Abstract
Colorectal cancer is commonly treated with 5-fluorouracil and 5-formyltetrahydrofolate (leucovorin). Metabolic action of leucovorin requires several enzymatic steps that are dependent on expression of corresponding coding genes. To identify folate pathway genes with possible impact on leucovorin metabolism, a retrospective study was performed on 193 patients with stage III colorectal cancer. Relative expression of 22 genes putatively involved in leucovorin transport, polyglutamation and metabolism was determined in tumor and mucosa samples using quantitative real-time polymerase chain reaction. After surgery, patients received adjuvant 5-fluorouracil-based bolus chemotherapy with leucovorin during six months, and were followed for 3 to 5 years. Cox regression analysis showed that high tumoral expression of the genes SLC46A1/PCFT (proton-coupled folate transporter) and SLC19A1/RFC-1 (reduced folate carrier 1) correlated significantly (p < 0.001 and p < 0.01, respectively) with a decreased risk of recurrent disease, measured as disease-free survival (DFS). These two genes are involved in the transport of folates into the cells and each functions optimally at a different pH. We conclude that SLC46A1/PCFT and SLC19A1/RFC-1 are associated with DFS of patients with colorectal cancer and hypothesize that poor response to 5-fluorouracil plus leucovorin therapy in some patients may be linked to low expression of these genes. Such patients might need a more intensified therapeutic approach than those with high gene expression. Future prospective studies will determine if the expression of any of these genes can be used to predict response to leucovorin.
INTRODUCTION
Patients with colorectal cancer are commonly treated with 5-fluorouracil (5-FU) in combination with 5-formyltetrahydrofolate (leucovorin; LV). The 5-FU+LV (FLV) treatment may be used alone or in combination with oxaliplatin or irinotecan as adjuvant treatment. Metaanalyses have shown that biochemical modulation of 5-FU with LV increases the treatment response of patients with colorectal cancer from 11% to about 21% (1). LV is considered to increase the concentration of the natural reduced folate cofactor [6R]-5,10-methylenetetrahydrofolate (methyleneTHF) in the cells (2). This cofactor forms a ternary complex with the enzyme thymidylate synthase (TS) in a reaction in which deoxyuridine monophosphate (dUMP) is transformed to deoxythymidine monophosphate (dTMP) (3). After administration with 5-FU, a fluorinated form of dUMP is formed intracellularly, which leads to inhibition of the TS enzyme. Stabilization of the ternary complex can be achieved by high levels of methyleneTHF. The inhibition of the TS enzyme impairs DNA synthesis by depleting the cells of dTMP, and is thought to be the major cause of the 5-FU-related antitumor effect.
Previous studies by our group (4) and by Houghton et al. (5) have shown that the tissue concentrations of methyleneTHF do not increase proportionately following administration of increasing doses of LV, whereas direct administration of methyleneTHF predictably leads to higher plasma and tissue concentrations. These findings are consistent with the suggestion that the level of LV activation is important for the therapeutic effect.
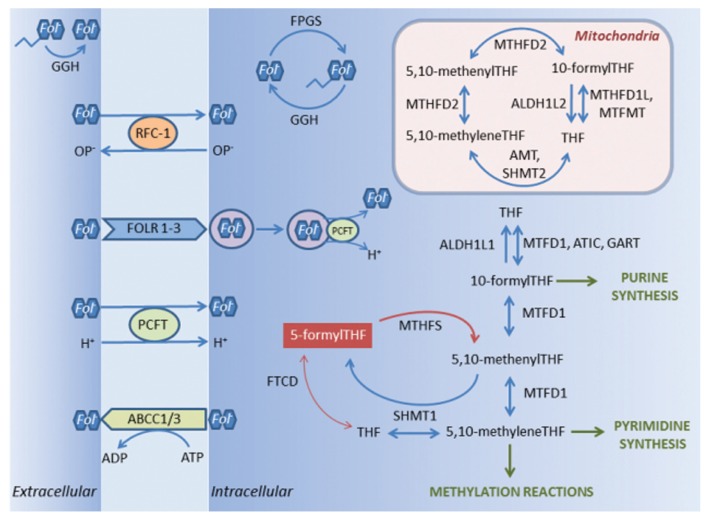
The metabolic activation of LV requires several enzymatic steps, which are likely dependent on the expression level of the corresponding coding genes, and which therefore possibly influence the level of response to therapy with LV. To identify genes in the folate pathway with possible impact on the metabolism of LV, the relative expression of 22 genes putatively involved in transport, polyglutamation and metabolism of LV was determined in tumor and mucosa tissue of patients with stage III colorectal cancer. A schematic representation of folate transport and folate-mediated 1-carbon metabolism in eukaryotic cells is shown in Figure 1 (6–19). The patients were subjected to adjuvant treatment with bolus FLV alone, or to bolus FLV plus oxaliplatin (FLOX), given according to the Nordic regimes (20,21). The gene expression was related to disease-free survival (DFS).
Figure 1.
Schematic representation of folate transport and folate mediated 1-carbon metabolism in eukaryotic cells (for gene abbreviations and enzyme commission numbers, see Table 2 and Supplementary Table S1, respectively). Before entering the cells, folate polyglutamates are converted to monoglutamates by the enzyme GGH. Transport of folate monoglutamates into the cells may occur through the proton-coupled folate transporter SLC46A1/PCFT, or SLC19A1/RFC-1, which utilizes the organic phosphate (OP−) gradient. Folates also may be transported via an endocytic mechanism by FOLR1, FOLR2 and FOLR3, possibly mediated by SLC46A1/PCFT. Export of folate monoglutamates occurs via ABCC1 and ABCC3. In the cytoplasm, the enzyme FPGS converts folate monoglutamates to polyglutamates, which is an essential step for folate homeostasis. The enzyme GGH is active also in the cytoplasm where it is primarily located to the lysosomes. Folate polyglutamates are considered to be better substrates than monoglutamates for enzymes involved in the folate metabolism. However, in eukaryotic cells the folate metabolism is highly compartmentalized and the distribution of folate cofactors and the extent of folate polyglutamation differ in cytosolic and mitochondrial pools. One-carbon metabolism in mitochondria is needed to generate formate for one-carbon metabolism in the cytoplasm. Two enzyme steps are required to convert 5-formylTHF (leucovorin) to methyleneTHF. The first step is usually catalyzed by MTHFS, which is considered to be “rate-limiting” with regard to the generation of methyleneTHF. 5-formylTHF also may be converted to 5,10-methyleneTHF by the actions of FTCD and SHMT1. Other enzymes involved in the folate metabolism are ALDH1L1, ALDH1L2, AMT, ATIC, FTCD, GART, MTFMT, MTHFD1, MTHFD1L, MTHFD2, SHMT1 and SHMT2. Note that only those proteins encoded by genes analyzed in the present study have been depicted in the figure. Also, some of the gene products, which in the figure are described as active in the cell membrane or cytoplasm, may also be active in mitochondria (for example, SLC19A1/RFC-1, FPGS and MTHFS), or may have dual actions. For instance, AMT in mitochondria also catalyzes the conversion of 5,10-methenylTHF to 5-formylTHF, which subsequently is converted back to 5,10-methenylTHF by MTHFS. THF = tetrahydrofolate,
 = folate polyglutamate,
= folate polyglutamate,
 = folate monoglutamate.
= folate monoglutamate.
MATERIALS AND METHODS
Patients
During the period 2001 to 2009, 542 patients with nonhereditary stage III colorectal cancer underwent surgery at the Sahlgrenska University Hospital/Östra and received adjuvant FLV (n = 383) or FLOX (n = 159) treatment. Of the 542 patients, 446 underwent elective surgery. Biopsy samples were obtained from 290 patients, and of these 193 were available for the current study. Patient and tumor characteristics are shown in Table 1. All tumors were classified according to the Tumor–Node–Metastasis (TNM) staging system (22). Patients were followed for 3 to 5 years after surgical removal of the primary tumor. The ethics committee of the University of Gothenburg approved the study and informed consent was obtained from all patients.
Table 1.
Patient and tumor characteristics (n = 193).
| Gender, n (%) | |
| Female | 92 (48) |
| Male | 101 (52) |
| Median age, years (range) | 65 (35–80) |
| Tumor differentiation, n (%) | |
| High/moderate | 152 (79) |
| Low | 32 (17) |
| Unknown | 9 (5) |
| Median number of assessed lymph nodes (range) | 18 (3–63) |
| Median number of positive lymph nodes (range) | 2 (1–31) |
| Median lymph node ratio (range) | 0.15 (0.016–0.86) |
Treatment
Patients received adjuvant treatment during six months with FLV (n = 151) or FLOX (n = 42). FLV treatment comprised intravenous bolus injections of 5-FU (500 mg/m2), followed by LV (60 mg/m2) 30–40 min later, on d 1 and d 2 every other week, according to the Nordic FLV regime (20). FLOX treatment comprised 5-FU (500 mg/m2) and LV (60 mg/m2) bolus on d 1 and d 2 every other week, plus an oxaliplatin infusion (85 mg/m2) over 120 min on d 1 every other week (21).
Selection of Genes
Based on recent scientific literature (23), 22 target genes with putative impact on LV metabolism were chosen for analysis (Table 2). Seven of these genes are involved in folate transport (ABCC1, ABCC3, FOLR1, FOLR2, FOLR3, SLC19A1/RFC-1 and SLC46A1/PCFT), whereas the other 15 genes encode enzymes involved in folate polyglutamation (FPGS and GGH) or folate metabolism (ALDH1L1, ALDH1L2, AMT, ATIC, FTCD, GART, MTFMT, MTHFD1, MTHFD1L, MTHFD2, MTHFS, SHMT1 and SHMT2). The Enzyme Commission (EC) numbers and locations of enzymes encoded by these 15 genes are shown in Supplementary Table S1.
Table 2.
List of 22 genes with putative impact on leucovorin metabolism chosen for analysis (plus 2 housekeeping genes).
| Gene category | Gene | Gene namea | Assay ID |
|---|---|---|---|
| Folate transport | ABCC1 | ATP-binding cassette, subfamily C (CFTR/MRP), member 1 | Hs00219905_m1 |
| ABCC3 | ATP-binding cassette, subfamily C (CFTR/MRP), member 3 | Hs00358656_m1 | |
| FOLR1 | Folate receptor 1 (adult) | Hs01124177_m1 | |
| FOLR2 | Folate receptor 2 (fetal) | Hs00265255_m1 | |
| FOLR3 | Folate receptor 3 (gamma) | Hs01549264_m1 | |
| SLC19A1/RFC-1 | Solute carrier family 19 (folate transporter), member 1 | Hs00953344_m1 | |
| SLC46A1/PCFT | Solute carrier family 46 (folate transporter), member 1 | Hs00611081_m1 | |
| Folate metabolism | ALDH1L1 | Aldehyde dehydrogenase 1 family, member L1 | Hs00201836_m1 |
| ALDH1L2 | Aldehyde dehydrogenase 1 family, member L2 | Hs00402876_m1 | |
| AMT | Aminomethyltransferase | Hs00166628_m1 | |
| ATIC | 5-Aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase | Hs00269671_m1 | |
| FTCD | Formiminotransferase cyclodeaminase | HS00198409_m1 | |
| GART | Phosphoribosylglycinamide formyltransferase, phosphoribosylglycinamide synthetase, phosphoribosylaminoimidazole synthetase | Hs00531926_m1 | |
| MTFMT | Mitochondrial methionyl-tRNA formyltransferase | Hs00373739_m1 | |
| MTHFD1 | Methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 1, methenyltetrahydrofolate cyclohydrolase, formyltetrahydrofolate synthetase | Hs00602830_m1 | |
| MTHFD1L | Methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 1-like | Hs00383616_m1 | |
| MTHFD2 | Methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 2, methenyltetrahydrofolate cyclohydrolase | Hs00741165_m1 | |
| MTHFS | 5,10-Methenyltetrahydrofolate synthetase (5-formyltetrahydrofolate cycloligase) | Hs00197574_m1 | |
| SHMT1 | Serine hydroxymethyltransferase 1 (soluble) | Hs00541038_m1 | |
| SHMT2 | Serine hydroxymethyltransferase 2 (mitochondrial) | Hs00193658_m1 | |
| Folate polyglutamation | FPGS | Folylpolyglutamate synthase | Hs00191956_m1 |
| GGH | γ-Glutamyl hydrolase (conjugase, folylpolygammaglutamyl hydrolase) | Hs00914163_m1 | |
| Housekeeping | ACTB | β-actin | Hs99999903_m1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Hs99999905_m1 |
Abbreviations: ATP, adenosine triphosphate; CFTR, cystic fibrosis transmembrane conductance regulator; IMP, inosine monophosphate; MRP, multidrug resistance protein; NADP, nicotinamide adenine dinucleotide phosphate.
Preparation of RNA and cDNA
Tumor and matched macroscopically normal-appearing mucosa (obtained approximately 10 cm from the tumor) were snap frozen in liquid nitrogen after removal and stored at –80°C until used. Total RNA was isolated from 10 to 30 mg of fresh-frozen tissue using the High Pure RNA Tissue Kit (# 12033674001, Roche Diagnostics Scandinavia AB) according to the manufacturer’s instructions. cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and run on Gene Amp PCR System 9600 (PerkinElmer). To optimize each run, the expression level of β-actin was determined in each sample in a pre-run. The variation between duplicates, calculated as [(standard deviation/mean) × 100], was no more than 0.5% for any sample. A second RNA extraction and cDNA synthesis were performed if the concentration was considered to be suboptimal.
Real-Time Quantitative PCR
The relative gene expression was quantified using TaqMan Low-Density Array (TLDA) cards (Applied Biosystems). Custom-designed TLDA cards containing 24 individual assays were used. Three samples and one calibrator (SK-N-AS) were loaded to each card according to the manufacturer’s instructions; each reservoir contained 83 ng of RNA converted to cDNA in a total volume of 100 μl. Two test runs were performed before the actual analysis. Quantitative polymerase chain reactions (QPCRs) were set up in duplicates in 384-well plates using the Biomek FX pipetting robot (Beckman Coulter) and were carried out in 10 μl reactions with 1× TaqMan Gene Expression Mastermix (Applied Biosystems), 1× gene-specific assay and 7.5 ng RNA converted into cDNA. Both TLDA cards and individual QPCR plates were run and analyzed by the ABI PRISM 7900HT Sequence Detection System (SDS 2.2, Applied Biosystems) according to the manufacturer’s protocol. The thresholds and baselines were set manually in SDS, and Ct values were extracted. Variations between runs were compensated for by normalization against a control sample. All Ct values were normalized to the mean of the endogenous housekeeping genes β-actin and GAPDH for each sample.
Statistics
Statistical analysis was performed using the “survival” package in the R statistical software (24). Cox proportional-hazards regression models were applied to the normalized data to examine the relationship between expression levels of chosen genes and DFS. To choose between the numerous clinical covariates, stepwise model selection by Akaike information criterion (AIC) was performed on Cox models excluding the expression values. AIC is a measure of goodness of fit and, as long as it improved the AIC-value, the covariate that gave the best AIC if removed was deleted. The selected covariates were then included in the final models.
Cox regression was performed individually for each gene and for expression values from mucosa and tumor separately. Furthermore, the difference between the mucosa and tumor was investigated in a separate model for each gene. Each of these models was fitted both with and without the additional selected clinical covariates to examine their influence on the models. Additionally, each pair of samples (tumor and mucosa) was fitted together to a Cox model in the same fashion, to examine possible interactions. All other calculations were performed using the ΔΔCt method. Differences in gene expression levels between groups were calculated using the Kruskal–Wallis test or the Pearson’s Chi-square test, and data were presented as the median and interquartile ranges (IQ ranges).
Values of p ≤ 0.05 were considered significant. Permutations were used to correct p values for multiple testing. There was a linear correlation between the two housekeeping genes β-actin and GAPDH. The expression of the target genes was related to a mean value representing both of these genes to keep variance to the minimum. DFS was calculated from the date of surgery to the last followup, or to the date of recurrence or death. All patients who were disease-free at 5 years were censored at 5 years.
All supplementary materials are available online at www.molmed.org.
RESULTS
The multivariate Cox regression analysis included gene expression and the parameters gender, age, lymph node ratio, tumor differentiation and adjuvant therapy (FLV or FLOX). One patient was considered to be an outlier and was excluded from the Cox regression analysis due to very low expression values of the housekeeping genes. When doing stepwise variable selection with AIC, the two variables that emerged and which were included in the model were lymph node ratio and adjuvant therapy. Both varied to some extent in the different models, with lymph node ratio constantly having values of p < 0.01, while adjuvant therapy was significant (at the p ≤ 0.05 level) in only 42% of the models, although it constantly had values of p < 0.1.
During followup, 59 of the 193 (31%) patients relapsed. A similar recurrence rate was found in 92 of the 253 (36%) patients who underwent elective surgery but from whom biopsy samples were not available. This indicates that the selected patient group was representative of the total cohort of patients treated with adjuvant FLV or FLOX therapy between 2001 and 2009.
There was a significant correlation between high expression of the genes SLC46A1/PCFT (p < 0.001), and SLC19A1/RFC-1 (p < 0.01) in tumor tissue and a decreased risk of recurrent disease, measured as DFS. After correction for multiple testing, SLC46A1/PCFT and SLC19A1/RFC-1 remained significant, with p values <0.05 (Table 3). Furthermore, the correlation between DFS and expression of the following genes showed nominally significant p values (although they did not remain significant after correction for multiple testing: ABCC3 (p < 0.01), FPGS (p < 0.01), ATIC (p < 0.05), GGH (p < 0.05), MTFMT (p < 0.05), MTHFD1 (p < 0.05) and MTHFD1L (p < 0.05) (Table 3). Almost identical survival functions were obtained when the expression levels of the SLC46A1/PCFT, ABCC3, FPGS and SLC19A1/RFC-1 genes were plotted against time. A representative survival curve is shown in Figure 2. Furthermore, IQ ranges for normalized Ct values were calculated (Table 3), to assure that acquired hazard ratios (HR) were large enough to be relevant. With HR of 1.3 and an IQ range of 1.6, SLC46A1/PCFT showed a 111% increased risk between the upper and the lower quartile. For SLC19A1/RFC-1, the increase was 82%. All other genes with nominal p values below 0.05 showed an increase of above 55%, except MTFMT (36%) and ATIC (25%).
Table 3.
Results of the Cox regression analysis testing the effect of gene expression (normalized Ct values) in tumor tissue on disease-free survival.
| Gene | Gene category | Hazard ratioa | IQ rangeb | p Value | Corrected p valuec |
|---|---|---|---|---|---|
| SLC46A1/PCFT | Transport | 1.30 | 9.1–10.7 | 0.00089 | 0.021 |
| SLC19A1/RFC-1 | Transport | 1.39 | 6.5–7.9 | 0.0016 | 0.035 |
| FPGS | Polyglutamation | 1.45 | 4.9–6.0 | 0.0038 | 0.071 |
| ABCC3 | Transport | 1.24 | 5.2–7.1 | 0.0051 | 0.093 |
| MTHFD1L | Metabolism | 1.30 | 6.1–7.5 | 0.016 | 0.24 |
| GGH | Polyglutamation | 1.18 | 5.3–7.7 | 0.025 | 0.34 |
| MTHFD1 | Metabolism | 1.32 | 5.8–7.2 | 0.038 | 0.45 |
| MTFMT | Metabolism | 1.38 | 7.1–8.1 | 0.038 | 0.46 |
| ATIC | Metabolism | 1.43 | 5.3–6.1 | 0.041 | 0.48 |
| GART | Metabolism | 1.16 | 3.9–4.9 | 0.17 | 0.92 |
| ABCC1 | Transport | 1.17 | 6.0–7.1 | 0.18 | 0.93 |
| SHMT1 | Metabolism | 0.92 | 4.9–6.8 | 0.26 | 0.98 |
| ALDH1L2 | Metabolism | 1.11 | 7.5–9.4 | 0.26 | 0.98 |
| MTHFD2 | Metabolism | 0.92 | 5.0–6.4 | 0.38 | 1.0 |
| FTCD | Metabolism | 0.96 | 14.3–17.3 | 0.51 | 1.0 |
| SHMT2 | Metabolism | 1.06 | 4.8–6.2 | 0.64 | 1.0 |
| FOLR1 | Transport | 1.02 | 8.1–12.0 | 0.66 | 1.0 |
| FOLR2 | Transport | 1.02 | 8.6–11.5 | 0.72 | 1.0 |
| MTHFS | Metabolism | 0.95 | 6.8–7.8 | 0.74 | 1.0 |
| ALDH1L1 | Metabolism | 0.99 | 7.7–12.1 | 0.79 | 1.0 |
| AMT | Metabolism | 1.00 | 6.9–8.8 | 0.96 | 1.0 |
| FOLR3 | Transport | 1.00 | 12.2–15.3 | 0.97 | 1.0 |
Hazard ratios are calculated from ΔCt values and correspond to a halving of the expression levels.
Interquartile range, calculated from normalized Ct values.
p Values corrected for multiple testing with permutations.
Figure 2.
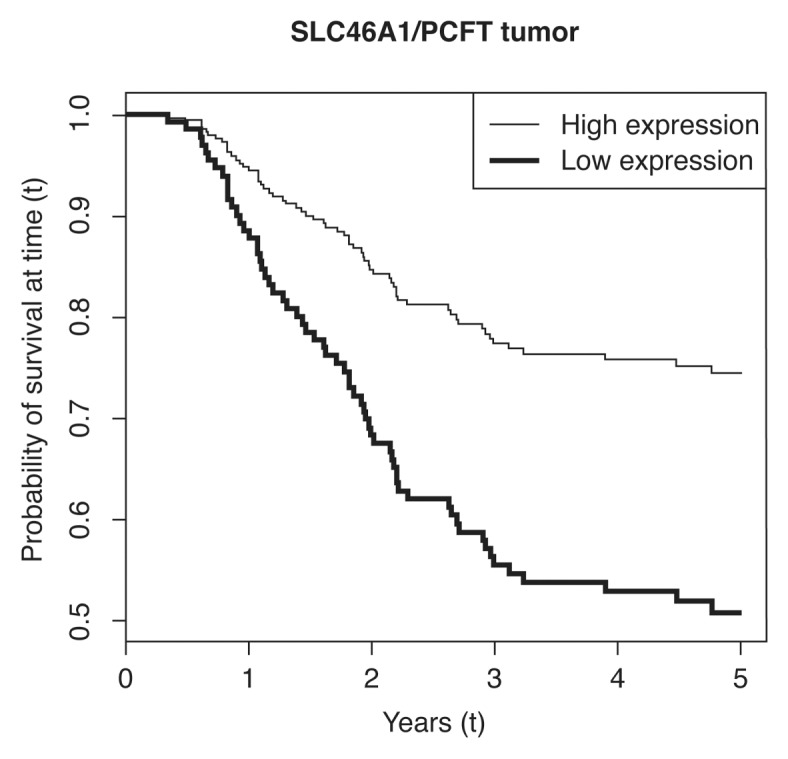
Estimated survival functions by level of SLC46A1/PCFT gene expression in colorectal cancer tissue. The two curves show predicted survival functions for the fitted Cox proportional hazards models. The expression levels for the two curves have been set one standard deviation above and one standard deviation below the mean, where one standard deviation corresponds to a fold change in log expression levels of 1.57. Lymph node ratio has been set to its mean, and adjuvant therapy has been set to 5-fluorouracil plus leucovorin, as this was the treatment given to most the patients. Standard deviations and mean for expression levels have been calculated from ΔCt values.
There were indications of a positive correlation between recurrent disease and the expression of MTHFD1 and FOLR2 (p < 0.05) in mucosa tissue. Furthermore, an increased tumor relative to mucosa expression of the following genes correlated with a lower risk of recurrent disease: MTHFD1 (p < 0.01), ATIC (p < 0.05), FPGS (p < 0.05), MTHFD1L (p < 0.05) and SLC19A1/RFC-1 (p < 0.05). However, after correction for multiple testing, none of these p values remained significant. When the relative gene expression levels in mucosa and tumor tissue were compared, significant differences were found (Table 4). The genes that were expressed differently in tumor and mucosa samples were associated with folate transport (ABCC3, FOLR1, FOLR3 and SLC46A1/PCFT), polyglutamation (GGH) and metabolism (GART, MTHFD1, MTHFD1L, MTHFD2 and SHMT2).
Table 4.
Comparison of gene expression levels, calculated using the ΔΔCt method, in mucosa and tumor tissue (n = 193).
| Gene | Gene category | Mucosa, median (IQ rangea) | Tumor, median (IQ rangea) | p Value | Corrected p valueb |
|---|---|---|---|---|---|
| ABCC1 | Transport | 0.58 (0.39–0.94) | 0.90 (0.61–1.3) | 0.79 | 1.0 |
| ABCC3 | Transport | 3.2 (2.3–4.6) | 1.2 (0.55–2.2) | <0.0001 | <0.0001 |
| ALDH1L1 | Metabolism | 0.24 (0.13–0.42) | 0.086 (0.015–0.36) | 0.56 | 1.0 |
| ALDH1L2 | Metabolism | 0.37 (0.22–0.66) | 0.24 (0.12–0.47) | 0.066 | 0.53 |
| AMT | Metabolism | 0.60 (0.39–0.99) | 0.37 (0.17–0.64) | 0.052 | 0.45 |
| ATIC | Metabolism | 0.95 (0.74–1.3) | 1.6 (1.2–2.2) | 0.072 | 0.58 |
| FOLR1 | Transport | 0.032 (0.018–0.055) | 0.079 (0.021–0.34) | <0.0001 | <0.0001 |
| FOLR2 | Transport | 0.37 (0.20–0.66) | 0.080 (0.027–0.20) | 0.25 | 0.98 |
| FOLR3 | Transport | 0.030 (0.019–0.055) | 0.0064 (0.0019–0.017) | <0.0001 | 0.0003 |
| FPGS | Polyglutamation | 1.4 (1.0–2.5) | 1.9 (1.3–2.7) | 0.19 | 0.92 |
| FTCD | Metabolism | 0.0050 (0.0024–0.013) | 0.0015 (0.0004–0.0034) | 0.012 | 0.12 |
| GART | Metabolism | 2.3 (1.7–3.4) | 3.9 (2.8–5.7) | <0.0001 | 0.00002 |
| GGH | Polyglutamation | 0.43 (0.23–0.70) | 0.91 (0.39–2.1) | <0.0001 | <0.0001 |
| MTFMT | Metabolism | 0.51 (0.36–0.71) | 0.43 (0.31–0.61) | 0.18 | 0.90 |
| MTHFD1 | Metabolism | 0.50 (0.35–0.71) | 0.90 (0.58–1.5) | <0.0001 | <0.0001 |
| MTHFD1L | Metabolism | 0.36 (0.18–0.67) | 0.76 (0.47–1.3) | 0.0004 | 0.003 |
| MTHFD2 | Metabolism | 1.0 (0.67–1.6) | 1.6 (1.1–2.9) | 0.0005 | 0.005 |
| MTHFS | Metabolism | 0.45 (0.33–0.62) | 0.53 (0.38–0.78) | 0.79 | 1.0 |
| SHMT1 | Metabolism | 1.5 (0.97–3.2) | 1.5 (0.87–3.3) | 0.92 | 1.0 |
| SHMT2 | Metabolism | 0.77 (0.58–1.1) | 1.8 (1.2–3.1) | <0.0001 | <0.0001 |
| SLC19A1/RFC-1 | Transport | 0.31 (0.21–0.50) | 0.57 (0.34–0.85) | 0.57 | 1.0 |
| SLC46A1/PCFT | Transport | 0.22 (0.13–0.49) | 0.089 (0.052–0.16) | <0.0001 | <0.0001 |
Interquartile range.
p Values corrected for multiple testing with permutations.
DISCUSSION
This retrospective study determined the relative expression of 22 genes with putative impact on LV metabolism in patients with stage III colorectal cancer treated with adjuvant bolus 5-FU-based chemotherapy and LV. The genes included those encoding folate transporters (ABCC1, ABCC3, FOLR1, FOLR2, FOLR3, SLC19A1/RFC-1 and SLC46A1/PCFT), and enzymes involved in folate polyglutamation (FPGS and GGH) and folate metabolism (ALDH1L1, ALDH1L2, AMT, ATIC, FTCD, GART, MTFMT, MTHFD1, MTHFD1L, MTHFD2, MTHFS, SHMT1 and SHMT2).
Transport of LV and other folates into the cells occurs through folate carriers and receptors, whereas outward transport occurs predominantly through active transporters (25). The results of the present study showed that high expression of the two folate transport genes SLC19A1/RFC-1 and SLC46A1/PCFT in tumor tissue correlated with decreased risk of recurrent disease, measured as DFS. Both SLC19A1/RFC-1 and SLC46A1/PCFT belong to the superfamily of solute carriers, but are functionally distinct (26). The SLC19A1/RFC-1 gene encodes the reduced folate carrier which functions optimally at physiological pH, transferring reduced folates, including LV, into the cells (27). Below pH 7, the transport of folates by SLC19A1/RFC-1 decreases dramatically and is mainly accomplished by SLC46A1/PCFT. Thus, in the upper GI, where the microenvironment is more acidic, the SLC46A1/PCFT protein has a critical role in intestinal absorption of dietary folates. Also in tumor tissue, where the pH is usually lower than in normal tissue, the influx of reduced folates is preferentially carried out by the SLC46A1/PCFT protein (28). High expression of the SLC19A1/RFC-1 and SLC46A1/PCFT genes would result in high levels of intracellular folates, and stabilization of the ternary complex comprising the active cofactor methyleneTHF, TS and fluorinated dUMP.
The ABCC1 and ABCC3 proteins are members of the superfamily of adenosine triphosphate (ATP)-binding cassette (ABC) transporters that transfer a variety of molecules, including monoglutamated forms of reduced folates, to the outside of the cell membrane (8). Based on recent publications, the ABCC1 protein seems to be a promising predictive factor for reduced folate levels after LV administration (23). However, in the present study, expression of the ABCC1 gene did not correlate with DFS, nor did expression of the ABCC3 gene.
The tumoral expression of the two genes FPGS and GGH, which are involved in folate polyglutamation, were pinpointed as determinants of the efficacy of LV in enhancing the antitumor activity of 5-FU in a previous study on human colon cancer cells (29). Indeed, high expression of FPGS and low expression of GGH seem to be predictive for high levels of reduced folates after LV administration in colorectal cancer (23). However, in the present study, neither FPGS nor GGH gene expression correlated significantly with DFS, after correction for multiple testing. The same was true for four genes involved in conversion of 10-formylTHF to THF (ATIC, MTHFD1, MTHFD1L and MTFMT).
The results regarding gene expression levels in mucosa tissue and DFS are hard to interpret because the significant results were few in number and quite weak. Furthermore, after correction for multiple testing, there was no significant correlation between mucosal expression of any gene and DFS. However, in agreement with previous studies (30,31), significant differences in the expression levels of several of the analyzed genes were found when mucosa and tumor tissues were compared. This indicates that in-and outward transport, polyglutamation and metabolism of folates differ greatly between mucosa and tumor tissue. These differences may be related to the folate content, the cellular proliferation rate or the pH of the tissue, which, in turn, may be related to the epigenetics (for example, the methylation status of the gene in question).
There are several limitations of the present study. Firstly, it is presently unknown whether the correlation between the SLC46A1/PCFT and SLC19A1/RFC-1 gene expression levels and DFS is prognostic, or predictive, of the given treatment. To elucidate a possible prognostic effect, we plan to compare the results of the present study with those obtained from untreated patients with stage III colorectal cancer. A predictive effect related to 5-FU or oxaliplatin seems unlikely, considering that these drugs enter the cells by transport mechanisms unrelated to SLC46A1/PCFT or SLC19A1/RFC-1 (32,33). Thus, any predictive effect pertaining to the expression of these two genes ought to be related to LV. Secondly, it is not known whether the gene expression levels correlate with the folate status in the tissue at surgery; or how, and if, they change in response to the FLV-treatment. However, the analyzed genes were selected because they have a known impact on the folate metabolism. We are now planning to analyze the folate levels in the tissue samples to be able to correlate these with gene expression. Thirdly, the number of patients included was relatively small, and the patient group consisted of an uneven mixture of FLV and FLOX-treated patients. It is thus important to confirm the results in a larger group of patients where subgrouping by treatment will be possible. Fourthly, other folate pathway genes than those analyzed are likely to be of importance. For instance, the expression of the gene TYMS which encodes the 5-FU target TS, is known to affect response to treatment (3). Also, combinations of genes, such as the ratio between SLC46A1/PCFT and SLC19A1/RFC-1, may be informative. These types of calculations, as well as gene expression analysis of TYMS and other relevant genes, will be performed in future studies.
CONCLUSION
The study showed that high tumoral expression of two genes involved in folate transport, SLC46A1/PCFT and SLC19A1/RFC-1, correlated positively with longer DFS of patients with colorectal cancer. We hypothesize that poor response to bolus FLV-based therapy in some patients with stage III colorectal cancer is linked to low expression of these genes. Such patients may warrant a different therapeutic approach than those with high gene expression. One approach would be to use natural methyleneTHF, which, unlike LV, does not require metabolic activation; its effects are therefore likely to be independent of these folate-associated genes. However, it is important to note that differences in clinical efficacy due to biochemical modulation of 5-FU-based treatment using LV or other folates may also relate to differences in drug administration (for example, whether bolus or continuous infusion of 5-FU or LV or a combination is being used). Thus, future prospective studies are needed to investigate the association between the levels of gene expression and specific folate metabolites in colorectal cancer tissue in relation to different treatment protocols. Additional studies are also needed to determine whether the expression level of any of the identified genes can be used to predict response to LV.
Supplemental Data
ACKNOWLEDGMENTS
We thank J Flach, I Palmgren and M Åkerström for their technical assistance, H Björkqvist and A-L Helminen for the collection of surgical samples and L Munro and B Sjöberg for their work with the clinical database. We also thank personnel at the Genomics Core Facility for performing the quantitative gene expression analysis, and personnel at the Bioinformatics Core Facility for their help with the statistical analyses. This work was supported by grants from the Swedish Cancer Society, the Health and Medical Care Committee of the Regional Executive Board Region Västra Götaland, Isofol Medical AB, the Assar Gabrielsson Foundation for Cancer Research, the Gustaf V Jubilee Clinic Foundation for Cancer Research, the Anna-Lisa and Bror Björnsson Foundation and the Ingabritt and Arne Lundberg Foundation.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The study was partially funded by Isofol Medical AB and a patent application related to the results of the study is pending. B Gustavsson is a shareholder in Isofol Medical AB.
Cite this article as: Odin E, Sondén A, Gustavsson B, Carlsson G, Wettergren Y. (2015) Expression of folate pathway genes in stage III colorectal cancer correlates with recurrence status following adjuvant bolus 5-FU-based chemotherapy. Mol. Med. 21:597–604.
REFERENCES
- 1.Thirion P, et al. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004;22:3766–75. doi: 10.1200/JCO.2004.03.104. [DOI] [PubMed] [Google Scholar]
- 2.Porcelli L, et al. The impact of folate status on the efficacy of colorectal cancer treatment. Curr Drug Metab. 2011;12:975–84. doi: 10.2174/138920011798062274. [DOI] [PubMed] [Google Scholar]
- 3.Kaiyawet N, Rungrotmongkol T, Hannongbua S. Effect of halogen substitutions on dUMP to stability of thymidylate synthase/dUMP/mTHF ternary complex using molecular dynamics simulation. J Chem Inf Model. 2013;53:1315–23. doi: 10.1021/ci400131y. [DOI] [PubMed] [Google Scholar]
- 4.Taflin H, Wettergren Y, Odin E, Derwinger K. Folate levels measured by LC-MS/MS in patients with colorectal cancer treated with different leucovorin dosages. Cancer Chemother Pharmacol. 2014;74:1167–74. doi: 10.1007/s00280-014-2591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houghton JA, et al. Influence of dose of [6RS]leucovorin on reduced folate pools and 5-fluorouracil-mediated thymidylate synthase inhibition in human colon adenocarcinoma xenografts. Cancer Res. 1990;50:3940–6. [PubMed] [Google Scholar]
- 6.Galivan J, et al. Glutamyl hydrolase. pharmacological role and enzymatic characterization. Pharmacol Ther. 2000;85:207–15. doi: 10.1016/s0163-7258(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 7.Shane B. Folate Chemistry and Metabolism. In: Bailey LB, editor. Folate in Health and Disease. 2nd edition. CRC Press, Taylor & Francis Group; Boca Raton, FL: 2010. pp. 1–24. [Google Scholar]
- 8.Hooijberg JH, et al. The role of multidrug resistance proteins MRP1, MRP2 and MRP3 in cellular folate homeostasis. Biochem Pharmacol. 2003;65:765–71. doi: 10.1016/s0006-2952(02)01615-5. [DOI] [PubMed] [Google Scholar]
- 9.Lowe KE, et al. Regulation of folate and one-carbon metabolism in mammalian cells. II. Effect of folylpoly-gamma-glutamate synthetase substrate specificity and level on folate metabolism and folylpoly-gamma-glutamate specificity of metabolic cycles of one-carbon metabolism. J Biol Chem. 1993;268:21665–73. [PubMed] [Google Scholar]
- 10.Tibbetts AS, Appling DR. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 11.Field MS, Szebenyi DM, Stover PJ. Regulation of de novo purine biosynthesis by methenyltetrahydrofolate synthetase in neuroblastoma. J Biol Chem. 2006;281:4215–21. doi: 10.1074/jbc.M510624200. [DOI] [PubMed] [Google Scholar]
- 12.Kohls D, Sulea T, Purisima EO, MacKenzie RE, Vrielink A. The crystal structure of the formiminotransferase domain of formiminotransferase-cyclodeaminase: implications for substrate channeling in a bifunctional enzyme. Structure. 2000;15:35–46. doi: 10.1016/s0969-2126(00)00078-2. [DOI] [PubMed] [Google Scholar]
- 13.Herbig K, et al. Cytoplasmic serine hydroxymethyltransferase mediates competition between folate-dependent deoxyribonucleotide and S-adenosylmethionine biosyntheses. J Biol Chem. 2002;277:38381–9. doi: 10.1074/jbc.M205000200. [DOI] [PubMed] [Google Scholar]
- 14.Krupenko NI, et al. ALDH1L2 is the mitochondrial homolog of 10-formyltetrahydrofolate dehydrogenase. J Biol Chem. 2010;285:23056–63. doi: 10.1074/jbc.M110.128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nzila A, Ward SA, Marsh K, Sims PF, Hyde JE. Comparative folate metabolism in humans and malaria parasites (part II): activities as yet untargeted or specific to Plasmodium. Trends Parasitol. 2005;21:334–9. doi: 10.1016/j.pt.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baggott JE, Tamura T. Metabolism of 10-formyldihydrofolate in humans. Biomed Pharmacother. 2001;55:454–7. doi: 10.1016/s0753-3322(01)00093-2. [DOI] [PubMed] [Google Scholar]
- 17.Lee SG, Lutz S, Benkovic SJ. On the structural and functional modularity of glycinamide ribonucleotide formyltransferases. Protein Sci. 2003;12:2206–14. doi: 10.1110/ps.03139603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinha A, et al. Biochemical characterization of pathogenic mutations in human mitochondrial methionyl-tRNA formyltransferase. J Biol Chem. 2014;289:32729–41. doi: 10.1074/jbc.M114.610626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, et al. Purification and properties of human cytosolic folylpoly-gamma-glutamate synthetase and organization, localization, and differential splicing of its gene. J Biol Chem. 1996;271:13077–87. doi: 10.1074/jbc.271.22.13077. [DOI] [PubMed] [Google Scholar]
- 20.Carlsson G, et al. Sequential 5-fluorouracil and leucovorin in patients with advanced symptomatic gastrointestinal cancer. Eur J Cancer. 1990;26:874–6. doi: 10.1016/0277-5379(90)90188-y. [DOI] [PubMed] [Google Scholar]
- 21.Sorbye H, Dahl O. Nordic 5-fluorouracil/leucovorin bolus schedule combined with oxaliplatin (Nordic FLOX) as first-line treatment of metastatic colorectal cancer. Acta Oncol. 2003;42:827–31. doi: 10.1080/02841860310018972. [DOI] [PubMed] [Google Scholar]
- 22.Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739–57. doi: 10.1002/(sici)1097-0142(20000401)88:7<1739::aid-cncr30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 23.Sadahiro S, et al. Molecular determinants of folate levels after leucovorin administration in colorectal cancer. Cancer Chemother Pharmacol. 2010;65:735–42. doi: 10.1007/s00280-009-1079-5. [DOI] [PubMed] [Google Scholar]
- 24.Therneau T. A Package for Survival Analysis in S. R package version 2.37-4 Version 2.38-3. 2013. available from: http://CRAN.R-project.org/package=survival.
- 25.Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med. 2009;11:e4. doi: 10.1017/S1462399409000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou Z, Matherly LH. Biology of the major facilitative folate transporters SLC19A1 and SLC46A1. Curr Top Membr. 2014;73:175–204. doi: 10.1016/B978-0-12-800223-0.00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao R, Goldman ID. Folate and thiamine transporters mediated by facilitative carriers (SLC19A1–3 and SLC46A1) and folate receptors. Mol Aspects Med. 2013;34:373–85. doi: 10.1016/j.mam.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao R, Goldman ID. The molecular identity and characterization of a Proton-coupled Folate Transporter—PCFT; biological ramifications and impact on the activity of pemetrexed. Cancer Metastasis Rev. 2007;26:129–39. doi: 10.1007/s10555-007-9047-1. [DOI] [PubMed] [Google Scholar]
- 29.Sakamoto E, et al. Folylpolyglutamate synthase and gamma-glutamyl hydrolase regulate leucovorin-enhanced 5-fluorouracil anticancer activity. Biochem Biophys Res Commun. 2008;365:801–7. doi: 10.1016/j.bbrc.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 30.Kidd EA, et al. Variance in the expression of 5-Fluorouracil pathway genes in colorectal cancer. Clin Cancer Res. 2005;11:2612–9. doi: 10.1158/1078-0432.CCR-04-1258. [DOI] [PubMed] [Google Scholar]
- 31.Odin E, et al. Altered gene expression of folate enzymes in adjacent mucosa is associated with outcome of colorectal cancer patients. Clin Cancer Res. 2003;9:6012–9. [PubMed] [Google Scholar]
- 32.Li H, Chung SJ, Shim CK. Characterization of the transport of uracil across Caco-2 and LLC-PK1 cell monolayers. Pharm Res. 2002;19:1495–501. doi: 10.1023/a:1020456632737. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, et al. Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Res. 2006;66:8847–57. doi: 10.1158/0008-5472.CAN-06-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.