.005 |
| C4 (mg/dL) | 14.9 | 22.1 | 0.017 |
| CRP (mg/L) | 23.4 | 3.1 | 0.008 |
| ESR (mm/hr) | 51 | 11 | <0.001 |
Plasmablast, IgG4, disease extent, and disease activity correlations
No differences were observed between the plasmablast counts of IgG4-RD patients with elevated as opposed to normal serum IgG4 values (medians 5,525/mL and 3,784/mL; P=0.24). Plasmablast counts were significantly different between those with multiorgan disease and those with ≤2 organs involved (medians 7,370/mL and 3,435/mL; P=0.01). Correlation between plasmablast counts and the baseline IgG4-RD RI score was modest (R=0.17, P=0.16).
IgG4+ Plasmablasts
IgG4+ plasmablast levels were assessed in 24 patients with active, untreated IgG4-RD. The median IgG4+ plasmablast level was 2,808/mL (range 203/mL – 57,012/mL). In this same group of 24 IgG4-RD patients, the median total number of plasmablasts was 4,083/mL (range 610/mL – 79,524/mL). Thus, IgG4+ plasmablasts accounted for a mean percentage of 61% of the total plasmablast concentrations (range 14%–90%). There appeared to be trends toward a higher median IgG4+ plasmablast level among patients with elevated as opposed to normal serum IgG4 concentrations (4,631/mL versus 1,372/mL; P=0.10). Similarly, the percentage of plasmablast that were IgG4+ plasmablasts was higher among those patients with serum IgG4 elevation, but this comparison fell short of statistical significance (67% versus 49%, respectively, P=0.15).
Test characteristics of elevated plasmablast concentrations
A receiver operator characteristic curve was created to analyze the utility of the plasmablast level as a diagnostic test for IgG4-RD. Overall, plasmablast levels demonstrated excellent performance as a test for IgG4-RD (AUC 0.96, P<0.05, 95% CI: 0.92–0.99). A plasmablast value of 900/mL had a sensitivity of 95%, a specificity of 82%, a positive predictive value of 86%, and a negative predictive value of 97%. A plasmablast value of 2,000/mL had a sensitivity of 87%, specificity of 91%, positive predictive value of 91%, and a negative predictive value of 87%.
Change in plasmablast counts and serum IgG4 concentrations with treatment
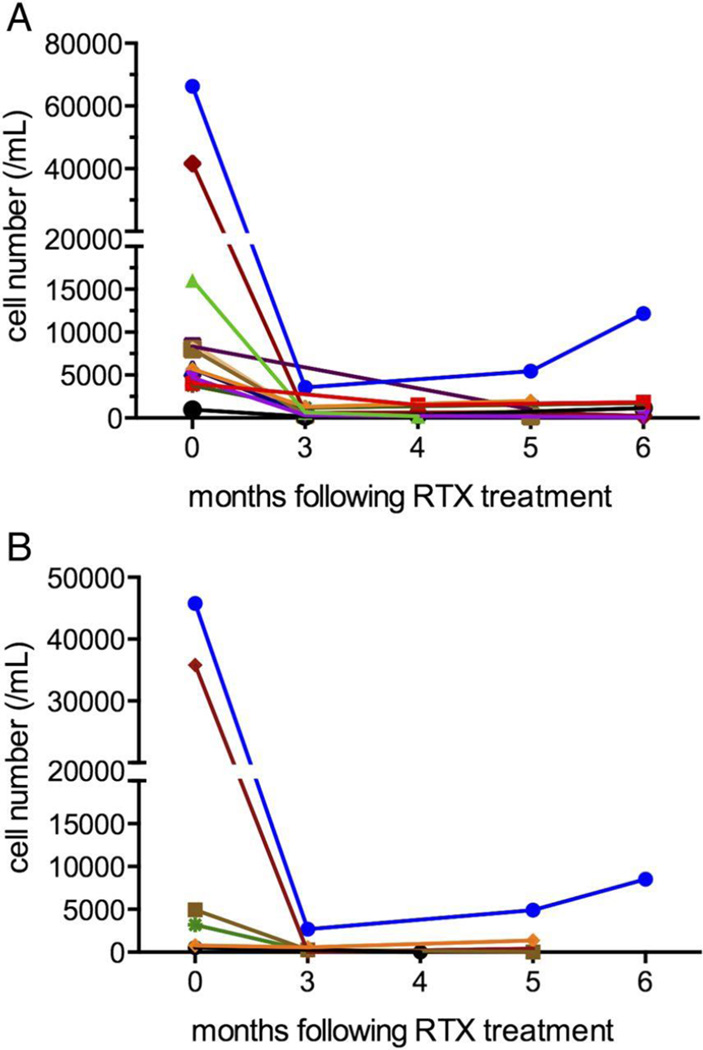
Of the patients evaluated with active, untreated disease, twelve received a course of rituximab (1 gram times two doses, separated by fifteen days). Flow cytometry was performed at baseline and at least one timepoint between months 3 and 6 after treatment in all patients (Figure 2A). The median plasmablast levels during periods of active disease, 6,356/mL (range: 1,123–41,589/mL),declined to 1,419/mL (range: 386–4,150/mL) during periods of complete or partial remission (P<0.01 by related samples Wilcoxon signed rank test) (Supplementary 1). In contrast, no significant difference in the mean serum IgG4 concentration was observed between these same periods (mean serum IgG4 concentration with active disease: 1,014 mg/dL [range: 26 – 4,780 mg/dL] compared with 521 mg/dL [range: 18 – 2,098 mg/dL]; P=0.12 by paired T-test)(Supplementary 2). The mean percentage change in plasmablast level between flare and remission was 66% (range: −15% to 98%) compared to 23% (range: −109% to 75%) for IgG4 concentrations (P=0.002 by paired T-test). The mean baseline and follow-up IgG4-RD RI scores were 13.8 during flare (range: 3–36) and 4.4 during periods of partial or complete remission (range: 0–10; P=0.001) following rituximab.
Figure 2.
(A) Absolute plasmablast count (/mL) at baseline (month 0) and following rituximab (RTX) infusions. Each line represents a different patient (n=12 patients). (B) Absolute IgG4+ plasmablast count (/mL) at baseline (month 0) and following RTX infusions. Each line represents a different patient (n=6 patients). The same colour is used for these six patients as in figure 2(A).
In six patients, IgG4+ and total plasmablast levels were assessed at baseline and 3–6 months following rituximab treatment (Figure 2B). The median IgG4+ plasmablast level at baseline among these patients was 4,077/mL (range: 534–45,752/mL). Three to six months following treatment, the median IgG4+ plasmablast level was 178 (range: 0–4,906/mL; P=0.046).
DISCUSSION
Our data reveal that patients with active, untreated IgG4-RD have significant elevations in their circulating plasmablast counts, regardless of their serum IgG4 concentrations, and that patients with multiorgan IgG4-RD have higher absolute plasmablast counts than do those with involvement of only one or two organs. Plasmablast counts decline swiftly following peripheral B-cell depletion, and this decline is accompanied by corresponding decreases in the IgG4-RD-RI. Plasmablast levels appear to be superior to serum IgG4 concentrations as a biomarker for IgG4-RD. This is a rare example of an immune-mediated condition in which measurement of a single cell type may play a central role in diagnosing, monitoring, and managing the disease.
The identification of circulating plasmablast expansion in IgG4-RD has substantial implications for the diagnosis and management of IgG4-RD. The diagnosis of IgG4-RD remains dependent upon biopsy and is therefore subject to the limitations of tissue accessibility (e.g., the aorta), small size of the biopsy specimen (the upper airways, retroperitoneum), and sampling error. Though elevated plasmablast levels can be found in patients with other inflammatory conditions, the expansion seen in patients with IgG4-RD is significantly greater such that levels over 2,000/ml have a high specificity and positive predictive value for this diagnosis. At the same time, blood plasmablast levels of this magnitude are associated with a high sensitivity for IgG4-RD. Further, the combination of an extreme elevation in plasmablast count and a substantial elevation in the serum IgG4 concentration suggests a high likelihood of multi-organ disease and should prompt providers to consider more comprehensive evaluations, e.g., with additional imaging, as appropriate. Alternatively, a low plasmablast level argues against active IgG4-RD and may suggest an another diagnosis.
Measurements of peripheral blood plasmablasts may be particularly useful in patients with normal serum IgG4 concentrations in whom there remains a high clinical suspicion for IgG4-RD and in whom appropriate measures have been undertaken to exclude malignancy. Moreover, the declines observed in circulating plasmablast counts following treatment and the rise before clinical flare in several patients suggests that serial measurements may have an important role in following response to treatment, determining if the intensity of treatment has been sufficient, and guiding the timing of repeat therapy. This final point is particularly critical in patients with organ-threatening disease (e.g., IgG4-related sclerosing cholangitis or tubulointerstitial nephritis) in whom disease recurrence may lead to further irreversible damage or organ failure. Other immune-mediated diseases that respond to B-cell depletion, e.g., ANCA-associated vasculitis and rheumatoid arthritis, typically require retreatment over the course of longitudinal follow-up, but there remain no reliable laboratory indicators that predict the timing of disease relapse in advance. Consequently, clinicians are confronted with the need to treat patients at regular intervals – risking overtreatment of some patients – or waiting for clinical disease flares to become evident – placing patients at some risk of irreversible organ damage.
All 37 patients with active, untreated IgG4-RD had elevated plasmablast counts despite the fact that thirteen (36%) had normal serum IgG4 concentrations. This finding is consistent with other studies of serum IgG4 concentrations, which have observed that between 10% and 30% of patients have normal serum IgG4 measurements concentrations in the presence of classic histopathological and immunohistochemical staining features of the disease[13, 30]. In our study, the median plasmablast count did not differ between the two groups with normal and elevated serum IgG4 concentrations, respectively, consistent with the concept that the circulating plasmablast count is a more robust diagnostic marker than are serum IgG4 concentrations. In several patients, rising plasmablast levels during periods of partial or complete remission preceded overt clinical flares (unpublished data). These results suggest a relationship between plasmablast levels and disease activity such that rising plasmablast levels after treatment may predict clinical flares.
The proportion of the expanded plasmablast population that is comprised of IgG4+ plasmablasts is variable, and there appears to be no consistent relationship between the concentrations of IgG4+ plasmablasts in the blood and the serum IgG4 concentration. The clinical implications of IgG4+ plasmablast concentrations remain uncertain. Further studies of IgG4+ plasmablast levels in patients with IgG4-RD and other immune-mediated conditions are an important avenue for future investigations.
Expansion in circulating plasmablasts in patients with immune-mediated disease has previously been studied in patients with RA, systemic lupus erythematosus, and ulcerative colitis[20, 25, 26, 31–33]. In RA, elevated levels of circulating plasmablasts, especially elevated levels of late-stage plasmablasts characterized by expression of IgJ mRNA, have been associated with a poor treatment response to peripheral B-cell depletion[32]. In pediatric and adult patients with ulcerative colitis, those with severe disease have been found to have significantly higher circulating plasmablasts (particularly IgA+ plasmablasts) than healthy controls[31, 33].
The observation of elevated plasmablasts in patients with active, untreated IgG4-RD has important implications for understanding the pathophysiology of IgG4-RD as well as the response to treatment. Successful treatment of IgG4-RD by the depletion of mature peripheral B-cells depletion has been described, but plasmablasts lack surface expression ofCD20 and are therefore resistant to direct depletion by anti-CD20 treatment approaches[17]. The steep decline in plasmablast counts after anti-CD20 treatment likely stems from the depletion of CD20+ precursors. We observed that IgG4-RD patients with elevated plasmablast counts on presentation demonstrate clinical responses to rituximab, coincident with a swift decline in both total and IgG4+ plasmablast levels. The utility of total and IgG4+ plasmablast levels as biomarkers of disease activity and predictors of flare requires further investigation.
Our study has both strengths and weaknesses. All patients had IgG4-RD confirmed by biopsy according to recent consensus criteria[7] and had active disease when their blood samples were evaluated. In addition, none had been treated before entry. A common challenge in studying patients with inflammatory conditions is the identification of patients before they have received any immunosuppressive therapy. The identification of such patients was a major focus of our study and the main reason why the number of patients studied was relatively small. Although it will be important to confirm and extend these findings in larger studies, our statistical analyses suggest robust differences across many of the comparisons made.
In summary, IgG4-RD is associated with elevated plasmablast counts in the blood. Our results suggest that the plasmablast count is superior to serum IgG4 concentrations as a biomarker for the diagnosis of IgG4-RD.Theplasmablastcount reflects disease extent and may also be useful for the timing of retreatment. Plasmablasts are an important focus for additional studies in IgG4-RD.
Supplementary Material
Acknowledgments
Competing interests, Funding.
This study was funded by grants AI 064930 and AI 076505 from the NIH and a pilot grant from the Harvard Institute of Translational Immunology supported by the Helmsley Foundation.
REFERENCES
- 1.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–551. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 2.Carruthers MN, Stone JH, Khosroshahi A. The latest on IgG4-RD: a rapidly emerging disease. Curr Opin Rheumatol. 2012;24:60–69. doi: 10.1097/BOR.0b013e32834ddb4a. [DOI] [PubMed] [Google Scholar]
- 3.Ebbo M, Daniel L, Pavic M, Seve P, Hamidou M, Andres E, et al. IgG4-related systemic disease: features and treatment response in a French cohort: results of a multicenter registry. Medicine (Baltimore) 2012;91:49–56. doi: 10.1097/MD.0b013e3182433d77. [DOI] [PubMed] [Google Scholar]
- 4.Ferry JA, Deshpande V. IgG4-related disease in the head and neck. Semin Diagn Pathol. 2012;29:235–244. doi: 10.1053/j.semdp.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Plaza JA, Garrity JA, Dogan A, Ananthamurthy A, Witzig TE, Salomao DR. Orbital inflammation with IgG4-positive plasma cells: manifestation of IgG4 systemic disease. Arch Ophthalmol. 2011;129:421–428. doi: 10.1001/archophthalmol.2011.16. [DOI] [PubMed] [Google Scholar]
- 6.Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, Aalberse R, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64:3061–3067. doi: 10.1002/art.34593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 8.Sato Y, Ohshima K, Ichimura K, Sato M, Yamadori I, Tanaka T, et al. Ocular adnexal IgG4-related disease has uniform clinicopathology. Pathol Int. 2008;58:465–470. doi: 10.1111/j.1440-1827.2008.02257.x. [DOI] [PubMed] [Google Scholar]
- 9.Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, et al. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22:1–14. doi: 10.1007/s10165-011-0508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu Y, Yamamoto M, Naishiro Y, Sudoh G, Ishigami K, Yajima H, et al. Necessity of early intervention for IgG4-related disease--delayed treatment induces fibrosis progression. Rheumatology (Oxford) 2013;52:679–683. doi: 10.1093/rheumatology/kes358. [DOI] [PubMed] [Google Scholar]
- 11.Brenner I, Roth S, Puppe B, Wobser M, Rosenwald A, Geissinger E. Primary cutaneous marginal zone lymphomas with plasmacytic differentiation show frequent IgG4 expression. Mod Pathol. 2013;26:1568–1576. doi: 10.1038/modpathol.2013.106. [DOI] [PubMed] [Google Scholar]
- 12.Strehl JD, Hartmann A, Agaimy A. Numerous IgG4-positive plasma cells are ubiquitous in diverse localised non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J Clin Pathol. 2011;64:237–243. doi: 10.1136/jcp.2010.085613. [DOI] [PubMed] [Google Scholar]
- 13.Carruthers M, Augustin T, Stone J, Khosroshahi A. Diagnostic Utility of Serum IgG4 in IgG4-Related Disease. Arthritis Rheum. 2012;64:S815–S816. doi: 10.1136/annrheumdis-2013-204907. [DOI] [PubMed] [Google Scholar]
- 14.Koike T. IgG4-related disease: why high IgG4 and fibrosis? Arthritis Res Ther. 2013;25:103. doi: 10.1186/ar4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuboi H, Matsuo N, Iizuka M, Tsuzuki S, Kondo Y, Tanaka A, et al. Analysis of IgG4 class switch-related molecules in IgG4-related disease. Arthritis Res Ther. 2012;14:R171. doi: 10.1186/ar3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zen Y, Nakanuma Y. Pathogenesis of IgG4-related disease. Curr Opin Rheumatol. 2011;23:114–118. doi: 10.1097/BOR.0b013e3283412f4a. [DOI] [PubMed] [Google Scholar]
- 17.Khosroshahi A, Carruthers MN, Deshpande V, Unizony S, Bloch DB, Stone JH. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine (Baltimore) 2012;91:57–66. doi: 10.1097/MD.0b013e3182431ef6. [DOI] [PubMed] [Google Scholar]
- 18.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroopinsky D, Katz T, Rowe JM, Melamed D, Avivi I. Rituximab-induced direct inhibition of T-cell activation. Cancer Immunol Immunother. 2012;61:1233–1241. doi: 10.1007/s00262-011-1168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harada Y, Kawano MM, Huang N, Mahmoud MS, Lisukov IA, Mihara K, et al. Identification of early plasma cells in peripheral blood and their clinical significance. Br J Haematol. 1996;92:184–191. doi: 10.1046/j.1365-2141.1996.300835.x. [DOI] [PubMed] [Google Scholar]
- 21.Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR, et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol. 2000;165:5970–5979. doi: 10.4049/jimmunol.165.10.5970. [DOI] [PubMed] [Google Scholar]
- 22.Fink K. Origin and function of circulating plasmablasts during acute viral infections. Front Immunol. 2012;3:1. doi: 10.3389/fimmu.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobi AM, Odendahl M, Reiter K, Bruns A, Burmester GR, Radbruch A, et al. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1332–1342. doi: 10.1002/art.10949. [DOI] [PubMed] [Google Scholar]
- 24.Odendahl M, Keitzer R, Wahn U, Hiepe F, Radbruch A, Dorner T, et al. Perturbations of peripheral B lymphocyte homoeostasis in children with systemic lupus erythematosus. Ann Rheum Dis. 2003;62:851–858. doi: 10.1136/ard.62.9.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerkman PF, Rombouts Y, van der Voort EI, Trouw LA, Huizinga TW, Toes RE, et al. Circulating plasmablasts/plasmacells as a source of anticitrullinated protein antibodies in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1259–1263. doi: 10.1136/annrheumdis-2012-202893. [DOI] [PubMed] [Google Scholar]
- 26.Vital EM, Dass S, Buch MH, Henshaw K, Pease CT, Martin MF, et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum. 2011;63:3038–3047. doi: 10.1002/art.30466. [DOI] [PubMed] [Google Scholar]
- 27.Carruthers MN, Stone JH, Deshpande V, Khosroshahi A. Development of an IgG4-RD Responder Index. Int J Rheumatol. 2012;2012:259408. doi: 10.1155/2012/259408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart PA, Topazian MD, Witzig TE, Clain JE, Gleeson FC, Klebig RR, et al. Treatment of relapsing autoimmune pancreatitis with immunomodulators and rituximab: the Mayo Clinic experience. Gut. 2013;62:1607–1615. doi: 10.1136/gutjnl-2012-302886. [DOI] [PubMed] [Google Scholar]
- 29.Khosroshahi A, Cheryk L, Carruthers M, Edwards J, Bloch D, Stone J. Spuriously Low Serum IgG4 Concentrations Caused by the Prozone Phenomenon in IgG4-Related Disease. Arthritis Rheum. 2013 doi: 10.1002/art.38193. (Accepted) [DOI] [PubMed] [Google Scholar]
- 30.Khosroshahi A, Stone JH. IgG4-related systemic disease: the age of discovery. Curr Opin Rheumatol. 2011;23:72–73. doi: 10.1097/BOR.0b013e328341a229. [DOI] [PubMed] [Google Scholar]
- 31.Hosomi S, Oshitani N, Kamata N, Sogawa M, Okazaki H, Tanigawa T, et al. Increased numbers of immature plasma cells in peripheral blood specifically overexpress chemokine receptor CXCR3 and CXCR4 in patients with ulcerative colitis. Clin Exp Immunol. 2011;163:215–224. doi: 10.1111/j.1365-2249.2010.04290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owczarczyk K, Lal P, Abbas AR, Wolslegel K, Holweg CT, Dummer W, et al. A plasmablast biomarker for nonresponse to antibody therapy to CD20 in rheumatoid arthritis. Sci Transl Med. 2011;3:101ra92. doi: 10.1126/scitranslmed.3002432. [DOI] [PubMed] [Google Scholar]
- 33.Tarlton NJ, Green CM, Lazarus NH, Rott L, Wong AP, Abramson ON, et al. Plasmablast frequency and trafficking receptor expression are altered in pediatric ulcerative colitis. Inflamm Bowel Dis. 2012;18:2381–2391. doi: 10.1002/ibd.22962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.