Abstract
AIM: To detect the expression pattern of FAK (focal adhesion kinase) and integrin α5 and β1 subunits in different kinds of cancerous tissues and to study their correlation with clinicopathological data including tumor type, grade and lymph node status.
METHODS: Using an immunohistochemical technique, we examined the expression of FAK and integrin and 1 subunits in cancerous and noncancerous tissues obtained from 75 patients with gastric carcinomas, 21 colorectal carcinomas, 16 hepatocellular carcinomas, 20 uterocervical carcinomas, and 20 breast carcinomas.
RESULTS: The staining of FAK was stronger in cancerous than in noncancerous areas. Enhanced expression of FAKwas detected in poor-differentiated carcinoma of the stomach and colorectum. Tumors with lymph node metastases had more FAK protein than those without metastases. In addition, the deeper the extent of tumor infiltration, the higher the FAK expression. The expression of integrin α5 and β1 subunits was lower in cancerous areas than in noncancerous areas, but it was higher in well-differentiated cancerous tissues than in poor differentiated tissues. The relationship between the expression of integrin α5 and β1 subunits and infiltration or metastasis was not significant. Cancerous tissues with stronger FAK expression (++ or +++) also had a higher expression of integrin α5 and β1 subunits in the tumor and its unaffected margins.
CONCLUSION: FAK is a better marker for carcinogenesis and the progression of cancer than integrin α5 or β1 subunit, and it may be not only a transformation-linked enzyme but also a progression-linked enzyme.
INTRODUCTION
In recent years, much attention has focused on cell adhesion molecules. These studies have shown that adhesion molecules affect cell behaviors and mediate signal transduction, especially in cancer cells. Integrins are a large family of cell surface receptors that are found in many animal species[1]. They can affect signal transduction, cell proliferation, differentiation, survival and apoptosis, cell cycle and invasion and metastasis of carcinoma cells[2-7]. Previous studies have provided a better understanding of the signaling pathways activated by integrins in adherent cells[1]. As integrins bind to the extra cellular matrix (ECM), they become clustered in the plane of the cell membrane and activate a variety of non-receptor protein tyrosine kinases (PTKs), such as FAK, Abl, Syk and Src-family PTKs[7-13]. Upon activation of FAK, a number of focal adhesion components, such as paxillion, tensin, Shc and P130cas, are phosphorylated and activate the ERK2/MAPK signaling pathway network[13-20]. Therefore, FAK may play a central role in integrin-stimulated signaling events[20], but it is a precondition that integrins bind to the ECM and cluster in the plane of the cell membrane. Integrin expression has been studied[21-34], but studies on the expression integrin α5 and β1 subunits and FAK in gastric cancer and uterocervical cancer are rare. Furthermore, the correlation between integrin and FAK expression has not been studied, and the difference of integrin or FAK expression among these five kinds of cancer has not been reported either.
In the present study, the authors used an immunohistochem-ical technique to examine the expression of integrin α5 and β1 subunits and FAK in gastric cancer, colorectal cancer, hepatocellular carcinomas, uterocervical cancer, and breast cancer. In addition, the authors correlated the expression of FAK and integrin α5, and β1 subunits with clinicopathologic data, including tumor differentiation, infiltration and metastasis.
MATERIALS AND METHODS
Specimens
Tissue samples were obtained from 75 cases of gastric carcinoma (40 males and 35 females), 21 colorectal cancer (11 males and 10 females), 16 hepatocellular carcinomas (10 males and 6 females), 20 uterocervical cancer, and 20 breast cancer that had undergone total or partial resection in Tumor Hospital, Shanghai Medical University from Jan. 1995 to Jan.1996. Patient age ranged from 20 to 85 years old. Of the specimens, 51 cases of gastric cancer, 16 colorectal cancer, 16 hepatocellular carcinomas, 18 uterocervical cancer and 17 breast cancer were with unaffected margins. The specimens were fixed in 4% methanal solution, embedded in wax, and cut in 5 μm serial sections.
Reagents
Antibodies used in this study were as follows: rabbit anti-mouse FAK polyclonal antibody, biotinylated sheep anti-rabbit IgG, and biotinylated sheep anti-mouse IgG from Santa Cruz Biotechnology, Inc. (California, USA.). Monoclonal mouse antibodies directed against human integrin α5 subunit and human integrin β1 subunit were provided by Dr. Mingzhe Zheng at University of Washington, Seattle, USA.
Immunohistochemistry
Immunohistochemical staining was performed using the avidin-biotin-peroxidase technique[35]. Anti-FAK antibody was diluted into 1:100, anti-α 5 1:15, and anti-β1 1:80. The avidin-biotin-peroxidase reagent kit was purchased from Vector Labratories Inc.
A semiquantitative evaluation system was used to determine antigen expression in tissue samples[36]. Expression was graded on the following scale: negative reaction, (-); mild positive, (+); moderate positive, (++); strong positive, (+++). Evaluation of cell-surface and cytoplasmic staining was performed independently by two of the authors. In the occasional instance of disagreement, the slide was reviewed by the third observer, and a consensus opinion was obtained. The protein expression was correlated with tumor grades, which was in accordance with WHO classification.
Statistical analysis
Was performed by using the χ² test. A P value of < 0.05 was considered significant.
RESULTS
Expression of FAK and integrin subunits α5 and β1 in human gastric carcinomas
The expression patterns of the integrin subunits α5 and β1 and FAK in the 75 cases of gastric carcinomas and 51 unaffected margins are summarized in Table 1. Of the 51 unaffected margin specimens, only 2 (4%) showed moderate (++) FAK, others showed either negative or minimal FAK, but 39 (76%) cases showed moderate (++) or strong (+++) integrin α5 immunoreactivity (Figure 1A), and 43 (85%) showed moderate (++) or strong (+++) integrin β1 immunoreactivity. Of the 75 cancer specimens, 43 (57%) samples showed moderate (++) or strong (+++) FAK immunoreactivity (Figure 2A), 22 (30%) samples had moderate integrin α5 immunoreactivity, and 19 (25%) showed moderate integrin immunoreactivity. None of 75 cancer specimens showed strong immunoreactivity (+++) and neither integrin α5 nor β1 subunit. In contrast with 51 unaffected margin specimens, 35 (69%) carcinoma specimens had reduced α5 immunoreactivity, 43 (85%) had reduced β1 immunoreactivity, and 46 (90%) had increased FAK immunoreactivity. So a significant difference was found between gastric carcinomas and their unaffected margins in the expression of FAK, integrin α5, and β1 subunit.
Table 1.
Expression of FAK and integrin subunits α5 and β1 in human gastric carcinomas and their relation to clinical and histological variables
|
FAK |
Integrin α5 |
Integrin β1 |
||||
| -/+ | ++/+++ | -/+ | ++/+++ | -/+ | ++/+++ | |
| Type of tissues | ||||||
| Unaffected margin specimen | 49 | 2 | 12 | 39 | 8 | 43 |
| Gastric carcinoma | 32 | 43 | 53 | 22 | 56 | 19 |
| P < 0.01 | P < 0.01 | P < 0.01 | ||||
| Differentiation | ||||||
| Poor | 14 | 30 | 36 | 8 | 37 | 7 |
| Moderate or well | 18 | 13 | 17 | 14 | 19 | 12 |
| P < 0.05 | P < 0.05 | P < 0.01 | ||||
| Extent of invasion | ||||||
| Mucosa or superficial stratum | 13 | 3 | 11 | 5 | 12 | 4 |
| Deep or full stratum | 19 | 40 | 42 | 17 | 44 | 15 |
| P < 0.01 | NS | NS | ||||
| Metastasis | ||||||
| Absent | 23 | 13 | 26 | 10 | 29 | 7 |
| Present | 19 | 30 | 27 | 12 | 27 | 12 |
| P < 0.01 | NS | NS | ||||
NS: No significant
Figure 1.

Integrin α5 subunit expressed in carcinoma and unaffected margin tissues, ABC-DAB, × 400. A. Positive expression of integrin α5 subunit in unaffected margin tissues of gastric carcinoma; B. Positive expression of integrin α5 subunit in unaffected margin tissues of colorectal carcinoma; C. Positive expression of integrin α5 subunit in unaffected margin tissues of hepatocellular carcinoma.
Figure 2.
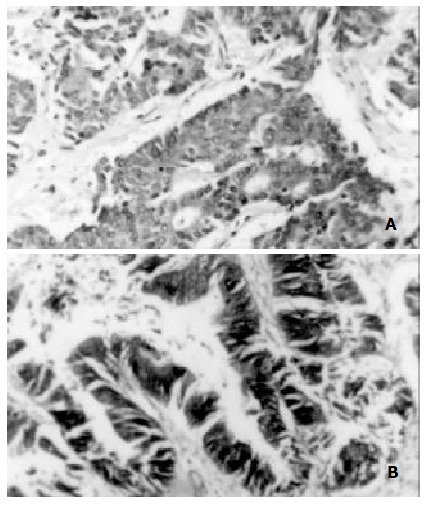
FAK expressed in carcinoma tissues, ABC-DAB, × 400. A. Positive expression of FAK in gastric carcinoma; B. Positive expression of FAK in colorectal carcinoma.
When FAK expression was compared with histopatholog-ical and clinical parameters, the authors found that 30 (68%) of 44 cases of poorly-differentiated cancer showed moderate or strong immunoreactivity, however, only 13 (42%) of 31 well-differentiated cancer showed moderate or strong; Of the 59 deep or full stratum invasive cancer, 40 (68%) showed moderate or strong, and only 3 (23%) of 16 superficial or mucous invasive cancer showed moderate or strong; 30 (77%) of 39 cases with lymph node metastasis showed moderate or strong, but 13 (36%) of 36 cases without lymph node metastasis showed moderate or strong activity. So, a significant association was found between enhanced expression of FAK and poor differentiation, deep invasion, and lymph node metastasis of gastric carcinomas.
In addition, of the 44 cases of poorly-differentiated cancer, 36 (81.8%) showed negative or minimal α5 and 37 (84%) showed negative or minimal β1, of the 31 moderate or well differentiated cancers, 17 (55%) showed negative or minimal α5 and 19 (61%) showed negative or minimal β1. Reduced expression of subunit α5 and β1 was significantly associated with poorly-differentiated carcinomas, but not with invasion and lymph node metastasis.
Finally, the authors found that 30 of the 43 gastric carcinomas with high FAK expression (++ or +++) had over expression of integrin subunit α5 and β1 in the tumor tissues and unaffected margin of the same specimens.
Expression of FAK and integrin subunits α5 and β1 in human colorectal carcinomas
The expression patterns of the integrin subunits α5 and β1 and FAK in the 21 colorectal carcinomas and 16 unaffected margins are summarized in Table 2.
Table 2.
Expression of FAK and integrin subunits α5 and β1 in human colorectal carcinomas and their relation to clinical and histological variables
|
FAK |
Integrin α5 |
Integrin β1 |
||||
| -/+ | ++/+++ | -/+ | ++/+++ | -/+ | ++/+++ | |
| Type of tissues | ||||||
| Unaffected margin specimen | 49 | 2 | 12 | 39 | 8 | 43 |
| Gastric carcinoma | 32 | 43 | 53 | 22 | 56 | 19 |
| P < 0.01 | P < 0.01 | P < 0.01 | ||||
| Differentiation | ||||||
| Poor | 14 | 30 | 36 | 8 | 37 | 7 |
| Moderate or well | 18 | 13 | 17 | 14 | 19 | 12 |
| P < 0.05 | P < 0.05 | P < 0.01 | ||||
| Extent of invasion | ||||||
| Mucosa or superficial stratum | 13 | 3 | 11 | 5 | 12 | 4 |
| Deep or full stratum | 19 | 40 | 42 | 17 | 44 | 15 |
| P < 0.01 | NS | NS | ||||
| Metastasis | ||||||
| Absent | 23 | 13 | 26 | 10 | 29 | 7 |
| Present | 19 | 30 | 27 | 12 | 27 | 12 |
| P < 0.01 | NS | NS | ||||
NS: No significant
In contrast with 16 unaffected margin specimens, 10 (62%) carcinoma specimens had reduced α5 immunoreactivity (Figure 1B), 6 (37%) had reduced β1 immunoreactivity (Figure 3A, Figure 3B), and 13 (81%) had increased FAK immunoreactivity (Figure 2B). So a significant difference was found between colorectal carcinomas and their unaffected margins in the expression of FAK, integrin α5, and β1 subunit.
Figure 3.

Integrin β1 subunit expressed in carcinoma and unaffected margin tissues. A. Positive expression of integrin β1 subunit in unaffected margin tissues of colorectal carcinoma, ABC-DAB, × 100; B. Negative expression of Integrin β1 subunit in colorectal carcinoma specimen, ABC-DAB, × 200; C. Positive expression of integrin β1 subunit in unaffected margin tissues of hepatocellular carcinoma, ABC-DAB, × 400.
When FAK expression was compared with histopatholog-ical and clinical parameters, A significant association was also found between over expression of FAK and poor differentiation, deep invasion, and present lymph node metastasis of colorectal carcinomas.
In addition, low expression of subunit α5 and β1 was significantly associated with poorly-differentiated colorectal carcinomas, but not with invasion and metastasis.
Expression of FAK and integrin subunits α5 and β1 in human hepatocellular carcinomas
The expression patterns of the integrin subunits α5 and β1 and FAK in the 16 hepatocellular carcinomas and their unaffected margins are summarized in Table 3.
Table 3.
Expression of FAK and integrin subunits α5 and β1 in human hepatocellular carcinomas and their relation to clinical and histological variables
|
FAK |
Integrin α5 |
Integrin β1 |
||||
| -/+ | ++/+++ | -/+ | ++/+++ | -/+ | ++/+++ | |
| Type of tissues | ||||||
| Unaffected margin specimen | 15 | 1 | 3 | 13 | 1 | 15 |
| Hepatocellular carcinoma | 4 | 12 | 11 | 5 | 10 | 6 |
| P < 0.01 | P < 0.01 | P < 0.01 | ||||
| Differentiation | ||||||
| Poor | 1 | 7 | 8 | 0 | 7 | 1 |
| Moderate or well | 2 | 5 | 3 | 4 | 4 | 5 |
| P < 0.05 | P < 0.05 | P < 0.01 | ||||
In contrast with 16 unaffected margin specimens, 11 (69%) carcinoma specimens had reduced α5 immunoreactivity, 7 (44%) had reduced β1 immunoreactivity, and 11 (69%) had increased FAK immunoreactivity. So a significant difference was found between hepatocellular carcinomas and their unaffected margins in the expression of FAK, integrin α5 and β1 subunit.
When these protein expression was compared with the degree of differentiation the authors found a significant association between these protein expression of FAK and poor differentiation in hepatocellular carcinomas.
Expression of FAK and integrin subunits α5 and β1 in human uterocervical carcinomas
The expression patterns of the integrin subunits α5 and β1 and FAK in the 20 uterocervical carcinomas and 18 unaffected margins are summarized in Table 4. Comparing with 18 unaffected margin specimens, 13 (72%) carcinoma specimens had reduced α5 immunoreactivity, 14 (78%) had reduced β1 immunoreactivity, and 3 (17%) had increased FAK immunoreactivity. So the positive percentage of integrin α5 or β1 had a significant difference between carcinomas and their unaffected margins. That of FAK was not apparent.
Table 4.
Expression of FAK and integrin subunits α5 and β1 in human uterocervical carcinomas and their relation to clinical and histological variables
|
FAK |
Integrin α5 |
Integrin β1 |
||||
| -/+ | ++/+++ | -/+ | ++/+++ | -/+ | ++/+++ | |
| Type of tissues | ||||||
| Unaffected margin specimen | 18 | 0 | 13 | 5 | 9 | 9 |
| adenocarcinoma | 9 | 1 | 9 | 9 | 1 | 9 |
| Squamous carcinoma | 10 | 0 | 10 | 0 | 9 | 1 |
| P < 0.05 | P < 0.05 | P < 0.05 | ||||
| Differentiation | ||||||
| Poor | 9 | 1 | 10 | 0 | 9 | 1 |
| Moderate or well | 10 | 0 | 9 | 1 | 9 | 1 |
| P < 0.05 | P < 0.05 | P < 0.05 | ||||
| Extent of invasion | ||||||
| Mucosa or superficial stratum | 5 | 0 | 8 | 0 | 4 | 1 |
| Deep or full stratum | 14 | 1 | 14 | 1 | 14 | 1 |
| P < 0.05 | NS | NS | ||||
NS: No significant
A significant association was not found between increased expression of FAK and poor differentiation or deep invasion of uterocervical carcinomas. Reduced expression of subunit α5 and β1 was significantly associated with poor differentiated carcinomas, but not with invasion.
Expression of FAK and integrin subunits α5 and β1 in human breast carcinomas
The expression patterns of the integrin subunits α5 and β1 and FAK in the 20 breast carcinomas and 17 unaffected margins are summarized in Table 4. Similarly, the increased FAK expression was significantly associated with breast carcinogenesis and lymph node metastasis of breast carcinomas. Reduced expression of subunit α5 and β1 was significantly associated with breast carcinogenesis and poorly-differentiated carcinomas, but not with lymph node metastasis. In contrast with 17 unaffected margin specimens, 14 (82%) carcinoma specimens had reduced α5 immunoreactivity, 16 (94%) had reduced β1 immunoreactivity, and 14 (82%) had increased FAK immunoreactivity.
DISCUSSION
This study indicated that the expression of the FAK antigen was lower in unaffected noncancerous margin tissues than in carcinoma tissues, whereas expression of integrin α5 and β1 subunits was higher in unaffected margin than in carcinoma tissues. The quantity of all three proteins related to the degree of differentiation of the cancer. The reasons for these findings are complex. First, the contents of fibronectin and laminin in the ECM is lower in normal tissues than in cancerous counterparts. However, the fibroblast is stimulated by cancer cells to produce more matrix proteins thereby increasing the concentration of matrix proteins[37]. Second, the increased expression of integrin α5 and β1 subunits in preliminary cancer tissue make the matrix proteins relatively much more deficient. Under these conditions, FAK can exist in a state with high tyrosine-phosphorylated on the Tyr-397/Src SH2 binding site[30-42]. However, the Src-family PTKs are not significantly associated with FAK that has only low levels of kinase activity and its expression is reduced[13,20,43-45]. In unaffected margin tissues, the negative regulators of integrin-stimulated signaling events include the FAK-associated PTP, PTEN and the p130cas that all reduced the kinase activity of FAK[46-48]. The quantity of matrix proteins is increased in the ECM of cancerous tissue. Integrins bind to the ECM, and become clustered in the plane of the cell membrane where they increase FAK tyrosine phosphorylation and kinase activity. Src-PTKs can significantly associate with FAK. Since FAK kinase activity is important for the FAK-enhanced increase in Src-PTK activity[49] and Src-PTKs can also phosphorylate FAK within the kinase activation loop (Tyr-576 and Tyr-577) to promote maximal FAK kinase activity, the transient complex formed between FAK and Src after integrin stimulation of cells may lead to the maximal activation of both PTKs[38,50]. The survival signals are continually magnified, and cells with overexpression of FAK can inhibit the effects of PTEN on PI3-K activity and partially inhibit its effects on PIP3 levels, Akt phosphorylation and cell apoptosis[51]. Cells proliferate extensively, Therefore the authors believe overexpression of FAK is required for carcinogenesis. The reduced expression of integrin α5, β1 subunit in carcinomas does not decrease the FAK kinase activity. Ca2+, PKC and Phorbol 12-myristate 13-acetate (PMA). can also increase FAK kinase activity[20].
This study indicates that the relationship between integrin α5, β1 subunits and metastasis is not significant in cancer cells, whereas FAK expression is significantly associated with metastasis or invasiveness. The process of cancer invasion or metastasis is complex, and involves attachment of the cancer cell to the ECM, decomposition of the ECM by proteases, and migration of cancer cells. The integrin subunits that aid cell adhesion are reduced in cancerous tissue, but integrin subunits that relate to cell migration are increased or constant[52]. On the basis of these findings, the expression of a single integrin subunit may not correlate with metastasis, but FAK is different because it can associate with integrin β2, β3 and some a subunits[20,53]. Several other cellular stimuli that generate signals through either G-protein linked receptors, transmembrane growth factor receptors or other unknown mechanisms can increase the level of FAK tyrosine phosphorylation in cells[20]. Thus, FAK may play a central role in signaling events stimulated by integrin or other molecules.
This study also indicates that the quantity change of integrin α5, β1 and their related signal molecule FAK is relate to the type of cancer. For integrin α5, breast carcinoma (82%) had the most reduced expression, then uterocervical carcinoma (72%), gastric carcinoma (69%), hepatocellular carcinoma (69%), colorectal carcinoma (62%) successively. For integrin α1, breast carcinoma (94%) also had the most reduced expression, then gastric carcinoma (85%), uterocervical carcinoma (78%), hepatocellular carcinoma (44%), colorectal carcinoma (37%) successively. For FAK, gastric carcinoma (90%) had the most increased expression, then colorectal carcinoma (80%), breast carcinoma (80%), hepatocellular carcinoma (69%), uterocervical carcinoma (37%) successively. From the above data, the authors find that the changes of integrin β1 and FAK is much more related with the type of cancer.
In summary, the authors have shown in the present study that expression of FAK is more significantly associated with carcinogenesis, differentiation and metastasis than that of integrin α5 and β1 subunits. FAK may be not only a transformation-linked enzyme but also a progression-linked enzyme. The level of FAK expression might be a valuable marker for the carcinogenesis and progression of some types of carcinoma. The expression of integrin α5, β1 and FAK is relate to the type of cancer.
ACKNOWLEDGMENTS
We are grateful to Dr. Ming-Zhe Zheng, University of Washington, Washington, USA, for providing the integrin antibody and to the Pathology Department of the Cancer Hospital of Shanghai Medical University for providing surgical specimens. We also thank Mrs. Xiu-Fong Zhang and Mrs. Yue-Zheng Dai for their instruction in preparing this manuscript.
Footnotes
Edited by Zhang JZ
Supported by National Natural Science Foundation of China, No.39970373 and the grant from the Science Committee of Shanghai, No.00JC14042
References
- 1.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 3.Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruoslahti E. Integrins as signaling molecules and targets for tumor therapy. Kidney Int. 1997;51:1413–1417. doi: 10.1038/ki.1997.193. [DOI] [PubMed] [Google Scholar]
- 5.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 6.Blau HM, Baltimore D. Differentiation requires continuous regulation. J Cell Biol. 1991;112:781–783. doi: 10.1083/jcb.112.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaller MD, Sasaki T. Differential signaling by the focal adhesion kinase and cell adhesion kinase beta. J Biol Chem. 1997;272:25319–25325. doi: 10.1074/jbc.272.40.25319. [DOI] [PubMed] [Google Scholar]
- 10.Guan JL. Role of focal adhesion kinase in integrin signaling. Int J Biochem Cell Biol. 1997;29:1085–1096. doi: 10.1016/s1357-2725(97)00051-4. [DOI] [PubMed] [Google Scholar]
- 11.Hanks SK, Polte TR. Signaling through focal adhesion kinase. Bioessays. 1997;19:137–145. doi: 10.1002/bies.950190208. [DOI] [PubMed] [Google Scholar]
- 12.Parsons JT, Parsons SJ. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol. 1997;9:187–192. doi: 10.1016/s0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- 13.Schlaepfer DD, Hunter T. Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- 14.Bellis SL, Miller JT, Turner CE. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J Biol Chem. 1995;270:17437–17441. doi: 10.1074/jbc.270.29.17437. [DOI] [PubMed] [Google Scholar]
- 15.Schaller MD, Parsons JT. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tachibana K, Urano T, Fujita H, Ohashi Y, Kamiguchi K, Iwata S, Hirai H, Morimoto C. Tyrosine phosphorylation of Crk-associated substrates by focal adhesion kinase. A putative mechanism for the integrin-mediated tyrosine phosphorylation of Crk-associated substrates. J Biol Chem. 1997;272:29083–29090. doi: 10.1074/jbc.272.46.29083. [DOI] [PubMed] [Google Scholar]
- 17.Vuori K, Hirai H, Aizawa S, Ruoslahti E. Introduction of p130cas signaling complex formation upon integrin-mediated cell adhesion: a role for Src family kinases. Mol Cell Biol. 1996;16:2606–2613. doi: 10.1128/mcb.16.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamasaki K, Mimura T, Morino N, Furuya H, Nakamoto T, Aizawa S, Morimoto C, Yazaki Y, Hirai H, Nojima Y. Src kinase plays an essential role in integrin-mediated tyrosine phosphorylation of Crk-associated substrate p130Cas. Biochem Biophys Res Commun. 1996;222:338–343. doi: 10.1006/bbrc.1996.0745. [DOI] [PubMed] [Google Scholar]
- 19.Sakai R, Nakamoto T, Ozawa K, Aizawa S, Hirai H. Characterization of the kinase activity essential for tyrosine phosphorylation of p130Cas in fibroblasts. Oncogene. 1997;14:1419–1426. doi: 10.1038/sj.onc.1200954. [DOI] [PubMed] [Google Scholar]
- 20.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen M, Castello R, Wayner E, Schwarting R. Distribution of VLA integrins in solid tumors. Emergence of tumor-type-related expression. Patterns in carcinomas and sarcomas. Am J Pathol. 1993;142:1009–1018. [PMC free article] [PubMed] [Google Scholar]
- 22.Stamp GW, Pignatelli M. Distribution of beta 1, alpha 1, alpha 2 and alpha 3 integrin chains in basal cell carcinomas. J Pathol. 1991;163:307–313. doi: 10.1002/path.1711630407. [DOI] [PubMed] [Google Scholar]
- 23.Pignatelli M, Hanby AM, Stamp GW. Low expression of beta 1, alpha 2 and alpha 3 subunits of VLA integrins in malignant mammary tumours. J Pathol. 1991;165:25–32. doi: 10.1002/path.1711650106. [DOI] [PubMed] [Google Scholar]
- 24.Pignatelli M, Smith ME, Bodmer WF. Low expression of collagen receptors in moderate and poorly differentiated colorectal adenocarcinomas. Br J Cancer. 1990;61:636–638. doi: 10.1038/bjc.1990.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koretz K, Schlag P, Boumsell L, Möller P. Expression of VLA-alpha 2, VLA-alpha 6, and VLA-beta 1 chains in normal mucosa and adenomas of the colon, and in colon carcinomas and their liver metastases. Am J Pathol. 1991;138:741–750. [PMC free article] [PubMed] [Google Scholar]
- 26.Liebert M, Washington R, Stein J, Wedemeyer G, Grossman HB. Expression of the VLA beta 1 integrin family in bladder cancer. Am J Pathol. 1994;144:1016–1022. [PMC free article] [PubMed] [Google Scholar]
- 27.Maragou P, Bazopoulou-Kyrkanidou E, Panotopoulou E, Kakarantza-Angelopoulou E, Sklavounou-Andrikopoulou A, Kotaridis S. Alteration of integrin expression in oral squamous cell carcinomas. Oral Dis. 1999;5:20–26. doi: 10.1111/j.1601-0825.1999.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 28.Shinohara M, Nakamura S, Sasaki M, Kurahara S, Ikebe T, Harada T, Shirasuna K. Expression of integrins in squamous cell carcinoma of the oral cavity. Correlations with tumor invasion and metastasis. Am J Clin Pathol. 1999;111:75–88. doi: 10.1093/ajcp/111.1.75. [DOI] [PubMed] [Google Scholar]
- 29.Häkkinen L, Kainulainen T, Salo T, Grenman R, Larjava H. Expression of integrin alpha9 subunit and tenascin in oral leukoplakia, lichen planus, and squamous cell carcinoma. Oral Dis. 1999;5:210–217. doi: 10.1111/j.1601-0825.1999.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez MA, Pinder SE, Wencyk PM, Bell JA, Elston CW, Nicholson RI, Robertson JF, Blamey RW, Ellis IO. An immunohistochemical examination of the expression of E-cadherin, alpha- and beta/gamma-catenins, and alpha2- and beta1-integrins in invasive breast cancer. J Pathol. 1999;187:523–529. doi: 10.1002/(SICI)1096-9896(199904)187:5<523::AID-PATH296>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Nejjari M, Hafdi Z, Dumortier J, Bringuier AF, Feldmann G, Scoazec JY. alpha6beta1 integrin expression in hepatocarcinoma cells: regulation and role in cell adhesion and migration. Int J Cancer. 1999;83:518–525. doi: 10.1002/(sici)1097-0215(19991112)83:4<518::aid-ijc14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Sordat I, Bosman FT, Dorta G, Rousselle P, Aberdam D, Blum AL, Sordat B. Differential expression of laminin-5 subunits and integrin receptors in human colorectal neoplasia. J Pathol. 1998;185:44–52. doi: 10.1002/(SICI)1096-9896(199805)185:1<44::AID-PATH46>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 33.Ensinger C, Obrist P, Bacher-Stier C, Mikuz G, Moncayo R, Riccabona G. beta 1-Integrin expression in papillary thyroid carcinoma. Anticancer Res. 1998;18:33–40. [PubMed] [Google Scholar]
- 34.Tagliabue E, Ghirelli C, Squicciarini P, Aiello P, Colnaghi MI, Ménard S. Prognostic value of alpha 6 beta 4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin Cancer Res. 1998;4:407–410. [PubMed] [Google Scholar]
- 35.Cui J, Zhou XD, Liu YK, Tang ZY, Zile MH. Abnormal beta-catenin gene expression with invasiveness of primary hepatocellular carcinoma in China. World J Gastroenterol. 2001;7:542–546. doi: 10.3748/wjg.v7.i4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su JM, Zhou YP, Zha XL. E-Cadherin expression in four kinds of carcinomas and their relations to differentiation and metastasis. Chin J Cancer Res. 2001;13:1–5. [Google Scholar]
- 37.Saiki I, Makabe T, Yoneda J, Murata J, Ishizaki Y, Kimizuka F, Kato I, Azuma I. Inhibitory effect of fibronectin and its recombinant polypeptides on the adhesion of metastatic melanoma cells to laminin. Jpn J Cancer Res. 1991;82:1112–1119. doi: 10.1111/j.1349-7006.1991.tb01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calalb MB, Zhang X, Polte TR, Hanks SK. Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem Biophys Res Commun. 1996;228:662–668. doi: 10.1006/bbrc.1996.1714. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 41.Schlaepfer DD, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardson A, Shannon JD, Adams RB, Schaller MD, Parsons J. Identification of integrin-stimulated sites of serine phosphorylation in FRNK, the separately expressed C-terminal domain of focal adhesion kinase: a potential role for protein kinase A. Biochem J. 1997;324(Pt 1):141–149. doi: 10.1042/bj3240141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan PY, Kanner SB, Whitney G, Aruffo A. A transmembrane-anchored chimeric focal adhesion kinase is constitutively activated and phosphorylated at tyrosine residues identical to pp125FAK. J Biol Chem. 1994;269:20567–20574. [PubMed] [Google Scholar]
- 44.Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- 45.Lin TH, Aplin AE, Shen Y, Chen Q, Schaller M, Romer L, Aukhil I, Juliano RL. Integrin-mediated activation of MAP kinase is independent of FAK: evidence for dual integrin signaling pathways in fibroblasts. J Cell Biol. 1997;136:1385–1395. doi: 10.1083/jcb.136.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 47.Garton AJ, Burnham MR, Bouton AH, Tonks NK. Association of PTP-PEST with the SH3 domain of p130cas; a novel mechanism of protein tyrosine phosphatase substrate recognition. Oncogene. 1997;15:877–885. doi: 10.1038/sj.onc.1201279. [DOI] [PubMed] [Google Scholar]
- 48.Shen Y, Schneider G, Cloutier JF, Veillette A, Schaller MD. Direct association of protein-tyrosine phosphatase PTP-PEST with paxillin. J Biol Chem. 1998;273:6474–6481. doi: 10.1074/jbc.273.11.6474. [DOI] [PubMed] [Google Scholar]
- 49.Schlaepfer DD, Hunter T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- 50.Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 51.Tamura M, Gu J, Danen EH, Takino T, Miyamoto S, Yamada KM. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1999;274:20693–20703. doi: 10.1074/jbc.274.29.20693. [DOI] [PubMed] [Google Scholar]
- 52.Zha XL. Structure and Function of Biomacromolecules. In: (Chen HL) editors Cell adhesion molecule., editor. Shanghai: Shanghai Med University; 1999. pp. 123–142. [Google Scholar]
- 53.Leong L, Hughes PE, Schwartz MA, Ginsberg MH, Shattil SJ. Integrin signaling: roles for the cytoplasmic tails of alpha IIb beta 3 in the tyrosine phosphorylation of pp125FAK. J Cell Sci. 1995;108(Pt 12):3817–3825. doi: 10.1242/jcs.108.12.3817. [DOI] [PubMed] [Google Scholar]


