Abstract
AIM: To investigate p27 expression in hepatocellular carcinoma (HCC), adjacent nontumoral and normal liver tissues, and to verify whether the subcellular localization of p27 was altered in HCC.
METHODS: The level of p27 in tumoral, nontumoral, and normal liver tissues were assessed by immunohistochemical (IHC) analysis. Parallel immunostaining was done for proliferating cell nuclear antigen (PCNA) to evaluate cell proliferation.
RESULTS: The labeling index (LI) of p27 in tumoral lesions was significantly lower than that in adjacent nontumoral lesions (t = 2.444, P = 0.017) and normal controls (t = 2.268, P = 0.029). The LI of p27 significantly decreased in patients with massive type (t = 2.227, P = 0.037) and infiltration (t = 2.197, P = 0.036). The prognosis of patients with higher p27 LI was longer than that of patients with lower p27 LI (P = 0.0247, log-rank test). The LI of PCNA was significantly higher in HCC than that in adjacent nontumoral lesions (t = 2.092, P = 0.041) and normal controls (t = 3.533, P = 0.002). There was no significant correlation between p27 expression and cell proliferation in tumor samples. The level of p27 in the cytoplasmic fraction was higher in tumoral and nontumoral liver tissues, and was associated with clinical stage (t = 2.520, P = 0.029) and the degree of invasion (t = 2.640, P = 0.019). Survival analysis showed that p27 was an independent prognosis marker for HCC patients.
CONCLUSION: These results suggest that p27 underexp-ressing in patients with HCC is closely associated with infiltration, metastasis, and prognosis. Alterations in the subcellular localization of p27 protein may occur early during hepatocarcinogenesis.
INTRODUCTION
Abnormalities in various regulators of cell cycle frequently occur in human cancers[1-4]. Cell cycle is tightly controlled by the regulators, including cyclins, cyclin-dependent kinases (CDKs), and their inhibitors. Cyclin-dependent kinase inhibitors (CKIs) are potent negative regulators of cell cycle, and have two families on the basis of sequence homology. The inhibitors of CDK4 (INK4) family, including p16Ink4a, p15Ink4b, p18Ink4c, and p19Ink4, specifically binds to CDK4 and CDK6, and inhibits cyclin D binding. The Cip/Kip family, including p21Cip1, p27Kip1, and p57Kip2, binds to and inhibits cyclin-bound CDKs[5]. Among CKIs, p27 is thought to have crucial[6] and important roles in a variety of fundamental cellular processes[7-10]. The underexpression of p27 assessed by IHC analysis has been associated with more severe tumor grade and a negative prognostic marker in different carcinomas[11-16].
Furthermore, studies showed that reduced p27 expression was correlated with advanced tumor stages and poorer disease-free survival in patients with HCC at a variety of evolutionary stages[17-20]. However, most precious investigations are based on immunostaining of tumoral liver samples without comparing with surrounding nontumoral liver, which should be the best control tissue to be used for comparison.
In addition, p27 functions at the nuclear level by binding to and inhibiting cyclin/CDK complexes. It was recently reported that cytoplasmic displacement was an alternative way to inhibit p27 activity and might also play a role in tumor development[21]. Indeed, positive immunostaining of cytoplasmic p27 has been previously reported in various human cancers[22-25]. To address this hypothesis, the expression of p27 in tumoral, adjacent nontumoral, and normal liver tissues was evaluated by IHC analysis in patients with HCC, and the subcellular distribution of p27 was also investigated in this study.
MATERIALS AND METHODS
Patients and tumor samples
From January 2000 to December 2001, 32 liver samples were collected from patients who had HCC and underwent surgery in our institution. All liver tumors were histologically diagnosed. Tumor gross type and stage were diagnosed using Eggel’s classification and Union International Contre Cancer (UICC) criteria. Tumor cellular differentiation was identified by Edmondson’s classification. No patient received pre-operative chemotherapy or chemoembolization. The clinicopathologic characteristics of 32 patients with HCC are listed in Table 1. Normal livers were prepared from 10 patients who were died from the accident as the controls.
Table 1.
Clinicopathologic characteristics of 32 Patients
| Clinicopathologic parameters | Number | % |
| Age | ||
| ≤ 45 years | 14 | 43.8 |
| > 45 years | 18 | 56.2 |
| Gender | ||
| Male | 26 | 81.2 |
| Female | 6 | 18.8 |
| Gross type | ||
| Massive | 15 | 46.9 |
| Nodular | 17 | 53.1 |
| Tumor size | ||
| ≤ 5 cm | 5 | 15.6 |
| > 5 cm | 27 | 84.4 |
| Cellular differentiation | ||
| I | 4 | 12.5 |
| II | 17 | 53.1 |
| III | 5 | 15.6 |
| IV | 6 | 18.8 |
| Degree of invasion | ||
| T1 + T2 | 12 | 37.5 |
| T3 + T4 | 20 | 62.5 |
| Stage | ||
| I + II | 10 | 31.2 |
| III + IV | 22 | 68.8 |
| Lymph node metastasis | ||
| Present | 4 | 87.5 |
| Absent | 28 | 12.5 |
| Portal invasion | ||
| With | 3 | 9.4 |
| Without | 29 | 90.6 |
| Infiltration | ||
| Present | 12 | 37.5 |
| Absent | 20 | 62.5 |
After curative surgery, all patients were followed up every 3 mo till death. They were followed from 2 to 32 mo (mean 15.2 mo). Actuarial survival was measured from the day of surgical operation to that of death or last follow-up.
Immunohistochemical study
Liver samples were routinely fixed in 40 g/L formaldehyde solution and embedded in paraffin. After slicing into 4 μm-thick sections, IHC analysis was performed using Dako ElivisionTM plus two-step System. In brief, the sections were dewaxed in xylene, and rinsed in alcohol and graded alcohol/water mixtures. Then, 30 mL/L hydrogen peroxide was applied to block endogenous peroxidase activity. The sections were subsequently treated in a microwave oven twice for 5 min in citrate buffer (pH6.0) at high power. After blocking with goat serum, the mouse monoclonal antibodies against p27 (ZM-0340) and PCNA (ZM-0213) (Zymed Biotechnology, Zymed, CA) were applied on the slides at the dilution of 1:120 and 1:50, respectively. After rising, staining was performed by the ElivisionTM plus two-step System (kit-9902, Dako, Carpinteria, CA). The color was developed by reacting with 3,3-diaminobenzidine. Slides were then counterstained with hematoxylin, dehydrated, cleared, and mounted. Negative controls were performed by replacing the primary antibody with nonimmune mouse serum or PBS (Figure 1). A human breast cancer specimen and the reactive tonsil were used as positive controls for p27 (Figure 2) and PCNA, respectively.
Figure 1.
Negative control of p27 (× 400).
Figure 2.
Positive control of p27 (× 400).
Immunostaining evaluation
Slides were mounted independently by 2 investigators without notifying any clinical or pathological information. The positive cells for p27 and PCNA were measured by counting at least 1000 HCC cells from at least 5 randomly selected fields under the microscope. Then, LI was calculated as a percentage for each protein.
Statistical analysis
Values were expressed as mean ± SD, The Student’s t test was performed to analyze the relationship between the expression of the proteins and various clinicopathologic parameters. The cumulative survival rate was computed and actuarial survival curves were constructed by the Kaplan-Meier method. The cumulative survival rate was compared between groups using the log-rank test. Relevant prognostic factors were identified by univariate and multivariate Cox proportional hazard regression analyses. Tests were considered significant when the P values were < 0.05.
RESULTS
Reduced expression of p27 in HCC
The mean LI of p27 in HCC was 27.7% ± 19.9%, which was lower than that in adjacent nontumoral lesions (42.3% ± 21.4%, t = 2.444, P = 0.017) and that in normal controls (39.1% ± 17.5%, t = 2.268, P = 0.029). Furthermore, the LI of p27 in adjacent nontumoral lesions was not significantly different from that in normal controls (Figure 3). Table 2 shows the relationship between p27 expression and clinicopathologic characteristics. The LI of p27 significantly decreased in patients with massive type (t = 2.227, P = 0.037) and infiltration (t = 2.197, P = 0.036).
Figure 3.
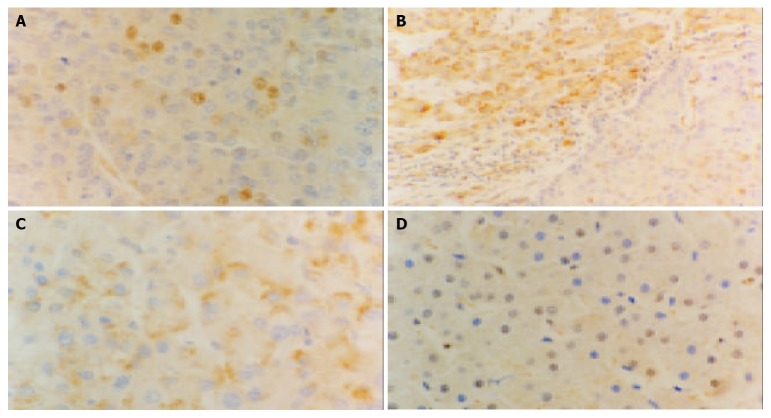
Immunohistochemical staining of p27 in different liver tissues (Elivision), A: p27 expression is decreased in HCC × 400, B: Decreased p27 expression in HCC (right), the p27 expression is mostly located in the cytoplasm in nontumoral liver tissues (left) × 200, C: Some tumor cells show cytoplasmic staining of p27 × 400, D: p27 staining in normal controls is mostly located in nuclear × 400.
Table 2.
Relationship among p27, PCNA, and cytoplasmic p27staining with clinicopathologic features
| Parameters | p27 LI (%) | P | PCNA LI (%) | P | Cytoplasmic p27 LI (%) | P |
| Gross type | ||||||
| Massive | 20.5 ± 13.1 | 0.037 | 46.4 ± 13.4 | 0.249 | 25.9 ± 18.9 | 0.221 |
| Nodular | 35.7 ± 23.4 | 52.3 ± 14.7 | 17.9 ± 17.3 | |||
| Tumor size | ||||||
| ≤ 5 cm | 23.8 ± 14.4 | 0.645 | 38.1 ± 10.7 | 0.047 | 27.0 ± 24.1 | 0.481 |
| > 5 cm | 28.4 ± 20.9 | 51.7 ± 13.9 | 20.6 ± 17.4 | |||
| Cellular differentiation | ||||||
| Well or moderate | 29.7 ± 21.4 | 0.438 | 45.7 ± 13.8 | 0.031 | 23.2 ± 18.0 | 0.498 |
| Poor | 23.8 ± 17.1 | 56.9 ± 12.6 | 18.6 ± 19.1 | |||
| Degree of invasion | ||||||
| T1 + T2 | 32.6 ± 23.3 | 0.285 | 51.1 ± 14.7 | 0.64 | 33.0 ± 22.3 | 0.019 |
| T3 + T4 | 24.7 ± 17.5 | 48.6 ± 14.4 | 14.8 ± 11.1 | |||
| Stage | ||||||
| I + II | 33.6 ± 25.6 | 0.261 | 48.0 ± 12.9 | 0.688 | 35.3 ± 23.9 | 0.029 |
| III + IV | 24.9 ± 16.7 | 50.2 ± 15.0 | 15.4 ± 10.7 | |||
| Infiltration | ||||||
| Present | 18.3 ± 18.8 | 0.036 | 44.4 ± 12.3 | 0.117 | 19.0 ± 18.3 | 0.536 |
| Absent | 33.3 ± 18.7 | 52.6 ± 14.7 | 23.2 ± 18.5 | |||
| Lymphocyte metastasis | ||||||
| With | 25.5 ± 2.6 | 0.565 | 56.0 ± 14.9 | 0.339 | 16.0 ± 10.6 | 0.518 |
| Without | 27.9 ± 21.3 | 48.6 ± 14.2 | 22.4 ± 19.1 | |||
| Portal invasion | ||||||
| Present | 14.3 ± 12.7 | 0.229 | 58.3 ± 12.0 | 0.267 | 13.0 ± 11.8 | 0.398 |
| Absent | 29.1 ± 20.1 | 48.6 ± 14.3 | 22.5 ± 18.7 | |||
Proliferating activity in HCC
The mean LI of PCNA was 49.5% ± 14.2% in HCC, which was higher than that in adjacent nontumoral lesions (42.8% ± 11.2%, t = 2.092, P = 0.041) and normal controls (36.2% ± 8.9%, t = 3.533, P = 0.002). The LI of PCNA in adjacent nontumoral lesions was not significantly different from that in normal controls (Figure 4). The LI of PCNA significantly increased in cases with poor differentiation (t = 2.259, P = 0.031, Table 2). The patients were divided into low p27 and high p27 groups with the cut-off value of the median LI. The LI of PCNA was 51.2% ± 15.6% and 48.1% ± 13.0% in low p27 and high p27 groups, respectively. There was no significant difference between these two groups (t = 0.578, P = 0.568).
Figure 4.
Expression of PCNA in different liver tissues (Elivision), A: Expression of PCNA in HCC × 400, B: Expression of PCNA in HCC (left) and in nontumoral liver tissues (right) × 200, C: Expression of PCNA in nontumoral liver tissues × 400, D: in normal controls × 400.
Altered subcellular localization of p27 in HCC
The cytoplasmic expression of p27 was found in the HCC, adjacent nontumoral, and normal liver tissues. Therefore, the cytoplasmic displacement of p27 might not be a specific phenomenon of tumor cells. However, the cytoplasmic sequestration of p27 was more frequent in HCC and adjacent nontumoral lesions (Figure 3).
The mean LI of cytoplamic p27 was 31.0% ± 12.6% in adjacent nontumoral lesions, which was higher than that in HCC (21.6% ± 18.2%, t = 2.378, P = 0.021) and normal controls (13.9% ± 5.6%, t = 5.994, P < 0.001). The LI of cytoplamic p27 in HCC was higher than that in normal controls (t = 2.106, P = 0.042). Whereas, the nuclear LI in normal controls (31.3% ± 12.6%) was higher than that in HCC (12.5% ± 9.7%, t = 4.968, P < 0.001) and nontumoral lesions (16.5% ± 10.4%, t = 3.731, P = 0.001). Altered subcellular localization of p27 was correlated with clinical stage (t = 2.520, P = 0.029) and the degree of invasion (t = 2.640, P = 0.019) (Table 2).
Expression of p27 and PCNA and survival
The survival rate of 6, 12, and 24 mo was 71.9%, 47.8%, and 25.1%, respectively. The patients were divided into 2 groups according to the median LI of p27, cytoplasmic p27, and PCNA. The survival analysis was performed on 32 patients and took into account for the following variables: age, gender, gross type, tumor size, UICC tumor stage, Edmondson’s grade, portal invasion, lymph node metastasis, depth of invasion, infiltration, p27 LI, cytoplasmic p27 LI, and PCNA LI. In univariate analysis, a significant correlation with short survival was found only for present portal invasion (P = 0.021) and low p27 expression (P = 0.033). The prognosis of patients with higher p27 LI was longer than that of patients with lower p27 LI (P = 0.0247, log-rank test, Figure 5). Multivariate analysis was performed by Cox proportional hazard regression model, age ≥ 45 years (P = 0.016) and low p27 expression (P = 0.009) were significantly associated with shorter survival. In addition, there was a trend of present lymphocyte metastasis (P = 0.05) and advanced stage (P = 0.08) correlated with poor prognosis (Table 3). For gross type, tumor size, Edmondson grade, depth of invasion, infiltration, cytoplasmic p27 LI, and PCNA LI, no significant correlations with overall survival were found.
Figure 5.

Kaplan-Meier curve for actuarial survival of patients in low p27 and high p27 groups.
Table 3.
Univariate and multivariate analyses of individual parameters
| Variables |
P values |
|
| Univariate | Multivariate | |
| Age | 0.150 | 0.016 |
| Tumor size | 0.513 | 0.678 |
| Carcinoma differentiation | 0.415 | 0.628 |
| Stage | 0.200 | 0.080 |
| Lymphocyte metastasis | 0.821 | 0.050 |
| Infiltration | 0.317 | 0.849 |
| Portal invasion | 0.021 | 0.619 |
| p27 | 0.033 | 0.009 |
| Cytoplasmic p27 | 0.114 | 0.624 |
| PCNA | 0.915 | 0.282 |
DISCUSSION
Hepatocellular carcinoma is one of the most common malignant tumors in China, and the incidence of HCC has increased in recent years[26]. Cell proliferating activity is one of the prominent parameters for evaluating the biological aggressiveness of tumors. The study in cell proliferating status of HCC is very important for evaluating the biological aggressiveness[27,28], because distant and lymph node metastasis are not very common.
The protein p27 is an important member of CKI family. It regulates G1 progression by binding to and inhibiting cyclin/CDKs[29,30]. Loss or reduced expression of p27 has been found in a variety of human cancers and associated with more aggressive tumor behavior. The present study showed that p27 expression significantly decreased in HCC. The finding of p27 underexpression in HCC was similar to the previous reports[31]. This study showed that the expression of p27 in HCC was associated with gross type and direct liver invasion. Univariate analysis showed that portal invasion and p27 LI were related to survival in this study. In multivariate analysis, p27 expression was the strongest predictor of survival for HCC patients, which was independent of the other variables. Similar findings have also been observed in other cancers[32-34]. It is therefore suggested that p27 should work as a negative regulator during hepatocarcinogenesis and may exert a useful prognostic marker. Interestingly, as reported by Milde-Langosch[35], there was no correlation between p27 expression and proliferating level, indicating that reduced expression of p27 did not merely reflect high proliferating activity during tumor progression.
To our knowledge, this was the first study to report the altered subcellular localization of p27 in HCC. The protein p27 can bind to and inhibit the active cyclin/CDK complexes in the nucleus, so the cytoplasmic displacement may play an important role in inactivating this protein in tumor cells and in contributing to tumor development. Indeed, cytoplasmic localization of p27 immunostaining has been reported in various types of human cancers. In our study, we found that the cytoplasmic localization of p27 was more frequent in HCC and surrounding nontumoral livers. Furthermore, the cytoplasmic staining for p27 was more frequently accompanied with nuclear staining in normal controls. However, the increase in the cytoplasmic staining for p27 was often observed in the absence of a concomitant increase in the nuclear staining and was sometimes associated with a decrease in the nuclear staining. Some tumors expressed an increased level of p27 mainly because of an increase in the cytoplasmic level of this protein. The increase in the amount of cytoplasmic p27 was more frequent in early stage (I and II) tumors. Altered subcellular localization of p27 was also reported in Barrett’s associated adenocarcinoma and colon cancer[22,23]. In agreement with our results, cytoplasmic localization of p27 was an early event during carcinogenesis.
Although the mechanism responsible for the abnormal subcellular localization was not known, it may be due to loss of the tuberous sclerosis complex gene-2 (TSC2), the HER/Grb2/MAPK pathway which leads to nuclear export of p27[36], overexpression of cyclin D3 which contributes to retaining p27 in the cytoplasm[37], or PKB/Akt which phosphorylates p27 to impair its nuclear import[38-40].
In conclusion, our results suggest that underexpression of p27 can contribute to the development of HCC. Cytoplasmic displacement is an alternative mechanism of inactivating p27 that acts early during hepatocarcinogenesis. Furthmore, studies on a large number of cases will reveal the significance of p27 subcellular localization in hepatocarcinogenesis and its relationship with clinicopathologic and prognostic parameters.
Footnotes
Edited by Chao JCJ Proofread by Xu FM
References
- 1.Ho A, Dowdy SF. Regulation of G(1) cell-cycle progression by oncogenes and tumor suppressor genes. Curr Opin Genet Dev. 2002;12:47–52. doi: 10.1016/s0959-437x(01)00263-5. [DOI] [PubMed] [Google Scholar]
- 2.Sánchez-Beato M, Sánchez-Aguilera A, Piris MA. Cell cycle deregulation in B-cell lymphomas. Blood. 2003;101:1220–1235. doi: 10.1182/blood-2002-07-2009. [DOI] [PubMed] [Google Scholar]
- 3.Todd R, Hinds PW, Munger K, Rustgi AK, Opitz OG, Suliman Y, Wong DT. Cell cycle dysregulation in oral cancer. Crit Rev Oral Biol Med. 2002;13:51–61. doi: 10.1177/154411130201300106. [DOI] [PubMed] [Google Scholar]
- 4.Fiano V, Ghimenti C, Schiffer D. Expression of cyclins, cyclin-dependent kinases and cyclin-dependent kinase inhibitors in oligodendrogliomas in humans. Neurosci Lett. 2003;347:111–115. doi: 10.1016/s0304-3940(03)00615-3. [DOI] [PubMed] [Google Scholar]
- 5.Polák J, Peková S, Schwarz J, Kozák T, Haskovec C. [Expression of cyclin-dependent kinase inhibitors in leukemia] Cas Lek Cesk. 2003;142:25–28. [PubMed] [Google Scholar]
- 6.Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Philipp-Staheli J, Payne SR, Kemp CJ. p27(Kip1): regulation and function of a haploinsufficient tumor suppressor and its misregulation in cancer. Exp Cell Res. 2001;264:148–168. doi: 10.1006/excr.2000.5143. [DOI] [PubMed] [Google Scholar]
- 8.Tsukiyama T, Ishida N, Shirane M, Minamishima YA, Hatakeyama S, Kitagawa M, Nakayama K, Nakayama K. Down-regulation of p27Kip1 expression is required for development and function of T cells. J Immunol. 2001;166:304–312. doi: 10.4049/jimmunol.166.1.304. [DOI] [PubMed] [Google Scholar]
- 9.Muraoka RS, Lenferink AE, Simpson J, Brantley DM, Roebuck LR, Yakes FM, Arteaga CL. Cyclin-dependent kinase inhibitor p27(Kip1) is required for mouse mammary gland morphogenesis and function. J Cell Biol. 2001;153:917–932. doi: 10.1083/jcb.153.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander K, Hinds PW. Requirement for p27(KIP1) in retinoblastoma protein-mediated senescence. Mol Cell Biol. 2001;21:3616–3631. doi: 10.1128/MCB.21.11.3616-3631.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitti D, Belluco C, Mammano E, Marchet A, Ambrosi A, Mencarelli R, Segato P, Lise M. Low level of p27(Kip1) protein expression in gastric adenocarcinoma is associated with disease progression and poor outcome. J Surg Oncol. 2002;81:167–175; discussion 175-176. doi: 10.1002/jso.10172. [DOI] [PubMed] [Google Scholar]
- 12.Hu YX, Watanabe H, Li P, Wang Y, Ohtsubo K, Yamaguchi Y, Sawabu N. An immunohistochemical analysis of p27 expression in human pancreatic carcinomas. Pancreas. 2000;21:226–230. doi: 10.1097/00006676-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Filipits M, Puhalla H, Wrba F. Low p27Kip1 expression is an independent prognostic factor in gallbladder carcinoma. Anticancer Res. 2003;23:675–679. [PubMed] [Google Scholar]
- 14.Anastasiadis AG, Calvo-Sanchez D, Franke KH, Ebert T, Heydthausen M, Schulz WA, Burchardt M, Gerharz CD. p27KIP1-expression in human renal cell cancers: implications for clinical outcome. Anticancer Res. 2003;23:217–221. [PubMed] [Google Scholar]
- 15.Tannapfel A, Grund D, Katalinic A, Uhlmann D, Köckerling F, Haugwitz U, Wasner M, Hauss J, Engeland K, Wittekind C. Decreased expression of p27 protein is associated with advanced tumor stage in hepatocellular carcinoma. Int J Cancer. 2000;89:350–355. doi: 10.1002/1097-0215(20000720)89:4<350::aid-ijc6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Yue H, Song FL, Zhang N, Feng XL, An TY, Yu JP. Expression of p27(kip1), Rb protein and proliferating cell nuclear antigen and its relationship with clinicopathology in human pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2003;2:142–146. [PubMed] [Google Scholar]
- 17.Fiorentino M, Altimari A, D'Errico A, Cukor B, Barozzi C, Loda M, Grigioni WF. Acquired expression of p27 is a favorable prognostic indicator in patients with hepatocellular carcinoma. Clin Cancer Res. 2000;6:3966–3972. [PubMed] [Google Scholar]
- 18.Zhou Q, He Q, Liang LJ. Expression of p27, cyclin E and cyclin A in hepatocellular carcinoma and its clinical significance. World J Gastroenterol. 2003;9:2450–2454. doi: 10.3748/wjg.v9.i11.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin LF, Ng IO. Expression of p27(KIP1) and p21(WAF1/CIP1) in primary hepatocellular carcinoma: clinicopathologic correlation and survival analysis. Hum Pathol. 2001;32:778–784. doi: 10.1053/hupa.2001.27105. [DOI] [PubMed] [Google Scholar]
- 20.Ito Y, Matsuura N, Sakon M, Miyoshi E, Noda K, Takeda T, Umeshita K, Nagano H, Nakamori S, Dono K, et al. Expression and prognostic roles of the G1-S modulators in hepatocellular carcinoma: p27 independently predicts the recurrence. Hepatology. 1999;30:90–99. doi: 10.1002/hep.510300114. [DOI] [PubMed] [Google Scholar]
- 21.Blagosklonny MV. Are p27 and p21 cytoplasmic oncoproteins? Cell Cycle. 2002;1:391–393. doi: 10.4161/cc.1.6.262. [DOI] [PubMed] [Google Scholar]
- 22.Sgambato A, Ratto C, Faraglia B, Merico M, Ardito R, Schinzari G, Romano G, Cittadini AR. Reduced expression and altered subcellular localization of the cyclin-dependent kinase inhibi-tor p27(Kip1) in human colon cancer. Mol Carcinog. 1999;26:172–179. doi: 10.1002/(sici)1098-2744(199911)26:3<172::aid-mc6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Singh SP, Lipman J, Goldman H, Ellis FH, Aizenman L, Cangi MG, Signoretti S, Chiaur DS, Pagano M, Loda M. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinoma. Cancer Res. 1998;58:1730–1735. [PubMed] [Google Scholar]
- 24.Masciullo V, Sgambato A, Pacilio C, Pucci B, Ferrandina G, Palazzo J, Carbone A, Cittadini A, Mancuso S, Scambia G, et al. Frequent loss of expression of the cyclin-dependent kinase inhibitor p27 in epithelial ovarian cancer. Cancer Res. 1999;59:3790–3794. [PubMed] [Google Scholar]
- 25.Masciullo V, Susini T, Zamparelli A, Bovicelli A, Minimo C, Massi D, Taddei G, Maggiano N, De Iaco P, Ceccaroni M, et al. Frequent loss of expression of the cyclin-dependent kinase inhibitor p27(Kip1) in estrogen-related Endometrial adenocarcinomas. Clin Cancer Res. 2003;9:5332–5338. [PubMed] [Google Scholar]
- 26.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol. 2002;8:193–199. doi: 10.3748/wjg.v8.i2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng WJ, Liu GY, Xu J, Zhou XD, Zhang YE, Zhang N. Pathological characteristics, PCNA labeling index and DNA index in prognostic evaluation of patients with moderately differentiated hepatocellular carcinoma. World J Gastroenterol. 2002;8:1040–1044. doi: 10.3748/wjg.v8.i6.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, Scheithauer BW. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154:313–323. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIntyre M, Desdouets C, Sénamaud-Beaufort C, Laurent-Winter C, Lamas E, Bréchot C. Differential expression of the cyclin-dependent kinase inhibitor P27 in primary hepatocytes in early-mid G1 and G1/S transitions. Oncogene. 1999;18:4577–4585. doi: 10.1038/sj.onc.1202815. [DOI] [PubMed] [Google Scholar]
- 31.Armengol C, Boix L, Bachs O, Solé M, Fuster J, Sala M, Llovet JM, Rodés J, Bruix J. p27(Kip1) is an independent predictor of recurrence after surgical resection in patients with small hepatocellular carcinoma. J Hepatol. 2003;38:591–597. doi: 10.1016/s0168-8278(03)00025-4. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Sun XF. Loss of p27 expression predicts poor prognosis in patients with Dukes' B stage or proximal colorectal cancer. Int J Oncol. 2001;19:49–52. [PubMed] [Google Scholar]
- 33.Hayashi H, Ogawa N, Ishiwa N, Yazawa T, Inayama Y, Ito T, Kitamura H. High cyclin E and low p27/Kip1 expressions are potentially poor prognostic factors in lung adenocarcinoma patients. Lung Cancer. 2001;34:59–65. doi: 10.1016/s0169-5002(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 34.Haitel A, Wiener HG, Neudert B, Marberger M, Susani M. Expression of the cell cycle proteins p21, p27, and pRb in clear cell renal cell carcinoma and their prognostic significance. Urology. 2001;58:477–481. doi: 10.1016/s0090-4295(01)01188-8. [DOI] [PubMed] [Google Scholar]
- 35.Milde-Langosch K, Hagen M, Bamberger AM, Löning T. Expression and prognostic value of the cell-cycle regulatory proteins, Rb, p16MTS1, p21WAF1, p27KIP1, cyclin E, and cyclin D2, in ovarian cancer. Int J Gynecol Pathol. 2003;22:168–174. doi: 10.1097/00004347-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Yang HY, Zhou BP, Hung MC, Lee MH. Oncogenic signals of HER-2/neu in regulating the stability of the cyclin-dependent kinase inhibitor p27. J Biol Chem. 2000;275:24735–24739. doi: 10.1074/jbc.C000147200. [DOI] [PubMed] [Google Scholar]
- 37.Baldassarre G, Belletti B, Bruni P, Boccia A, Trapasso F, Pentimalli F, Barone MV, Chiappetta G, Vento MT, Spiezia S, et al. Overexpressed cyclin D3 contributes to retaining the growth inhibitor p27 in the cytoplasm of thyroid tumor cells. J Clin Invest. 1999;104:865–874. doi: 10.1172/JCI6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 39.Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 40.Viglietto G, Motti ML, Bruni P, Melillo RM, D'Alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P, Bellacosa A, et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8:1136–1144. doi: 10.1038/nm762. [DOI] [PubMed] [Google Scholar]