Abstract
AIM: To observe the change of tumor microcirculation after transcatheter arterial chemoembolization (TACE) with bletilla microspheres by using first pass perfusion MR imaging (FP) and Chinese ink casting.
METHODS: VX2 carcinoma cells were surgically implanted into the left and right lobes of liver of 30 New Zealand white rabbits, which were divided into 3 groups at random. Emulsion of lipiodol mixed with mitomycin C, and 5-FU bletilla microspheres were injected into the hepatic artery respectively, and saline was used as control agent. MR imaging was performed with turbo-flash sequence 14 d after tumor implantation and 7 d after interventional therapy. The steepest slopes (SS) of the signal intensity versus time curves were created for quantitative analysis, 7.5% Chinese ink gelatin solution was injected through ascending artery (17 cases) or portal vein (2 cases) for lesion microvessel area (MVA) measurement after the last MRI examination.The correlation between perfusion imaging and MVA was studied blindly.
RESULTS: The SS values at the rim of tumor in lipiodol group (mean, 49% per second) and bletilla group (mean, 35% per second) were significantly decreased (P < 0.05) as compared with control group (mean, 124% per second), no difference was found between lipiodol and bletilla groups (P > 0.05). In lipiodol group, the MVAs (24974 ± 11836 μm2) in the center of the tumor were significantly smaller than those of the control group (35510 ± 15675 μm2) (P < 0.05), while the MVAs (80031 ± 22745 μm2) around the tumor were significantly increased because small and dense plexuses appeared around the tumor which correlated to intense reaction of granulation tissue. None of the vessels was seen in the tumor in bletilla group, the peripheral MVAs of the tumor were significantly smaller than those of the control group (P < 0.05) and lipiodol group (P < 0.05). There was a good correlation between SS and MVAs in control group (rs, 0.985, P < 0.0001) and bletilla group (rs, 0.743, P < 0.05), the correlation was not significant in lipiodol group (rs, 0.527, P > 0.05).
CONCLUSION: TACE with bletilla microspheres may enhance its anti-tumor effect by inhibiting the angiogenesis, and FP-MRI provides useful information to assess the TACE effect by depicting tumor vascularization and perfusion.
INTRODUCTION
Transcatheter arterial chemoembolization (TACE) has been widely used and is considered to be an effective conservative treatment for hepatocellular carcinoma[1,2]. Rapid development of small vessels after TACE resulting in incomplete necrosis, however, reduces therapeutic effectiveness[1-9]. Therefore, inhibition of the development of arterial collaterals may be important in enhancing the therapeutic efficacy of this treatment. Recently, TACE with microspheres, microcapsules, cyanoacrylate and bletilla has been shown to improve the therapeutic results[1,3,10-12]. Bletilla microspheres have also been shown to improve the therapeutic results because of its embolization of the hepatic artery and portal vein as well as controlled release systems, although changes at the level of hepatic microcirculation are not completely elucidated.
The purpose of this study was to observe the change of tumor microcirculation after TACE with bletilla microspheres by using first pass perfusion MR imaging (FP) and Chinese ink casting.
MATERIALS AND METHODS
Animals and tumor models
The VX2 tumor model used in this study was initially a virus-induced papilloma first seen in domestic rabbit in 1937. With sequential transplantations, the tumor line became increasingly anaplastic[13]. VX2 carcinoma (Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China) was retained for approximately 4 years by repeated passage of tumor every 14-21 d by way of intramuscular or subcutaneous implantation into the thighs of male New Zealand white rabbits.
Twenty-five male and five female New Zealand white rabbits (Laboratory Center, Tongji Medical College) weighing 1-1.5 kg were used. Prior to all procedures, including tumor implantation and imaging, rabbits received an intramuscular injection of 1.0 mL/kg of body weight of ketamine hydrochloride injection (Shanghai Sino-west Pharmaceutical Company), intravenous access was then acquired via a marginal ear vein. Anesthesia was maintained by using the same dose of intravenously administered ketamine hydrochloride.
The technique for tumor implantation was basically similar to that described by Li et al[14]. Briefly, the liver was exposed by performing midline laparotomy, VX2 tumor fragments of approximately 1 mm3 were injected intraparenchymally into the right and left lobes of the rabbit liver. Thirty New Zealand white rabbits with tumors were divided into three groups at random. They were group A (control group, 8 rabbits), group B (lipiodol group, 12 rabbits) and group C (bletilla group, 10 rabbits). All three groups received their interventional therapy 14-21 d after tumor inoculation when MRI confirmed the successful implantation.
Interventional therapy
A polyethylene catheter (inner diameter 0.3 mm, outer diameter 0.5 mm) was retrogradely inserted into the gastroduodenal artery, the following agents were manually injected into the gastroduodenal artery, namely, saline only into group A, 0.4-0.5 mL lipiodol (Aulnay Sous-Bios, France) emulsion (lipiodol mixed with MMC) into group B, 0.3 mL ultravist mixed with 10 mg bletilla microspheres (Department of Radiology, Union Hospital, Wuhan, China, 40-200 μm in diameter, combined with 5-FU) into group C.
MR imaging and data analysis
Fourteen days after tumor implantation and 7 d after interventional therapy, MR imaging was undertaken by using a 1.5 T system (Magnetom Vision, Siemens Medical Systems) with a head coil. Transverse spin-echo T1-weighted images (repetition time was 525 ms, the echo time was 14 ms) and HASTE T2-weighted images (repetition time was 4.4 ms, the echo time was 90 ms) were obtained by using a 3 mm thick section, 0.5 mm intersection space, and four signals were acquired.
For the FP, a strongly T1-weighted, turbo-FLASH sequence was used with a high temporal resolution of 1.196 s per section, a single image was acquired sequentially with 65 repetitions, which resulted in an acquisition time of 2 min. The repetition time was 3.3 ms, the echo time was 1.4 ms, and time interval was 300 ms. At the end of the fourth acquisition, a dose of 0.1 mmol/kg body weight of gadopentetate dimeglumine (Bellona, Beijing, China) was administered via a marginal ear vein.
To quantitatively analyze FP, four circular ROIs were hand drawn, covering the center and rim of the lesion, signal intensity time curve was obtained over ROIs, and the steepest slope of the curve (SS) was calculated according to the previously described method[15].
Evaluation of anti-tumor effect
Tumor size was measured with calipers. The size of each tumor on the liver surface was measured immediately before treatment and seven days after treatment on MR imaging. We evaluated the anti-tumor effect based on the tumor volume, which was calculated as follows: tumor volume (mm3) = 0.5 × a × b2, where a is the length of major axis and b is the length of minor axis measured with calipers.
Chinese ink casting and histological evaluation
The tumor microvessels were demonstrated by perfusion of 75 g/L Chinese ink (Beijing Chinese Ink Company) gelatin solution into the ascending artery (17 cases) or portal vein (2 cases) after the last MR imaging examination. Before perfused with 75 g/L Chinese ink gelatin solution, 500 units of heparin was administered intravenously. After the rabbits were killed, the livers were removed and stored at -20 °C for 24 h. The specimens were immersed in 250-995 mL/L ethyl alcohol at increasing concentrations, and finally in a solution of methyl salicylate. When the oil penetrated the tissue, the specimens became transparent. The sections (50 μm) were observed first under a low power microscope (× 40), then the sections with most dense area of microvessels were selected and observed under a high power (× 200). Micro-vessels filled with Chinese ink gelatin were evaluated under a microscope, micro-vessel areas (MVAs) in the center and rim of the lesion were calculated by an imaging analysis system (Beijing Aeronautic University). A 5 μm sections of the same specimens were stained with hematoxylin and eosin for light microscopy.
Statistical analysis
All data were expressed as mean ± SD, the statistical differences between different groups were analyzed by ANOVA, and the correlation between SS and MVA was assessed by Spearman correlation analysis. Significance was accepted when P < 0.05.
RESULTS
MR imaging
MR imaging depicted all tumors on pretreatment images. All untreated tumors had a low signal intensity on T1-weighted images and an intermediate signal intensity on T2-weighted images. Central areas of high signal intensity on T2-weighted images and low signal intensity on T1-weighted images were compatible with the central necrosis when tumors were larger than 1 cm. T1-weighted images after injection of gadolinium showed a slight enhancement in the center of lesion and a rim enhancement around the center of lesion in all tumors (Figure 1). No significant difference in tumor volume was observed among the three groups before therapy.
Figure 1.
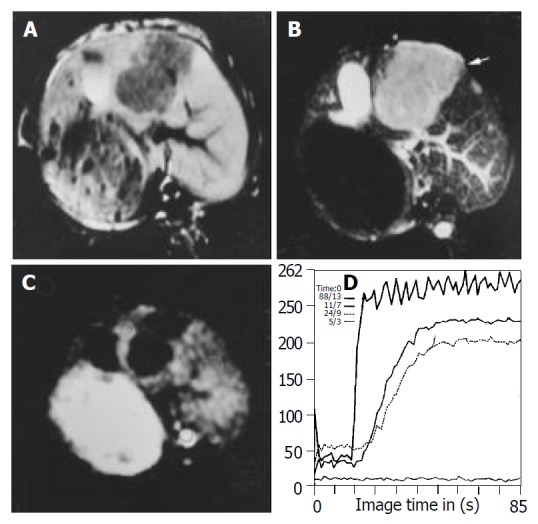
Images obtained before treatment in control group. A: T1WI shows hypointense lesions in left lobe of the liver. B: T2WI shows homogeneous hyperintense lesions in left lobe of the liver. C: FP T1-weighted image with gadolinium shows rim enhance-ment and no enhancement in the center of lesion. D: Signal intensity time curve derived from FP, SS of the curve in the border of the lesion is steeper than that of the normal liver.
Tumor volumes in the lipiodol group and bletilla group after TACE were significantly decreased compared with the control group on d 7 after treatment, no significant difference in tumor volumes was observed between lipiodol group and bletilla group (Figure 2, Table 1). The central slight enhancement area was larger, and the peripheral arterial phase rim enhancement in the two groups was thinner. The SS values at the rim of tumor in lipiodol group (mean, 49% per second) and bletilla group (mean, 35% per second) were significantly decreased (P < 0.05), as compared with the control group (mean, 124% per second), no difference was found between lipiodol group and bletilla one (P > 0.05) (Table 2).
Figure 2.
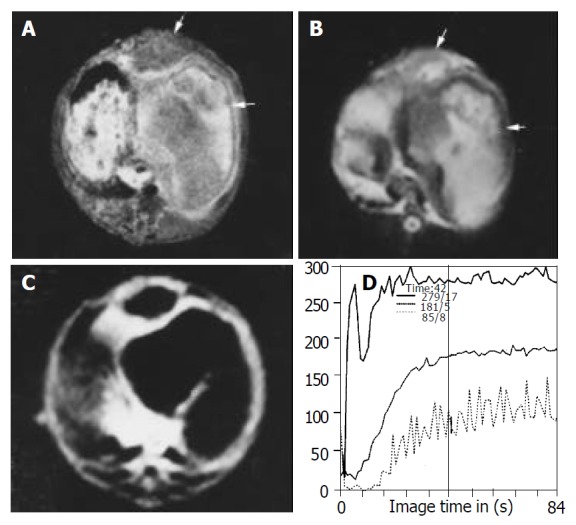
Images obtained after treatment in lipiodol group. Inhomogeneous hyperintense lesions with intermediate intense rim (arrow head) can be seen on T1WI (A) and T2WI (B), indicating the necrosis of the lesion and intratumor retention of lipiodol. (C) FP T1-weighted image with gadolinium shows thinner rim enhancement and no enhancement in the center of the lesion com-pared with control group although the lesion volume is increased. (D) SS of the curve in center of the lesion is decreased compared with those of the border.
Table 1.
Tumor volumes pre- and post-treatment (cm3)
| Group | Pre-treatment | Post treatment |
| Control | 0.430 ± 0.067 | 1.620 ± 0.327 |
| Lipiodol | 0.465 ± 0.120 | 0.971 ± 0.285a |
| Bletilla | 0.402 ± 0.171 | 0.736 ± 0.145a |
P < 0.05 vs control.
Table 2.
SS values at the rim of tumor in different groups
P < 0.05 vs control.
Chinese ink casting
Investigation under microscope (× 40) of the livers filled with Chinese ink in control group revealed networks of vessels or plexuses of dilated and tortuous course around and within the tumor originated from the arterioles, some sinusoid vessels were observed in the tumors, all these vessels were clearly distinct from the lobular architecture. In lipiodol group after TACE, the MVAs within the tumor (24974 ± 11836 μm2) were significantly smaller than those of the control group (35510 ± 15675 μm2) (P < 0.05), while the MVAs around the tumor (80031 ± 22745 μm2) were significantly increased, as compared with the control group (35510 ± 15675 μm2) (P < 0.05). Small and dense plexuses appeared around the tumor, with unknown origin. None of the vessels was seen in the tumor in bletilla group after TACE (Figure 3). The peripheral MVAs of the tumor (15530 ± 7973 μm2) in bletilla group were significantly smaller than those of the control group (35510 ± 15675 μm2) (P < 0.05) and lipiodol group (80031 ± 22745 μm2) (P < 0.05). There was a good correlation between SS and MVA at the rim of tumor in control group (rs, 0.985, P < 0.0001) and bletilla group (rs, 0.743, P < 0.05), the correlation was not significant in lipiodol group(rs, 0.527, P > 0.05) (Table 3).
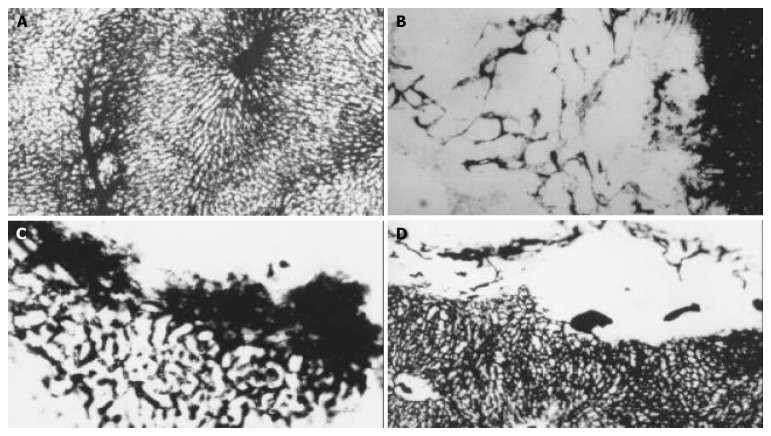
Figure 3.
Micro-vessel casting with Chinese ink through ascending artery. A: The specimens show a lobular architecture of normal liver, magnification × 100. B: In control group, hepatic artery perfusion shows networks of micro-vessels or plexuses of dilated and tortuous course around and within the tumor originated from the arterioles, some sinusoid vessels can be observed in this tumor, magnification × 100. C: The original micro-vessels of the tumor are remarkably diminished, small and dense new plexuses appear around the tumor in lipiodol group, which are correlated to intense reaction of granulation tissue, magnification × 100. D: micro-vessels are decreased in bletilla group, no new micro-vessels can be seen at all, magnification × 100.
Table 3.
Correlation between SS values and MVA in different groups
| Group | SS (%·s-1) | MVA (μm2) | rs | P |
| Control | 124 ± 62 | 35510 ± 15675 | 0.985 | < 0.05 |
| Lipiodol | 49 ± 15 | 80031 ± 22745 | 0.527 | > 0.05 |
| Bletilla | 35 ± 9 | 15530 ± 7973 | 0.743 | < 0.05 |
Pathologic correlation
The specimens did not show necrosis or infarct when the lesion was less than 1 cm in control group. In lipiodol group none lesions underwent complete necrosis, coagulation necrosis was demonstrated at the center of all lesions. Viable or residual cancerous tissues as well as an intense reaction of granulation tissue were seen at the periphery of all lesions in lipiodol groups after TACE, small and dense vessels were scattered at the border of the lesions, correlating with the irregular thick rim enhancement on MR image. In bletilla group after TACE, all lesions demonstrated complete coagulation necrosis, large infarcts involving multiple adjacent lobules were seen as well, few vessels were seen at the border of the lesion, no granulation tissue was seen (Figure 4).
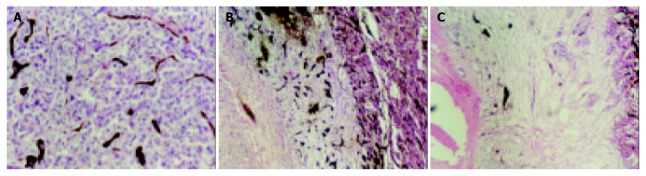
Figure 4.
Histological examinations of hepatic lesion before and after treatment. A: HE stain shows nidal arrangement of VX2 cells as well as micro-vessels filled with Chinese ink before treatment, magnification × 200. B: In lipiodol group, histological examina-tion shows coagulation necrosis at the center of lesion, very few micro-vessels can be seen within this zone, residual tumor cells are seen at the periphery of lesions, moreover, an intense reaction of granulation tissue is seen within this area, small dense vessels are scattered at border of the lesion, and congested sinusoids are seen in the periphery of the tumor, magnification × 200. C: Coagulation necrosis as well as large infarcts involving multiple adjacent lobules is seen in bletilla group, few vessels are seen at the border of the lesion, magnification × 200.
DISCUSSION
Bletilla inhibition of tumor micro-vessels
A previous study reported an intense reaction of granulation tissue at the periphery of all lesions in lipiodol group[16], the dense angiogenic plexus in the granulation tissue is, to our knowledge, a new finding. Granulation tissue which resulted in capillary proliferation and permeability increase was correlated to arterial phase rim enhancement on MR image. Angiogenesis was central to tumor stroma formation and offered nutrition to the remaining viable tumor cells, which limited lipiodol embolization and anti-tumor effect. As a result, local tumor recurrence would occur sooner or later[7-9,16].
TNP-470, cyanoacrylate and Plcg-mitomycin-microsphere have been used to inhibit capillary networks or to prolong first-pass effect and to enhance the anti-tumor effect of TACE[1,17-20]. Our experimental study indicated that bletilla could enhance the anti-tumor effect of TACE as well. Bletilla, as an anti-tumor and anti-inflammation agent, is a Chinese medicine[10,12]. Its microspheres (mixed with 5-FU, 40-200 μm in diameter) can result in dual embolization (embolization of hepatic arterial and portal venous blood supply) and inhibit the microvessels around and in the tumor completely. The reason why no microvessels developed after TACE was partly due to its anti-tumor and anti-inflammation effect, partly through inhibition of the binding of vascular endothelial growth factor to its receptor[10]. As a result, treatment with bletilla could necrose the tumor thoroughly. This was why bletilla could enhance the anti-tumor effect of TACE even the lesion was very large[12].
VX2 microcirculation morphology
Many materials, including Chinese ink and microfil, have been used to demonstrate the vascular morphology of tumors[17,21]. Among them, microfil is commonly used because of its different colors, which could identify the origin of vessels. But microfil was too viscous to get into the sinusoid, no vessel was visible inside the tumors less than 0.25 mm in diameter[17].So we selected less viscous materials to reveal microvessel morphology of VX2. Three percent of Chinese ink gelatin solution was used to explore the vascular architecture of cheek pouch carcinoma[21]. To avoid overfilling the microvessels, we added gelatin to increase the viscosity of the solution to obtain 7.5% solution. As a result, all the microvessels of normal liver including arterioles and sinusoid were filled with black Chinese ink, so did those of tumor. Moreover, the pathologic change of the other part of the same specimens stained with HE could be observed. So microvessel casting correlating with pathologic change could assess the changes of tumor microcirculation at the same time. We believe that Chinese ink gelatin solution casting may be used as the golden standard to evaluate the microvessels in perfusion study with CT and MR imaging.
Value of FP-MRI
Although power Doppler US has been used to assess vascularity of tumors[23-25], before contrast enhanced harmonic power Doppler US has widely been used, the major problems in the use of power Doppler are as follows. The detected velocities were too slow in the tumor, there were too many blooming artifacts associated with micro-bubble injection, the duration of enhancement was short and there were artifacts resulted from respiration[23]. So the vascularity of tumors could not be evaluated in detail by power Doppler US. FP-MRI, with a high time resolution, can monitor the contrast agent first passing the target tissue by using signal intensity time curve, the steepest slope of which (SS, i.e. the maximum upward slope of the curve) correlates linearly with flow velocity and angiogenesis, and has been successfully used to quantify the myocardial perfusion reserve and to depict the tumor vascularization[15,22], but no study has assessed the tumor microcirculation after TACE by FP[26,27]. Our experimental result revealed that areas with the fastest contrast medium uptake (SS) colocalized significantly with focal hot spots of MVA in control group and bletilla group, but no significant correlation was observed between SS and MVA in lipiodol group, suggesting that factors beyond the MVA are involved in the gadopentetate dimeglumine -related signals enhanced in microvessels. We believed that the discrepancy could reflect the difference between small dense vessels newly developed at the border of the lesion after lipiodol treatment and tumor microvessels before treatment. The new vessels were hyperpermeable[28], part of the small molecular contrast media (gadopentetate dimeglumine) might leak out of microvessels even at first pass course. On the other hand, the new vessels might be too small to have the same flow velocity like those before treatment. So we believed that when the same categoric vessels were assessed by FP, the flow velocity might be constant, the SS was correlated linearly with MVA. When different categoric vessels were evaluated by FP, the flow velocity and permeability were different, and not dependent only on MVA.
In this study, bletilla microspheres were administered for 7 d only, and further studies of extended duration are needed. We conclude that tumor microvessels can be markedly inhibited by using bletilla microspheres in combination with 5-FU, which can explain and confirm its effectiveness in clinic. The SS derived from FP has a good correlation with MVA and may be used as a noninvasive method to quantitatively evaluate tumor angiogenesis after TACE.
Footnotes
Edited by Wang XL and Zhu LH Proofread by Xu FM
References
- 1.Loewe C, Cejna M, Schoder M, Thurnher MM, Lammer J, Thurnher SA. Arterial embolization of unresectable hepatocellular carcinoma with use of cyanoacrylate and lipiodol. J Vasc Interv Radiol. 2002;13:61–69. doi: 10.1016/s1051-0443(07)60010-4. [DOI] [PubMed] [Google Scholar]
- 2.Pacella CM, Bizzarri G, Cecconi P, Caspani B, Magnolfi F, Bianchini A, Anelli V, Pacella S, Rossi Z. Hepatocellular carcinoma: long-term results of combined treatment with laser thermal ablation and transcatheter arterial chemoembolization. Radiology. 2001;219:669–678. doi: 10.1148/radiology.219.3.r01ma02669. [DOI] [PubMed] [Google Scholar]
- 3.Tancredi T, McCuskey PA, Kan Z, Wallace S. Changes in rat liver microcirculation after experimental hepatic arterial embolization: comparison of different embolic agents. Radiology. 1999;211:177–181. doi: 10.1148/radiology.211.1.r99ap09177. [DOI] [PubMed] [Google Scholar]
- 4.Park SI, Lee DY, Won JY, Lee JT. Extrahepatic collateral supply of hepatocellular carcinoma by the intercostal arteries. J Vasc Interv Radiol. 2003;14:461–468. doi: 10.1097/01.rvi.0000064856.87207.1e. [DOI] [PubMed] [Google Scholar]
- 5.Tan LL, Li YB, Chen DJ, Li SX, Jiang JD, Li ZM. [Helical dual-phase CT scan in evaluating blood supply of primary heptocellular carcinoma after transcatheter hepatic artery chemoembolization with lipiodol] Zhonghua Zhongliu Zazhi. 2003;25:82–84. [PubMed] [Google Scholar]
- 6.Won JY, Lee DY, Lee JT, Park SI, Kim MJ, Yoo HS, Suh SH, Park SJ. Supplemental transcatheter arterial chemoembolization through a collateral omental artery: treatment for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2003;26:136–140. doi: 10.1007/s00270-002-2629-y. [DOI] [PubMed] [Google Scholar]
- 7.Yi J, Liao X, Yang Z, Li X. Study on the changes in microvessel density in hepatocellular carcinoma following transcatheter arterial chemoembolization. J Tongji Med Univ. 2001;21:321–322, 331. doi: 10.1007/BF02886568. [DOI] [PubMed] [Google Scholar]
- 8.Shao G, Wang J, Zhou K, Yan Z. [Intratumoral microvessel density and expression of vascular endothelial growth factor in hepatocellular carcinoma after chemoembolization] Zhonghua Ganzangbing Zazhi. 2002;10:170–173. [PubMed] [Google Scholar]
- 9.Huang J, He X, Lin X, Zhang C, Li J. Effect of preoperative transcatheter arterial chemoembolization on tumor cell activity in hepatocellular carcinoma. Chin Med J (Engl) 2000;113:446–448. [PubMed] [Google Scholar]
- 10.Feng GS, Li X, Zheng CS, Zhou CK, Liu X, Wu HP. [Mechanism of inhibition of tumor angiogenesis by Bletilla colloid: an experimental study] Zhonghua Yixue Zazhi. 2003;83:412–416. [PubMed] [Google Scholar]
- 11.Furuse J, Ishii H, Satake M, Onaya H, Nose H, Mikami S, Sakai H, Mera K, Maru Y, Yoshino M. Pilot study of transcatheter arterial chemoembolization with degradable starch microspheres in patients with hepatocellular carcinoma. Am J Clin Oncol. 2003;26:159–164. doi: 10.1097/00000421-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Feng GS, Zheng CS, Zhou RM, Liang B, Zhang YF. Arterial em-bolization of hepatocellular carcinoma with use of bletilla and gelatin powder. Zhonghua Fangshexue Zazhi. 1996;30:135–137. [Google Scholar]
- 13.Kuszyk BS, Boitnott JK, Choti MA, Bluemke DA, Sheth S, Magee CA, Horton KM, Eng J, Fishman EK. Local tumor recurrence following hepatic cryoablation: radiologic-histopathologic correlation in a rabbit model. Radiology. 2000;217:477–486. doi: 10.1148/radiology.217.2.r00nv41477. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Zheng CS, Feng GS, Zhuo CK, Zhao JG, Liu X. An implantable rat liver tumor model for experimental transarterial chemoembolization therapy and its imaging features. World J Gastroenterol. 2002;8:1035–1039. doi: 10.3748/wjg.v8.i6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verstraete KL, De Deene Y, Roels H, Dierick A, Uyttendaele D, Kunnen M. Benign and malignant musculoskeletal lesions: dynamic contrast-enhanced MR imaging--parametric "first-pass" images depict tissue vascularization and perfusion. Radiology. 1994;192:835–843. doi: 10.1148/radiology.192.3.8058957. [DOI] [PubMed] [Google Scholar]
- 16.Han GH, Guo QL, Huang GS, Guo YL. Distribution of lipiodol in hepatocellular carcinoma after hepatic arterial injection and its significance. Zhonghua Fangshexue Zazhi. 1993;27:828–831. [Google Scholar]
- 17.Mugitani T, Taniguchi H, Takada A, Yamaguchi A, Masuyama M, Hoshima M, Takahashi T. TNP-470 inhibits collateralization to complement the anti-tumour effect of hepatic artery ligation. Br J Cancer. 1998;77:638–642. doi: 10.1038/bjc.1998.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka H, Taniguchi H, Mugitani T, Koishi Y, Masuyama M, Higashida T, Koyama H, Suganuma Y, Miyata K, Takeuchi K. Intra-arterial administration of the angiogenesis inhibitor TNP-470 blocks liver metastasis in a rabbit model. Br J Cancer. 1995;72:650–653. doi: 10.1038/bjc.1995.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund EL, Bastholm L, Kristjansen PE. Therapeutic synergy of TNP-470 and ionizing radiation: effects on tumor growth, vessel morphology, and angiogenesis in human glioblastoma multiforme xenografts. Clin Cancer Res. 2000;6:971–978. [PubMed] [Google Scholar]
- 20.Qian J, Truebenbach J, Graepler F, Pereira P, Huppert P, Eul T, Wiemann G, Claussen C. Application of poly-lactide-co-glycolide-microspheres in the transarterial chemoembolization in an animal model of hepatocellular carcinoma. World J Gastroenterol. 2003;9:94–98. doi: 10.3748/wjg.v9.i1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou ZT, Jin ZG, Zhang SL, Wang Z, Li WG. A morphic study on erigeron breviscapus (vant) hand-mazz affecting angiogen-esis of golden hamster cheek pouch. Linchuang Kouqiang Yixue Zazhi. 2000;16:166–169. [Google Scholar]
- 22.Wilke N, Jerosch-Herold M, Wang Y, Huang Y, Christensen BV, Stillman AE, Ugurbil K, McDonald K, Wilson RF. Myocardial perfusion reserve: assessment with multisection, quantitative, first-pass MR imaging. Radiology. 1997;204:373–384. doi: 10.1148/radiology.204.2.9240523. [DOI] [PubMed] [Google Scholar]
- 23.Du WH, Yang WX, Wang X, Xiong XQ, Zhou Y, Li T. Vascularity of hepatic VX2 tumors of rabbits: assessment with conventional power Doppler US and contrast enhanced harmonic power Doppler US. World J Gastroenterol. 2003;9:258–261. doi: 10.3748/wjg.v9.i2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubota K, Hisa N, Nishikawa T, Fujiwara Y, Murata Y, Itoh S, Yoshida D, Yoshida S. Evaluation of hepatocellular carcinoma after treatment with transcatheter arterial chemoembolization: comparison of Lipiodol-CT, power Doppler sonography, and dynamic MRI. Abdom Imaging. 2001;26:184–190. doi: 10.1007/s002610000139. [DOI] [PubMed] [Google Scholar]
- 25.Hosoki T, Yosioka Y, Matsubara T, Minamitani K, Higashi M, Ohtani M, Choi S, Mitomo M, Tono T. Power Doppler sonography of hepatocellular carcinoma treated by transcatheter arterial chemoembolization. Assessment of the therapeutic effect. Acta Radiol. 1999;40:639–643. doi: 10.3109/02841859909175602. [DOI] [PubMed] [Google Scholar]
- 26.Tsui EY, Chan JH, Cheung YK, Cheung CC, Tsui WC, Szeto ML, Lau KW, Yuen MK, Luk SH. Evaluation of therapeutic effective-ness of transarterial chemoembolization for hepatocellular carcinoma: correlation of dynamic susceptibility contrast-en-hanced echoplanar imaging and hepatic angiography. Clin Im-aging. 2000;24:210–216. doi: 10.1016/s0899-7071(00)00204-7. [DOI] [PubMed] [Google Scholar]
- 27.Chan JH, Tsui EY, Luk SH, Yuen MK, Cheung YK, Wong KP. Detection of hepatic tumor perfusion following transcatheter arterial chemoembolization with dynamic susceptibility con-trast-enhanced echoplanar imaging. Clin Imaging. 1999;23:190–194. doi: 10.1016/s0899-7071(99)00119-9. [DOI] [PubMed] [Google Scholar]
- 28.Du JR, Jiang Y, Zhang YM, Fu H. Vascular endothelial growth factor and microvascular density in esophageal and gastric carcinomas. World J Gastroenterol. 2003;9:1604–1606. doi: 10.3748/wjg.v9.i7.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]