SUMMARY
Inhibitory neurons are critical for proper brain function, and their dysfunction is implicated in several disorders, including autism, schizophrenia, and Rett syndrome. These neurons are heterogeneous, and it is unclear which subtypes contribute to specific neurological phenotypes. We deleted Mecp2, the mouse homolog of the gene that causes Rett syndrome, from the two most populous subtypes, parvalbumin-positive (PV+) and somatostatin-positive (SOM+) neurons. Loss of MeCP2 partially impairs the affected neuron, allowing us to assess the function of each subtype without profound disruption of neuronal circuitry. We found that mice lacking MeCP2 in either PV+ or SOM+ neurons have distinct, non-overlapping neurological features: mice lacking MeCP2 in PV+ neurons developed motor, sensory, memory, and social deficits, whereas those lacking MeCP2 in SOM+ neurons exhibited seizures and stereotypies. Our findings indicate that PV+ and SOM+ neurons contribute complementary aspects of the Rett phenotype and may have modular roles in regulating specific behaviors.
INTRODUCTION
Rett syndrome (RTT) is a devastating postnatal neurodevelopmental disorder caused by mutations in the gene encoding methyl-CpG binding protein 2 (MeCP2) (Amir et al., 1999). Affected children appear to develop normally during the first year of life but quickly regress, losing language and acquired motor skills and developing ataxia, respiratory dysrhythmias, seizures, cognitive deficits, and stereotyped hand movements (Chahrour and Zoghbi, 2007). Because the RTT phenotype is so broad, several conditional knockout mice have been generated to dissect the contribution of various brain regions and neuronal subtypes to the pathogenesis of the disorder. For instance, mice with Mecp2 conditionally deleted from the medulla and spinal cord reproduce the respiratory dysrhythmias and premature death (Ward et al., 2011), while a forebrain excitatory neuron deletion mouse develops seizures (Zhang et al., 2014). Unexpectedly, depleting MeCP2 in inhibitory neurons using a Viaat-Cre allele that targets GABAergic neurons recapitulates almost the entire spectrum of RTT features, including motor, sensory, memory, social, and autonomic dysfunction as well as stereotyped behaviors (Chao et al., 2010). These results underscore the importance of MeCP2 for the function of inhibitory neurons and the breadth of neurological consequences that follow moderate compromise of inhibitory signaling.
The various interneuron subtypes, which exhibit a striking range of morphological, electrophysiological, and molecular properties, seem to possess distinct circuit functions (Kepecs and Fishell, 2014; Klausberger and Somogyi, 2008) and appear to be recruited by different behavioral events (Kvitsiani et al., 2013; Wolff et al., 2014). For instance, parvalbumin-positive (PV+) and somatostatin-positive (SOM+) neurons, which each constitute 30%–40% of the entire inhibitory neuronal population in the cortex (Rudy et al., 2011), have been implicated in different stages of foraging behavior, regulated by the anterior cingulate cortex, and have different functions in the amygdala during cued memory (Kvitsiani et al., 2013; Wolff et al., 2014). To better understand how these two subpopulations individually contribute to complex behaviors in vivo, it would be ideal to partially disable their function rather than ablate them, so as to avoid the confound of widespread cell death or complete collapse of the neural circuit.
Because loss of MeCP2 creates just such a partial dysfunction of the affected neuron, we conditionally deleted Mecp2 from PV+ neurons and from SOM+ neurons to explore the behavioral consequences. While neither conditional knockout mouse developed the full array of constitutive Mecp2-deletion features, the two lines together recapitulated many of the Rett-like phenotypes: the PV conditional knockouts developed motor, sensory, social, and cognitive deficits, while the SOM conditional knockout mice exhibited repetitive behaviors and seizures. These two models reinforce the importance of a robust functionality of these neurons for normal behavior.
RESULTS
MeCP2 Is Expressed Both in PV+ and SOM+ Neurons in Various Brain Regions
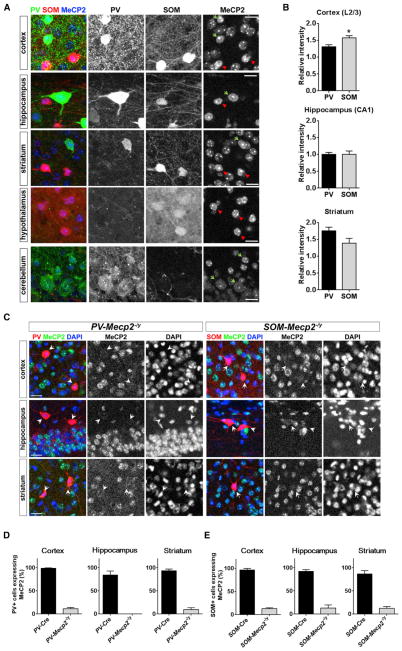
To confirm that MeCP2 is expressed in PV+ and SOM+ neurons, we stained brain sections from adult SOM-Cre: Ai9 mice, which express a tdTomato reporter in SOM+ neurons, with antibodies for MeCP2 and PV. Both PV+ and SOM+ neurons expressed MeCP2 in various brain regions (Figure 1A). MeCP2 signal was comparable between PV+ and SOM+ neurons in the CA1 region of the hippocampus (n = 5 mice, p = 0.96, paired t test) and in the striatum (n = 5 mice, p = 0.19, paired t test), while the signal was somewhat stronger in SOM+ neurons in the cortex (layer 2/3, n = 5 mice, p = 0.013, paired t test) (Figure 1B). We also confirmed that there was little overlap between PV+ and SOM+ cells, which was consistent with previous reports (Rudy et al., 2011); cells expressing both PV and SOM accounted for 3.7% ± 2.8% of neurons in the cortex (layer 2/3) and 5.7% ± 2.8% in the hippocampus (CA1) (n = 5 mice); however, these values may be overestimated, as the SOM-Cre:Ai9 line labels SOM+ cells throughout development.
Figure 1. MeCP2 Is Expressed Both in PV+ and SOM+ Neurons.
(A) MeCP2 is expressed by both PV+ neurons (green arrows) and SOM+ neurons (red arrowheads) in the cortex (layer 2/3), hippocampus (CA1), striatum, hypothalamus, and cerebellum of 4-month-old SOM-Cre:Ai9 mice expressing tdTomato in SOM+ neurons (red) and immunostained for MeCP2 (blue) and PV (green).
(B) MeCP2 levels in PV+ and SOM+ neurons in three brain regions. Relative MeCP2 intensity in PV+ and SOM+ cells was quantified by normalizing the intensity to PV− and SOM-negative cells. MeCP2 intensity was slightly higher in SOM+ neurons in the cortex, while the intensity was similar in PV+ and SOM+ neurons in the hippocampus and the striatum. n = 5 mice per genotype.
(C) Representative images of brain slices from 4-month-old PV− and SOM-Mecp2-/y mice, immunostained for MeCP2. PV+ neurons were visualized by immunofluorescence. SOM+ neurons were identified by crossing mice to Ai9 mice to induce TdTomato expression. MeCP2 signal was significantly reduced in either PV+ or SOM+ cells (arrows).
(D and E) Ratio of MeCP2-positive cells among PV+ (D) and SOM+ (E) neurons in PV− and SOM-Mecp2-/y mice, respectively. n = 3 mice per genotype. Bars represent mean ± SEM. *p < 0.05. Paired t test. See also Figure S1. Scale bar = 20 μm.
PV+ and SOM+ Neurons Lacking MeCP2 Produce Distinct Neurological Phenotypes
To determine the contributions of PV+ and SOM+ neurons to the behavioral features observed in Viaat-Mecp2-/y mice (Chao et al., 2010), we generated two lines of conditional knockout mice lacking MeCP2 in either PV+ neurons (PV-Mecp2-/y) or SOM+ neurons (SOM-Mecp2-/y) by crossing Mecp2flox/+ mice (Flox) (Guy et al., 2001) to PV-Cre (Madisen et al., 2010) and SOM-Cre (Taniguchi et al., 2011) mice, respectively. PV-Mecp2-/y and SOM-Mecp2-/y mice initially appeared similar to littermate control mice. Immunofluorescence staining confirmed MeCP2 expression was effectively depleted in PV+ and SOM+ cells, respectively (Figures 1C–1E). The expression of MeCP2 in SOM+ cells of PV-Mecp2-/y brains and in PV+ cells of SOM-Mecp2-/y mice was unaltered, indicating no compensation of Mecp2 expression in the unmanipulated inhibitory neuron subtypes (Figure S1).
We next characterized PV-Mecp2-/y and SOM-Mecp2-/y mice using multiple behavioral assays. Because the Mecp2flox/y conditional allele causes a constitutive 50% reduction of MeCP2 levels (Samaco et al., 2008), we expected the Flox mice to differ slightly from wild-type as the animals age; we therefore analyzed all data by comparing conditional knockout mice with male littermates of three control groups: wild-type (WT), PV-Cre alone or SOM-Cre alone, and Mecp2flox/y alone (Flox).
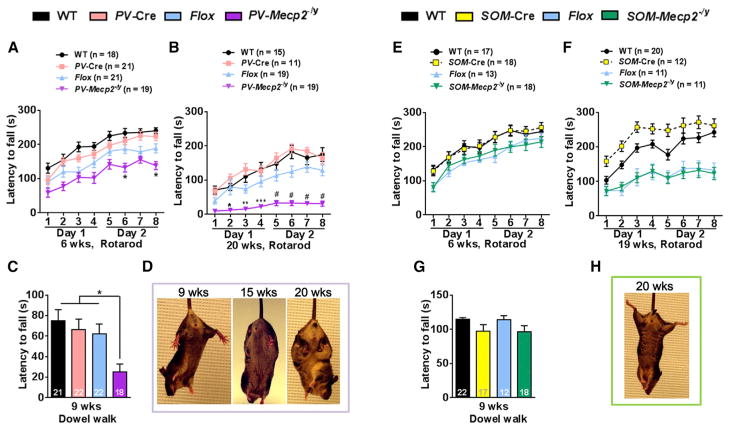
Interestingly, PV-Mecp2-/y and SOM-Mecp2-/y mice developed completely different neurological deficits. PV-Mecp2-/y mice developed progressive ataxia, as shown by reduced latency to fall in both the rotarod (Figures 2A and 2B) and dowel walk assays (Figure 2C). The ataxia was apparent at 6 weeks and worsened by 20 weeks (Figures 2A and 2B). Consistent with this observation, PV-Mecp2-/y mice developed widely splayed hind limbs after 10 weeks (Figures S2A and S2B) and hind limb retraction after 15 weeks of age (Figure 2D); they also showed impaired performance on the marble-burying test, suggesting forelimb incoordination (Figure S2C). The SOM-Mecp2-/y mice displayed none of these motor deficits (Figures 2E–2H and Figure S2D).
Figure 2. Depletion of MeCP2 in PV+ but Not in SOM+ Neurons Causes Motor Dysfunction.
(A and B) PV-Mecp2-/y mice showed progressive impairment in rotarod performance. The latency to fall was decreased in PV-Mecp2-/y mice at 6 weeks but was statistically significant for only two trials (A). The decrease was significant between PV-Mecp2-/y and controls for all trials at 20 weeks (B).
(C) Time on the dowel was decreased at 9 weeks in PV-Mecp2-/y mice.
(D) PV-Mecp2-/y mice developed clear hind limb retraction after 15 weeks.
(E–G) SOM-Mecp2-/y mice displayed no motor dysfunction on the rotarod (E and F) or dowel (G). The latency to fall from the rotarod was significantly decreased in both Flox and SOM-Mecp2-/y at 19 weeks while there was no difference between Flox and SOM-Mecp2-/y (F). The differing baselines at 19–20 weeks is likely due to two independent cohorts of PV-Mecp2-/y mice being used to test rotarod at 6 weeks and 20 weeks versus the same cohort of SOM-Mecp2-/y mice used for both time points.
(H) SOM-Mecp2-/y mice showed no hindlimb clasping.
Bars represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.0001. Numbers in graphs represent number of mice (n) per genotype. See also Figure S2.
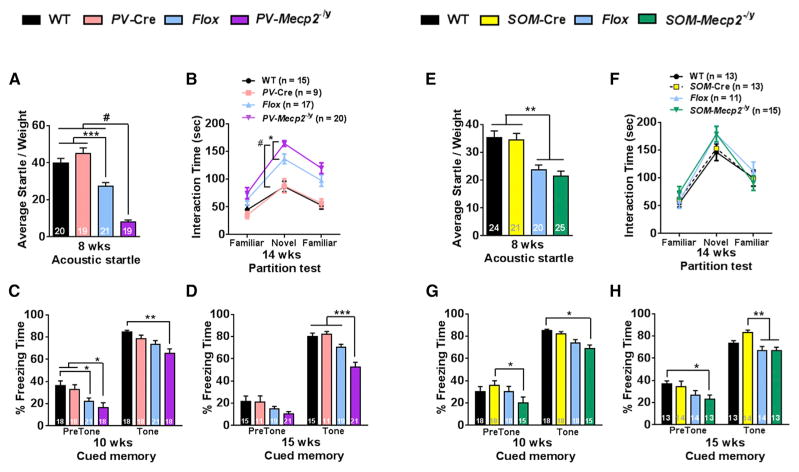
PV-Mecp2-/y mice also exhibited sensory, memory, and cognitive impairments that were not apparent in SOM-Mecp2-/y mice (Figure 3). PV-Mecp2-/y mice had a significantly diminished acoustic startle response (Figure 3A) and spent significantly more time interacting with novel partner mice in a social behavior assay (Figure 3B). In addition, PV-Mecp2-/y mice developed progressive cued memory deficits (Figures 3C and 3D). The difference in freezing response to a conditioned tone between PV-Mecp2-/y and each of the three control groups became statistically significant at 15 weeks of age (Figures 3C and 3D). In contrast, SOM-Mecp2-/y mice did not show alterations in acoustic startle response, partition, or cued memory tests (Figures 3E–3H).
Figure 3. Depletion of MeCP2 in PV+ but Not in SOM+ Neurons Causes Sensory, Social, and Memory Deficits.
(A) PV-Mecp2-/y mice showed decreased acoustic startle response. Average startle response was decreased in Flox compared to WT and PV-Cre. It was further decreased in PV-Mecp2-/y mice, and the difference was significant between all three control groups.
(B) PV-Mecp2-/y mice spent more time interacting with novel partners than their littermates in the partition test. While the interaction time of Flox was higher than those of WT or PV-Cre mice, PV-Mecp2-/y mice showed an increase in the interaction time when compared to all three control groups, including Flox.
(C and D) PV-Mecp2-/y mice showed progressive cued memory deficits. Freezing response of PV-Mecp2-/y mice was decreased compared to WT but was similar to PV-Cre and Flox at 10 weeks (C). The response was significantly decreased in PV-Mecp2-/y compared to all control groups at 15 weeks (D).
(E) SOM-Mecp2-/y mice did not show deficits in acoustic startle response. Average startle response of SOM-Mecp2-/y was lower compared to WT and SOM-Cre but was similar to Flox.
(F–H) SOM-Mecp2-/y mice did not show deficits in social interaction (F) or cued memory (G and H). Freezing response to tone was decreased in SOM-Mecp2-/y compared to WT at 10 weeks and SOM-Cre at 15 weeks but was similar to Flox at both 10 weeks and 15 weeks (G and H).
Bars represent mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.0001. Numbers in graphs represent number of mice (n) per genotype.
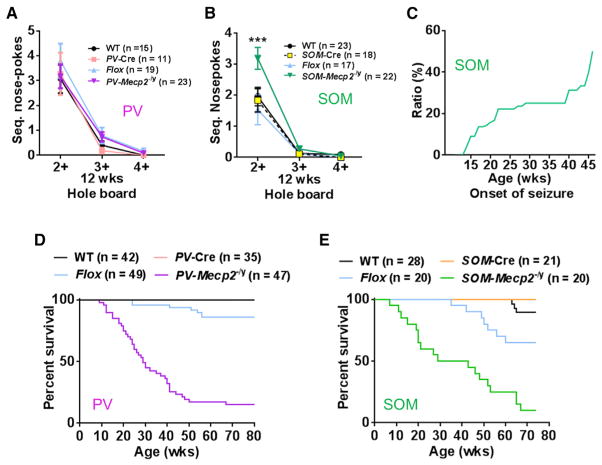
The PV-Mecp2-/y mice thus reproduced many of the features observed in the Mecp2 null and Viaat-Mecp2-/y male mice (Chao et al., 2010), but they did not reproduce the stereotyped behaviors (Figure 4A) or seizures. These two features were prominent, however, in the SOM-Mecp2-/y mice (Figures 4B and 4C). Like the Viaat-Mecp2-/y mice, SOM-Mecp2-/y mice showed repetitive nose-poking behavior in the hole board test (Figure 4B) (Chao et al., 2010). Fifty percent of SOM-Mecp2-/y mice developed spontaneous epileptic seizures starting at 12 weeks (Figure 4C), with generalized tonic clonic seizures observed during routine handling (Movie S1). There was only one RTT-related feature that the PV-Mecp2-/y and SOM-Mecp2-/y mice shared: both conditional knockout lines died prematurely, with 50% mortality by 29–35 weeks of age (Figures 4D and 4E).
Figure 4. Depletion of MeCP2 in SOM+ Neurons Causes Seizures and Stereotyped Behavior, whereas Loss of MeCP2 in either PV+ or SOM+ Neurons Causes Premature Lethality.
(A) PV-Mecp2-/y mice did not show repetitive nose poking in the hole board assay.
(B) SOM-Mecp2-/y mice showed repetitive nose poking to the same holes in the hole board assay. The frequency of sequential nose pokes was significantly higher in SOM-Mecp2-/y than in all three control groups.
(C) SOM-Mecp2-/y mice showed generalized seizures after 12 weeks. The graph shows incidence of seizures (n = 14–25 mice per time point).
(D and E) Both PV-Mecp2-/y (D) and SOM-Mecp2-/y (E) male mice die prematurely. Half of the mice died by 30 weeks of age.
Bars represent mean ± SEM. ***p < 0.001. See also Movie S1.
PV+ and SOM+ Neurons Are Responsible for the Majority of RTT-like Features Observed in Viaat-Mecp2-/y Mice
The above behavioral studies reveal that PV-Mecp2-/y and SOM-Mecp2-/y mice develop complementary aspects of the RTT phenotype, together recapitulating the majority of the Viaat-Mecp2-/y mouse phenotype (Table 1). Neither the PV-Mecp2-/y nor the SOM-Mecp2-/y mice developed any deficits that were not observed in Viaat-Mecp2-/y mice, such as anxiety behaviors as measured in the light/dark and elevated plus maze tests (Figures S3A–S3D). It is worth noting, however, that these two lines failed to develop three features exhibited by the Viaat-Mecp2-/y mice (Chao et al., 2010): increased pre-pulse inhibition, reduced locomotor activity, or increased body weight (Figures S3E–S3J). We also found that long-term potentiation (LTP) in the CA1 region of hippocampus was not altered in either conditional knockout (data not shown). It thus seems likely that some features of Viaat-Mecp2-/y mice are mediated by another inhibitory neuron subtype (such as glycinergic neurons, which also express the Viaat transporter) or that synergistic impairment of multiple inhibitory neurons is necessary for some RTT symptoms to appear.
Table 1.
Phenotypic Consequences of Depleting MeCP2 in Inhibitory Neurons
| Feature | Viaat-Mecp2-/y | PV-Mecp2-/y | SOM-Mecp2-/y |
|---|---|---|---|
| Median Survival | 26 weeks | 30 weeks | 35 weeks |
| Motor Incoordination | ↓ | ↓ | − |
| Spasticity | + | + | − |
| Altered Startle Response | ↓ | ↓ | − |
| Learning and Memory Deficit | ↓ | ↓ | − |
| Altered Social Interaction | ↑ | ↑ | − |
| Stereotypy | ↑ | − | ↑ |
| Seizures | + | − | + |
| Activity Changes | ↓ | − | − |
| Sensorimotor Gating Defect | ↑ | − | − |
| Obesity | + | − | − |
PV-Mecp2-y and SOM-Mecp2-y showed specific subsets of neurological features observed in Viaat-Mecp2-y mice. See also Figures S2 and S3.
DISCUSSION
Loss-of-function mutations in MECP2 cause a broad array of neuropsychiatric symptoms, indicating that MeCP2 is critical for normal brain function and behavior (Chahrour and Zoghbi, 2007; Chao and Zoghbi, 2012). The Mecp2-heterozygous female mouse reproduces many features of the human disease, such as ataxia, breathing abnormalities, tremor, learning and memory deficits, abnormalities in social behavior, stereotypies, and premature death (Samaco et al., 2013); the Mecp2 null male mouse develops an accelerated and more severe version of the disorder (Akbarian et al., 2001; Baker et al., 2013; Chen et al., 2001; Guy et al., 2001; Heckman et al., 2014). Strikingly, most of these RTT-like features can be replicated by eliminating MeCP2 from GABAergic neurons (Chao et al., 2010), which make up approximately 20% of the neurons in the brain. The heterogeneity of this cellular population led us to investigate the contributions of the different subtypes of inhibitory neurons to the pathogenesis of RTT. Here, we show that the two largest subtypes of GABAergic neurons, PV+ and SOM+ neurons, each contribute distinct features to the phenotype of the Viaat-Mecp2-/y mice (Chao et al., 2010) and thus to the major features of RTT (Table 1). It is possible that some subtle phenotypic abnormalities are present in both conditional knockout models but were obscured due to comparison with the Flox mice, which express ~50% less MeCP2 and show some behavioral changes when compared to wild-type mice, especially as they age. However, even when taking this into consideration, the contrasting features of the PV− and SOM− Mecp2-/y were clear and confirmed using multiple behavioral tests, suggesting that these two neuronal subtypes play distinct roles in the pathogenesis of RTT.
Disruptions in inhibitory neuron function have been postulated to contribute to several neurodevelopmental disorders, including autism and schizophrenia (Le Magueresse and Monyer, 2013; Penzes et al., 2013); our studies suggest that dysfunction of specific inhibitory neuron subtypes probably underlies specific symptoms in these disorders. Recent work using optogenetics has revealed that PV+ and SOM+ cells in a local circuit are activated differently during foraging behavior and acquisition of fear conditioning (Kvitsiani et al., 2013; Wolff et al., 2014). Our findings using partial impairment of these neurons suggest additional functional roles, with PV+ neurons critical for motor and higher cognitive functions and SOM+ neurons contributing to seizure susceptibility and stereotypic behaviors. Given that most neuropsychiatric disorders do not involve total loss of a neuronal subtype and/or its function but rather are due to subtle impairment of one or more neuronal subtypes, our study sheds light on the neuroanatomical bases of some human neuropsychiatric phenotypes. Neuropsychiatric disorders that share symptoms with RTT might well involve dysfunction of PV+ and/or SOM+ neurons.
PV+ and SOM+ inhibitory neurons are both widely distributed throughout the brain but differ in their firing patterns and innervation targets: PV+ neurons tend to be fast spiking and synapse onto the soma and axon initial segment of their target neurons, while SOM+ neurons spike more slowly and target dendrites (Kepecs and Fishell, 2014). Because MeCP2 is expressed in both neuronal subtypes and throughout the brain (Figures 1A and 1B), the distinct behavioral features of PV-Mecp2-/y and SOM-Mecp2-/y mice cannot be explained by regional differences in MeCP2 expression. It is, however, intriguing that MeCP2 may be more highly expressed in SOM+ neurons in the cortex, suggesting that MeCP2 expression levels may contribute to different properties of neuronal subtypes (Figure 1B). It is also worth noting that PV-Mecp2-/y mice developed a wide range of RTT-related features (motor, sensory, social, and cognitive deficits), while SOM-Mecp2-/y mice seemed to be deficient in the modulatory functions of inhibitory neurons, developing seizures and stereotypies. That PV-Mecp2-/y mice seem to be more severely affected could reflect the greater role of PV+ cells in suppressing pyramidal cell output (Atallah et al., 2012). The stronger neurological phenotype of PV-Mecp2-/y mice could also reflect the integral role of PV+ neurons in maintaining excitatory/inhibitory balance in the cortex, a role that SOM+ neurons do not share (Xue et al., 2014).
Recent work by He et al. showed that loss of MeCP2 from PV+ neurons resulted in cells with immature neuronal properties, leading to decreased sensitivity to excitatory input (He et al., 2014); a Mecp2 null PV+ neuron may be unable to assess downstream excitatory neuronal activity and modulate its inhibitory activity appropriately. The authors also performed a limited number of behavioral analyses of mice lacking MeCP2 in PV+ cells, but their results showed only a mild ataxia and no effect on social interaction or cued memory. The more extensive phenotypes seen in our study are most likely due to the difference in the animal’s age: He et al. evaluated the mice at a younger age (8–10 weeks), while we studied them longitudinally over an extended period and observed memory and social deficits at 14 and 15 weeks, respectively. It is also important to consider the differences in expression patterns between the utilized Cre lines: He et al. used a Pvalb-IRES-Cre mouse line, which expresses Cre only in neurons that strongly express PV but does not cover a large population of more weakly PV-expressing neurons, such as those in the inner nuclei of the thalamus, which are targeted by the Pvalb-2A-Cre line used here (Madisen et al., 2010). It has been shown that cells expressing a low level of PV, including some layer 5 pyramidal cells, also express Cre in the Pvalb-2A-Cre line (Madisen et al., 2010), and we cannot exclude the possibility that some features observed were confounded by the deficit in layer 5 pyramidal cells. This possibility is unlikely, however, as CamKII-Cre; Mecp2flox/y mice (CamKII-Mecp2-/y) (Chen et al., 2001; Gemelli et al., 2006), which lack Mecp2 in forebrain excitatory neurons, show only limited behavioral abnormalities, whereas Viaat-Mecp2-/y mice recapitulate the majority of RTT-related features without affecting Mecp2 expression in layer 5. In addition, Viaat- and PV-Mecp2-/y show increased social interaction, a severe motor deficit, but no anxiety, whereas CamKII-Mecp2-/y mice show anxiety, decreased social interaction, and only mild ataxia in the rotarod assay.
Although neurological features in PV-Mecp2-/y and SOM-Mecp2-/y mice recapitulated the majority of behavioral deficits observed in Viaat-Mecp2-/y mice, this replication was not complete (Table 1 and Figure S3). It thus seems likely that either another inhibitory neuron subtype mediates the remaining three features or that synergistic impairment of multiple cell types is necessary for some RTT symptoms to appear. Some of the features absent from both PV-Mecp2-/y and SOM-Mecp2-/y mice could be attributed to the medium spiny neurons in the striatum, which are inhibitory projection neurons expressing Viaat but not PV or SOM, or to 5HT3a receptor-expressing inhibitory neurons, which constitute about 30% of the interneurons in the cortex but do not overlap with PV+ or SOM+ interneurons (Férézou et al., 2002; Rudy et al., 2011). One subtype of 5HT3a receptor+ cells, vasoactive intestinal peptide-expressing cells, is activated during auditory discrimination tasks, whisking, and other behaviors and serves primarily to innervate SOM+ cells in the cortex to disinhibit local circuitry (Lee et al., 2013; Pi et al., 2013). It would be of great interest to investigate the role of 5HT3a receptor+ cells in behavior and in the pathogenesis of RTT.
The only common feature observed both in PV-Mecp2-/y and SOM-Mecp2-/y mice was early death, suggesting that robust function of both PV+ and SOM+ neurons are critical for survival (Figures 4D and 4E). Re-expressing MeCP2 only in PV+ or in SOM+ cells of Mecp2 null mice improves gross phenotypic score and survival rate, while such improvement is not observed in mice expressing MeCP2 only in the forebrain inhibitory neurons (Goffin et al., 2014). Loss of MeCP2 in the brain stem and spinal cord causes abnormal respiration and heart rate and is associated with premature death (Ward et al., 2011). It thus seems likely that PV+ or SOM+ cells in the hindbrain regions or the spinal cord are critical for normal lifespan, although the direct cause of premature lethality in Mecp2 null mice is still unclear. Further study is necessary to distinguish the physiological functions of PV+ and SOM+ cells in these brain regions.
How does MeCP2 loss in PV+ and SOM+ cells lead to neurological deficit? Because MeCP2 regulates expression of glutamic acid decarboxylase (GAD), the phenotypes of PV− and SOM− Mecp2-/y mice may be caused by partial loss of GAD function (Chao et al., 2010). This seems unlikely, as only a few features in the inhibitory neuron Mecp2 conditional knockout are present in GAD2 knockout mice, and other phenotypes do not overlap with either GAD1- or GAD2-deficient mice. While GAD1 knockout mice die postnatally from a severe reduction in GABA and cleft palate (Asada et al., 1997), GAD1 heterozygous mice are viable and have reduced sociability (Sandhu et al., 2014). GAD2 knockout mice are viable with only mild reduction in GABA and develop spontaneous seizures, anxiety-related features, impaired cued and contextual memory, and decreased prepulse inhibition (Heldt et al., 2004; Kash et al., 1997, 1999; Stork et al., 2003), but GAD2 heterozygotes are grossly normal (Heldt et al., 2004; Kash et al., 1997). Therefore, the behavioral effects must be due to some other cause beyond the partial reduction in GAD1/2 levels. MeCP2 binds to DNA genome-wide and regulates the expression of thousands of genes (Chahrour et al., 2008; Skene et al., 2010). It is thus likely that alteration of many genes in addition to GAD1/2 causes widespread neuronal dysfunction that leads to the behavioral effects of MeCP2 loss.
In conclusion, our data indicate that individual inhibitory inter-neuron subtypes contribute to the RTT phenotype in a somewhat modular manner. Further characterization of the contribution of these two subtypes to behavior in health and disease should shed light on a number of neuropsychiatric conditions.
EXPERIMENTAL PROCEDURES
A full description of methods is found in the Supplemental Experimental Procedures.
Supplementary Material
Highlights.
Mecp2 loss in PV+ and SOM+ neurons leads to non-overlapping neurological phenotypes
Mecp2 deletion in PV+ neurons causes motor, sensory, memory, and social deficits
Mecp2 deletion in SOM+ neurons causes seizure activity and stereotypies
Acknowledgments
This research was supported by NIH/NINDS 5R01NS057819-08, the International Rett Syndrome Foundation (H.Y.Z.), the Japan Society for the Promotion of Science (A.I.-I.), NIH F32NS083137, NIH T325 HD055200 (K.U.), and NIH/ 1U54HD083092-01 (Neurovisualization, Neuroconnectivity and Neurobehavioral Cores) at the BCM Intellectual and Developmental Disabilities Research Center. H.Y.Z. is an investigator with the Howard Hughes Medical Institute.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, one table, and one movie and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2015.10.029.
AUTHOR CONTRIBUTIONS
A.I.-I., K.U., and H.Y.Z. conceived the project, designed the experiments, and wrote the manuscript. A.I.-I. and K.U. performed and analyzed the behavioral and histological experiments. H.C. and J.W.S. designed electrophysiological experiments that were performed by H.C. but are not shown in the paper.
References
- Akbarian S, Chen RZ, Gribnau J, Rasmussen TP, Fong H, Jaenisch R, Jones EG. Expression pattern of the Rett syndrome gene MeCP2 in primate prefrontal cortex. Neurobiol Dis. 2001;8:784–791. doi: 10.1006/nbdi.2001.0420. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah BV, Bruns W, Carandini M, Scanziani M. Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron. 2012;73:159–170. doi: 10.1016/j.neuron.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Chen L, Wilkins AD, Yu P, Lichtarge O, Zoghbi HY. An AT-hook domain in MeCP2 determines the clinical course of Rett syndrome and related disorders. Cell. 2013;152:984–996. doi: 10.1016/j.cell.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Zoghbi HY. MeCP2: only 100% will do. Nat Neurosci. 2012;15:176–177. doi: 10.1038/nn.3027. [DOI] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Férézou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J Neurosci. 2002;22:7389–7397. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemelli T, Berton O, Nelson ED, Perrotti LI, Jaenisch R, Monteggia LM. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biol Psychiatry. 2006;59:468–476. doi: 10.1016/j.biopsych.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Goffin D, Brodkin ES, Blendy JA, Siegel SJ, Zhou Z. Cellular origins of auditory event-related potential deficits in Rett syndrome. Nat Neurosci. 2014;17:804–806. doi: 10.1038/nn.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- He LJ, Liu N, Cheng TL, Chen XJ, Li YD, Shu YS, Qiu ZL, Zhang XH. Conditional deletion of Mecp2 in parvalbumin-expressing GABAergic cells results in the absence of critical period plasticity. Nat Commun. 2014;5:5036. doi: 10.1038/ncomms6036. [DOI] [PubMed] [Google Scholar]
- Heckman LD, Chahrour MH, Zoghbi HY. Rett-causing mutations reveal two domains critical for MeCP2 function and for toxicity in MECP2 duplication syndrome mice. eLife. 2014;3:3. doi: 10.7554/eLife.02676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Green A, Ressler KJ. Prepulse inhibition deficits in GAD65 knockout mice and the effect of antipsychotic treatment. Neuropsychopharmacology. 2004;29:1610–1619. doi: 10.1038/sj.npp.1300468. [DOI] [PubMed] [Google Scholar]
- Kash SF, Johnson RS, Tecott LH, Noebels JL, Mayfield RD, Hanahan D, Baekkeskov S. Epilepsy in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA. 1997;94:14060–14065. doi: 10.1073/pnas.94.25.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash SF, Tecott LH, Hodge C, Baekkeskov S. Increased anxiety and altered responses to anxiolytics in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA. 1999;96:1698–1703. doi: 10.1073/pnas.96.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505:318–326. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013;498:363–366. doi: 10.1038/nature12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013;77:388–405. doi: 10.1016/j.neuron.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16:1662–1670. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Buonanno A, Passafaro M, Sala C, Sweet RA. Developmental vulnerability of synapses and circuits associated with neuropsychiatric disorders. J Neurochem. 2013;126:165–182. doi: 10.1111/jnc.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Fryer JD, Ren J, Fyffe S, Chao HT, Sun Y, Greer JJ, Zoghbi HY, Neul JL. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum Mol Genet. 2008;17:1718–1727. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, McGraw CM, Ward CS, Sun Y, Neul JL, Zoghbi HY. Female Mecp2(+/−) mice display robust behavioral deficits on two different genetic backgrounds providing a framework for pre-clinical studies. Hum Mol Genet. 2013;22:96–109. doi: 10.1093/hmg/dds406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu KV, Lang D, Müller B, Nullmeier S, Yanagawa Y, Schwegler H, Stork O. Glutamic acid decarboxylase 67 haplodeficiency impairs social behavior in mice. Genes Brain Behav. 2014;13:439–450. doi: 10.1111/gbb.12131. [DOI] [PubMed] [Google Scholar]
- Skene PJ, Illingworth RS, Webb S, Kerr AR, James KD, Turner DJ, Andrews R, Bird AP. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork O, Yamanaka H, Stork S, Kume N, Obata K. Altered conditioned fear behavior in glutamate decarboxylase 65 null mutant mice. Genes Brain Behav. 2003;2:65–70. doi: 10.1034/j.1601-183x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CS, Arvide EM, Huang TW, Yoo J, Noebels JL, Neul JL. MeCP2 is critical within HoxB1-derived tissues of mice for normal lifespan. J Neurosci. 2011;31:10359–10370. doi: 10.1523/JNEUROSCI.0057-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff SB, Gründemann J, Tovote P, Krabbe S, Jacobson GA, Müller C, Herry C, Ehrlich I, Friedrich RW, Letzkus JJ, Lüthi A. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–458. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- Xue M, Atallah BV, Scanziani M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature. 2014;511:596–600. doi: 10.1038/nature13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Peterson M, Beyer B, Frankel WN, Zhang ZW. Loss of MeCP2 from forebrain excitatory neurons leads to cortical hyperexcitation and seizures. J Neurosci. 2014;34:2754–2763. doi: 10.1523/JNEUROSCI.4900-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.