Abstract
AIM: To investigate the expression of cancer related genes in gastric carcinoma (GC) through the use of Atlas Human Cancer Array membranes with 588 well-characterized human genes related to cancer and tumor biology.
METHODS: Hybridization of cDNA blotting membrane was performed with 32P-labeled cDNA probes synthesized from RNA isolated from gastric carcinoma and adjacent noncancerous gastric epithelial tissue. AtlasImage, which is a software specific to array, was used to analyze the result.
RESULTS: The differentially expression cell cycle/growth regulator in GC showed a stronger tendency toward cell proliferation with 2.7-fold up-regulation of CK1. The promoter genes of apoptosis were down-regulated, including caspase-8 precursor, caspase-9 and caspase-10. Among the oncogene/tumor suppressor genes, ABL2 was down-regulated. In addition, some genes were up-regulated, including matrix metalloproteinse 2 (MMP-2), MMP-16 (MT3-MMP), SKY, CD9 and semaphorin V. A number of genes were down-regulated, including neuroendocrine-dlg (NE-dlg), retinoic acid receptor gamma and tumor suppressor DCC colorectal. In general, The expression of the cancer progression genes were up-regulated, while the expression of anti-cancer progression genes were down-regulated.
CONCLUSION: Investigation of these genes should help to disclose the molecular mechanism of the onset, progression and prognosis of GC. Several genes are reported herein to be altered in GC for the first time. The quick and high-throughout method of profiling gene expression by cDNA array provides us with an overview of key factors that may involved in GC, and may aid the study of GC carcinogenesis and provide molecular targets for diagnosis and therapy. The precise relationship between the altered genes and gastric carcinogenesis is a matter for further investigation.
INTRODUCTION
Gastric carcinoma (GC) is one of the most common malignant tumors worldwide, which ranks the first in frequency among human cancer in China[1]. However, the molecular mechanism underlying GC are currently unknown. Some genes may play a significant role in carcinogenesis, such as p53, bcl-2, bax, and c-myc[2-4]. However, these genetic changes do not precisely reflect the biological nature of tumor cells or the clinical characteristics of GC patients.
Tumor development and progression involves a cascade of genetic alterations. Techniques frequently used to study gene expression alterations, such as Reverse Transcription Polymerase Chain Reaction (RT-PCR), Differential Display Polymerase Chain Reaction (DD-PCR) and Northern blot analysis, have their limitations: some need large amounts of RNA, others are time-consuming and can only study a small number of genes simultaneously. Hence, analysis of expression profiles of a large number of genes in clinical GC material is an essential step toward clarifying the detailed mechanisms of carcinogenesis and discovering target molecules for the development of novel therapeutic drugs.
The cDNA microarray technology, which has recently undergone rapid development, enables investigators to study the gene expression profile and gene activation of thousands of genes and sequences[5,6]. This technique allows the large-scale comparison of multiple genes in a single hybridization and has been used successfully to explore the gene expression profile of various carcinomas and diseases[7-11].
In this study, we used cDNA expression microarray technology containing 588 genes related to carcinoma to analyze genes that are differentially expressed in human GC and normal gastric epithelial tissues. This large body of information not only furthers our understanding of the mechanism of carcinogenesis but also reveals novel features of known genes and identifies potential candidates for GC diagnosis and therapy.
MATERIALS AND METHODS
Tissues and specimens
Sixteen pairs of GC and corresponding noncancerous gastric epithelial tissue were obtained with informed consent from patients who underwent gastrectomy at the First Clinical College of Harbin Medical University and Cancer Hospital of Chinese Academy of Medical Science. Tumor tissue was dissected from the resected specimen. The normal tissue block was taken from the distal resection margin. The specimens were immediately frozen in liquid nitrogen. Histopathological classification was performed by a single pathologist.
RNA isolation and purification
Total RNA were obtained by extracting frozen tissues in Trizol (Life Technologies Inc., Gaitherbur, MD, USA) according to the manufacturer’s instructions. Normal gastric epithelial tissue and GC tissue were morsellated and homogenized in Trizol solution (1 mL/100 mg). Homogenates were incubated for 15 min on ice, and then a 1/5 volume of choloform was added to the homogenates. After vigorous agitation for 5 min, the inorganic phase was separated by centrifugation at 12000 × g for 20 min at 4 °C. RNA were then precipitated in the presence of 1 volume of isopropanol and centrifuged at 10000 × g for 15 min at 4 °C. RNA pellets were washed with 70% ice-cold ethanol and then dissolved in diethyl pyrocarbonate (DEPC)- reated H2O. Total RNA concentration and quantity was assessed by absorbency at 260 nm using an Nucleic Acid and Protein Analyzer (BECKMAN 470, USA).
cDNA microarray membrane
Atlas Human Cancer cDNA Expression Array (7742-1) was purchased from Clontech Laboratories Inc (Palo Alto, USA). The membrane contained 10 ng of each gene-specific cDNA from 588 known genes and 9 housekeeping genes (Figure 1A). Several plasmid and bacteriophage DNAs and blank spots are also included as negative and blank controls to confirm hybridization specificity. The cancer-related genes analyzed in this study are divided into six groups: (A) cell cycle regulators, growth regulators intermediate filament markers; (B) apoptosis, oncogenes, and tumor suppressors; (C) DNA damage response/repair and recombination; cell fate and development; and receptors; (D) cell adhesion and motility; and angiogenesis; (E) invasion regulators and cell-cell interactions; (F) growth factors and cytokines. A complete list of the 588 genes with the array positions and GeneBank accession number spotted on the array is available at Clontech’s web site (http://www.clontech.com).
Figure 1.
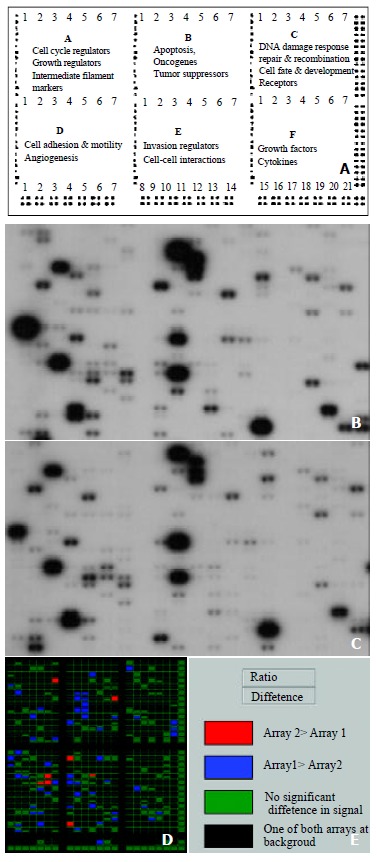
Parallel analysis of gene expression profiles in human gastric carcinoma and adjacent normal gastric mucosa. The schematic diagram of Atlas human cancer expression array contains 588 cancer-related human cDNA spotted as duplicates. Nine housekeeping genes are spotted at the bottom line to serve as positive controls. Dark grey spots at the outer end of the array represent genomic DNA spots, which serve as orientation marks (A). Atlas human cancer cDNA expression array (Clontech, USA) was hybridized with 32P-labeled cDNA probes obtained from total RNA of human gastric carcinoma (B) and adjacent normal gastric tissues (C). The colorful compare diagram between human gastric carcinoma and adjacent normal gastric tissues was got when you aligned two arrays to AtlasImage Grid Temples and adjusted the alignments and background calculations (D). Definitions of colors in the array comparison were showed (E).
cDNA synthesis, labeling and purification
Total RNA was reverse-transcribed into cDNA and labeled with α-32P dCTP using SuperscriptTM Preamplification system for First Strand cDNA Synthesis (Life Technologies, Gaithersburg, MD, USA). Before labeling, 5 μg total RNA of each type was treated with 2 μL DNase I (10 μnits/μL, Boehringer Mannheim, Mannheim, Germany), 1 μL RNasin (40 μnits/μL, Promega, Madison, WI, USA) at 37 °C for 15 min to remove contaminated DNA. For each labeling, the treated 5 μg total RNA was raised in volume to 6 μL with diethyl pyrocarbonate (DEPC)-treated H2O and first incubated with 4 μL oligo dT (0.5 μg/μL, Life Technologies, Gaithersburg, MD, USA) at 70 °C for 10 min. Then, 6 μL 5 × first strand reaction buffer, 1 μL 0.1 M DTT (Life Technologies, Gaithersburg, MD, USA), 1.5 μL dNTP mixture containing dATP, dGTP, dTTP at 20 μM (Promega, Madison, WI, USA), 10 μL α-32P dCTP (10 μCi/μL, NEN Life Science, Boston, MA, USA) and 1.5 μL SuperScript II reverse transcriptase (200 μnits/μL, Life Technologies, Gaithersburg, MD, USA) were added and incubated at 37 °C for 90 min. The labeled first strand cDNA probes were purified by Spin 200 column (Clontech, Palo Alto, CA, USA) to remove the unincorporated nucleotides. After purification, labeled cDNAs were denatured in a boiling water bath for 3 min before use.
Membrane hybridization and exposure
Five milliliters ExpressHyb hybridization solution (Clontech, Palo Alto, CA, USA) and 5 μg Cot-1 DNA (Life Technologies, Gaithersburg, MD, USA) were added to the tube containing Atlas human cancer cDNA expression array, which were pre-hybridized at 68 °C for 2 h. Then different incubated probes were added to different tubes and hybridization was performed at 68 °C for 18 h in rolling bottles. The membranes were washed twice at 68 °C in 2 × SSC, 0.1% SDS for 20 min, once at 50 °C in 1 × SSC, 0.5% SDS for 20 min, once at room temperature in 0.5 × SSC, 1% SDS for 20 min and 0.5 × SSC 10 min. Membrane were then exposed to X-ray films (Fuji Films, Tokyo, Japan) at -70 °C for 24-48 h.
Images and analysis
The images were scanned with Fluor-S MultiImager (Bio-Rad, Hercules, CA, USA) and saved as TIFF format files. The TIFF images were imported into the AtlasImage analysis software Version 1.01 a (Clontech, Palo Alto, CA, USA) and analyzed step by step with the guide. Housekeeping gene Ubiquitin was selected for normalization, because its expression was constant in cancer array hybridization system. Then the normalized intensity of each spot representing a unique gene expression level was acquired. Genes were considered to be up-regulated when the intensity ratio was ≥ 1.5 and the difference was ≥ 10000 between the expressions of GC tissues and normal gastric tissues.
RESULTS
Using a cDNA expression microarray technique we established the expression profile of 588 genes, selected from different areas of cancer research, in human GC and normal gastric tissue (Figure 1B, Figure 1C). No signals were visible in the blank spots and negative control spots indicating that the Atlas human cancer array hybridization was highly specific. The housekeeping genes’ density were very similar at the same time which indicate that the results were credible (Figure 1B, Figure 1C). We used the housekeeping gene, Ubiquitin, to normalize the intensities. The color image of the difference between GC and normal gastric tissue was produced by AtlasImage array software (Figure 1D). The comparison results analyzed by AtlasImage software showed that there were 63 genes altered, 8 were up-regulated and 55 down-regulated in GC versus normal gastric tissue, using the criteria that the ratio was = 1.5 or the difference was = 10000 (Table 1). There were pronounced differences in the gene expression profile between the two tissues.
Table 1.
Genes differentially expressed between HCC and adjacent noamal liver tissues generated by atlasImage software (Version 1.01a)
| Gene | Ratio | Difference | Protein/gene |
| B2f | -42675 | TRAF6 | |
| E2h | 0.171 | -31204 | T-plasminogen activator (T-PA) |
| B3h | 0.444 | -31180 | caspase-8 precursor; MACH; FLICE; (CAP4) (CASP8) |
| D1b | 0.334 | -30676 | byglycan |
| B3i | 0.445 | -30287 | caspase-9 precursor; ICE-LAP6; apoptotic protease MCH-6 |
| B2g | 0.493 | -28449 | TRAF-interacting protein (TRIP) |
| B3g | -27610 | caspase-8 precursor; MCH-5 isoform alpha | |
| E4j | 0.346 | -27534 | CDC42 GTPase-activating protein |
| C7m | 0.336 | -27032 | retinoid X receptor beta (RXR-beta) |
| B1k | 0.438 | -26170 | serine/threonine protein kinase NIK; |
| B3j | 0.486 | -25940 | ICE-like apoptotic protease 4 precursor; caspase-10 |
| B3f | -25206 | caspase-7 precursor; apoptotic protease MCH-3; LICE2 | |
| F2g | 0.428 | -23730 | endothelin ET2 |
| D2a | 0.176 | -22099 | collagen type VIII alpha-1 |
| D7f | 0.47 | -21445 | PLGF1 & 2 precursor (placenta growth factor) |
| C7l | 0.593 | -21145 | retinoic acid receptor gamma |
| A4j | 0.326 | -20847 | extracellular signal-regulated kinase 6 (ERK6) (ERK5) |
| E5b | 0.299 | -20388 | rho GDP-dissociation inhibitor 1 |
| E2f | 0.638 | -20244 | extracellular matrix metalloproteinase inducer emmprin |
| D4j | 0.624 | -20147 | integrin beta7 |
| D2n | 0.646 | -17013 | TENASCIN-R |
| F4l | 0.626 | -16150 | interleukin-6 (IL-6) precursor; BSF-2; interferon beta-2; |
| B5l | 0.633 | -16122 | proto-oncogene rhoA multidrug resistance protein; |
| B7j | 0.639 | -15630 | tyrosine-protein kinase ABL2; tyrosine kinase ARG (ABLL) |
| E7l | 0.721 | -15615 | ephrin type-B receptor 2 precursor; tyrosine-protein eph-3 |
| E1f | 0.318 | -15559 | MMP-9; gelatinase B |
| B2i | 0.245 | -15356 | CD40 receptor associated factor 1 (CRAF1) |
| D5g | 0.569 | -15106 | ezrin (cytovillin 2) |
| E7k | -14688 | ephrin type-B receptor 1 precursor; NET | |
| E1j | -14540 | MMP-13; collagenase-3 | |
| A2f | 0.403 | -14368 | serine/threonine protein kinase PITALRE |
| D5m | 0.38 | -14156 | tumor suppressor DCC colorectal |
| E1e | 0.3 | -13906 | MMP-8; collagenase-2 |
| D2e | 0.295 | -13706 | collagen type XVIII alpha |
| D2m | 0.624 | -12991 | tenascin-C |
| F3j | 0.77 | -12411 | transcription factor ETR103; early growth response protein 1 |
| C2j | 0.648 | -12053 | Rad |
| F5k | 0.68 | -11371 | leukocyte interferon-gamma (IFN-gamma) |
| D5k | 0.643 | -11064 | ninjurin-1 |
| C7k | 0.356 | -11056 | retinoic acid receptor epsilon; retinoic acid receptor beta-2 |
| C1b | 0.392 | -10834 | ataxia telangiectasia (ATM) |
| D4k | 0.799 | -10645 | integrin beta8 |
| D1c | -10630 | CD34 | |
| D3e | 0.798 | -10529 | vitronectin precursor; serum spreading factor; |
| B4c | 0.347 | -10506 | WSL-LR +WSL-S1+WSL-S2+TRAMP (Apo-3) |
| D6c | 0.264 | -10460 | semaphorin E |
| E2n | 0.483 | -9167 | low-density lipoprotein receptor-related protein 1 precursor |
| E5m | 0.351 | -8076 | truncated-cadherin; H-cadherin; heart-cadherin |
| A4n | 0.363 | -7958 | stress-activated protein kinase JNK3; JNK3; |
| A5i | 0.282 | -7204 | E2F-3 |
| E6f | 0.287 | -7197 | neuroendocrine-dlg (NE-dlg); |
| A1e | 0.343 | -7043 | cell division protein kinase 5; kinase PSSALRE |
| E4h | 0.419 | -6025 | rhoHP1 |
| D6h | 0.451 | -4647 | LAR |
| A7n | 0.466 | -4426 | desmin |
| E4e | 2.185 | 4701 | RAS-related C3 botulinum toxin substrate 1 (P21-RAC2) |
| D5f | 1.447 | 10249 | CD9 |
| B7g | 2.841 | 11419 | SKY (DTK) (TYRO3) (RSE) |
| A7d | 2.705 | 13394 | type II cytoskeletal 11 keratin; cytokeratin 1 (K1; CK 1) |
| D6f | 2.772 | 14015 | semaphorin-1 |
| D6e | 3.182 | 15879 | semaphorin V |
| E1b | 3.079 | 15956 | MMP-2; gelatinase A |
| E1m | 2 | 17032 | MMP-16 (MT3-MMP) |
DISCUSSION
In this study, we have explored the gene expression profiles in human GC and adjacent noncancerous normal gastric tissues using Atlas human cancer array which contains 588 genes that were classified according to their function to be relevant for cancer. cDNA array technology is used to examine simultaneously the expression of specific genes on a single hybridization. Although human genome projects have generated large-scale sequence data for millions of genes, the biological functions of such genes remain to be clarified. It is very important to define differential gene expression profiles of tumors and normal tissues before understanding the functional significance of specific gene products. Although expression analysis techniques such as Differential Display Polymerase Chain Reaction (DD-PCR), northern blot, and serial analysis of gene expression (SAGE) and RT-PCR have been widely used in the past, these studies are time-consuming and can only be used to deal with a limited number of genes.
The microarray technique was first reported in 1995 by Schena et al[5] and allows the simultaneous parallel expression analysis of thousands of genes. There is considerable interest in the potential application of cDNA microarray analysis for gene expression profiles in human cancers[12]. Such information might be useful for tumor classification, for elucidation of key factor in tumors and for the identification of genes which might be useful for diagnostic purposes or as therapeutic targets[13-15]. The Atlas Human cDNA Expression system provides a convenient and quick method for simultaneously profiling the expression of 588 genes related to cancer at the same time.
Among the genes showing differential expression in human GC, compared with the adjacent normal gastric tissue, several different classes of genes were up-regulated. Cytokeratin 1 (CK1), which is an intermediate filament protein, has a 2.7-fold up-regulation. The cytokeratins assembly in vivo, with obligatory heterologous dimeric combinations of different cytokeratins from each of the two major groups, comprising together more than 20 different individual cytokeratins. The keratin polypeptide expression common to all melanoma cells include K1 protein expression. A measure of keratin expression universality in malignant melanoma cells may have implications regarding their invasive and metastatic behaviors[16]. However it has never been reported in GC. Sky is a member of a subfamily of related receptor tyrosine kinases. Sky may function as a cell adhesion molecule and mediate cell-to-cell or cell-matrix interactions between hematopoietic cells and their respective microenvironments[17]. CD9 belongs to the tetraspanin superfamily of cell-surface proteins that span the membrane four times, forming two extracellular loops[18]. A low CD9 expression by tumors of the lung may be associated with poor prognosis[19]. Although the expression of CD9 was markedly down-regulated in basal cell carcinoma[20], it was up-regulated in GC. CD9 was found to be useful for prognosis of patients with colorectal cancer[21] or pancreatic cancer[22]. CD9 gene is a useful indicator of a poor prognosis in breast cancer patients[23].
Matrix metalloproteinases (MMPs) are members of a multigene family of zinc- and calcium-dependent enzymes involved in the degradation of numerous extracellular matrix (ECM) components[24,25]. MMP-2 and MT3-MMP were up-regulated in GC. MMP-2 may initiate and promote angiogenesis[26] and is considered to play a critical role in cell migration and invasion[27]. It is also thought to play an important role in tumour progression[28]. It has been reported that some kinds of malignant tumor tissues, including lung and stomach carcinomas, contain activated MMP-2[29]. MT3-MMP plays a role in extracellular matrix (ECM) turnover by activating proMMP-2 and also by acting directly on ECM macromolecules[30].
As described above, numerous genes were up-regulated, however more genes were down-regulated. Caspases are essential components of the mammalian cell death machinery. Caspase-8, 9 and 10 were all down-regulated in GC. Caspase-9 is the member of the apoptotic protease cascade that is triggered by cytochrome c and dATP[31]. Caspase-9 in the presence of cytochrome c and dATP can form an initiating complex for an apoptotic protease cascade[32]. Activated caspase-9 in turn cleaves and activates caspase-3, -6 and -7 zymogens[33]. Activation of the above caspases is blocked by a dominant negative form of caspase-9[32]. The Fas/APO-1-receptor associated cysteine protease Mch5 (caspase-8), a member of the interleukin-1beta-converting enzyme family, is believed to be the enzyme responsible for activating a protease cascade after Fas-receptor ligation, leading to cell death[34,35]. A total of eight different isoforms of caspase-8 have been described. Only two of the caspase-8 isoforms (caspase-8/a and caspase-8/b) were predominantly expressed in cells of different origin. Both isoforms were recruited to the CD95 death-inducing signaling complex and were activated upon CD95 stimulation with similar kinetics[36]. Caspase-10 is involved in CD95 and p55 signal transduction. Caspase-10 is recruited to both the CD95 and p55 tumor necrosis factor receptor signaling complexes in a FADD-dependent manner[37] and may be responsible for activation of the ICE-like proteases[38].
Retinoic acid receptors (RARs) are members of the steroid/thyroid hormone receptor superfamily. RARs serve as ligand-activated transcription factors[39]. RARs are found in all tissues but predominantly in the developing fetus, dividing tumor cells and adult skin[40]. Retinoic acid (RA) exerts its effects by differentially regulating its own receptor gene expression, including RAR alpha, beta, and gamma[41]. RAR-gamma selectively binding retinoids are potent inhibitors of breast cancer cell proliferation alone and in combination with interferon-gamma (IFN-gamma)[42]. RAR-gamma plays a critical role in mediating growth suppression by RA in ovarian cancer cells[43]. Reduced endogenous RAR gamma expression may contribute to the malignant phenotype of human NB[44]. RAR-gamma plays a critical role in a genetic switch between melanocytes and melanoma and induction of ligand-dependent apoptosis[45]. It is still not know if RAR plays a similar role in GC.
The DCC (deleted in colorectal cancer) gene was originally identified as a candidate tumour suppressor gene in colonic carcinogenesis on the basis of allelic losses in chromosome 18q.21 in 70% of colon cancers. DCC appears likely to play a significant role in differentiation, cell fate determination, and migration in the nervous system and perhaps other tissues as well[46]. Absence of DCC expression however is not associated with colonic tumour progression[47,48].
Neuroendocrine-Dlg (NE-Dlg, neuronal and endocrine dig) is a member of the discs-large-related (DLG) subfamily of the membrane-associated guanylate kinase-related proteins[49]. NE-dlg is a human homolog of the Drosophila discs large (dig) tumor suppressor protein[50]. The NE-dlg appears to be critical for synaptogenesis, acting as a site-specific organizational center for integral membrane proteins and their downstream signaling molecules associated with the cytoskeleton[50]. NE-Dlg directly interacts with the colorectal tumor suppressor gene adenomatous polyposis coli (APC), suggesting that it may play a role in regulating cell proliferation in epithelial cells[50].
In conclusion, our study demonstrates that cDNA array is a powerful tool to explore gene expression profiles in cancer. The genes described in this study should therefore be a valuable resource for basic research, into molecular mechanism of carcinogenesis and the progression and prognosis of tumors. In addition, the clinical application of this work, may include the development of new diagnostic markers and the identification of novel therapeutic strategies for GC.
ACKNOWLEDGMENTS
We thank Dr. Michael Kelly (Department of Surgery, St. George Hospital, University of New South Wales, Sydney, Australia) for his review of the manuscript and helpful suggestions.
Footnotes
Edited by KellyMD
Supported by China Key Program on Basic Research, Grant Number: Z19-01-01-02; Chinese Climbing Project No.18; Youth Natural Scientific Foundation of Heilongjiang Province
References
- 1.Deng DJ. progress of gastric cancer etiology: N-nitrosamides 1999s. World J Gastroenterol. 2000;6:613–618. doi: 10.3748/wjg.v6.i4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba H, Korenaga D, Kakeji Y, Haraguchi M, Okamura T, Maehara Y. DNA ploidy and its clinical implications in gastric cancer. Surgery. 2002;131:S63–S70. doi: 10.1067/msy.2002.119306. [DOI] [PubMed] [Google Scholar]
- 3.Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403–406. doi: 10.3748/wjg.v7.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu HF, Liu WW, Fang DC, Men RP. Expression and significance of proapoptotic gene Bax in gastric carcinoma. World J Gastroenterol. 1999;5:15–17. doi: 10.3748/wjg.v5.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 6.Wang K, Gan L, Jeffery E, Gayle M, Gown AM, Skelly M, Nelson PS, Ng WV, Schummer M, Hood L, et al. Monitoring gene expression profile changes in ovarian carcinomas using cDNA microarray. Gene. 1999;229:101–108. doi: 10.1016/s0378-1119(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 7.Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, et al. Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet. 2000;24:227–235. doi: 10.1038/73432. [DOI] [PubMed] [Google Scholar]
- 8.Khan J, Simon R, Bittner M, Chen Y, Leighton SB, Pohida T, Smith PD, Jiang Y, Gooden GC, Trent JM, et al. Gene expression profiling of alveolar rhabdomyosarcoma with cDNA microarrays. Cancer Res. 1998;58:5009–5013. [PubMed] [Google Scholar]
- 9.Lu J, Liu Z, Xiong M, Wang Q, Wang X, Yang G, Zhao L, Qiu Z, Zhou C, Wu M. Gene expression profile changes in initiation and progression of squamous cell carcinoma of esophagus. Int J Cancer. 2001;91:288–294. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1063>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Kallioniemi OP. Biochip technologies in cancer research. Ann Med. 2001;33:142–147. doi: 10.3109/07853890109002069. [DOI] [PubMed] [Google Scholar]
- 11.Khan J, Saal LH, Bittner ML, Chen Y, Trent JM, Meltzer PS. Expression profiling in cancer using cDNA microarrays. Electrophoresis. 1999;20:223–229. doi: 10.1002/(SICI)1522-2683(19990201)20:2<223::AID-ELPS223>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 12.Hu YC, Lam KY, Law S, Wong J, Srivastava G. Identification of differentially expressed genes in esophageal squamous cell carcinoma (ESCC) by cDNA expression array: overexpression of Fra-1, Neogenin, Id-1, and CDC25B genes in ESCC. Clin Cancer Res. 2001;7:2213–2221. [PubMed] [Google Scholar]
- 13.Selaru FM, Zou T, Xu Y, Shustova V, Yin J, Mori Y, Sato F, Wang S, Olaru A, Shibata D, et al. Global gene expression profiling in Barrett's esophagus and esophageal cancer: a comparative analysis using cDNA microarrays. Oncogene. 2002;21:475–478. doi: 10.1038/sj.onc.1205111. [DOI] [PubMed] [Google Scholar]
- 14.Unger MA, Rishi M, Clemmer VB, Hartman JL, Keiper EA, Greshock JD, Chodosh LA, Liebman MN, Weber BL. Characterization of adjacent breast tumors using oligonucleotide microarrays. Breast Cancer Res. 2001;3:336–341. doi: 10.1186/bcr317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rew DA. DNA microarray technology in cancer research. Eur J Surg Oncol. 2001;27:504–508. doi: 10.1053/ejso.2001.1116. [DOI] [PubMed] [Google Scholar]
- 16.Katagata Y, Aoki T, Hozumi Y, Yoshida T, Kondo S. Identification of K1/K10 and K5/K14 keratin pairs in human melanoma cell lines. J Dermatol Sci. 1996;13:219–227. doi: 10.1016/s0923-1811(96)00538-5. [DOI] [PubMed] [Google Scholar]
- 17.Crosier PS, Hall LR, Vitas MR, Lewis PM, Crosier KE. Identification of a novel receptor tyrosine kinase expressed in acute myeloid leukemic blasts. Leuk Lymphoma. 1995;18:443–449. doi: 10.3109/10428199509059643. [DOI] [PubMed] [Google Scholar]
- 18.Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- 19.Adachi M, Taki T, Konishi T, Huang CI, Higashiyama M, Miyake M. Novel staging protocol for non-small-cell lung cancers according to MRP-1/CD9 and KAI1/CD82 gene expression. J Clin Oncol. 1998;16:1397–1406. doi: 10.1200/JCO.1998.16.4.1397. [DOI] [PubMed] [Google Scholar]
- 20.Okochi H, Kato M, Nashiro K, Yoshie O, Miyazono K, Furue M. Expression of tetra-spans transmembrane family (CD9, CD37, CD53, CD63, CD81 and CD82) in normal and neoplastic human keratinocytes: an association of CD9 with alpha 3 beta 1 integrin. Br J Dermatol. 1997;137:856–863. [PubMed] [Google Scholar]
- 21.Mori M, Mimori K, Shiraishi T, Haraguchi M, Ueo H, Barnard GF, Akiyoshi T. Motility related protein 1 (MRP1/CD9) expression in colon cancer. Clin Cancer Res. 1998;4:1507–1510. [PubMed] [Google Scholar]
- 22.Sho M, Adachi M, Taki T, Hashida H, Konishi T, Huang CL, Ikeda N, Nakajima Y, Kanehiro H, Hisanaga M, et al. Transmembrane 4 superfamily as a prognostic factor in pancreatic cancer. Int J Cancer. 1998;79:509–516. doi: 10.1002/(sici)1097-0215(19981023)79:5<509::aid-ijc11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 23.Huang CI, Kohno N, Ogawa E, Adachi M, Taki T, Miyake M. Correlation of reduction in MRP-1/CD9 and KAI1/CD82 expression with recurrences in breast cancer patients. Am J Pathol. 1998;153:973–983. doi: 10.1016/s0002-9440(10)65639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polette M, Birembaut P. Membrane-type metalloproteinases in tumor invasion. Int J Biochem Cell Biol. 1998;30:1195–1202. doi: 10.1016/s1357-2725(98)00083-1. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds JJ. Collagenases and tissue inhibitors of metalloproteinases: a functional balance in tissue degradation. Oral Dis. 1996;2:70–76. doi: 10.1111/j.1601-0825.1996.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 26.Sang QX. Complex role of matrix metalloproteinases in angiogenesis. Cell Res. 1998;8:171–177. doi: 10.1038/cr.1998.17. [DOI] [PubMed] [Google Scholar]
- 27.Nagase H. Cell surface activation of progelatinase A (proMMP-2) and cell migration. Cell Res. 1998;8:179–186. doi: 10.1038/cr.1998.18. [DOI] [PubMed] [Google Scholar]
- 28.Rooprai HK, McCormick D. Proteases and their inhibitors in human brain tumours: a review. Anticancer Res. 1997;17:4151–4162. [PubMed] [Google Scholar]
- 29.Ueno H, Nakamura H, Inoue M, Imai K, Noguchi M, Sato H, Seiki M, Okada Y. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res. 1997;57:2055–2060. [PubMed] [Google Scholar]
- 30.Matsumoto S, Katoh M, Saito S, Watanabe T, Masuho Y. Identification of soluble type of membrane-type matrix metalloproteinase-3 formed by alternatively spliced mRNA. Biochim Biophys Acta. 1997;1354:159–170. doi: 10.1016/s0167-4781(97)00120-6. [DOI] [PubMed] [Google Scholar]
- 31.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 32.Pan G, Humke EW, Dixit VM. Activation of caspases triggered by cytochrome c in vitro. FEBS Lett. 1998;426:151–154. doi: 10.1016/s0014-5793(98)00330-5. [DOI] [PubMed] [Google Scholar]
- 33.Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell. 1998;1:949–957. doi: 10.1016/s1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- 34.Yuan J. Transducing signals of life and death. Curr Opin Cell Biol. 1997;9:247–251. doi: 10.1016/s0955-0674(97)80069-5. [DOI] [PubMed] [Google Scholar]
- 35.Muzio M, Salvesen GS, Dixit VM. FLICE induced apoptosis in a cell-free system. Cleavage of caspase zymogens. J Biol Chem. 1997;272:2952–2956. doi: 10.1074/jbc.272.5.2952. [DOI] [PubMed] [Google Scholar]
- 36.Scaffidi C, Medema JP, Krammer PH, Peter ME. FLICE is predominantly expressed as two functionally active isoforms, caspase-8/a and caspase-8/b. J Biol Chem. 1997;272:26953–26958. doi: 10.1074/jbc.272.43.26953. [DOI] [PubMed] [Google Scholar]
- 37.Vincenz C, Dixit VM. Fas-associated death domain protein interleukin-1beta-converting enzyme 2 (FLICE2), an ICE/Ced-3 homologue, is proximally involved in CD95- and p55-mediated death signaling. J Biol Chem. 1997;272:6578–6583. doi: 10.1074/jbc.272.10.6578. [DOI] [PubMed] [Google Scholar]
- 38.Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri ES. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Repa JJ, Berg JA, Kaiser ME, Hanson KK, Strugnell SA, Clagett-Dame M. One-step immunoaffinity purification of recombinant human retinoic acid receptor gamma. Protein Expr Purif. 1997;9:319–330. doi: 10.1006/prep.1996.0696. [DOI] [PubMed] [Google Scholar]
- 40.Sharma RP, Kim YW. Localization of retinoic acid receptors in anterior-human embryo. Exp Mol Pathol. 1995;62:180–189. doi: 10.1006/exmp.1995.1020. [DOI] [PubMed] [Google Scholar]
- 41.Wan YJ, Cai Y, Magee TR. Retinoic acid differentially regulates retinoic acid receptor-mediated pathways in the Hep3B cell line. Exp Cell Res. 1998;238:241–247. doi: 10.1006/excr.1997.3851. [DOI] [PubMed] [Google Scholar]
- 42.Widschwendter M, Daxenbichler G, Culig Z, Michel S, Zeimet AG, Mörtl MG, Widschwendter A, Marth C. Activity of retinoic acid receptor-gamma selectively binding retinoids alone and in combination with interferon-gamma in breast cancer cell lines. Int J Cancer. 1997;71:497–504. doi: 10.1002/(sici)1097-0215(19970502)71:3<497::aid-ijc31>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 43.Wu S, Zhang D, Zhang ZP, Soprano DR, Soprano KJ. Critical role of both retinoid nuclear receptors and retinoid-X-receptors in mediating growth inhibition of ovarian cancer cells by all-trans retinoic acid. Oncogene. 1998;17:2839–2849. doi: 10.1038/sj.onc.1202208. [DOI] [PubMed] [Google Scholar]
- 44.Marshall GM, Cheung B, Stacey KP, Camacho ML, Simpson AM, Kwan E, Smith S, Haber M, Norris MD. Increased retinoic acid receptor gamma expression suppresses the malignant phenotype and alters the differentiation potential of human neuroblastoma cells. Oncogene. 1995;11:485–491. [PubMed] [Google Scholar]
- 45.Spanjaard RA, Ikeda M, Lee PJ, Charpentier B, Chin WW, Eberlein TJ. Specific activation of retinoic acid receptors (RARs) and retinoid X receptors reveals a unique role for RARgamma in induction of differentiation and apoptosis of S91 melanoma cells. J Biol Chem. 1997;272:18990–18999. doi: 10.1074/jbc.272.30.18990. [DOI] [PubMed] [Google Scholar]
- 46.Fearon ER. DCC: is there a connection between tumorigenesis and cell guidance molecules? Biochim Biophys Acta. 1996;1288:M17–M23. doi: 10.1016/0304-419x(96)00023-6. [DOI] [PubMed] [Google Scholar]
- 47.Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, Kinzler KW, Vogelstein B. Scrambled exons. Cell. 1991;64:607–613. doi: 10.1016/0092-8674(91)90244-s. [DOI] [PubMed] [Google Scholar]
- 48.Reymond MA, Dworak O, Remke S, Hohenberger W, Kirchner T, Köckerling F. DCC protein as a predictor of distant metastases after curative surgery for rectal cancer. Dis Colon Rectum. 1998;41:755–760. doi: 10.1007/BF02236264. [DOI] [PubMed] [Google Scholar]
- 49.Ciardiello F, Dono R, Kim N, Persico MG, Salomon DS. Expression of cripto, a novel gene of the epidermal growth factor gene family, leads to in vitro transformation of a normal mouse mammary epithelial cell line. Cancer Res. 1991;51:1051–1054. [PubMed] [Google Scholar]
- 50.Baldassarre G, Romano A, Armenante F, Rambaldi M, Paoletti I, Sandomenico C, Pepe S, Staibano S, Salvatore G, De Rosa G, et al. Expression of teratocarcinoma-derived growth factor-1 (TDGF-1) in testis germ cell tumors and its effects on growth and differentiation of embryonal carcinoma cell line NTERA2/D1. Oncogene. 1997;15:927–936. doi: 10.1038/sj.onc.1201260. [DOI] [PubMed] [Google Scholar]


