Abstract
AIM: To observe the effect of compound Biejiaruangan decoction (CBJRGC) (composite prescription of Carapax trionycis for softening the liver) on proliferation, activation, excretion of collagen and cytokine of hepatic stellate cells (HSCs) and to find the mechanism of prevention and treatment of hepatic fibrosis by CBJRGC.
METHODS: Using MTT, immunohistochemistry and image analysis technology, the related indexes for proliferation, activation, excretion of collagen and cytokine of hepatic stellate cells were detected in 24 h, 48 h, and 72 h after adminstration of different dosages of CBJRGC.
RESULTS: Statistical analysis showed that serum collected from rat perfused with CBJRGC could restrain the proliferation of HSC in 48 h and 72 h especially in high and medium dosage groups, markedly decrease the expression of desmin, synapsin and platelet derived growth factor (PDGF) in HSC in 24 h, 48 h and 72 h, as well as the expression of α-SMA, collagen III, TIMP and TGFβ1 in 48 h and 72 h, decrease the excretion of collagen I in 72 h. CBJRGC serum had no significant effect on collagens I, III and TIMP in 24 h.
CONCLUSION: CBJRGC serum has a good curative effect on hepatic fibrosis. Its main mechanism may be related to the following factors. The drug serum can restrain the proliferation and activation of HSC, decrease the number of activated HSC and the total number of HSC, the excretion of collagens I, III, enhance the degradation of collagen and restore the balance of synthesis and degradation of collagen, inhibit the expression of transforming growth factor β1 (TGFβ1) and platelet derived growth factor (PDGF) in HSC, block and delay the process of hepatic fibrosis. Synapsin is a new marker of activation of HSC, which provides a theoretical and testing basis for neural regulation in the developing process of hepatic fibrosis.
INTRODUCTION
Hepatic fibrosis is an inevitable pathological process of chronic liver disease to hepatic cirrosis. Hepatic fibrosis is caused by excessive deposition of extracelluar matrix (ECM), which is the result of more synthesis and less degradation of ECM. A clinical and experimental study has found that liver cells, hepatic stellate cells (HSC), kupffer cells and sinus endothelial cells all take part in the formation of hepatic fibrosis, in which HSC plays a very important role[1]. Activation of HSC is commonly regarded as the major link of hepatic fibrosis and the main resource of synthesis of ECM[2]. The main characteristic of activation of HSC is excessive proliferation of HSC[3]. In addition, desmin is regard as a marking protein of HSC and α-SMA is regarded as a marker of activation of HSC[4]. A foreign study has reported that in process of activation of HSC, the expression of synapsin can increase[5]. Activation of HSC can lead to excessive synthesis of collagen. MMP and TIMP also jointly take part in the synthesis and degradation of collagen[6]. Multiple cytokines including TGFβ1 and PDGF play a very important role in proliferation, activation of HSC and synthesis of ECM[7]. Therefore, it may be a good strategy to restrain the amount of activated HSC, decrease the synthesis and excretion of collagens I, III and TIMPs, and promote the synthesis and excretion of MMPs. Aiming at HSC, it has become a control issue in anti-hepatic fibrosis to restrain its proliferation, decrease the synthesis of ECM and accelerate the degradation of ECM, and even inverse activated HSC to silent HSC. Traditional Chinese medicine has shown its own advantage in treating some difficult diseases. Approved by the government, compound Biejiaruangan decoction (CBJRGC) has been used as the first traditional Chinese medicine for treating hepatic fibrosis (approval document number: 1999 2-102). Clinical observations in Beijng, Shanghai and Hubei Province proved that its effective rate was 78.9% in 121 patients by the first hepatic puncture and in 52 patients by the second hepatic puncture. However, further study of its detailed anti-hepatic fibrosis mechanism is still needed. On the basis of the above-mentioned theory and research developments, our study with the cell culture as technical platform, was to observe the influence of serum collected from rats perfused with CBJRGC on activation and proliferation of HSC in vitro, and using immunohistochemistry and image analysis technology to observe its influence on the expressions of desmin, α-SMA, synapsin, collagens I, III, TIMP, TGFβ1 and PDGF of HSC in vitro.
MATERIALS AND METHODS
Main reagents
RPMI1640 was produced by Gibco, fetal bovine serum was produced by Hyclone, and 96-well plates by Costa. Dimethyl sulfoxide (DMSO), ethylenediaminetetra-aceticacid (EDTA), 3-(4,5-dimethy1-thiazol-2-yl), 2,5-diphenyl tetrazlium bromide (MTT), N-2-Hydroxyethlpiperazine-N’-2-ehtane sulfonine acid (HEPES), and trypin were all products of Sigma. Rat desmin monoclonal antibody and rat α-SMA monoclonal antibody were bought from DAKO, rat synapsin monoclonal antibody was bought from Santa Cruz. Rat collagen I and III monoclonal antibodies, rat TIMP and PDGF as well as TGF-β1 monoclonal antibodies, ABC and DAB test kits were all bought from Beijing Zhongshan Biotechnology Inc.
SD rat HSC line
The HSC line was established in our laboratory and prepared after long-term generation.
Preparation of SD rat serum[8]
Normal SD rat serum A normal rat weighting 350 g fasted for 12 h was injected with diethylether for anesthesia. Under the sterile condition, 10 mL blood was obtained from abdominal aorta, then held for 2 h at room temperature. Blood serum was made by centrifugation at 427 g for 10 min, inactivated at 56 °C for 60 min, and frozen at -60 °C.
Hepatic fibrosis model SD rat serum Adapted Hernandez-Munoz method was used to establish animal model of hepatic fibrosis[9], 0.2 mL CCl4 (Olive oil, 1:6 dilution) was injected into abdominal cavity, three times each week for 7 wk. Serum preparation and preservation were the same as those of the normal SD rat serum.
Drug serum With 3.5, 7 and 14 times of human body dosage as low, medium and high dosage groups respectively, CBJRGC was perfused into rat stomach 3 times at 12 h intervals. Rats were fasted for 12 h before the third perfusion and blood sampling was conducted from abdominal aorta 2 h after the third perfusion. Serum preparation and preservation were the same as those of the normal SD rat serum.
Cell culture and grouping
Rat HSCs were inoculated in RPMI1640 with 100 g/L fetal bovine serum, and cultivated at 37 °C in an incubator containing 50 mL/L CO2 to logarithm growth time. After treatment with digestive fluid, HSCs were suspended by adding D-Hank’s fluid, deposited by centrifugation at 190 g with 5 min, and then counted. Using RPMI1640 containing 100 g/L fetal bovine serum, HSCs were adjusted to a density of 5 × 104/mL and added to a 24-well plate containing flying sheet, 0.2 mL each well. According to intervening factors, HSCs were divided into 5 groups, i.e. control group, model group, high dosage serum group, medium dosage serum group and low dosage serum group. Each group had 4 wells. Upper culture medium was removed after cultivated for 12 h. Control group serum, model group serum, high dosage group serum, medium dosage group serum and low dosage group serum were accordingly added. Flying sheets were taken out respectively at 24 h, 48 h and 72 h, fixed with cold acetone for 10 min, dried and preserved at -60 °C.
Using MTT method to detect effect of drug serum on proliferation of HSC
HSCs with a density of 5 × 104/mL were added to a 96-well plate, 0.2 mL each well. Each group contained 8 wells. Upper medium was removed after cultivated for 12 h. Control group serum, model group serum, high dosage group, medium dosage group serum and low dosage group serum were accordingly added. 50 μL cultivating fluid was taken out respectively at 24 h, 48 h and 72 h. A 50 μL of MTT (50 μg MTT) was then added and the culture continued for 4 h at 37 °C. After upper fluid was removed, 150 μL DMSO was added to each well. After concussed and dissolved, Absorbency value with wave-length of 450 nm was detected by enzyme labeled instrument (BioRad 2250, Japan). The test was repeated 5 times.
Immunohistochemical staining
α-SMA staining Preserved cell flying sheets were immersed with PBC for 5 min, blocked with 10 mL/L H2O2 for 10 min, washed 3 times with PBC, 5 min each time, and then incubated with 10% goat serum for 30 min. Rat α-SMA, desmin, synapsin collagens I and II, TIMP, TGFβ1 and PDGF antibodies were diluted at a concentration of 1:100 and added as the first antibodies, staying overnight at 4 °C. After washed 3 times with PBC, 5 min each time, biotin goat anti-rat IgM was added, staying overnight at 4 °C, washed with PBC 3 times, 5 min each time. Streptavidin was added for 30 min, stained with DAB. The first antibody was replaced with fetal bovine serum as negative control, α-SMA male staining Absorbency value and relative area occupied by α-SMA male cells in the reference system were detected with TN-8502 image analysis system (Tractor Northern Co, USA). Data were treated with SPSS for ANOVA test. Results were presented mean ± SD. P < 0.05 was considered statistically significant.
RESULTS
Influence of each group serum on proliferation of HSC at different time points
HSC just after digesting phase showed a global form under contrast microscope. After cultivated for 12 h, HSCs were pasted to the wall, changing into the oblate form. There were obvious lipid droplets in cytoplasm. Few cells started to show the extension of cytoplasm. After cultivated for 24 h, most cells showed the extension of cytoplasm, and some cells showed multi-angle pseudopodium and typical star-like form. The influence on proliferation of HSC detected by MTT method is presented in Table 1.
Table 1.
Influence of each group serum on proliferation of HSC at different time points (A value: mean ± SD)
| N | M | G | Z | D | |
| 24 h | 0.40 ± 0.06 | 0.40 ± 0.03 | 0.38 ± 0.03 | 0.391 ± 0.02 | 0.38 ± 0.03 |
| 48 h | 0.52 ± 0.03c | 0.66 ± 0.05a | 0.47 ± 0.02ac | 0.460 ± 0.02ac | 0.51 ± 0.03c |
| 72 h | 0.75 ± 0.09c | 0.96 ± 0.05a | 0.55 ± 0.07ac | 0.580 ± 0.08ac | 0.69 ± 0.07c |
N: Control group; M: Model group; G: High dosage group; Z: Medium dosage group; D: Low dosage group,
P < 0.05 vs normal group;
P < 0.05 vs model group.
Influence of each group serum on α-SMA, desmin, synapsin of HSC at different time points
Table 2, Table 3, Table 4 and Figure 1, Figure 2 show the influence of each group serum on α-SMA, desmin, synapsin of HSC at different time points, which were detected with TN-8502 image analysis system.
Table 2.
Influence of each group serum on α-SMA of HSC at different time points (A value: mean ± SD)
|
N |
M |
G |
Z |
D |
||||||
| A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | |
| 24 h | 619.89 ± 163.84c | 7.49 ± 2.06c | 778.93 ± 190.26a | 9.64 ± 0.26a | 110.95 ± 45.21ac | 0.90 ± 0.22ac | 243.83 ± 62.97ac | 2.72 ± 1.48ac | 417.46 ± 126.72c | 3.56 ± 0.91c |
| 48 h | 812.51 ± 123.39c | 8.28 ± 4.58c | 1032.69 ± 106.64a | 10.56 ± 1.83a | 333.71 ± 57.9ac | 1.55 ± 0.29ac | 345.08 ± 51.85ac | 3.66 ± 1.67ac | 675.35 ± 202.20c | 5.45 ± 2.50c |
| 72 h | 989.77 ± 166.32c | 9.36 ± 2.39c | 1255.30 ± 150.39a | 15.66 ± 2.34a | 448.36 ± 55.80ac | 3.25 ± 0.56ac | 556.27 ± 80.32ac | 4.66 ± 1.24ac | 759.38 ± 109.32ac | 6.99 ± 1.58ac |
N: Control group; M: Model group; G: High dosage group; Z: Medium dosage group; D: Low dosage group,
P < 0.05 vs normal group;
P < 0.05 vs model group.
Table 3.
Influence of each group serum on desmin of HSC at different time points (A value: mean ± SD)
|
N |
M |
G |
Z |
D |
||||||
| A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | |
| 24 h | 78.93 ± 18.56c | 17.49 ± 2.06c | 119.69 ± 23.84a | 20.64 ± 2.26a | 58.95 ± 5.21ac | 12.90 ± 3.22ac | 63.83 ± 12.97ac | 12.72 ± 1.48ac | 75.46 ± 26.72c | 13.56 ± 0.91c |
| 48 h | 103.78 ± 36.64c | 10.28 ± 4.58c | 182.51 ± 28.43a | 14.56 ± 1.83a | 63.71 ± 7.94ac | 8.55 ± 0.29ac | 65.08 ± 18.85ac | 7.66 ± 1.67ac | 79.35 ± 22.28c | 9.35 ± 2.50c |
| 72 h | 125.30 ± 40.39c | 9.36 ± 2.39c | 1255.30 ± 150.39a | 15.66 ± 2.34a | 448.36 ± 55.80ac | 3.25 ± 0.56ac | 556.27 ± 80.32ac | 4.66 ± 1.24ac | 759.38 ± 109.32ac | 6.99 ± 1.58ac |
N: Control group; M: Model group; G: High dosage group; Z: Medium dosage group; D: Low dosage group,
P < 0.05 vs normal group;
P < 0.05 vs model group.
Table 4.
Influence of each group serum on synapsin of HSC at different time points (A value: mean ± SD)
|
N |
M |
G |
Z |
D |
||||||
| A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | |
| 24 h | 396.29 ± 78.56c | 5.49 ± 2.06c | 528.79 ± 26.84c | 12.93 ± 2.33c | 138.92 ± 20.21ac | 2.64 ± 0.22ac | 190.87 ± 52.97ac | 3.69 ± 1.24ac | 284.54 ± 66.72c | 4.05 ± 0.91c |
| 48 h | 577.78 ± 86.84c | 8.28 ± 4.58c | 748.57 ± 29.73c | 10.56 ± 1.83c | 228.71 ± 71.94ac | 1.55 ± 0.29ac | 338.08 ± 108.66ac | 3.66 ± 1.67ac | 675.35 ± 202.20c | 5.45 ± 2.50c |
| 72 h | 989.77 ± 166.32c | 9.36 ± 2.39c | 1255.30 ± 150.39c | 15.66 ± 2.34c | 448.36 ± 55.80ac | 3.25 ± 0.56ac | 556.27 ± 80.32ac | 4.66 ± 1.24ac | 759.38 ± 109.32ac | 6.99 ± 1.58ac |
N: Control group; M: Model group; G: High dosage group; Z: Medium dosage group; D: Low dosage group,
P < 0.05 vs normal group;
P < 0.05 vs model group.
Figure 1.
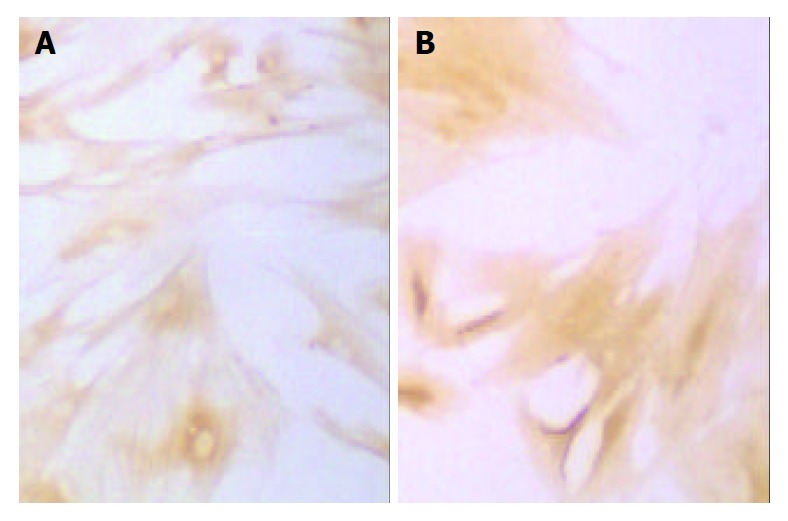
Immunohistochemical staining of α-SMA of SD rat HSC line in model group and medium dosage group (× 66). A: Immunohistochemical staining of α-SMA of SD rat HSC line in model group, B: Immunohistochemical staining of α-SMA of SD rat HSC line in medium dosage group.
Figure 2.
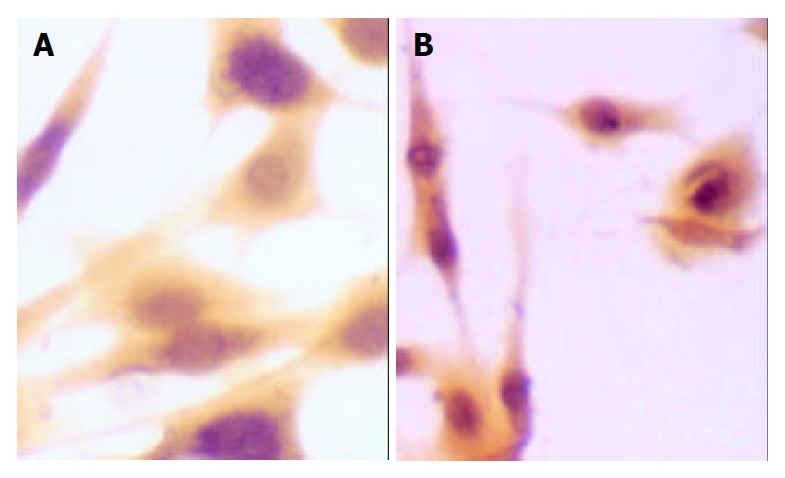
Immunohistochemical staining of synapsin of SD rat HSC line in model group and medium dosage group (× 66). A: Immunohistochemical staining of synapsin of SD rat HSC line in model group, B: Immunohistochemical staining of synapsin of SD rat HSC line in medium dosage group.
Influence of each group serum on collagen I, III and TIMP of HSC at different time points
The influence of each group serum detected with TN-8502 image analysis system on collagens I, III and TIMP of HSC at different time points is shown in Table 5, Table 6, Table 7 and Figure 3, Figure 4.
Table 5.
Influence of each group serum on collagen I of HSC at different time points (A value: mean ± SD)
|
N |
M |
G |
Z |
D |
||||||
| A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | |
| 24 h | 50.87 ± 9.84 | 5.49 ± 2.06c | 60.63 ± 9.26 | 4.64 ± 0.26 | 46.95 ± 5.21 | 2.90 ± 0.22 | 44.83 ± 6.97 | 3.72 ± 0.48 | 49.46 ± 7.36 | 3.89 ± 0.79 |
| 48 h | 270.51 ± 13.56 | 5.28 ± 0.58 | 456.78 ± 106.64 | 5.56 ± 1.83 | 107.79 ± 17.9 | 4.45 ± 0.29 | 115.28 ± 51.85 | 4.66 ± 1.67 | 129.35 ± 22.20 | 4.45 ± 0.50 |
| 72 h | 507.77 ± 66.38c | 11.36 ± 2.39c | 800.30 ± 150.39a | 15.66 ± 2.34a | 300.23 ± 25.80ac | 5.25 ± 0.56ac | 329.37 ± 45.98ac | 6.62 ± 1.33ac | 425.38 ± 19.32c | 7.99 ± 1.25c |
N: Control group; M: Model group; G: High dosage group; Z: Mdium dosage group; D: Low dosage group,
P < 0.05 vs normal group;
P < 0.05 vs model group.
Table 6.
Influence of each group serum on collagen III of HSC at different time points (A value: mean ± SD)
|
N |
M |
G |
Z |
D |
||||||
| A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | |
| 24 h | 60.93 ± 18.56 | 12.49 ± 3.16 | 65.69 ± 23.841 | 3.64 ± 2.36 | 40.95 ± 5.21 | 8.90 ± 3.82 | 45.83 ± 12.97 | 9.72 ± 1.47 | 52.46 ± 26.72 | 10.56 ± 0.56 |
| 48 h | 203.78 ± 36.64c | 25.28 ± 4.27c | 282.51 ± 28.43a | 31.56 ± 3.69a | 63.71 ± 7.94ac | 15.55 ± 3.59ac | 65.08 ± 18.85ac | 17.66 ± 1.65ac | 79.35 ± 22.28ac | 21.35 ± 2.36ac |
| 72 h | 325.30 ± 40.39c | 30.36 ± 2.50c | 501.77 ± 56.42a | 40.66 ± 4.82a | 178.36 ± 5.82ac | 23.25 ± 4.26ac | 182.27 ± 20.32ac | 25.66 ± 1.29ac | 220.38 ± 19.32c | 29.70 ± 1.72c |
N: Control group; M: Model group; G: High dosage group; Z: Medium dosage group; D: Low dosage group,
P < 0.05 vs normal group;
P < 0.05 vs model group.
Table 7.
Influence of each group serum on TIMP of HSC at different time points (A value: mean ± SD)
|
N |
M |
G |
Z |
D |
||||||
| A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | |
| 24 h | 60.93 ± 18.56 | 12.49 ± 3.16 | 65.69 ± 23.84 | 13.64 ± 2.36 | 40.95 ± 5.21 | 8.90 ± 3.82 | 45.83 ± 12.97 | 9.72 ± 1.47 | 52.46 ± 26.72 | 10.56 ± 0.56 |
| 48 h | 203.78 ± 36.64c | 25.28 ± 4.27c | 282.51 ± 28.43a | 31.56 ± 3.69a | 63.71 ± 7.94ac | 15.55 ± 3.59ac | 65.08 ± 18.85ac | 17.66 ± 1.65ac | 79.35 ± 22.28ac | 21.35 ± 2.36ac |
| 72 h | 325.30 ± 40.39c | 30.36 ± 2.50c | 501.77 ± 56.42a | 40.66 ± 4.82a | 178.36 ± 5.82ac | 23.25 ± 4.26ac | 182.27 ± 20.32ac | 25.66 ± 1.29ac | 220.38 ± 19.32c | 29.70 ± 1.72c |
N: Control group; M: Model group; G: High dosage group; Z: Medium dosage group; D: Low dosage group,
P < 0.05 vs normal group;
P < 0.05 vs model group.
Figure 3.
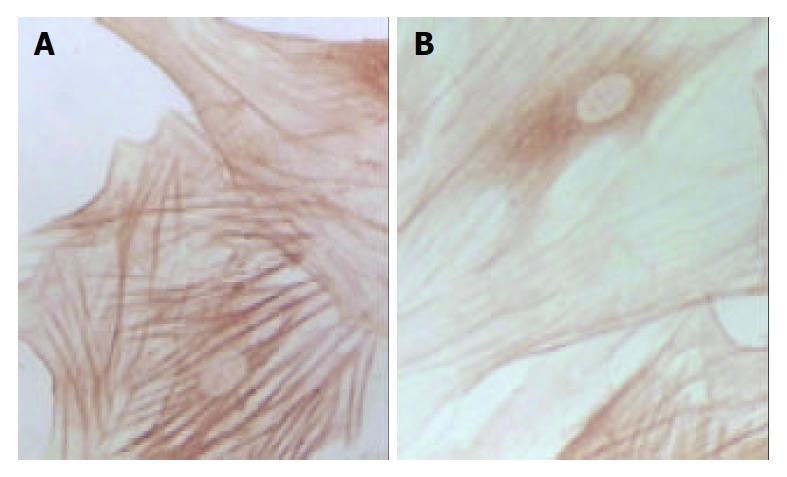
Immunohistochemical staining of type I collagen of SD rat HSC line in model group and medium dosage group(× 132). A: Immunohistochemical staining of type I collage of SD rat HSC line in model group, B: Immunohistochemical staining of type I collage of SD rat HSC line in medium dosage group.
Figure 4.
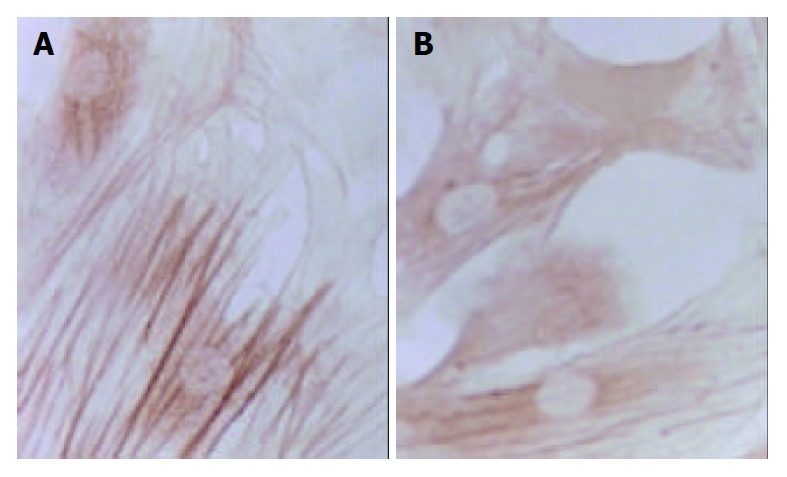
Immunohistochemical staining of type III collagen of SD rat HSC line in model group and medium dosage group (× 132). A: Immunohistochemical staining of type III collage of SD rat HSC line in model group, B: Immunohistochemical staining of type III collage of SD rat HSC line in medium dosage group.
Influence of each group serum on TGFβ1 and PDGF of HSC at different time points
The influence of each group serum detected with TN-8502 image analysis system on TGFβ1 and PDGF of HSC at different time points is shown in Table 8, Table 9 and Figure 5, Figure 6.
Table 8.
Influence of each group serum on TGFβ1 of HSC at different time points (A value: mean ± SD)
|
N |
M |
G |
Z |
D |
||||||
| A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | |
| 24 h | 126.56 ± 21.69 | 5.14 ± 0.69 | 158.79 ± 27.63 | 9.67 ± 2.59 | 98.92 ± 29.25 | 5.48 ± 0.49 | 105.69 ± 27.96 | 6.72 ± 1.74 | 122.56 ± 67.84 | 6.21 ± 0.98 |
| 48 h | 570.78 ± 57.61c | 15.78 ± 0.78c | 733.58 ± 189.73a | 19.39 ± 3.76a | 428.71 ± 51.30ac | 7.36 ± 0.87ac | 458.08 ± 37.98ac | 9.87 ± 1.98ac | 549.35 ± 124.29c | 13.27 ± 2.77a |
| 72 h | 1279.30 ± 147.78c | 21.98 ± 2.38c | 1509.87 ± 77.50a | 27.58 ± 5.65a | 609.37 ± 72.85ac | 9.25 ± 0.67ac | 823.75 ± 158.49ac | 12.35 ± 1.75ac | 971.57 ± 127.83c | 19.30 ± 1.36c |
N: Control group; M: Model group; G: High dosage group; Z: Medium dosage group; D: Low dosage group,
P < 0.05 vs normal group;
P < 0.05 vs model group.
Table 9.
Influence of each group serum on PDGF of HSC at different time points (A value: mean ± SD)
|
N |
M |
G |
Z |
D |
||||||
| A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | A | Male cell area (%) | |
| 24 h | 326.47 ± 15.31c | 7.14 ± 0.69 | 447.69 ± 25.47a | 12.05 ± 3.87 | 128.87 ± 35.14ac | 4.21 ± 0.29 | 187.39 ± 36.67ac | 7.83 ± 0.67 | 227.87 ± 54.39c | 9.64 ± 0.87 |
| 48 h | 479.65 ± 38.49c | 24.78 ± 5.90c | 659.45 ± 76.56a | 37.25 ± 4.89c | 214.17 ± 54.67ac | 9.28 ± 0.70ac | 264.58 ± 49.57ac | 14.02 ± 2.06ac | 379.27 ± 75.02c | 18.56 ± 3.27ac |
| 72 h | 805.30 ± 121.04c | 32.98 ± 5.49c | 1208.02 ± 132.21a | 45.94 ± 5.37c | 427.21 ± 39.72ac | 15.07 ± 2.78ac | 560.41 ± 132.02ac | 19.28 ± 2.09ac | 720.34 ± 102.07c | 27.19 ± 3.29ac |
N: Control group; M: Model group; G: High dosage group; Z: Medium dosage group; D: Low dosage group,
P < 0.05 vs normal group;
P < 0.05 vs model group.
Figure 5.
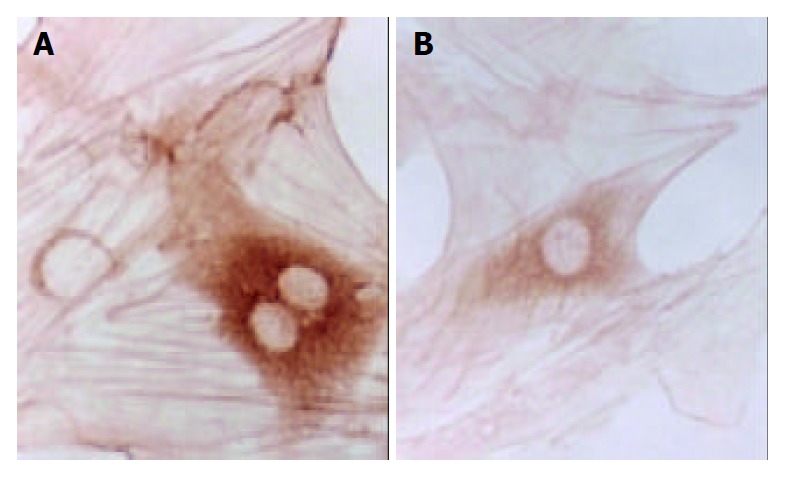
Immunohistochemical staining of TGF-β1 of SD rat HSC line in model group and medium dosage group (× 132). A: Immunohistochemical staining of TGF-β1 of SD rat HSC line in model group, B: Immunohistochemical staining of TGF-β1 of SD rat HSC line in medium dosage group.
Figure 6.
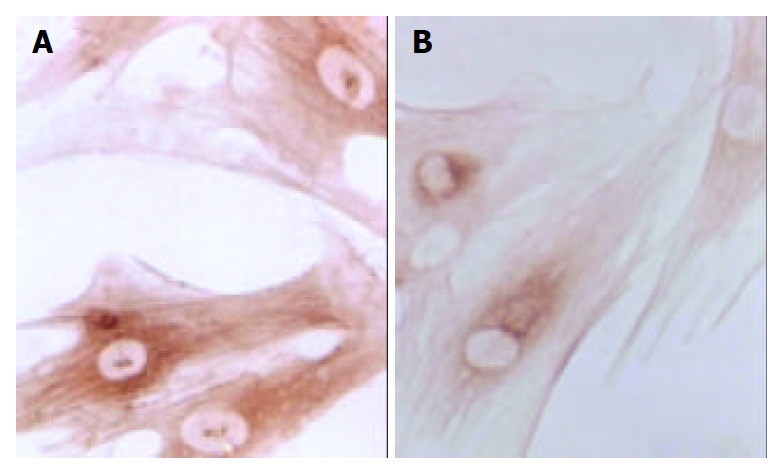
Immunohistochemical staining of PDGF of SD rat HSC line in model group and medium dosage group (× 132). A: Immunohistochemical staining of PDGF of SD rat HSC line in model group, B: Immunohistochemical staining of PDGF of SD rat HSC line in medium dosage group.
DISCUSSION
In past studies about traditional Chinese medicine, the method of adding directly coarse extract of the medicine to environment of cells was adopted in most experiments in vitro. Because of the complicated components of traditional Chinese medicine, it could not effectively reflect its pharmacological role. Therefore, we adopted the blood serum pharmacological method in our experiment[8]. After giving the drug to the animal orally, we took the drug serum as the drug source to add to the response system in vitro. This method could not only present the biotransformation in vivo, but also overcome confusion of other substances, aiding to makea pharmacokinetic study by finding the effective locus and the activity of components of traditional Chinese medicine. To avoid influence of different species animal sera on cells, animals were adopted in our experiment which were the same species with cultivated HSCs. Considering that the tested object of the drug was cells and the drug with biological reactivity in organism, we gave the drug to the animal orally with an equivalent dosage, to assure the maximum homeostasis concentration of the drug after bioconversion in vivo. The results of our experiment indicated that our experiment method was effective, stable and reliable.
A recent investigation has shown that proliferation and activation of HSCs are not only the central link of hepatic fibrosis, but also the cytological background of hepatic fibrosis[10,11]. Therefore, inhibiting the proliferation and activation of HSC has important significance in prevention and treatment of chronic liver disease and anit-hepatic fibrosis. Cultivated in a non-coating plastic Petri dish, HSC could be automatically activated, thus possessing the biological characteristics of activation in vivo, and becoming an ideal anti-hepatic fibrosis cell model[12]. Just as shown by the results of our experiment, with elongation of cultivating time, HSC showed multi-angle pseudopodium and typical star form. Cell proliferation and collagen synthesis were two important behaviors in activation of HSC. MTT chromatometry methods could be employed to detect cell ability of proliferation, which was based on the principle that succinic dehydrogenase in mitochondria of living cells could make ectogenical MTT recover to non-dissoluable blue and purple crystals and deposit in cells. DMSO could dissolve blue and purple crystals in cells. Adopting enzyme labled instruments to detect the Absorbency value at some wave-length, the quantity of cells could be reflected indirectly.
The results of our experiment showed that there was no significant difference (P > 0.05) between all the groups 24 h after serum was added. It means that adding serum to cultivate for 24 h did not exert any effective influence and HSCs still proliferated at its original rate, suggesting that intervention in proliferation of HSCs needs a time process, which from accepting excitation of cell signal to changing proliferation quantity of HSC, is undoubtedly longer than 24 h. Thus, how to restrain the activation of HSC at earlier time to hold back quick proliferation of HSC, has undoubtedly important significance in prevention and cure of hepatic fibrosis. Our results showed that proliferation of HSC in model group in 48 h and 72 h had a significant difference compared with other groups, indicating that the model group might contain substances which could promote quick proliferation of HSC. The reason may be that after cultivated for 48 h and 72 h, the PDGF of HSC in the model group increased to the effective content, leading to excessive proliferation of HSC. But a 24 h cultivation was not enough for PDGF to increase to the effective content. This could explain our results that there was no significant proliferation of HSC in the model group after 24 h serum culture. The results of our experiment also showed that after cultivated for 48 h and 72 h, there were significant differences among the high, medium and low dosage drug serum groups and the model group (P < 0.05), and between the high and medium dosage drug serum groups and the control group (P < 0.05), indicating that different dosage drug sera could inhibit the proliferation of HSC, and had an especially significant effect on the high and medium dosage groups. CBJRGC serum could significantly decrease and inhibit the proliferation of HSC. Its mechanism may be related to the inhibition of self-excretion or para-excretion of PDGF and TGF-β1[13], thus inhibiting the proliferation of HSC by decreasing the conversion from HSC to α-SMA.
Changes in form and function after HSC is activated could lead to production increase and degradation decrease of ECM, eventually resulting in deposition of hepatic collagens and hepatic fibrosis. Desmin could be regarded as a marker of HSC[14], and α-SMA as the marker of activation of HSC[15]. α-SMA is a kind of filament protein which is about 7 nm in diameter and mainly exists in smooth muscle cells as a portion of cytoplasm framework and a functional unit of cell contraction. Under normal conditions, this protein mainly exists in smooth muscle cells and myofibroblasts. There is a very small amount of this protein in HSCs of rats. In the animal model of liver diseases, HSC lost desmin expression and was changed to the expression of α-SMA in its activation process. So, α-SMA could be regarded as the marker of activation of HSC[16]. In the process of inducing rat hepatic fibrosis by CCl4[17], the dynamic change of desmin male cells was a mono-cusp curve, increasing in number in the earlier period, reaching the peak in 12 wk and then gradually decreasing. A foreign study has found that increase of expression of synapsin could be regarded as another marker of activation of HSC[18]. Our results showed that the dynamic changes in A value of male staining of α-SMA, desmin and synapsin and in the area occupied by α-SMA and synapsin male cells were almost synchronical. The area occupied by desmin male cells showed a tendency to decrease with elongation of cultivating time. This result was consistent with that reported by Li et al[19] that HSC lost the expression of desmin in its activation process, suggesting that in the process of hepatic fibrosis, α-SMA, desmin and synapsin all can be regarded as the markers of activation of HSC, but each has its own characteristics. The expression of desmin in the earlier period of activation was significant, but weakened with elongation of activating time, while the expression of α-SMA and synapsin developed with elongation of activating time. It is estimated that activation and proliferation of HSC can synchronically occur. Our results also showed that after cultivated for 24 h, the expression of α-SMA had no significant difference among all groups, and after cultivated for 48 h, α-SMA male cell area was smaller than that of desmin, indicating that in the period of cultivation for 24 h and 48 h, proliferation was the main form of HSC growth, but with elongation of cultivating time, HSC began to be partly activated and changed to expression of α-SMA. Seventy-tow h after cultivation, α-SMA male cell area was bigger than desmin male cell area, the amount of activated HSCs was more than that of silent HSCs and lots of HSCs altered in phenotype, further indicating that the activation of HSC could be synchronically expressed as proliferation and transformation of HSC. The results of our experiment showed that CBJRGC serum could inhibit the expressions of α-SMA, desmin and synapsin in HSC and inhibit the activation of HSC, whose drug effect was closely related to the concentration of drug serum.
Synapsin can be regarded as a new marker of activation of HSC. Its significance is to provide an important theoretic and testing basis for neuroregulation in the process of hepatic fibrosis. So far, no such study has been found in China. Our experiment firstly showed that after cultivated for 24 h, synapsin could express in HSC, and after cultivated for 48 h and 72 h, synapsin could continue to express. Expression of synapsin occurred before it was cultivated for 24 h and continued to maintain a high level. CBJRGC serum could significantly inhibit expression of synapsin in HSC. Nevertheless, further studies such as the precise time of synapsin expression, the essential significance of the increase in its lasting expression, whether the nervous system involves regulation of stress condition of liver, and whether CBJRGC serum takes part in nervous regulation in hepatic fibrosis, are still needed.
Under normal conditions, liver contains collagen of types I, III, IV, V, and VI. Collagens I and III take the most proportion of collagen, accounting for about 60% of total collagen in liver. When hepatic fibrosis occured, the proportion of collagens I and III might reach 95% of total collagen in liver[20]. Thus, in hepatic fibrosis, deposited ECM mainly consists of collagens I and III.
Synthesis of collagen could reflect alterative ability of individual fiber hyperplasia of cells[21]. Our experiment showed that after cultivated for 24 h, there was no significant difference of collagens I and III among all groups, indicating that within 24 h after acceptance of activating signal, HSC did not yet achieve enough time to excrete collagen. The outcome of our experiment showed that activated time should be longer than 24 h, so that activated HSC and transcription of collagen and cytokine could be altered. In further investigations we should observe and analyze changes in collagen-mRNA in this time process to test whether CBJRGC serum can influence collagen-mRNA. The results also suggested that to inhibit collagen in its transcriptional stage might have important significance in clinical treatment. In our experiment, collagen III began to markedly express after cultivated for 48 h, but collagen I started to markedly express after cultivated for 72 h, showing that expression of collagen III was earlier than that of collagen I, and that in the early time of hepatic fibrosis, collagen III expression took the most part of expression of collagen. Therefore collagen III can be the testing index of earlier hepatic fibrosis. The outcome of our experiment also showed that high and medium dosage groups could significantly inhibit the expression of collagen III in 48 h and 72 h and the expression of collagen I in 72 h. It means CBJRGC serum could play a role in anti-hepatic fibrosis at the earlier time when collagen was significantly expressed. It further suggested that CBJRGC serum could help quickly recover equilibration of synthesis and degradation of ECM in hepatic fibrosis, thus helping to cure hepatic fibrosis. Besides, low dosage group could only significantly inhibit the expression of collagen III in 48 h, but there was no significant difference compared with control group in 72 h. It is estimated that the effect of CBJRGC serum in inhibiting collagen III expression is related to the serum concentration of the drug.
MMP and TIMP mainly take part in regulation of equilibration in synthesis and degradation of collagen[22]. Among numerous MMPs, MMP1 is the chief MMP, decomposing collagens I and III in liver[23]. TIMP1 is an inhibiting factor of MMP, which plays its role by irreversibly binding to activated MMP1. Therefore, the imbalance of ratio of MMP/TIMP 1 plays a very important role in hepatic fibrosis. MMP can be inhibited by many specific or non-specific inhibitors, which at present, mainly include TIMP and α2- macroglobulin. TIMP 1 is the most important inhibitor of MMP and is negatively correlated to the activity of MMP.
TIMP is a kind of coding protein of multigene family[24]. It could irreversibly bind to activated MMP and inhibit the degradation of ECM[25]. So far, there are four kinds of TIMP isolated from tissues and cloned[26]. TIMP-1 is a kind of 28.5 ku glycoprotein, mainly inhibiting the activity of MMPs-1 and MMP-9. As the specific inhibitor of MMP, TIMP plays a very important role in hepatic fibrosis. TIMP could inhibit MMP, which is the important reason for specific descent of degradation of ECM[27]. HSC is the main source cell of TIMP and MMP.
The results of our experiment showed that after cultivated for 24 h, there was no significant expression of TIMP in all groups, indicating that HSC did not have enough time to excrete excessive TIMP. The activated time should be longer than 24 h, and within this time, transcription of TIMP of activated HSC might be altered. In further studies, we should observe and analyze the change of TIMP-mRNA within this time, so as to find whether CBJRGC serum can influence TIMP-mRNA. It is also suggested that to inhibit TIMP in transcriptional stage might have important significance in clinical treatment. In this experiment, TIMP of the model group maintained high expression in 48 h and 72 h, indicating that there was some substance to promote high expression of TIMP in the model group. Further study on the precise characteristics of the substance is suggested.
Researches have found that HSC is the key cell in hepatic fibrosis. Under the stimulation of chronic injury and inflammation, HSC can be activated from normal silent behavior to MFB, and meanwhile can secrete and synthetize excessive ECM, forming the foundation of hepatic fibrosis. Previous studies have indicated that activation and phenotype conversion of HSC are closely related to TGF-β1. TGF-β is a kind of polypeptide molecule of hormone activity, which is produced from kupffer cells by self-excretion and para-excretion and could take part in many pathological and physiological processes[28]. TGF-β has at least 5 sub-types, but there are only TGF-β1,TGF-β2 and TGF-β3 in human tissue cells. After binding to the recipient on the membrane, TGF-β could phosphorate and activate its signal transduction molecule (SMAD protein) of intracytoplasmic downstream, which could subsequently enter the nucleus, regulating the transcription of related target gene[29]. TGF-β1 exhibits the significant biological activity of TGF-β and is the main cytokine inducing the production of collagen. Through the mechanism of para-excretion and self-excretion, TGF-β1 could start and maintain the activation of HSC, regulating cell proliferation, accelerating transcription of collagen and proliferation of ECM[30].
The results of our experiment showed that after cultivated for 24 h, there was no significant expression of TGF-β1 among all groups. It showed that it was not enough for HSC to secrete TGF-β1 24 h after it received the activated signal. It is suggested that in further studies we should observe and analyze the change in TGF-β1-mRNA within this time, so as to find whether CBJRGC serum can influence TGF-β1-mRNA. Our results also showed that after cultivated for 48 h and 72 h, TGF-β1 in the model group maintained high expression, suggesting that it could continuously stimulate activated HSC to produce collagen and accelerate hepatic fibrosis. There might be some substance in the model group which could promote high expression of TGF-β1. Study on the precise characteristics of this substance is still needed. The results also showed that the high and medium dosage groups could markedly inhibit the expression of TGF-β1 in 48 h and 72 h, but the low dosage group did not obviously inbibit the expression of TGF-β1 in 48 h and 72 h, suggesting that CBJRGC serum can inhibit the expression of TGF-β1 and its effectiveness is related to the concentration of the drug serum.
PDGF is a kind of splitting agent and can promote activation and proliferation of HSC. It has been found PI3-K is the important pathway of intramembrane signal transduction[31]. The outcome of our experiment showed that after cultivated for 24 h, the A value and male cell area of PDGF were not completely consistent. In 24 h, the A value of PDGF in the model group was significantly higher than that in the other groups, but the male cell area did not show any significant difference compared with the other groups. Although PDGF in the model group achieved significant expression, the PDGF might not entirely come from the excretion of HSC and might include original PDGF existing in the model group. While PDGF bound to PDGF recipient of HSC and accelerated the proliferation of HSC, the absolute proportion of HSC in the situation of excretion of PDGF did not significantly increase. The outcome of our experiment showed that the high, medium and low dosage groups all could obviously inhibit the excretion of PDGF by HSC.
In summary, we suggest that further studies on the mechanism of anti-hepatic fibrosis of CBJRGC serum should focus on mRNA expression of TIMP1, collagens I, III and TGF-β1 and signal transduction within the cell.
Footnotes
Supported by the National Natural Science Foundation of China for Key Project, No. 30130220
Edited by Wang XL and Zhang JZ Proofread by Xu FM
References
- 1.Burt AD. Pathobiology of hepatic stellate cells. J Gastroenterol. 1999;34:299–304. doi: 10.1007/s005350050264. [DOI] [PubMed] [Google Scholar]
- 2.Calès P. Apoptosis and liver fibrosis: antifibrotic strategies. Biomed Pharmacother. 1998;52:259–263. doi: 10.1016/S0753-3322(98)80011-5. [DOI] [PubMed] [Google Scholar]
- 3.Desmouliere A, Xu G, Costa AM, Yousef IM, Gabbiani G, Tuchweber B. Effect of pentoxifylline on early proliferation and phenotypic modulation of fibrogenic cells in two rat models of liver fibrosis and on cultured hepatic stellate cells. J Hepatol. 1999;30:621–631. doi: 10.1016/s0168-8278(99)80192-5. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Zhang Z, Yang L, Chen D, Wang Y. Inhibition of the activation and collagen production of cultured rat hepatic stel-late cells by antisense oligonucleotides against transforming growth factor-beta 1 is enhanced by cationic liposome delivery. Huaxi Yike Daxue Xuebao. 2000;31:133–135. [PubMed] [Google Scholar]
- 5.Yu WP, Brenner S, Venkatesh B. Duplication, degeneration and subfunctionalization of the nested synapsin-Timp genes in Fugu. Trends Genet. 2003;19:180–183. doi: 10.1016/S0168-9525(03)00048-9. [DOI] [PubMed] [Google Scholar]
- 6.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Yanase K, Namisaki T, Imazu H, et al. Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology. 2002;36:850–860. doi: 10.1053/jhep.2002.35625. [DOI] [PubMed] [Google Scholar]
- 7.Tsukamoto H. Cytokine regulation of hepatic stellate cells in liver fibrosis. Alcohol Clin Exp Res. 1999;23:911–916. [PubMed] [Google Scholar]
- 8.Liu C, Liu P, Liu CH, Zhu XQ, Ji G. Effects of Fuzhenghuayu decoction on collagen synthesis of cultured hepatic stellate cells, hepatocytes and fibroblasts in rats. World J Gastroenterol. 1998;4:548–549. doi: 10.3748/wjg.v4.i6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernández-Muñoz R, Díaz-Muñoz M, Suárez-Cuenca JA, Trejo-Solís C, López V, Sánchez-Sevilla L, Yáñez L, De Sánchez VC. Adenosine reverses a preestablished CCl4-induced micronodular cirrhosis through enhancing collagenolytic activity and stimulating hepatocyte cell proliferation in rats. Hepatology. 2001;34:677–687. doi: 10.1053/jhep.2001.27949. [DOI] [PubMed] [Google Scholar]
- 10.Woo SW, Nan JX, Lee SH, Park EJ, Zhao YZ, Sohn DH. Aloe emodin suppresses myofibroblastic differentiation of rat hepatic stellate cells in primary culture. Pharmacol Toxicol. 2002;90:193–198. doi: 10.1034/j.1600-0773.2002.900404.x. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki C, Kayano K, Uchida K, Sakaida I, Okita K. Charac-teristics of the cell proliferation profile of activated rat hepatic stellate cells in vitro in contrast to their fibrogenesis activity. J Gastroenterol. 2001;36:322–329. doi: 10.1007/s005350170098. [DOI] [PubMed] [Google Scholar]
- 12.Levy MT, McCaughan GW, Marinos G, Gorrell MD. Intrahe-patic expression of the hepatic stellate cell marker fibroblast activation protein correlates with the degree of fibrosis in hepa-titis C virus infection. Liver. 2002;22:93–101. doi: 10.1034/j.1600-0676.2002.01503.x. [DOI] [PubMed] [Google Scholar]
- 13.Yuan N, Wang P, Wang X, Wang Z. [Expression and significance of platelet derived growth factor and its receptor in liver tissues of patients with liver fibrosis] Zhonghua Ganzangbing Zazhi. 2002;10:58–60. [PubMed] [Google Scholar]
- 14.Nitou M, Ishikawa K, Shiojiri N. Immunohistochemical analysis of development of desmin-positive hepatic stellate cells in mouse liver. J Anat. 2000;197 Pt 4:635–646. doi: 10.1046/j.1469-7580.2000.19740635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guma FCR TG, Mermelstein CS, Fortuna VA, Wofchuk ST, Gottfried C, Guaragna RM, Costa ML, Borojevic R. Intermediate filaments modulation in an in vitro model of the hepatic stellate cell activation or conversion into the lipocyte phenotype. Biochem Cell Biol. 2001;79:409–417. [PubMed] [Google Scholar]
- 16.Buniatian GH, Gebhardt R, Mecke D, Traub P, Wiesinger H. Common myofibroblastic features of newborn rat astrocytes and cirrhotic rat liver stellate cells in early cultures and in vivo. Neurochem Int. 1999;35:317–327. doi: 10.1016/s0197-0186(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Zhang Y, Zhang J, Zhu H, Zhou X, Du W, Zhang X, Chen Q. Expression of fibronectin receptor, integrin alpha 5 beta 1 of hepatic stellate cells in rat liver fibrosis. Chin Med J (Engl) 2000;113:272–276. [PubMed] [Google Scholar]
- 18.Cheetham JJ, Hilfiker S, Benfenati F, Weber T, Greengard P, Czernik AJ. Identification of synapsin I peptides that insert into lipid membranes. Biochem J. 2001;354:57–66. doi: 10.1042/0264-6021:3540057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Zhang J, Huang G, Zhang N, Chen Q, Zhang X. Effect of retinoid kappa receptor alpha (RXRalpha) transfection on the proliferation and phenotype of rat hepatic stellate cells in vitro. Chin Med J (Engl) 2002;115:928–932. [PubMed] [Google Scholar]
- 20.Zhang Q, Wang J, Hu M. [Effects of interferon-alpha on the mRNA expression of procollagen type I and III of hepatic stellate cells and on the deposition of collagen type I and III in fibrotic liver of rats] Zhonghua Yixue Zazhi. 1999;79:695–698. [PubMed] [Google Scholar]
- 21.Liu WB, Yang CQ, Jiang W, Wang YQ, Guo JS, He BM, Wang JY. Inhibition on the production of collagen type I, III of activated hepatic stellate cells by antisense TIMP-1 recombinant plasmid. World J Gastroenterol. 2003;9:316–319. doi: 10.3748/wjg.v9.i2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069–11076. doi: 10.1074/jbc.M111490200. [DOI] [PubMed] [Google Scholar]
- 23.Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427–436. doi: 10.1055/s-2001-17557. [DOI] [PubMed] [Google Scholar]
- 24.Yoshiji H, Kuriyama S, Miyamoto Y, Thorgeirsson UP, Gomez DE, Kawata M, Yoshii J, Ikenaka Y, Noguchi R, Tsujinoue H, et al. Tissue inhibitor of metalloproteinases-1 promotes liver fibrosis development in a transgenic mouse model. Hepatology. 2000;32:1248–1254. doi: 10.1053/jhep.2000.20521. [DOI] [PubMed] [Google Scholar]
- 25.Yang C, Zeisberg M, Mosterman B, Sudhakar A, Yerramalla U, Holthaus K, Xu L, Eng F, Afdhal N, Kalluri R. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124:147–159. doi: 10.1053/gast.2003.50012. [DOI] [PubMed] [Google Scholar]
- 26.Bennett RG, Kharbanda KK, Tuma DJ. Inhibition of markers of hepatic stellate cell activation by the hormone relaxin. Biochem Pharmacol. 2003;66:867–874. doi: 10.1016/s0006-2952(03)00403-9. [DOI] [PubMed] [Google Scholar]
- 27.Lichtinghagen R, Michels D, Haberkorn CI, Arndt B, Bahr M, Flemming P, Manns MP, Boeker KH. Matrix metalloproteinase (MMP)-2, MMP-7, and tissue inhibitor of metalloproteinase-1 are closely related to the fibroproliferative process in the liver during chronic hepatitis C. J Hepatol. 2001;34:239–247. doi: 10.1016/s0168-8278(00)00037-4. [DOI] [PubMed] [Google Scholar]
- 28.Bissell DM. Chronic liver injury, TGF-beta, and cancer. Exp Mol Med. 2001;33:179–190. doi: 10.1038/emm.2001.31. [DOI] [PubMed] [Google Scholar]
- 29.Okuno M, Akita K, Moriwaki H, Kawada N, Ikeda K, Kaneda K, Suzuki Y, Kojima S. Prevention of rat hepatic fibrosis by the protease inhibitor, camostat mesilate, via reduced generation of active TGF-beta. Gastroenterology. 2001;120:1784–1800. doi: 10.1053/gast.2001.24832. [DOI] [PubMed] [Google Scholar]
- 30.Arias M, Lahme B, Van de Leur E, Gressner AM, Weiskirchen R. Adenoviral delivery of an antisense RNA complementary to the 3' coding sequence of transforming growth factor-beta1 inhibits fibrogenic activities of hepatic stellate cells. Cell Growth Differ. 2002;13:265–273. [PubMed] [Google Scholar]
- 31.Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, Poupon R, Housset C. He myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Laboratory Invest. 2003;83:163–173. doi: 10.1097/01.lab.0000054178.01162.e4. [DOI] [PubMed] [Google Scholar]


