Abstract
AIM: To observe the expression of Human telomerase reverse transcriptase (hTERT) in gastric carcinomas and precancerosis lesions, to evaluate the immune state of such patients, and to then study the clinical significance of hTERT and immune state for the diagnosis, treatment and prognosis of gastric cancer.
METHODS: In situ hybridization was used to detect the expression of hTERT mRNA in 116 endoscopic of gastric mucosa. Analyzed tissue samples were as follows: 30 cases of chronic superficial gastritis (CSG), 44 of precancerosis lesions (including 27 of chronic atrophic gastritis, 8 of adenomatous polyp and 9 of gastric ulcer) and 42 of gastric cancer (GC). In addition, the T lymphocyte subsets (CD3+, CD4+, CD8+, CD4+/CD8+) and natural killer cells (NK) in peripheral blood were determined by flow cytometric analysis (FCM) in 30 cases of CSG, 27 of precancerosis (chronic atrophic gastritis, CAG), and 42 of GC. The data were compared with those of normal control (NC).
RESULTS: The detected positive rate of hTERT varied as follows: 86% (36/42) in GC, 36% (16/44) in precancerosis lesions and 0% (0/30) in CSG. The expression of hTERT mRNA was not associated with patient gender, tumor location, macroscopic type, lymph node metastasis, or degree of differentiation. It was found that the CD3+, CD4+ of the CSG group were lower than that of NC (P < 0.05). Meanwhile, the T lymphocyte subsets (CD3+, CD4+, CD4+/CD8+ ratio) and NK cells of CAG were remarkably lower than that of NC and CSG groups (P < 0.05-0.01). Values of T cells and NK cells of the GC group were significantly abnormal when compared with the CAG group (P < 0.05-0.01). Furthermore, with tumor progression, the function of T cells was weakened gradually.
CONCLUSION: The expression of telomerase may be a crucial step in gastric carcinogenesis and increased hTERT mRNA may serve as a novel marker for diagnosis of GC. The immune state of patients with GC and precancerosis was somewhat depressed, which indicates the importance of cellular immunological assays in cancer patients.
INTRODUCTION
There are many factors that contribute to gastric carcinogenesis[1-10]. Currently, telomerase has been a major focus[11-23]. Telomerase activation is associated with an early stage of stomach carcinogenesis[24-33]. Human telomerase reverse transcriptase (hTERT) has been identified as a catalytic subunit of human telomerase. Recent studies have demonstrated a close correlation between telomerase activity and hTERT expression[32-41]. In this study, in situ hybridization (ISH) was used to detect hTERT expression in patients with GC and precancerosis, which could help us better understand the role of telomerase in the carcinogenesis and development of GC.
In the meantime, many studies have suggested that immune responses to tumor cells play an important role in GC[42-54]. We detected tumor specific lymphocytes (T lymphocytes) and nonspecific natural killer (NK) cells in order to clarify the correlation between cell mediated immunity and the clinicopathologic factors of GC.
MATERIALS AND METHODS
Tissue samples
Gastroscopic removal tissues were obtained from the second affiliated Hospital of Heibei Medical University from January 2000 to July 2000. Totally, 116 gastroscopic samples were analyzed. The patients (mean age, 48.3 years; range, 40-56 years) are as follows: 42 cases of GC, 44 chronic gastritis with atypical hyperplasia (including 27 of CAG, 8 of adenomatous polyp and 9 of gastric ulcer), and 30 CSG. In case selection, the disease of liver, circulatory, endocrinopathy and rheumatic systems were excluded. The gastroscopic samples were fixed into formalin with 1% DEPC to prevent mRNA degradation. For FCM of T lymphocytes and NK cells, 1 mL samples of venous blood from each patient was put into a heparinized tube. The value of T lymphocytes and NK cells of 20 blood donors in the same age range were run as NC.
hTERT assay
ISH was carried out by using an hTERT ISH Detection Kit (produced by Wuhan Boster Biological Technology Ltd.). The antisense poly-oligonucleotide probe was digoxin-labeled.
Formalin-fixed, paraffin-embedded samples were cut at 5 μm and adhered to poly-l-lysine treated slides. Samples were deparaffinized and rehydrated through a graded series of ethanol, and endogenous peroxidase was blocked using 3% hydrogen peroxide for 10 min. The slides were digested with pepsin at 37 °C for 15-20 min. Twenty μL of probe was hybridized to each slide for 16-20 h at 40 °C. After hybridization, excess probe was removed by washing in 2 × SSC at 37 °C. Tissue sections were preblocked for 20 min with blocking reagent, then the primary antibody (rabbit anti-digoxin antibody) was added for 60 min at 37 °C. After washing with 0.5 M PBS three times at 5 min each, the slides were incubated with the secondary goat anti-rabbit immunoglobulin (IgG) antibody conjugated with biotin for 20 min at 37 °C, then washed with 0.5 M PBS again as previously described. Samples were next incubated with SABC for 20 min at room temperature and rinsed with 0.5 M PBS for four times at 5 min each. The reaction products of peroxidase were visualized by incubation with chromogen diaminobenzidine for 15-20 min. Finally, the slides were counterstained for nuclei by haematoxylin stain. A negative control was prepared for each sample using a hybridization solution without probe. The positive signals of hTERT mRNA expression were stains with the color of brown-yellow located in cell plasma. The average percentage of positive cells was determined in at least 5 areas at × 400 and assigned to one of four categories: (-)-negative or equivocal staining; (+)-weak positive, cells were stained in 1%-25%; (++)-middle positive, cells were stained in 25%-50%; and (+++)-strong positive expression, cells were stained over 50%.
Flow cytometric analysis of cellular immunity
The heparinized venous blood samples were made into suspensions of single cells, then plated in reaction tubes. Monoclonal antibodies of mature T cells (CD3+), TH (CD4+), Ts (CD8+), and NK cells (CD+) were added, then shaken into a well-distributed solution. The solution was incubated for 30 min at room temperature, then rinsed with distilled water for 10 min. The cells were collected after centrifugation at 1000 rpm for 10 min and kept at 4 °C. Measurement of T cells and NK cells was performed by using a FACS Calibur flow cytometer (Becton Dickinson).
Statistical analyses
The χ² test was used for statistical analysis of the frequency of positive hTERT among each group. The data of T cells and NK cells were expressed as mean ± standard deviation (¯x ± s), and the differences between the value of different groups were analysed by the t test. The criterion of significance was set at P < 0.05.
RESULTS
Expression levels of hTERT
The positive signals of hTERT mRNA expression were brown-yellow stains located in the cell plasma. (Figure 1 and Figure 2). The expression levels of hTERT in different gastric mucosae are summarized in Table 1. There was no hTERT mRNA expression in CSG (0/30). Positive hTERT was detected in 16 of 44 (36%) of precancerosis lesions and 36 of 42 (86%) GC.
Figure 1.
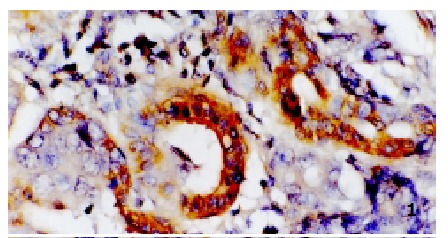
Positive signal of hTERT in gastric adenocancer, localized in plasma. ISH, × 400
Figure 2.
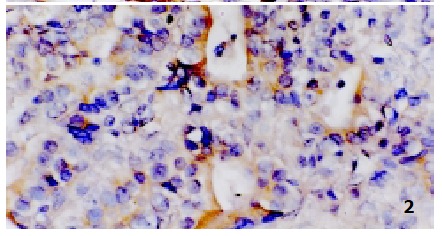
Weak positive signal of hTERT in precancerosis, localized in plasma. ISH, × 400
Table 1.
The expression of hTERT mRNA in 116 cases of gastric mucosas
| groups | n |
hTERT mRNA |
positive (%) | ||
| + | ++ | +++ | |||
| CSG | 30 | 0 | 0 | 0 | 0 |
| CAG | 27 | 10 | 0 | 0 | 37 |
| AP | 8 | 2 | 1 | 0 | 38 |
| GU | 9 | 3 | 0 | 0 | 33 |
| GC | 42 | 15 | 18 | 3 | 86 |
Carcinomas exhibited positive hTERT significantly more frequently than did precancerosis lesions (P < 0.05, by χ² test). The positive rate of three groups with dysplasia of CAG, adenomatous polyps (AP) and gastric ulcers (GU) are 37% (10 of 27), 38% (3 of 8) and 3 3% (3 of 9) respectively. There was no significant difference among the three groups (P > 0.05). Positive rate of early-stage GC and advanced-stage GC are 88% (7 of 8) and 85% (29/34) respectively. There was no significant difference between the two groups (P > 0.05). We also grouped the cancer patients by gender, tumour location, macroscopic type, histological grade, and lymph node metastasis, and found the positive rates of hTERT were not correlated with these clinicopathological factors.
The detection results of T cells and NK cells
The results are summarized in Table 2 and Table 3.
Table 2.
T lymphocyte subsets and NK cells in different gastric disease (¯x ± s)
| groups | n | CD3+ (%) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ | NK |
| NC | 20 | 68.0 ± 2.0 | 39.9 ± 4.5 | 27.1 ± 4.5 | 1.4 ± 0.2 | 21.4 ± 3.7 |
| CSG | 30 | 61.6 ± 4.4a | 35.9 ± 3.3a | 26.7 ± 4.2 | 1.4 ± 0.1 | 20.1 ± 5.1 |
| CAG | 27 | 57.6 ± 3.1ac | 33.5 ± 2.8ac | 26.8 ± 2.8 | 1.2 ± 0.1bd | 13.5 ± 3.4bd |
| GC | 42 | 53.4 ± 10.6e | 29.4 ± 7.6e | 35.6 ± 8.6e | 0.9 ± 0.4e | 9.4 ± 4.4f |
P < 0.05,
P < 0.01 vs NC;
P < 0.05
P < 0.01 vs CSG;
P < 0.05,
P < 0.01 vs CAG
Table 3.
T lymphocyte subsets and NK cells in different stages of gastric cancer (¯x ± s)
| groups | n | CD3+ (%) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ | NK |
| tumor gross type | ||||||
| early | 8 | 57.5 ± 5.5 | 32.1 ± 9.1 | 28.2 ± 10.0 | 1.1 ± 0.6 | 8.7 ± 3.9 |
| advanced | 34 | 52.9 ± 9.8b | 26.8 ± 6.0b | 35.5 ± 8.1 | 0.8 ± 0.3a | 9.6 ± 4.5 |
| lymph node metastasis | ||||||
| without | 13 | 57.3 ± 7.8 | 31.2 ± 7.4 | 32.4 ± 8.5 | 1.1 ± 0.4 | 9.7 ± 4.7 |
| with | 29 | 52.5 ± 10.1c | 25.7 ± 5.8c | 36.7 ± 8.1 | 0.7 ± 0.3c | 9.2 ± 4.3 |
P < 0.05,
P < 0.01vs early-stage cancer;
P < 0.05 vs the cancer patients without lymph node metastasis
The results of T cells examination were as follows: (1) The values of CD3+ and CD4+ in CSG were significantly lower than that in NC (P < 0.05 t test). CD8+ and CD4+/CD8+ ratio were slightly lower in CSG than in NC, but the change was not significant (P > 0.05). (2) The values of CD3+, CD4+, CD4+/CD8+ in CAG were significantly lower compared with CSG and NC (P < 0.05-0.01). There was no significant change in CD8+ (P > 0.05). (3) Compared with CAG, all the values of T cells had remarkable changes (P < 0.05-0.01). (4) The values of CD3+, CD4+, and CD4+/CD8+ in advanced cancer were remarkably lower than in early-stage cancers (P < 0.05-0.01). (5) The values of CD3+, CD4+, and CD4+/CD8+ in the cancer patients with lymph node metastasis were significantly lower than that of cancer patients without lymph node metastasis (P < 0.05).
The results of NK cell examination were as follows: (1) There was no significant difference between CSG and NC (P > 0.05). (2) The value of NK was significantly lower in CAG than in CSG (P < 0.01). (3) The value of NK is also significantly lower in GC than in CAG (P < 0.01). (4) There was no significant difference between early-stage cancer and advanced cancer (P > 0.05). The value of NK was not associated with lymph node metastasis (P > 0.05).
DISCUSSION
Most current studies have proposed that reactivation of telomerse is a critical step in tumorigenesis[24-41]. There is a close correlation between hTERT and telomerase activity[32,33]. Several researchers have reported high levels of hTERT expression in malignant tissues but not in non-malignant tissues by using ISH techniques[14,15,34]. In this study, we used the ISH method to analyze the localization of hTERT mRNA expression in formalin-fixed, paraffin-embedded tissues and got similar results as those previously reported. High levels of hTERT expression were found in the plasma of most carcinoma cells (Figure 1), and the positive rate was 86%. hTERT expression also increased in precancerosis lesions with the positive rate of 36%, most of them were weakly positive (Figure 2). No expression was observed in CSG. There is great significance among the three groups above (P < 0.01). Very high levels of hTERT were detected in 7 of 8 early-stage GC (88%), which is remarkably greater than that of the precancerosis group (36%). These data indicate that hTERT overexpression may be not only an early event but also a critical step in carcinogenesis of the stomach. Our result is good evidence that telomerase can be an important marker for diagnosis of early-stage cancers.
It is now almost axiomatic that host immunological reaction is an important factor in resistance to tumors. Immunological cells, either sensitized or nonspecifically activated, are considered necessary to inhibit or kill tumor cells[42-54]. The cell-mediated immunity (CMI) in contact with tumor cells plays an important role in host immune defense of GC patients. Immune response to cancer cells may be mediated mainly by T lymphocyte subsets and nonspecific natural killer (NK) cells. Many studies indicate that T lymphocyte activity is correlated closely with carcinogenesis and development of GC. Critically, the TH/TS ratio is a more important index to evaluate the state of cellular immunity and anti-tumor activity[46-50]. NK cells are the main components of nonspecific immune surveillance, which is capable of killing tumor cells without being sensitized previously, and makes up the first defense line to eliminate cancer cells[51-53]. In order to realize the importance of immune state in the process of gastric carciogenesis, we used FCM to detect the mature T lymphocyte, TH, Ts, TH/Ts, and NK cells in peripheral blood, whose superficial markers are CD3+, CD4+, CD8+, CD4+/CD8+, and CD (16 + 56)+, respectively.
Our results suggested that T lymphocyte subsets and NK cells have significant changes in every stage of the progression from normal gastric mucosa to GC: (1) In NC the values are as follows: CD3+, 68.02 ± 2.01; CD4+, 39.89 ± 4.50; CD8+, 27.14 ± 4.51; CD4+/CD8+, 1.42 ± 0.21; and NK, 21.44 ± 3.74. (2) CD3+ (61.61 ± 4.39) and CD4+ (35.92 ± 3.30) in CSG were significantly lower than that of NC, which indicated that CSG patients not only had the infiltrating lymphocytes in local pathological change but also had a remarkable imbalance in the T cell immunity of the host body. In the CAG group, CD3+ (57.55 ± 3.13), CD4+ (33.54 ± 2.82), CD4+/CD8+ (1.22 ± 0.13), and NK (13.48 ± 3.44) were all remarkably decreased, which indicated the cellular immunity of CAG patients had a distinct abnormality. In the GC group, CD3+ (53.37 ± 10.55), CD4+ (29.37 ± 7.61), CD4+/CD8+ (0.90 ± 0.39) and NK (9.40 ± 4.38) became much lower than ever. Furthermore, the impairment of cellular immunity became more and more critical with tumor progression and metastasis (Table 3). All these results indicated: (1) The state of cellular immunity of patients with GC was correlated with prognosis. The deficiency of cellular immunity may play an important role in the continuing growth and metastasis of GC; and (2) Using a combination of immunotherapy with other modalities, e.g. chemotherapy or radiation, may lead to effective antitumor therapy.
Reports regarding the relationship between hTERT and local pathological lesions, and the cellular immunity of the host in the process of gastric carcinogenesis are still rare. We found that hTERT had a certain expression in precancerosis lesions while the immunity state of most of those patients decreased. In early-stages of GC, the expression of hTERT had remarkably increased and the cellular immunity had decreased at the same time. These situations are worthy of more attention. According to previous studies, the main mechanism of cellular immunological insufficiency of GC patients is that tumor cells can produce a large amount of immunosuppressive factors. Before the metamorphoses of precancerosis cells, the components of their antigens have had the characteristics of cancer cells in the process of transition from precancerosis to cancer. Such cells can also produce immunosuppressive factors to impair host's cellular immunity. Thus, the insufficiency of the immune system may lead the cancer to worsen, which may cause a vicious cycle. It has been demonstrated that telomerase activation is a crucial step in gastric carcinogenesis. When the expression of telomerase changes the antigenic components of cells in the process of gastric carcinogenesis, then can cancer cells avoid immune surveillance? When telomerase activation affects the role of apoptosis, can this cause T cell or NK cell damage? Such questions need further research. Discussing such questions will be helpful to realize the mechanism of further reduction of cellular immunity in the process of carcinogenesis and cancer proliferation, and also will provide good prospects of gene therapy which combine anti-tumor telomerase and immunotherapy.
Footnotes
Edited by Pagliarini R
Supported by Science and Technology Fund, Governmental Department of Health, Hebei Province, No.2K007
References
- 1.Zhang XY. Some recent works on diagnosis and treatment of gastric cancer. World J Gastroenterol. 1999;5:1–3. doi: 10.3748/wjg.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xue XC, Fang GE, Hua JD. Gastric cancer and apoptosis. Shijie Huaren Xiaohua Zazhi. 1999;7:359–361. [Google Scholar]
- 3.Cai L, Yu SZ, Ye WM, Yi YN. Fish sauce and gastric cancer: an ecological study in Fujian Province, China. World J Gastroenterol. 2000;6:671–675. doi: 10.3748/wjg.v6.i5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhuang ZH, Chen YL, Wang CD, Chen YG. Expression of TGF-1 and TGF-A receptor 1 in gastric carcinoma and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 1999;7:507–509. [Google Scholar]
- 5.Wu YA, Lu B, Liu J, Li J, Chen JR, Hu SX. Consequence alimentary reconstruction in nutritional status after total gastrectomy for gastric cancer. World J Gastroenterol. 1999;5:34–37. doi: 10.3748/wjg.v5.i1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou HP, Wang X, Zhang NZ. Early apoptosis in intestinal and diffuse gastric carcinomas. World J Gastroenterol. 2000;6:898–901. doi: 10.3748/wjg.v6.i6.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu HF, Liu WW, Fang DC, Men RF, Wang ZH. Apoptosis and its relationship with Fas ligand expression in gastric carcinoma and its precancerous lesion. Shijie Huaren Xiaohua Zazhi. 1999;7:561–563. [Google Scholar]
- 8.Tuo BG, Yan YH, Ge ZL, Ou GW, Zhao K. Ascorbic acid secretion in the human stomach and the effect of gastrin. World J Gastroenterol. 2000;6:704–708. doi: 10.3748/wjg.v6.i5.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu HF, Liu WW, Fang DC. Study of the relationship between apoptosis and proliferation in gastric carcinoma and its precancerous lesion. Shijie Huaren Xiaohua Zazhi. 1999;7:649–651. [Google Scholar]
- 10.Zou SC, Qiu HS, Zhang CW, Tao HQ. A clinical and long-term follow-up study of peri-operative sequential triple therapy for gastric cancer. World J Gastroenterol. 2000;6:284–286. doi: 10.3748/wjg.v6.i2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan JL, Zhang JP, Zhou TH. Relationship between telomerase, Helicobacter pylori and stomach cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:910–911. [Google Scholar]
- 12.Yang SM, Fang DC, Luo YH, Lu R, Liu WW. Telomerase activity in gastroenterological submucous tumors and its clinical significance. Shijie Huaren Xiaohua Zazhi. 1998;6:765–767. [Google Scholar]
- 13.Ma JP, Cai SR, Zhan WH. Telomerase activity in human gastric carcinoma by TRAP silver stain assay. Zhonghua Weichang Waike Zazhi. 1998;1:103–105. [Google Scholar]
- 14.Hiyama T, Yokozaki H, Kitadai Y, Tahara E, Tahara H, Ide T, Haruma K, Yasui W, Kajiyama G, Tahara E. In situ mRNA hybridization technique for analysis of human telomerase RNA in gastric precancerous and cancerous lesions. Jpn J Cancer Res. 1998;89:1187–1194. doi: 10.1111/j.1349-7006.1998.tb00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasui W, Tahara H, Tahara E, Fujimoto J, Nakayama J, Ishikawa F, Ide T, Tahara E. Expression of telomerase catalytic component, telomerase reverse transcriptase, in human gastric carcinomas. Jpn J Cancer Res. 1998;89:1099–1103. doi: 10.1111/j.1349-7006.1998.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tahara H, Kuniyasu H, Yokozaki H, Yasui W, Shay JW, Ide T, Tahara E. Telomerase activity in preneoplastic and neoplastic gastric and colorectal lesions. Clin Cancer Res. 1995;1:1245–1251. [PubMed] [Google Scholar]
- 17.Ahn MJ, Noh YH, Lee YS, Lee JH, Chung TJ, Kim IS, Choi IY, Kim SH, Lee JS, Lee KH. Telomerase activity and its clinicopathological significance in gastric cancer. Eur J Cancer. 1997;33:1309–1313. doi: 10.1016/s0959-8049(97)00113-5. [DOI] [PubMed] [Google Scholar]
- 18.Maruyama Y, Hanai H, Fujita M, Kaneko E. Telomere length and telomerase activity in carcinogenesis of the stomach. Jpn J Clin Oncol. 1997;27:216–220. doi: 10.1093/jjco/27.4.216. [DOI] [PubMed] [Google Scholar]
- 19.Hiyama E, Yokoyama T, Tatsumoto N, Hiyama K, Imamura Y, Murakami Y, Kodama T, Piatyszek MA, Shay JW, Matsuura Y. Telomerase activity in gastric cancer. Cancer Res. 1995;55:3258–3262. [PubMed] [Google Scholar]
- 20.He XX, Wang JL, Wu JL, Yuan SY, Ai L. Telomere, cellular DNA content and gastric mucosal carcinogenesis. Shijie Huaren Xiaohua Zazhi. 2000;8:509–512. [Google Scholar]
- 21.Zhang FX, Zhang XY, Fan DM, Yan Y, Xu ZK. Telomerase activity in gastric cancer and precancerosis. DiSi Junyi Daxue Xuebao. 1998;19:457–459. [Google Scholar]
- 22.Yang SM, Fang DC, Luo YH, Wang ZH, Lu R, Liu WW. Telomerase Activity in Gastric Mucosa From Endoscopy. Zhonghua Xiaohua Neijing Zazhi. 1997;14:298–300. [Google Scholar]
- 23.He XX, Wang JL, Wu JL, Yuan SY, Ai L. Telomerase expression, Hp infection and gastric mucosal carcinogenesis. Shijie Huaren Xiaohua Zazhi. 2000;8:505–508. [Google Scholar]
- 24.Naka K, Yokozaki H, Yasui W, Tahara H, Tahara E, Tahara E. Effect of antisense human telomerase RNA transfection on the growth of human gastric cancer cell lines. Biochem Biophys Res Commun. 1999;255:753–758. doi: 10.1006/bbrc.1998.9938. [DOI] [PubMed] [Google Scholar]
- 25.Zhang FX, Deng ZY, Zhang XY, Dang SC, Wang Y, Yu XL, Wang H, Bian XH. Telomeric length associated with prognosis in human primary and metastatic gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:153–155. [Google Scholar]
- 26.Kuniyasu H, Domen T, Hamamoto T, Yokozaki H, Yasui W, Tahara H, Tahara E. Expression of human telomerase RNA is an early event of stomach carcinogenesis. Jpn J Cancer Res. 1997;88:103–107. doi: 10.1111/j.1349-7006.1997.tb00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jong HS, Park YI, Kim S, Sohn JH, Kang SH, Song SH, Bang YJ, Kim NK. Up-regulation of human telomerase catalytic subunit during gastric carcinogenesis. Cancer. 1999;86:559–565. [PubMed] [Google Scholar]
- 28.Yasui W, Tahara E, Tahara H, Fujimoto J, Naka K, Nakayama J, Ishikawa F, Ide T, Tahara E. Immunohistochemical detection of human telomerase reverse transcriptase in normal mucosa and precancerous lesions of the stomach. Jpn J Cancer Res. 1999;90:589–595. doi: 10.1111/j.1349-7006.1999.tb00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang SM, Fang DC, Luo YH, Lu R, Liu WW. Effect of antisense gene to hum an telomerase reverse transcriptase on telomerase activity and expression of apo ptosis-associated gene. Shijie Huaren Xiaohua Zazhi. 2002;10:149–152. [Google Scholar]
- 30.Wang W, Luo HS, Yu BP. Expression of human telomerase reverse transcriptase gene and c-myc protein in gastric carcinogenesis. Shijie Huaren Xiaohua Zazhi. 2002;10:258–261. [Google Scholar]
- 31.Chiu CP, Dragowska W, Kim NW, Vaziri H, Yui J, Thomas TE, Harley CB, Lansdorp PM. Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem Cells. 1996;14:239–248. doi: 10.1002/stem.140239. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 33.Luo F, Sun JL, Ren DM, Cai D, Shen M. Effect of hyperthermia on telomera se activity and genes expression in human gastric cancer cell line. Shijie Huaren Xiaohua Zazhi. 2001;9:1261–1264. [Google Scholar]
- 34.Yao XX, Yin L, Zhang JY, Bai WY, Li YM, Sun ZC. hTERT expression and cellular immunity in gastric cancer and precancerosis. Shijie Huaren Xiaohua Zazhi. 2001;9:508–512. [Google Scholar]
- 35.Meng ZQ, Guo WJ, Yu EX, Song MZ, Huang WX. Inhibition of telomerase activity and induced apoptosis of liver cancer cell SMMC-7721 by drug serum of Jianpi Liqi herbs. Shijie Huaren Xiaohua Zazhi. 2000;8:879–882. [Google Scholar]
- 36.Suda T, Isokawa O, Aoyagi Y, Nomoto M, Tsukada K, Shimizu T, Suzuki Y, Naito A, Igarashi H, Yanagi M, et al. Quantitation of telomerase activity in hepatocellular carcinoma: a possible aid for a prediction of recurrent diseases in the remnant liver. Hepatology. 1998;27:402–406. doi: 10.1002/hep.510270213. [DOI] [PubMed] [Google Scholar]
- 37.Fang XM, Yu JP, Luo HS. Relationship between hTERT and P16 gene expe ssions and telomerase activity in colorectal cancer. Shijie Huaren Xiaohua Zazhi. 2002;10:12–14. [Google Scholar]
- 38.Shen ZY, Xu LY, Li EM, Cai WJ, Chen MH, Shen J, Zeng Y. Telomere and telomerase in the initial stage of immortalization of esophageal epithelial cell. World J Gastroenterol. 2002;8:357–362. doi: 10.3748/wjg.v8.i2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng RH, Li JF, Liu BY, Zhu ZG, Yin HR. hTR gene cloning from human g astric cancer cells and the construction of its sense and antisense eukaryotic e xpression vector. Shijie Huaren Xiaohua Zazhi. 2001;9:1409–1414. [Google Scholar]
- 40.Nakashio R, Kitamoto M, Tahara H, Nakanishi T, Ide T, Kajiyama G. Significance of telomerase activity in the diagnosis of small differentiated hepatocellular carcinoma. Int J Cancer. 1997;74:141–147. doi: 10.1002/(sici)1097-0215(19970422)74:2<141::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 41.Yang JL, Fang DC, Yang SM, Luo YH, Lu R, Luo KL, Liu WW. Construction of sense and antisense hTR eukaryotic expression vector. Shijie Huaren Xiaohua Zazhi. 2000;8:491–493. [Google Scholar]
- 42.Xin Y, Zhao FK, Zhang SM, Wu DY, Wang YP, Xu L. Relationship between CD44v6 expression and prognosis in gastric carcinoma patients. Shijie Huaren Xiaohua Zazhi. 1999;7:210–214. [Google Scholar]
- 43.Chen ZF, Deng CS, Xia B, Zhu YQ, Zeng J, Gong LL. Expression of heat shock protein 60, CD44 splice variant V6 in human gastric cancer. Shijie Huaren Xiaohua Zazhi. 2001;9:988–991. [Google Scholar]
- 44.Morgner A, Miehlke S, Stolte M, Neubauer A, Alpen B, Thiede C, Klann H, Hierlmeier FX, Ell C, Ehninger G, et al. Development of early gastric cancer 4 and 5 years after complete remission of Helicobacter pylori associated gastric low grade marginal zone B cell lymphoma of MALT type. World J Gastroenterol. 2001;7:248–253. doi: 10.3748/wjg.v7.i2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagashima S, Kashii Y, Reichert TE, Suminami Y, Suzuki T, Whiteside TL. Human gastric carcinoma transduced with the IL-2 gene: increased sensitivity to immune effector cells in vitro and in vivo. Int J Cancer. 1997;72:174–183. doi: 10.1002/(sici)1097-0215(19970703)72:1<174::aid-ijc25>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 46.Kume T, Oshima K, Yamashita Y, Shirakusa T, Kikuchi M. Relationship between Fas-ligand expression on carcinoma cell and cytotoxic T-lymphocyte response in lymphoepithelioma-like cancer of the stomach. Int J Cancer. 1999;84:339–343. doi: 10.1002/(sici)1097-0215(19990820)84:4<339::aid-ijc1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 47.Zhong LM, Li JY, Xiao GX, Chen DD. The relationship between lymphocyte immune function and lipid peroxidative injury in gastic cancer patients. Chin J Gen Surg. 2000;15:361–363. [Google Scholar]
- 48.Bai DJ, Yang GL, Yuan HY, Li Y, Wang K. Effects of cimetidine on T lymphocyte subsets in perioperative gsatrointinal cancer patients. Shijie Huaren Xiaohua Zazhi. 2000;8:147–149. [Google Scholar]
- 49.Zhang H, Ren XL, Yao XX. T lymphocyte subsets, nitric oxide, hexosamine and Helicobacter pylori infection in patients with chronic gastric diseases. Shijie Huaren Xiaohua Zazhi. 1999;7:127–129. doi: 10.3748/wjg.v6.i4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nie ZH, Zhu Y, Zhang M, Feng FB, Chen Y, Wang BZ, Geng YL, Chen XZ. Relationship of T lymphocyte subsets in peripheral blood with gastric mucosal active inflammation, Gas and SS contents in patients with duodenal ulcer. Shijie Huaren Xiaohua Zazhi. 1999;7:338–340. [Google Scholar]
- 51.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, Aridome K, Hokita S, Aikou T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–583. [PubMed] [Google Scholar]
- 52.Zhou YM, Lan ZF, Zhao JC. Detection of Cell-Mediated Immunity of Patients with Chronicity Atrophic Gastritis Patients and Gastric Cancer Pationts. Zhongliu Fangzhi Yanjiu. 1999;26:104–105. [Google Scholar]
- 53.Adachi T, Hinoda Y, Nishimori I, Adachi M, Imai K. Increased sensitivity of gastric cancer cells to natural killer and lymphokine-activated killer cells by antisense suppression of N-acetylgalactosaminyltransferase. J Immunol. 1997;159:2645–2651. [PubMed] [Google Scholar]
- 54.Mi JQ, Zhang ZH and Shen MC. Significance of CD44v6 protein expression in gastric carcinoma and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:156–158. [Google Scholar]


