Abstract
AIM: To study the feasibility of panning and screening phage-displaying recombinant single-chain variable fragment (ScFv) of anti-tumor monoclonal antibodies for fixed whole cells as the carriers of mAb-binding antigens.
METHODS: The recombinant phage displaying libraries for anti-colorectal tumor mAb MC3Ab, MC5Ab and anti-gastric tumor mAb MGD1 was constructed. Panning and screening were carried out by means of modified fixation of colorectal and gastric tumor cells expressed the mAb-binding antigens. Concordance of binding specificity to tumor cells between phage clones and parent antibodies was analyzed. The phage of positive clones was identified with competitive ELISA, and infected by E. coli HB2151 to express soluble ScFv.
RESULTS: The ratio of positive clones to MC3-ScF-MC5-ScFv and MGD1-ScFv were 60%, 24% and 30%. MC3-ScFv had Mr 32000 confirmed by Western blot. The specificity to antigen had no difference between 4 positive recombinant phage antibodies and MC3Ab.
CONCLUSION: The modified process of fixing whole tumor cells is efficient, convenient and feasible to pan and screen the phage-displaying ScFv of anti-tumor monoclonal antibodies.
INTRODUCTION
Tumor specific or associated antibodies in vivo diagnosis and treatment in tumor patients have been sought recently[1-4]. Most tumor-specific or tumor-associated antibodies have been obtained by the approach to immunizing animals with tumor cells, which inevitably cause allergic reaction against animal antibodies[5,6]. To miniaturize animal antibodies is an efficient way to decrease the rejection and allergy reaction. Gene engineering methods, especially phage display (PD), have great advantages[7-9]. It is the best way that purified tumor antigens (TA) were coated to capture recombinant antibodies in phage antibody library[10-12]. Unfortunately, many TA corresponding to tumor specific antibodies have not yet been isolated and purified, even not yet identified[13,14]. It hinders the production of miniaturizing tumor-specific antibodies specific to unisolated TA. It was speculated that the whole tumor cells which expressed TA might have been considered for replacement of TA. However, It was reported that the panning and screening of PD was non-specific by means of replacing TA with whole tumor cells[8]. This could be attributed to the much lower antigen density and much complicated antigens. Nevertheless, significant progress on the methods has been made, allowing the utilization of PD using whole tumor cells[15,16]. But this utilization is just limited to screen new unknown recombinant antibodies. In this study, we modified the fixing conditions of whole cells for panning and screening phage libraries constructed for the unique monoclonal antibodies such as anti-colon cancer MC3, MC5mAb and anti-gastric cancer MGD1 mAb[13,17-21], and cell ELISA for screening ScFv clone. The results were satisfactory.
MATERIALS AND METHODS
Cell lines
Gastric tumor cell lines[2,10] KATO-III, AGS, MKN-45, GC803, SGC7901, colorectal tumor cell lines W480, HT-29, CoCa-2, and human fibroblast cells were grown in RPMI 1640 or DMEM supplemented with 100 mL·L-1 new born bovine serum (NBS). All cell lines were grown adherently except KATO-III.
Construction of phage ScFv libraries
mRNA was isolated from the corresponding antibodies hybridoma cells. VH and VL cDNA were amplified with RT-PCR and linked with ScFv by linker DNA to form ScFc DNA, which then were inserted into plasmid PCANBSE. Plasmid DNA was transformed into E. coli strain TG1. ScFv-phage was induced by superinfection with helper phage M13KO7.
Cells fixation
The fixed cells were used for libraries panning and as antigens of cell ELISA. Methods reported by Ridgway et al[8] were used with the following modifications. Fixation of suspending cells: the cells were washed with PBS, resuspended, and transferred to 96-well enzyme-labeled plates at (4-5) × 105 cells/well. The volume of cell suspension was no less than 300 μL each well. Otherwise, the cells would be distributed unevenly during centrifugation. The plates were centrifuged for 12 min at 1200 r·min-1, and the supernatants were discarded immediately without disturbing the pellets. The plates were allowed to dry at 37 °C for 15-20 min. Into each well, 2.5 g•L-1 glutaraldehyde prepared with 60 μL of 0.1 mol•L-1 PBS was added. Twelve min later, the fixative solution was discarded. The cells were washed 5 times by PBS. The plates were blocked with 100 g•L-1 skimmed milk powder overnight at 4 °C. Coating of suspending cells for library panning: the cells were plated into 6-well plates at (1-1.5) × 107 cells/well. The cell suspension volume was no less than 7 mL in each well. The rest procedures were as described above. Fixation of adherent cells: the cells were plated into 96-well plates at 0.2 × 104 cells/well. The cells were allowed to incubate 48-72 h. When the cells were 80% confluent, the medium was removed. The plates were washed twice with prewarmed PBS and dried at 37 °C for 20 min. The cells were fixed for 8 min as described above.
Detection of intracellular peroxidase
The fixed cells were divided into 2 groups. Cells in one group were treated with 3 mL•L-1 H2O2 prepared with methanol and washed 3 times with PBS. The plates were blocked with 50 g•L-1 skimmed milk powder overnight at 4 °C (or 37 °C for 2 h). Cells in another group were treated with blocking solution directly. After the blocking solution was removed, the plates were washed 3 times with PBS containing 0.5 g•L-1 Tween 20, and OPD substrate (50 μL/well) was added to develop color. Thirty min later, the color development was terminated with 2 mol•L-1 sulfuric acid. A490 were read. The well without substrate was designed as background control. Negative control and blank control were also designed.
Panning of phage libraries
The TG1 recombinant phage antibodies and soluble ScFv secreted by E. coli HB2151 were obtained according to the kit instructions (Pharmacia Biotech)[7]. Wash the 6 well plate coated with tumor cells three times with PBS, empty it completely after each wash. Fill the plate completely with blocking buffer to block any remaining sites on the plate surface. Incubate at room temperature for 1 h. Wash the flask three times with PBS, and empty it completely after each wash. Prepare 14 mL of blocking buffer containing 1 mL•L-1 thimerosal or 100 mL•L-1 sodium azide as a preservative. Dilute the 16 mL of PEG-precipitated recombinant phage with 14 mL of blocking buffer and incubate at room temperature for 10-15 min. Add 20 mL of the diluted recombinant phage to the plate and incubate for 2 h at 37 °C. Wash the plate 20 times with PBS and 20 times with PBS containing 10 mL•L-1 Tween 20. Empty the plate completely each time. To isolate colonies for small-scale rescue, reinfect E. coli TG1 cells with bound phage directly in the panning vessel and plate the reinfected cells. Subsequent rounds of panning are to be performed. Add the entire 10 mL of log-phase TG1 cells to the flask or panning vessel. Incubate with shaking at 37 °C for 1 h. Transfer the entire 10 mL from the panning vessel into a sterile 50 mL disposable polypropylene centrifuge tube. To the cell suspension, add ampicillin to 0.1 g•L-1, and glucose to a final concentration of 200 g•L-1. Also add 4 × 1010 pfu of M13KO7 volume of stock to add = 4 × 1013 pfu/M13KO7 pfu/L. Incubate the culture for 1 h at 37 °C with shaking. Sediment the cells by centrifugation and complete the rescue. The selection produre was repeated 4 times before isolated clones were tested by ELISA.
Cell ELISA
Detection of parent antibodies activity[22,23]: cellular endogenous peroxidase activity was rather low, so treatment of cells with H2O2 was unnecessary. The procedures were as follows. mAb were added to the blocked wells (50 μL/well). The plates stood for 1 h at 37 °C. The cells were washed 5 times by PBST. fiftyμL horseradish peroxidase (HRP) conjugated sheep anti-mice IgG was added to each blocked well. The plates stood for 1 h at 37 °C. Cells were washed 5 times with PBST. OPD substrate (50 μL/well) was added to develop color. Ten to twenty min later, A490 was read. Normal mice IgG and PBS served as negative and blank controls respectively.
Screening of recombinant phage antibodies or recombinant soluble ScFv clones: recombinant phage antibodies (M13KO7 as negative control) or soluble antibodies bearing anti-E-tag label protein displayed on phage served as the first antibody. Incubation condition was set as 2 h at room temperature, gently shaking at 120 r•min⁻¹ with cradle. Correspondently, rabbit anti-M13-HRP polyclonal antibody (1:5000, Pharmacia) or mouse anti-E-tag IgG (1:1000, Pharmacia) were chosen as the second antibody, for the latter sheep anti-mouse IgG HRP, should be used. The rest procedures were as described above.
Concordance of the specificity to tumor cells between the positive recombinant phage clones and parent mAb: the blocked cells were coated as described above (including tumor cells or normal cells expressing antigen highly, lowly and blankly). Pairs of ScFv and parent antibodies were added to all cell plates respectively. A values for each well were read by the same method. Correlation coefficient was obtained through correlation analysis[24-26].
Competitive ELISA
The aim was to screen positive clones with high affinity[27-29]. The cells were coated as described previously. ScFv-phage supernatants or recombinant soluble ScFv (50 μL/well) were added to plates after co-incubation with 1/4 volume of 200 g•L-1 skimmed milk powder for 15 min. The plates stood for 1 h at 37 °C. Twenty-five μL corresponding mAb was added to each well. After standing for 1 h at 37 °C, the plates were washed. Fifty μL HRP conjugated sheep anti-mouse IgG were added to each well. After standing for 1 h at 37 °C, the plates were washed 5 times with PBST. Fifty μL OPD substrate were added to each well to develop color. A490 were read 10-20 min later. M13KO7 served as negative control. Inhibition rate (%) = 1 - (A490 for experimental well/A490 for control well) × 100%.
Western blot
Statistical methods
The data were analyzed with SPSS software package.
RESULTS
Detection of parent antibody activity with cell ELISA
The least dilution for MC3mAb was 25 mg•L-1, for MC5mAb was 50 mg•L-1, and for MGD1mAb was 12.5 mg•L-1. A490 were all more than 0.600, indicating that the mAb used in the experiment were active.
Construction of the ScFv antibody libraries
The VH, VL and ScFv DNAs were about 340, 320 and 750 bp respectively (Figure 1, Figure 2).
Figure 1.
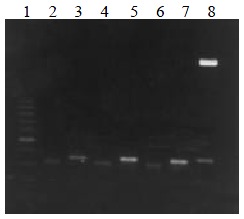
VH and VL DNA amplified by RT-PCR. Lane 1: 100 bp DNA ladder (100-1000 bp); lane 2, 4, 6: VL DNA; lane 3, 5, 7: VH DNA; lane 8: VH DNA marker (2.7 kb, 350 bp)
Figure 2.
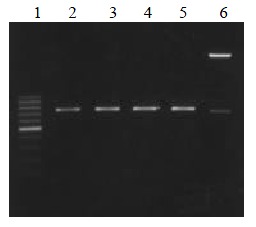
ScFv DNA amplified by RT-PCR. Lane 1: 100 bp DNA ladder (100-1000 bp); lane 2, 3, 4, 5: MC5, MC3, MGD1, MGD1 ScFv DNA; lane 6 ScFv DNA marker (2.7 kb, 750 bp)
Panning of phage libraries with coated cells
When suspending cells were plated, if the cell suspensions adding to each well were not enough (< 5 mL) or the cell number in each well was too many (> 2 × 107 cells/well), the cells would be distributed unevenly during centrifugation. In 4 outer wells, the cells were found to be distributed in a half-moon shape, which was inconvenient for next fixation and panning steps. To avoid the above problems, we added no less than 7 mL cell suspensions to each well with (1-1.5) × 107 cells/well, and speeded centrifugation to 1200 r•min⁻¹ gradually. The TG1 containing recombinant phage recovered after 4 rounds of panning were plated on SOB-AG (A: ampicillin, G: glucose). After 14 h, dense clones were observed.
Screening of recombinant phage antibodies or recombinant soluble clones with cell ELISA
Sixty clones were selected randomly from each panned library. The positive clones, whose A490 were over 0.300 (positive control), were counted. The ratio of positive clones were obtained. Soluble expression of E. coli HB2151 was carried out using the positive clones. Its positive rate was determined by cell ELISA and Western blot. The result is shown in Table 1.
Table 1.
ratio of positive recombinant phage antibody clone and soluble ScFv
|
Recombinant phage antibodies |
soluble ScFv |
|||
| Ratio of positive clone (%) | Ratio of competitive clone (%) | Ratio of positive clone (%) | Ratio of competitive clone (%) | |
| MC3 ScFv | 60 | 8 | 30 | 8 |
| MC5 ScFv | 24 | 6 | 18 | 4 |
| MGD1 ScFv | 30 | 4 | 10 | 4 |
Concordance of binding specificity of phage library and parent antibody to tumor cells
Taking MC3-ScFv clone19 as example, the binding specificity of ScFv and parent antibody MC3 to AGS, SW480, SGC7901 fibroblasts were analyzed as shown in Table 2. A490 of parent antibodies were set as Y axis, and that of ScFv as X axis. Correlation analysis revealed r = 0.991, P < 0.01, primarily indicating the concordance of binding specificity of MC3-ScFv clone 19 and parent antibody to tumor cells. Other clones of MC3-ScFv displayed similar characteristics to MC3-ScFv clone 19.
Table 2.
Concordance for MC3-ScFv-19 and it's parental mAb specificity to cells
| Cell lines | MC3-ScFv 19 | MC3 Ab |
| AGS | 1.403 ± 0.132 | 0.929 ± 0.187 |
| SW480 | 0.921 ± 0.201 | 0.647 ± 0.132 |
| SGC7901 | 0.257 ± 0.045 | 0.259 ± 0.078 |
| Fibroblast | 0.325 ± 0.106 | 0.297 ± 0.056 |
Screening of positive ScFv clones with competitive ELISA
Inhibition rate over 30% was put as criteria[8]. Positive clones from all libraries were obtained as shown in Table 1. As an example, inhibition rates of 4 positive recombinant phage MC3 antibodies were 56.2%, 53.6%, 49.7% and 46.7%. And inhibition rates of their soluble ScFvs were 41.5%, 36.9%, 33.7% and 21.6% respectively.
Western blot
Soluble products expressed by E. coli HB2151 were all about Mr 32000, consistent with the expected molecule weight of soluble ScFv. The result is shown in Figure 3.
Figure 3.
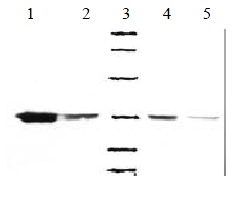
Western bloting of soluble MC3-ScFv clones. 1, 2, 4, 5: Four posotive clones of MC3 ScFv, 3: Low molecular weight marker (14.4, 20.1, 31.0, 43.0, 66.2, 97.4) KD
DISCUSSION
Phage display (PD) has many advantages in preparing minimized mouse and man antibodies. But the operation has met great difficulties due to severe conditions. The most difficult to satisfy is purified antigen, which is necessary for library panning and clone screening[32-35]. Recently, whole cells have been used in PD to obtain tumor-associated or tumor-specific antibodies. The results are encouraging. Kupsch et al[32] obtained human melanoma specific antibody by panning of a phage library using melanoma cells as negative screening, peripheral mononuclear cells as negative screening. Ridgway et al[8] cloned a human anti-CD55 ScFv by subtractive panning of a phage library using tumor and nontumor cells. In their studies, they panned the known libraries to obtain new tumor specific ScFv, so most possible integrity of tumor antigen should have been retained, so they used live cells in panning and cell ELISA. However, the procedures were complicated, and the specialized equipments were required. Particularly, only tumor-associated membrane antigens could be obtained, as for intracellular antigens, the method was powerless[36-38]. The defects could be avoided by using fixed cells. But fixation procedures could destroy antigens partially, resulting in loss of some information. In the present report, we have studied the amenability of fixed cells to phage panning in PD technique.
Since development of mAb technique in the 1980s, many tumor specific or associated mAb have been cloned. However, most tumor antigens have not been identified and purified, and most mouse mAb had great molecular weight, and heterogeneity, which limited the application[39-42]. So, it is necessary to minimize some specific mAb. We constructed recombinant phage displaying libraries for anti-colorectal tumor mAb MC3Ab, MC5Ab and anti-gastric tumor mAb MGD1. Panning was carried out using fixed coated colorectal and gastric tumor cell lines. The positive recombinant phage clones were screened by means of cell ELISA. The concordance of the specificity to tumor cells with parent mAb was analyzed. The affinity of positive clones with tumor antigens was detected with competitive ELISA. The molecules and product amounts were determined by Western blot. All results suggested that fixed cells could be used to panning tumor-associated mouse ScFv using PD.
However, only the following conditions were satisfactory, it successful manipulation was confirmed. Activity of antigen should not be influenced during cell fixation procedures[43-45]. If the structure of antigen determinant cluster were changed during the fixation procedures, the fixation procedures should be modified or the fixative should be changed. We got satisfactory results using coating cells and cell ELISA with the following modifications. Cell lines highly expressing target antigens were used. Adherently or half-adherently grown cells were used because they easily plated and distributed evenly after plating. Fixation with glutaraldehyde could be half shortened. Cell lines highly expressing endogenous peroxidase were excluded to avoid destruction of lipoprotein antigens caused by methanol and H2O2 blocking[46-48]. So previous detection of endogeneous peroxidase activity of cell lines is very important; Fixation condition with glutaraldehyde should be modified. Kupsch et al[32] recommended that the cells should be fixed with glutaraldehyde at concentration of 5 g•L-1 for 30 min in cell ELISA. But we found that the results were more satisfactory with glutaraldehyde at concentration of 2.5 g•L-1 for 12 min for suspending cells and for 9 min for adherent cells. Other precise conditions, such as centrifugation condition of suspending cells, drying prior to fixation, should also be paid great attention to[49,50]. Application limits: Firstly, fixed cells could be applied to minimization and gene engineering of known mouse mAb. The phage libraries were constructed by using hybridoma. Panning of phage libraries and screening of positive clones were feasible theoretically and practically by using cell lines which strongly expressed these mAb-binding antigens. Secondly, fixed cells might be also applied to panning of large human, mouse phage libraries to obtain unknown tumor specific antibodies. Although fixation procedures might destroy antigens partially so as to lose some information, application of live cells also had disadvantages, such as unfeasibility of screening of intracellular antigens. In present study, gastric tumor specific antigen corresponding to MDG1-ScFv did belong to intracellular antigens, which further indicating the amenability of the fixed cells.
ACKNOWLEDGMENTS
We would like to thank Prof. Bo-Rong Pan from Oncology Center, Xijing Hospital for his great contributions to this article.
Footnotes
Edited by Ma JY
Supported by the National “63” Strategy for Science (102-10-01-06) and National Natural Science Foundation of China, No.39525020
References
- 1.McCall AM, Shahied L, Amoroso AR, Horak EM, Simmons HH, Nielson U, Adams GP, Schier R, Marks JD, Weiner LM. Increasing the affinity for tumor antigen enhances bispecific antibody cytotoxicity. J Immunol. 2001;166:6112–6117. doi: 10.4049/jimmunol.166.10.6112. [DOI] [PubMed] [Google Scholar]
- 2.Zhang XY. Some recent works on diagnosis and treatment of gastric cancer. World J Gastroenterol. 1999;5:1–3. doi: 10.3748/wjg.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan DM, Xiao B, Shi YQ, Ming F, Qiao TD, Chen BJ, Chen Z. A novel cDNA fragment associated with gastric cancer drug resistance was screened out from a library by monoclonal antibody MGr-1. World J Gastroenterol. 1998;4(suppl 2):110–111. [Google Scholar]
- 4.Ji F, Wang WL, Yang ZL, Li YM, Huang HD, Chen WD. Study on the expression of matrix metallo proteinase-2 mRNA in human gastric cancer. World J Gastroenterol. 1999;5:455–457. doi: 10.3748/wjg.v5.i5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou C, Jiang M, Xu L, Zhen Y. [Construction and secretory expression of single-chain Fv fragment M97 with therapeutic potential against tumor invasion and metastasis] Zhongguo Yixue Kexueyuan Xuebao. 1998;20:81–88. [PubMed] [Google Scholar]
- 6.Liu HF, Liu WW, Fang DC, Men RP. Expression and significance of proapoptotic gene Bax in gastric carcinoma. World J Gastroenterol. 1999;5:15–17. doi: 10.3748/wjg.v5.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams GP, Schier R. Generating improved single-chain Fv molecules for tumor targeting. J Immunol Methods. 1999;231:249–260. doi: 10.1016/s0022-1759(99)00161-1. [DOI] [PubMed] [Google Scholar]
- 8.Ridgway JB, Ng E, Kern JA, Lee J, Brush J, Goddard A, Carter P. Identification of a human anti-CD55 single-chain Fv by subtractive panning of a phage library using tumor and nontumor cell lines. Cancer Res. 1999;59:2718–2723. [PubMed] [Google Scholar]
- 9.Rozemuller H, Chowdhury PS, Pastan I, Kreitman RJ. Isolation of new anti-CD30 scFvs from DNA-immunized mice by phage display and biologic activity of recombinant immunotoxins produced by fusion with truncated pseudomonas exotoxin. Int J Cancer. 2001;92:861–870. doi: 10.1002/ijc.1266. [DOI] [PubMed] [Google Scholar]
- 10.Roovers RC, van der Linden E, de Bruïne AP, Arends JW, Hoogenboom HR. In vitro characterisation of a monovalent and bivalent form of a fully human anti Ep-CAM phage antibody. Cancer Immunol Immunother. 2001;50:51–59. doi: 10.1007/s002620000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powers DB, Amersdorfer P, Poul M, Nielsen UB, Shalaby MR, Adams GP, Weiner LM, Marks JD. Expression of single-chain Fv-Fc fusions in Pichia pastoris. J Immunol Methods. 2001;251:123–135. doi: 10.1016/s0022-1759(00)00290-8. [DOI] [PubMed] [Google Scholar]
- 12.Yuan Y, Cong W, Xu RT, Wang XJ, Gao H. Gastric cancer screening in 16 villages of Zhuanghe region: a high risk area of stomach cancer in China. World J Gastroenterol. 1998;4(Suppl 2):111–112. [Google Scholar]
- 13.Shi YQ, Xiao B, Miao JY, Zhao YQ, You H, Fan DM. Construction of eukaryotic expression vector pBK-fas and MDR reversal test of drug-resistant gastric cancer cells. Shijie Huaren Xiaohua Zazhi. 1999;7:309–312. [Google Scholar]
- 14.Wei TY, Wei MX, Yang SM. Significance of expression of cyclin D1, P16 and preneoplastic lesion tissues. Shijie Huaren Xiaohua Zazhi. 2000;8:234–235. [Google Scholar]
- 15.Van Ewijk W, de Kruif J, Germeraad WT, Berendes P, Röpke C, Platenburg PP, Logtenberg T. Subtractive isolation of phage-displayed single-chain antibodies to thymic stromal cells by using intact thymic fragments. Proc Natl Acad Sci USA. 1997;94:3903–3908. doi: 10.1073/pnas.94.8.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai X, Garen A. Comparison of fusion phage libraries displaying VH or single-chain Fv antibody fragments derived from the antibody repertoire of a vaccinated melanoma patient as a source of melanoma-specific targeting molecules. Proc Natl Acad Sci USA. 1997;94:9261–9266. doi: 10.1073/pnas.94.17.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo JC, Li JC, Fan DM, Qiao TD, Zhang XY. Regulation of HSP70 expression in human gastric cancer cell line SGC7901 by gene transfection. Shijie Huaren Xiaohua Zazhi. 1999;7:773–776. [Google Scholar]
- 18.Liu HF, Liu WW, Fang DC, Liu FX, He GY. Clinical significance of Fas antigen expression in gastric carcinoma. World J Gastroenterol. 1999;5:90–91. doi: 10.3748/wjg.v5.i1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao CY, Lie J, Fan DM. Immunohistochemical study of monoclonal antibody MGD-1 in gastric carcinoma. Histopathology. 1989;15:523–529. doi: 10.1111/j.1365-2559.1989.tb01612.x. [DOI] [PubMed] [Google Scholar]
- 20.Xiao B, Shi YQ, Zhao YQ, You H, Wang ZY, Liu XL, Yin F, Qiao TD, Fan DM. Transduction of Fas gene or Bcl-2 antisense RNA sensitizes cultured drug resistant gastric cancer cells to chemotherapeutic drugs. World J Gastroenterol. 1998;4:421–425. doi: 10.3748/wjg.v4.i5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao GD, Wang SW, Wu SS, Li HF, Zhang WG. Retrovirus mediated antisense RNA to bcl-2 alter the biological behavior of stomach carcinoma MGC-803 cell lines. World J Gastroenterol. 1998;4(suppl 2):45–48. [Google Scholar]
- 22.Luo ZB, Luo YH, Lu R, Jim HY, Zhang PB, Xu CP. Immunohistochemical study on dendritic cells in gastric mucosa of patients with gastric cancer and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:400–402. [Google Scholar]
- 23.Xu SH, Feng JG. Relationship between CD44 in the peripheral blood of patients with colorectal cancer and clinico-pathological features. Shijie Huaren Xiaohua Zazhi. 2000;8:432–435. [Google Scholar]
- 24.Zhang J, Wang WL, Li Q, Qiao Q. Expression and significance of transforming growth factor-α and its receptor in human primary hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:939–941. [Google Scholar]
- 25.Du DW, Zhou YX, Feng ZH, Li GY, Yao ZQ. Study on immunization of anti subcutaneous transplanting tumor induced by gene vaccine. Shijie Huaren Xiaohua Zazhi. 1999;7:955–957. [Google Scholar]
- 26.Zheng CS, Wang WL, Reng WD, Hu PZ, Chai YB, Ma FC. Promotion of apoptosis of SMMC7721 cells by Bcl-2 ribozyme. Shijie Huaren Xiaohua Zazhi. 2000;8:417–419. [Google Scholar]
- 27.Wang CD, Chen YL, Wu T, Liu YR. Association between lowe expression of somatostatin receptor II gene and lymphoid metastasis in patients with gastric cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:864–866. [Google Scholar]
- 28.Han FC, Yan XJ, Hou Y, Xiao LY, Guo YH, Su CZ. Gold immunochromatographic assay for anti-Helicobacter pylori antibody. Shijie Huaren Xiaohua Zazhi. 1999;7:743–746. [Google Scholar]
- 29.Cui DX, Yan XJ, Zhang L, Zhao JR, Jiang M, Guo YH, Zhang LX, Bai XP, Su CZ. Screening and its clinical significance of 6 fragments of highly expressing genes in gastric cancer and precancerous mucosa. Shijie Huaren Xiaohua Zazhi. 1999;7:770–773. [Google Scholar]
- 30.Wang YF, Wu XN, Zhang XQ, Chen XF. Circulating soluble intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in patients with gastric cancer. Shijie Huaren Xiaohua Zazhi. 1998;6:1017–1019. [Google Scholar]
- 31.Sun YX, Chen CJ, Zhou HG, Shi YQ, Pan BR, Feng WY. Expression of c-myc and p53 in colorectal adenoma and adenocarcinoma. Shijie Huaren Xiaohua Zazhi. 1998;6:1054–1056. [Google Scholar]
- 32.Kupsch JM, Tidman NH, Kang NV, Truman H, Hamilton S, Patel N, Newton Bishop JA, Leigh IM, Crowe JS. Isolation of human tumor-specific antibodies by selection of an antibody phage library on melanoma cells. Clin Cancer Res. 1999;5:925–931. [PubMed] [Google Scholar]
- 33.Tordsson JM, Ohlsson LG, Abrahmsén LB, Karlström PJ, Lando PA, Brodin TN. Phage-selected primate antibodies fused to superantigens for immunotherapy of malignant melanoma. Cancer Immunol Immunother. 2000;48:691–702. doi: 10.1007/s002620050018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marty C, Scheidegger P, Ballmer-Hofer K, Klemenz R, Schwendener RA. Production of functionalized single-chain Fv antibody fragments binding to the ED-B domain of the B-isoform of fibronectin in Pichia pastoris. Protein Expr Purif. 2001;21:156–164. doi: 10.1006/prep.2000.1362. [DOI] [PubMed] [Google Scholar]
- 35.Sakai A. Inhibition of endothelial cell adhesion molecule expression with SJC13, an azaindolidine derivative, in vitro. Inflamm Res. 1996;45:224–229. doi: 10.1007/BF02259607. [DOI] [PubMed] [Google Scholar]
- 36.Goel A, Colcher D, Baranowska-Kortylewicz J, Augustine S, Booth BJ, Pavlinkova G, Batra SK. Genetically engineered tetravalent single-chain Fv of the pancarcinoma monoclonal antibody CC49: improved biodistribution and potential for therapeutic application. Cancer Res. 2000;60:6964–6971. [PubMed] [Google Scholar]
- 37.Kang N, Hamilton S, Odili J, Wilson G, Kupsch J. In vivo targeting of malignant melanoma by 125Iodine- and 99mTechnetium-labeled single-chain Fv fragments against high molecular weight melanoma-associated antigen. Clin Cancer Res. 2000;6:4921–4931. [PubMed] [Google Scholar]
- 38.Kuan CT, Wikstrand CJ, Archer G, Beers R, Pastan I, Zalutsky MR, Bigner DD. Increased binding affinity enhances targeting of glioma xenografts by EGFRvIII-specific scFv. Int J Cancer. 2000;88:962–969. doi: 10.1002/1097-0215(20001215)88:6<962::aid-ijc20>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 39.Tur MK, Huhn M, Sasse S, Engert A, Barth S. Selection of scFv phages on intact cells under low pH conditions leads to a significant loss of insert-free phages. Biotechniques. 2001;30:404–408, 410, 404-408. doi: 10.2144/01302rr04. [DOI] [PubMed] [Google Scholar]
- 40.Goel A, Augustine S, Baranowska-Kortylewicz J, Colcher D, Booth BJ, Pavlinkova G, Tempero M, Batra SK. Single-Dose versus fractionated radioimmunotherapy of human colon carcinoma xenografts using 131I-labeled multivalent CC49 single-chain fvs. Clin Cancer Res. 2001;7:175–184. [PubMed] [Google Scholar]
- 41.Khare PD, Shao-Xi L, Kuroki M, Hirose Y, Arakawa F, Nakamura K, Tomita Y, Kuroki M. Specifically targeted killing of carcinoembryonic antigen (CEA)-expressing cells by a retroviral vector displaying single-chain variable fragmented antibody to CEA and carrying the gene for inducible nitric oxide synthase. Cancer Res. 2001;61:370–375. [PubMed] [Google Scholar]
- 42.Böldicke T, Tesar M, Griesel C, Rohde M, Gröne HJ, Waltenberger J, Kollet O, Lapidot T, Yayon A, Weich H. Anti-VEGFR-2 scFvs for cell isolation. Single-chain antibodies recognizing the human vascular endothelial growth factor receptor-2 (VEGFR-2/flk-1) on the surface of primary endothelial cells and preselected CD34+ cells from cord blood. Stem Cells. 2001;19:24–36. doi: 10.1634/stemcells.19-1-24. [DOI] [PubMed] [Google Scholar]
- 43.Matsumura R, Umemiya K, Goto T, Nakazawa T, Ochiai K, Kagami M, Tomioka H, Tanabe E, Sugiyama T, Sueishi M. Interferon gamma and tumor necrosis factor alpha induce Fas expression and anti-Fas mediated apoptosis in a salivary ductal cell line. Clin Exp Rheumatol. 2000;18:311–318. [PubMed] [Google Scholar]
- 44.Yuan QA, Yu WY, Huang CF. [Construction and expression of a hepatocellular carcinoma specific rodent and its humanized single-chain Fv fragments in Escherichia coli] Shengwu Gongcheng Xuebao. 2000;16:86–90. [PubMed] [Google Scholar]
- 45.Ozaki H, Ishii K, Horiuchi H, Arai H, Kawamoto T, Okawa K, Iwamatsu A, Kita T. Cutting edge: combined treatment of TNF-alpha and IFN-gamma causes redistribution of junctional adhesion molecule in human endothelial cells. J Immunol. 1999;163:553–557. [PubMed] [Google Scholar]
- 46.Ohizumi I, Tsunoda S, Taniguchi K, Saito H, Esaki K, Koizumi K, Makimoto H, Wakai Y, Matsui J, Tsutsumi Y, et al. Identification of tumor vascular antigens by monoclonal antibodies prepared from rat-tumor-derived endothelial cells. Int J Cancer. 1998;77:561–566. doi: 10.1002/(sici)1097-0215(19980812)77:4<561::aid-ijc15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 47.Takami S, Yamashita S, Kihara S, Ishigami M, Takemura K, Kume N, Kita T, Matsuzawa Y. Lipoprotein (a) enhances the expression of intercellular adhesion molecule-1 in cultured human umbilical vein endothelial cells. Circulation. 1998;97:721–728. doi: 10.1161/01.cir.97.8.721. [DOI] [PubMed] [Google Scholar]
- 48.Strindhall J, Lundblad A, Påhlsson P. Interferon-gamma enhancement of E-selectin expression on endothelial cells is inhibited by monensin. Scand J Immunol. 1997;46:338–343. doi: 10.1046/j.1365-3083.1997.d01-135.x. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi M, Suwa H, Miyasaka M, Kumada K. Selective inhibition of vascular cell adhesion molecule-1 expression by verapamil in human vascular endothelial cells. Transplantation. 1997;63:759–764. doi: 10.1097/00007890-199703150-00024. [DOI] [PubMed] [Google Scholar]
- 50.Zünd G, Nelson DP, Neufeld EJ, Dzus AL, Bischoff J, Mayer JE, Colgan SP. Hypoxia enhances stimulus-dependent induction of E-selectin on aortic endothelial cells. Proc Natl Acad Sci USA. 1996;93:7075–7080. doi: 10.1073/pnas.93.14.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]


