Abstract
AIM: To study local therapeutic efficacy, side effects, and complications of radiofrequency ablation (RFA), which is emerging as a new method for the treatment of patients with hepatocellular carcinoma (HCC) with cirrhosis or chronic hepatitis and metastatic liver cancer.
METHODS: Thirty-six patients with primary and secondary liver cancers (21 with primary hepatocellular carcinoma, 12 with colorectal cancer liver metastases and 3 with other malignant liver metastases), which were considered not suitable for curative resection, were include in this study. They were treated either with RFA (RITA2000, Mountain View, California, USA) percutaneously (n = 20) or intraoperatively (n = 16). The procedures were performed using the ultrasound guidance. The quality of RFA were based on monitoring of equipments and subject feeling of the practitioners. Patients treated with RFA was followed according to clinical findings, radiographic images, and tumor markers.
RESULTS: Thirty-six patients underwent RFA for 48 nodules. RFA was used to treat an average 1.3 lesions per patient, and the median size of treated lesions was 2.5 cm (range, 0.5-9 cm). The average hospital stay was 5.6 d overall (2.8 d for percutaneous cases and 7.9 d for open operations). Seven patients underwent a second RFA procedure (sequential ablations). Sixteen HCC patients with a high level of alpha fetoprotein (AFP) and 9 colorectal cancer liver metastases patients with a high level of serum carcinoembryonic antigen (CEA) have a great reduction benefited from RFA. Four (11.1%) patients had complications: one skin burn; one postoperative hemorrhage; one cholecystitis and one hepatic abscess associated with percutaneous ablations of a large lesion. There were 4 deaths: 3 patients died from local and system diseases (1 at 7 month, 1 at 9 month, and 1 at 12 month), 1 patients died from cardiovascular shock, but no RFA-related death. At a median follow-up of 10 mo (range, 1-24 mo), 6 patients (16.7%) had recurrences at an RFA site, and 20 patients (56.7%) remained clinically free of disease.
CONCLUSION: RF ablation appears to be an effective, safe, and relatively simple procedure for the treatment of unresectable liver cancers. The rate and severity of complications appear acceptable. However, further study is necessary to assess combination with other therapies, long-term recurrence rates and effect on overall survival.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common solid cancers in the world, with an annual incidence estimated to be at least one million new patients, especially in Eastern Asian and South African[1]. Furthermore, the liver is second only to lymph nodes as a common site of metastasis from other solid cancers, particularly in patients with colorectal adenocarcinoma[2]. Surgical resection of HCC, colorectal cancer hepatic metastases, and patients with other types of primary tumors with liver-only metastases can result in significant long-term survival benefit in 20% to 35% of patients[3-5]. However, the majority of hepatic cancers are not suitable for curative resection at the time of diagnosis. Only 5% to 15% of newly diagnosed HCC or colorectal cancer liver metastasis patients undergo a potentially curative resection. Difficulties related to surgical resection are those of the size, site, number of tumors, adjacent to vascular and biliary structures, extrahepatic involvement, poor general condition, and poor liver function especially in HCC with inadequate functional hepatic reserve related to coexistent cirrhosis[6,7]. Thus, for the majority of patients with primary or metastatic hepatic malignancies who are not candidates for surgical resection, novel treatment approaches to control and potentially cure the unresectable tumors in the liver must be explored. In the last few years, minimal invasive techniques have become available for destruction of hepatic carcinomas. These include percutaneous ethanol injection (PEI), hepatic arterial embolization and thermoablation with cryoprobes, microwave, laser or radiofrequency[8-18].
Radiofrequency ablation (RFA) is a relatively new procedure for treatment of hepatic tumors not amenable to resection. RFA employs a high-frequency alternating current to cause thermal coagulation and protein denaturation. The procedure is carried out using a needle electrode connected to a radiofrequency generator. As ions attempt to follow the change in direction of the alternating current, there is frictional heating within the tissue. The result is a coagulative necrosis of the targeted tissue. Previous studies have employed both imaging studies and pathologic evaluation of ablated lesions to show complete tumor eradication[19-28].
The purpose of this analysis was to evaluate our initial experience with RFA of hepatic tumors, and examine the associated complications and local recurrence rate. Although the number of patients is limited and the follow-up is short, we provide some insight for the future wider application of this method.
MATERIALS AND METHODS
Patients
The study was performed with approval from the institutional ethics committees at the First Clinical College of Harbin Medical University. Informed consent was obtained from all patients at the time of enrollment.
Between January 2000 and December 2001, 36 consecutive patients (22 men, 14 women; mean age, 48.1 years; age range, 35-74 years) with HCC and liver metastases underwent the RF procedure. Twenty-one patients suffered HCC and 3 was recurrent HCC. Twelve patients got liver metastases from colorectal carcinoma, 2 patients from breast cancers, 1 from gastric carcinoma. The cirrhosis, which existed not only in HCC but also in liver metastases, were in 26 patients. It was related to hepatitis B in 15 patients, related to hepatitis C in 3, related to hepatitis B or C in 6 and related to alcohol use in 2. The Child-Pugh class of cirrhosis was determined in each patient, and at the time of the ablation procedure, Twenty-five patients were judged to have Child-Pugh class A; and 11 class B. Pathologic result of HCC was obtained by means of ultrasonographically (US) guided biopsy with a 21-gauge cutting needle or viral hepatitis or cirrhosis history with a high level of AFP. Biopsy was not performed in patients in whom US and CT findings consistently indicated HCC and AFP levels were more than 200 ng/mL (> 200 μg/L). The pathologic result of liver metastases was got by the same way and a history of primary colorectal carcinoma or other cancer with an increasing of CEA and other serum makers. Biopsy was only performed in patients in whom US and CT findings not consistently indicated metastasis and CEA levels were normal. The characteristics of 36 patients treated with RFA are summarized in Table 1.
Table 1.
Characteristics of 36 patients treated with radiofrequence ablation
| Characteristics | HCC | CLM | OLM |
| No. of patients | 21 | 12 | 3 |
| No. of nodules | 28 | 17 | 3 |
| Mean age | 45.9 (35-70) | 52.3 (41-74) | 50.6 (48-54) |
| Male/Famale | 15/6 | 9/3 | 0/3 |
| Child-Pugh cirrhosis | |||
| Class A | 11 | 11 | 3 |
| Class B | 10 | 1 | 0 |
| Class C | 0 | 0 | 0 |
| Positive HBsAg | 10 | 4 | 1 |
| Positive Antibody against Hepatitis C | 2 | 1 | 0 |
| Positive HBsAg and Hepatitis C | 6 | 0 | 0 |
| Lesion size | |||
| < 3.0 cm | 15 | 10 | 2 |
| > 3.0 cm and < 5.0 cm | 8 | 4 | 1 |
| > 5.0 cm | 5 | 3 | 0 |
| Serum AFP > 200 μg/L | 20 | 1 | 0 |
| Serum CEA > 20 μg/L | 1 | 12 | 0 |
HCC = Hepatocellular carcinoma, CLM = Colorectal cancer liver metastases, OLM = Other malignancies liver metastases, AFP = α - fetoprotein, CEA = carcinoembryonic antigen
All the patients had been examined by three experienced surgeon and were considered to be unsuitable for surgical intervention. Patients with coexistent morbidity-related poor life expectancy, multinodular or diffuse intrahepatic tumor, extrahepatic spread, portal thrombosis, Child-Pugh class C cirrhosis, refractory ascites, prothrombin activity less than 50%, or a platelet count lower than 50 × 109/L were excluded from this study.
Before enrollment, the stage of intrahepatic disease was determined by using US and contrast material-enhanced spiral computed tomography (CT) in all the patients. Selective hepatic angiography and magnetic resonance (MR) images were acquired in 11 patients. Dual-phase spiral CT was performed with injection of 150 mL of contrast. Selective hepatic angiography and MR were only conducted in patients whose images of ultrasound and CT were not clear. The size and location of the HCC nodules were assessed with Couinaud nomenclature by means of consensus between at least two observers who compared the images obtained with each of the radiological techniques.
All patients who underwent RF ablation, the following serologic values were measured before treatment and 24 h, 48 h, 7 d, and 1 month after treatment, induding AFP, CEA, CA19-9, transaminases, alkaline phosphatase, bilirubin, electrolytes, hemoglobin, fibrinogen, haptoglobin, creatinine, prothrombin activity, and complete blood cell count.
Equipments
Multielectrode, 15-gauge and radiofrequency probes, which was expandable by 8 hooks, were supplied by RITA Medical Systems (Mountain View, California). The hook-shaped retractable electrodes are deployed to a maximum diameter of 5.0 cm. The probe is insulated to within 1 cm of the tip to prevent cauterization along the shaft. Each electrode tip contains a thermistor that allows temperature monitoring in the tissue around the needle. The radiofrequency generator (RITA Medical Systems) delivers a 460 kHz continuous unmodulated sinusoidal waveform in the monopolar output mode (50-150 W). A computer (IBM ThinkPad, USA) with dedicated software (Micro Interactive, USA) connected to the generator recorded the power delivered, impedance values, thermistor temperatures, and timing of each procedure.
RFA procedures
The percutaneous procedure was performed in a hospital procedure room by one radiologist, one surgeon and one nurse. One hour before treatment, the patient received an orally administered sedative and an intravenously administered analgesic. The patient was monitored continuously before, during, and after the procedure. A 20-cm-long, 15-gauge and multielectrode RF probe with an expansion at 2, 3, 4, 5 cm (Starburst XL, RITA, California, USA) was used to deliver RF energy. Grounding was achieved by attaching a dispersive pad with a surface area greater than 400 cm2 to each of the patient's thighs. The patients were under local anesthesia induces by using 10 mL of 1% Lidocaine injected from the skin into the peritoneum along the predetermined puncture line. The electrode was then attached to a 460-kHz RF generator.
Celiotomy was performed if the percutaneous method was not feasible or the leisions located near diaphragm and major hepatic vessel or bile ducts. The lesions were identified by a combination of manual palpation and handheld intraoperative ultrasonography. During the period of RFA using open laprotomy, Pringle's occlusion was applied to increase the effectiveness by preventing heat conduction from surrounding vessels.
All procedures were under the guidance of ultrasound. During lesion ablation, a thermocouple embedded in the electrode tip continuously measured the local temperature. Tissue impedance was monitored continuously by means of circuitry incorporated in the generator. For each treatment session, a single RF probe was positioned at the center of the tumor less than 3 cm in diameter. Otherwise, RF probe was introduced into a 1.0 cm deep position from the center of the lesion more than 3.0 cm diameter. Following satisfactory deployment of the multiple array, the initial power was applied at 50 W and then increased in 10 W increments at 1, 2, 3, and 4 min to a maximum power of 90 W. Power and tissue impedance was monitored continuously from the RF generator. Treatment started automatically when the temperatures of each tips were above 95 °C. Treatment continued until power "roll-off"occured, indicating a precipitous drop in power output as tissue impedance increased markedly from coagulative necrosis. Before took out the probe, the ablation of the puncture line was necessary to prevent tumor cell metastasis.
During energy deposition, a hyperechoic patch due to vaporization and cavitation effects was observed around the electrode tip; the patch progressively increased to cover all of the neoplastic area or a larger area. Several minutes after the end of treatment, the hyperechoic patch cleared and was replaced by a hyperechoic ring, which was usually smaller. RF energy was applied for 10-12 min. After RF therapy, patients were hospitalized for 2-3 d for percutaneous, whereas were 7-10 d in open celiotomy, unless complications necessitated longer hospitalization.
Effectiveness of RFA
All treatment sessions were completed within 1 mo after the beginning of the therapies. Dynamic CT was performed 1 wk and 1 month after the initial treatments. The CT scans were interpreted by the same radiologist. When a nonenhancing area with a diameter equal to or greater than that of the treated nodule was detected, tumor necrosis was considered to be complete. When nodule enhancement was seen at dynamic CT, tumor necrosis was considered to be incomplete. Additional RFA was performed in nodules that showed incomplete necrosis at dynamic CT performed 1 wk after the initial treatment. The therapeutic effect was evaluated with dynamic CT 1 mo after the initial treatment, and when no enhancing lesion was seen, the therapeutic effect was considered to be complete. When nodule enhancement was still seen, the therapeutic effect was considered to be incomplete and one more procedure was offered to these patients.
Follow-up dynamic CT was performed every 3 mo. A newly appeared and enhanced lesion in or near the treated nodule or an enlargement of the treated nodule was considered to be residual foci of the disease. The follow-up periods ranged from 1 to 24 mo (mean, 10 mo).
RESULTS
Therapeutic efficacy
The results of RF treatment, according to tumor size and pathology, are summarized in Table 2. Seven lesions required repeat treatment within 1 month after the initial ablation, and one lesion was re-treated after 6 mo of follow-up owing to the identification of small foci of persistent viable tumor that were undetectable at the previous examination. Overall, when compared with tumor pathology, tumor size was highly significant in predicting treatment success. Tumors less than 3.0 cm in diameter were fully ablated once with recurrence. Tumors 3.1-5.0 cm in diameter were more successfully treated significantly than those greater than 5.0 cm in diameter. At 6-month follow-up, abnormal α-fetoprotein levels normalized (< 20 ng/mL [20 μg/L]) in 6 of 21 patients, decreased in 10 patients, and increased in 5 patients. CEA levels normalized in 4 of 12 patients, decreased in 5 patients, and increased in 2 patients. There were 4 deaths: 3 patients died from local and system diseases (1 at 7 month, and 1 at 9 month and 1 at 12 month), and 1 patients died from cardiovascular shock.
Table 2.
Summary of results of RFA in liver cancers
| Results |
HCC |
CLM |
OLM |
||||||
| < 3.0 cm | > 3.0 cm < 5.0 cm | > 5.0 cm | < 3.0 cm | > 3.0 cm < 5.0 cm | > 5.0 cm | < 3.0 cm | > 3.0 cm < 5.0 cm | > 5.0 cm | |
| Nodules necrosis | 16 | 5 | 2 | 9 | 4 | 2 | 0 | 2 | 1 |
| Retreated nodulars | 0 | 2 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| Normal AFP | 5 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Decreased AFP | 6 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Normal CEA | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| Decreased CEA | 0 | 0 | 0 | 3 | 1 | 1 | 0 | 0 | 0 |
HCC = Hepatocellular carcinoma, CLM = Colorectal cancer liver metastases, OLM = Other malignancies liver metastases, AFP = α - fetoprotein, CEA = carcinoembryonic antigen
Imaging findings
A rim of hyperattenuation surrounding the region of coagulated tumor was apparent in the majority of cases during the portal phase of contrast enhancement on CT scans obtained 24 h after RF treatment. This was attributed to reactive hyperemia rather than residual viable tumor and disappeared progressively on subsequent follow-up studies. Thickening of the hepatic capsule was sometimes observed on CT scans obtained 1 month after ablation and beyond, particularly when the tumor was located close to the surface of the liver. This finding also progressively disappeared on subsequent follow-up studies.
Immediately following RF therapy, treated areas of tumor appeared as areas of hypoattenuation, compared with both surrounding liver and residual tumor. However, tumor size was unchanged, compared with pretreatment tumor size, regardless of treatment response. On subsequent follow-up CT scans, areas of successfully treated tumor remained either unchanged in size or diminished at unpredictable rates.
Although the diameter of the hyperechoic focus seen during treatment was used to guide the duration of RF application, and grossly corresponded to the diameter of necrosis demonstrated at CT, conventional US was not used for the evaluation of therapeutic response. This was principally because of the heterogeneous and variable extent of hyperechogenicity observed after treatment (Figure 1, Figure 2).
Figure 1.
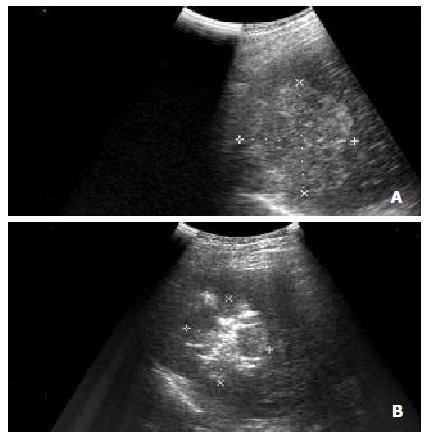
(A) Intercostal oblique sonogram shows a 5.7 cm diameter nodular HCC in segment 8. (B) The RF electrode has been placed in the lesion, and the electrode tip is recognizable as an echogenic area in the tumor. Sonogram obtained after several minutes of energy deposition shows a hyperechoic patch around the electrode tip.
Figure 2.
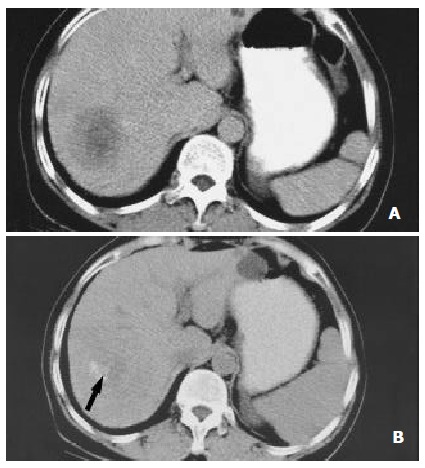
Nearly complete necrosis in a large HCC located in segment 7 and treated with RF therapy in a 57-year-old colorectal cancer liver metastases. (A) CT scan obtained prior to therapy demonstrates a large, 5.3 cm nodules. (B) CT scan obtained 6 mo after RF therapy shows nearly complete tumor necrosis. A small hypervascularized area of viable neoplastic tissue (arrow) remains at the posteromedial aspect of the tumor.
Side effects
The majority of patients treated with the use of sedation or analgesia had mild or moderate pain during the percutaneous procedure. This disappeared 1-2 d following the cessation of RF application. However, in 2 cases, propofol and additional analgesia with assisted ventilation were administered to complete the procedure. In 1 of 25 patients, RF application had to be interrupted temporarily because of severe pain and transfer to open operation. In the majority of patients, a small asymptomatic right pleural effusion that lasted for less than 1 month was observed.
In the majority of patients, transaminase levels increased two to nine times over baseline during the first 3 d following therapy. Moreover, a slight increase in leukocytes and bilirubin and a decrease in platelets were observed. All of these test results returned to baseline levels by 7 d following RF ablation. No significant changes in other test results were observed.
Complications
Four (11.1%) patients had complications: one liver abscess; one skin burn; one postoperative hemorrhage and one cholecystitis.
Major complications
One 51 year old patient with cirrhosis and diabetes and two hepatocellular carcinoma nodules of 5.0 and 2.5 cm diameter had a liver abscess (2.7%). On day 15 after the procedure, this patient had fever with leukocytosis. US showed a big liver abscess in the remnant of big tumor. The patient was treated successfully with percutaneous drainage of the abscess. This complication was attributed to a break in sterile technique and not to the application of RF. As a result of this complication, however, antibiotic prophylaxis with 1000 mg of ceftriaxone sodium (Rocephin, Roche, Shanghai, China) is currently administered to all patients.
Minor complications
One (2.7%) of 36 patients developed self-limited intraperitoneal hemorrhage (the appearance of peritoneal effusion with an associated 1-2 g/dL decrease in serum hemoglobin). This patient did not require blood transfusion or other interventions. Another had a mild cholecystitis with a severe leukocytosis. The patient had been given antibiotic for 5 d and recovered quickly. One patient got a skin burn due to incorrectly grounding for not attaching the dispersive pad to skin in the early stage of performing RFA. None of these complications required further treatment, although hospital discharge was delayed.
DISCUSSION
The management of HCC and liver metastases is challenging. HCC and liver metastases from other solid tumors are major causes of morbidity and cancer-related death worldwide. The poor prognosis of HCC is underscored by a mortality index of 0.94. That is mean that 94% of the patients diagnosed with HCC will die as a result of this disease[1]. Similarly, the development of solid tumor liver metastases is frequently a sign of rapidly death if not be treated. Although resection of primary or metastatic hepatic lesions can be curative in some cases, most patients have unresectable tumors. Unfortunately, systemic chemotherapy is rarely effective and often associated with significant toxicity, with few complete responses and rare long-term progression-free survival, and usually do not significantly improve overall patient survival[29-31]. As previously noted, the majority of patients with primary or metastatic liver tumors are not candidates for resection; however, because potentially curative or palliative benefit may be derived from destruction of the liver tumors, in situ ablative techniques like PEI, cryoablation and RFA were developed.
The mechanism of RF is that a high-frequency alternating current (100 to 500 kHz), mostly 460 kHz, passes from an uninsulated electrode tip into the surrounding tissues and causes ionic vibration as the ions attempt to follow the change in the direction of the rapidly alternating current. This ionic vibration causes frictional heating of the tissues surrounding the electrode, rather than the heat being generated from the probe itself. The goal of RFA is to achieve local temperatures so that tissue destruction occurs. In general, thermal damage to cells begins at 42 °C, with exposure times required for cell death at this temperature ranging from 3 to 50 h depending on the nature of the tissue. As the temperature is increased, there is an exponential decrease in the exposure time needed for cellular destruction. At temperatures above 60 °C, intracellular proteins including collagen denature, the lipid bilayer melts and cell death becomes inevitable. Thermal coagulation begins at 70 °C and tissue desiccation at 100 °C, producing coagulation necrosis of tumor tissue and surrounding hepatic parenchyma. Tissue heating also drives extracellular and intracellular water out of the tissue and results in further destruction of the tissue due to coagulative necrosis[19,20,32-34].
The first probe used in RF was a monopolar which only destroyed 1.5 cm tissues in diameter. Advances in RF technology led to the development of monopolar and bipolar tissue ablation devices designed to destroy larger areas of tissue, particularly malignant tumors. For tumor ablation, a needle electrode is placed in the tumor via a percutaneous or intraoperative approach, and an indifferent dispersive electrode pad is applied to the patient's outer skin surface, much like the grounding pads used for hemostatic electrocautery during surgical procedures. The final size of the sphere of heat-ablated tissue is proportional to the square of the radiofrequency current, also known as the radiofrequency power density. The radiofrequency power delivered via a monopolar electrode decreases in proportion to the square of the distance from the electrode. Therefore, the tissue temperature falls rapidly with increasing distance away from the electrode. The zone of tissue necrosis can be increased to 2.0 to 3.0 cm by the use of bipolar needle electrodes. New needles have been developed with multiple array hook electrodes used in our patients. The needle electrode shaft is placed into the tumor with the array retracted. Using real-time ultrasound guidance, the array is then deployed from the needle tip into the tumor. These deployed multiple array hooks create a series of electrodes with a diameter up to 5 cm, across which the radiofrequency current can be passed. Using a radiofrequency current generator with a 50 to 150 watt power output for 5 to 15 min, a 4.0 to 5.0 cm diameter tumor, can be ablated with 3.5 cm diameter hook electrodes fully deployed. In larger tumors, the needle electrode can be repositioned and the hook electrodes redeployed to create overlapping zones of coagulative necrosis to produce complete ablation of the tumor and a surrounding rim of hepatic parenchyma[35-38].
RF thermal ablation has proved to be safe and effective for the treatment of hepatic tumors in patients who are considered to be unsuitable for surgical intervention and recently has attracted much attention. It has some merits compared with the other percutaneous techniques: The treatment time is shorter than that with the more popular percutaneous ethanol injection[39], the thermal lesions are larger than those obtained with a microwave electrode[40], and it is less expensive and easier to perform than interstitial laser photocoagulation, in which multiple fiber insertions are always required[41], and It is more safer and lower complications than cryotherapy[42].
HCC with cirrhosis responded best to treatment with RFA than liver metastases. Large lesions over 3 cm were treated showing lesion stability on CT scan and a dramatic fall in AFP levels. The reason for a good response in this group of tumors is probably due to the characteristic features of hepatocellular carcinomas, which offer better heat conduction. It is clear that RFA is a useful primary therapy in patients with unresectable HCC, especially in cases with a poor liver reserve from cirrhosis and with multiple and deep-sited lesions. Prior experience treating small HCC described the "oven effect"[39], whereby cirrhotic liver surrounding individual HCC nodules acts as a thermal insulator that increases tissue heating during RF therapy. The results of this study further support the importance of this effect.
Colorectal liver metastases are usually hard. Patients with colorectal liver metastases usually have no cirrhosis, and as they have good liver reserve, most patients without extrahepatic spread are suitable for liver resection. Unresectability is commonly due to extensive multiple, bilateral disease or central lesions. For patients with colorectal metastases, we found that those with large liver deposits over 5 cm in diameter often required two applications of RFA because of the large diameter in relation to the radius of the thermal coagulation.
The local recurrence rate in the present study was 19.5%. Previous studies have reported highly variable local recurrence rates associated with RFA, ranging from 0% to 100%. Curley et al[43] reported a local recurrence rate of 1.8% in one of the largest series published to date. In our experience, local recurrences were evident at a median follow-up time of 6 mo and were associated with ablation of larger tumors. It is possible that the rate of local recurrence may be reduced by specific interventions. For example, newer probes have been developed to produce larger spheres of coagulation necrosis. We are now more likely to deploy the electrode in several overlapping ablation sites to encompass tumors greater than 5 cm in diameter. For larger tumors requiring more than one deployment, we have used open operation to perform the procedure and prolonged the acting time. Selective use of the Pringle maneuver in patients with larger tumors or tumors in the vicinity of large vessels may provide larger zones of ablation. Additionally, based on our limited experience, repeat RFA seems to be well tolerated.
However, recurrence in the liver is frequently accompanied by extrahepatic disease, and careful preoperative staging is essential to select patients who could potentially benefit from repeat RFA. More recently, it has been understood that HCC is a multifocal disease in due time. In other words, the first lesion to be detected is only the prelude to other neoplasms. For this reason, we believe that it is appropriate to offer repeat RFA for selected patients with isolated hepatic recurrence[44-46].
There is growing interest in applying RFA technology via minimally invasive approaches. Some have reported on the use of percutaneous and laparoscopic RFA procedures. In suitable patients, the percutaneous approach can be performed without general anesthesia. The laparoscopic approach can be used to evaluate for extrahepatic disease and spares the patient a laparotomy. However there are limitations with the more minimally invasive approaches, so patients must be carefully selected. We do not have experience with laparoscopic procedures for RFA, and long-term follow-up is necessary to determine whether this approach is equivalent to an open procedure. We have limited experience with percutaneous RFA. Difficult localization of the lesion under transcutaneous ultrasound guidance was thought to be the cause of failure. Few data are available regarding long-term follow-up of patients undergoing percutaneous RFA. Major complications, such as bilioperitoneum, intrahepatic abscesses, acute thrombosis of the portal vein, and a death related to necrosis of the diaphragm have been reported following percutaneous RFA. We have been hesitant to perform percutaneous RFA except in cases of relatively small tumors that are well within the hepatic parenchyma, away from adjacent organs. The potential for inadvertent ablation of the diaphragm, colon, stomach or other organs is an important consideration. In our experience, there is one patient who have liver abscess after percutaneous RFA due to instrict sterilization. But, minimal invasive do have less harm to patients with cancers, especially in HCC with cirrhosis and late-stage liver metastases. We have chosen to perform RFA as a percutaneous procedure in the vast majority of cases. We therefore believe that the decision to perform percutaneous ablation should be a multidisciplinary effort[47,48].
Resection or ablation of liver tumors will not cure most patients with primary or metastatic malignant disease, although long-term disease-free survival rates of 20% to 40%. We are very encouraged by our initial experience with RFA as a treatment for malignant liver tumors because it is safe, well-tolerated, associated with few complications, and usually effective in controlling grossly or ultrasonographically evident in liver tumors. If there are a combination of cryotherapy, percutaneous ethanol injection, RFA with regional and systemic chemotherapy, such a multimodality treatment approach will reduce hepatic and extrahepatic recurrence rates, and thus enhance long-term survival rates.
Footnotes
Edited by Zhang JZ
Supported by Youth Natural Scientific Foundation of Heilongjiang Province, Youth Natural Scientific Foundation of Harbin and Heilongjiang Province Education Government Grant
References
- 1.Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol. 2002;8:193–199. doi: 10.3748/wjg.v8.i2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss L, Grundmann E, Torhorst J, Hartveit F, Moberg I, Eder M, Fenoglio-Preiser CM, Napier J, Horne CH, Lopez MJ. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195–203. doi: 10.1002/path.1711500308. [DOI] [PubMed] [Google Scholar]
- 3.Yamanaka N, Okamoto E, Oriyama T, Fujimoto J, Furukawa K, Kawamura E, Tanaka T, Tomoda F. A prediction scoring system to select the surgical treatment of liver cancer. Further refinement based on 10 years of use. Ann Surg. 1994;219:342–346. doi: 10.1097/00000658-199404000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagorney DM, van Heerden JA, Ilstrup DM, Adson MA. Primary hepatic malignancy: surgical management and determinants of survival. Surgery. 1989;106:740–78; discussion 740-748;. [PubMed] [Google Scholar]
- 5.Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 6.Calvet X, Bruix J, Brú C, Ginés P, Vilana R, Solé M, Ayuso MC, Bruguera M, Rodes J. Natural history of hepatocellular carcinoma in Spain. Five year's experience in 249 cases. J Hepatol. 1990;10:311–317. doi: 10.1016/0168-8278(90)90138-h. [DOI] [PubMed] [Google Scholar]
- 7.Buscarini L, Fornari F, Canaletti R, Sbolli G, Civardi G, Cavanna L, Di Stasi M. Diagnostic aspects and follow-up of 174 cases of hepatocellular carcinoma. Second report. Oncology. 1991;48:26–30. doi: 10.1159/000226889. [DOI] [PubMed] [Google Scholar]
- 8.Zhou XD, Tang ZY, Yu YQ, Ma ZC. Clinical evaluation of cryosurgery in the treatment of primary liver cancer. Report of 60 cases. Cancer. 1988;61:1889–1892. doi: 10.1002/1097-0142(19880501)61:9<1889::aid-cncr2820610928>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 9.McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990;25:267–270. doi: 10.1097/00004424-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Onik GM, Atkinson D, Zemel R, Weaver ML. Cryosurgery of liver cancer. Semin Surg Oncol. 1993;9:309–317. doi: 10.1002/ssu.2980090406. [DOI] [PubMed] [Google Scholar]
- 11.Castells A, Bruix J, Bru C, Fuster J, Vilana R, Navasa M, Ayuso C, Boix L, Visa J, Rodés J. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology. 1993;18:1121–1126. [PubMed] [Google Scholar]
- 12.Kotoh K, Sakai H, Sakamoto S, Nakayama S, Satoh M, Morotomi I, Nawata H. The effect of percutaneous ethanol injection therapy on small solitary hepatocellular carcinoma is comparable to that of hepatectomy. Am J Gastroenterol. 1994;89:194–198. [PubMed] [Google Scholar]
- 13.Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, Pompili M, Brunello F, Lazzaroni S, Torzilli G. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101–108. doi: 10.1148/radiology.197.1.7568806. [DOI] [PubMed] [Google Scholar]
- 14.Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K, Sato M, Uchiyama S, Inoue K. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74:817–825. doi: 10.1002/1097-0142(19940801)74:3<817::aid-cncr2820740306>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Murakami R, Yoshimatsu S, Yamashita Y, Matsukawa T, Takahashi M, Sagara K. Treatment of hepatocellular carcinoma: value of percutaneous microwave coagulation. AJR Am J Roentgenol. 1995;164:1159–1164. doi: 10.2214/ajr.164.5.7717224. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi T, Kikuchi M, Okazaki M. Hepatocellular carcinoma after transcatheter hepatic arterial embolization. A histopathologic study of 84 resected cases. Cancer. 1994;73:2259–2267. doi: 10.1002/1097-0142(19940501)73:9<2259::aid-cncr2820730905>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Sato M, Watanabe Y, Ueda S, Iseki S, Abe Y, Sato N, Kimura S, Okubo K, Onji M. Microwave coagulation therapy for hepatocellular carcinoma. Gastroenterology. 1996;110:1507–1514. doi: 10.1053/gast.1996.v110.pm8613057. [DOI] [PubMed] [Google Scholar]
- 18.Shibata T, Murakami T, Ogata N. Percutaneous microwave coagulation therapy for patients with primary and metastatic hepatic tumors during interruption of hepatic blood flow. Cancer. 2000;88:302–311. [PubMed] [Google Scholar]
- 19.Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, Paties CT, Silverman DE, Buscarini L. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759–768. doi: 10.2214/ajr.167.3.8751696. [DOI] [PubMed] [Google Scholar]
- 20.Solbiati L, Ierace T, Goldberg SN, Sironi S, Livraghi T, Fiocca R, Servadio G, Rizzatto G, Mueller PR, Del Maschio A, et al. Percutaneous US-guided radio-frequency tissue ablation of liver metastases: treatment and follow-up in 16 patients. Radiology. 1997;202:195–203. doi: 10.1148/radiology.202.1.8988211. [DOI] [PubMed] [Google Scholar]
- 21.Livraghi T, Goldberg SN, Monti F, Bizzini A, Lazzaroni S, Meloni F, Pellicanò S, Solbiati L, Gazelle GS. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology. 1997;202:205–210. doi: 10.1148/radiology.202.1.8988212. [DOI] [PubMed] [Google Scholar]
- 22.Solbiati L, Goldberg SN, Ierace T, Livraghi T, Meloni F, Dellanoce M, Sironi S, Gazelle GS. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997;205:367–373. doi: 10.1148/radiology.205.2.9356616. [DOI] [PubMed] [Google Scholar]
- 23.Rossi S, Buscarini E, Garbagnati F, Di Stasi M, Quaretti P, Rago M, Zangrandi A, Andreola S, Silverman D, Buscarini L. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998;170:1015–1022. doi: 10.2214/ajr.170.4.9530052. [DOI] [PubMed] [Google Scholar]
- 24.Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dodd GD, Soulen MC, Kane RA, Livraghi T, Lees WR, Yamashita Y, Gillams AR, Karahan OI, Rhim H. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics. 2000;20:9–27. doi: 10.1148/radiographics.20.1.g00ja019. [DOI] [PubMed] [Google Scholar]
- 26.Parikh AA, Curley SA, Fornage BD, Ellis LM. Radiofrequency ablation of hepatic metastases. Semin Oncol. 2002;29:168–182. doi: 10.1053/sonc.2002.31673. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996;3:636–644. doi: 10.1016/s1076-6332(96)80188-7. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg SN, Solbiati L, Hahn PF, Cosman E, Conrad JE, Fogle R, Gazelle GS. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998;209:371–379. doi: 10.1148/radiology.209.2.9807561. [DOI] [PubMed] [Google Scholar]
- 29.Doci R, Gennari L, Bignami P, Montalto F, Morabito A, Bozzetti F, Bonalumi MG. Morbidity and mortality after hepatic resection of metastases from colorectal cancer. Br J Surg. 1995;82:377–381. doi: 10.1002/bjs.1800820332. [DOI] [PubMed] [Google Scholar]
- 30.Jamison RL, Donohue JH, Nagorney DM, Rosen CB, Harmsen WS, Ilstrup DM. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg. 1997;132:505–510; discussion 511. doi: 10.1001/archsurg.1997.01430290051008. [DOI] [PubMed] [Google Scholar]
- 31.Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–1410. doi: 10.1016/s0140-6736(94)92529-1. [DOI] [PubMed] [Google Scholar]
- 32.McGahan JP, Brock JM, Tesluk H, Gu WZ, Schneider P, Browning PD. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol. 1992;3:291–297. doi: 10.1016/s1051-0443(92)72028-4. [DOI] [PubMed] [Google Scholar]
- 33.McGahan JP, Griffey SM, Budenz RW, Brock JM. Percutaneous ultrasound-guided radiofrequency electrocautery ablation of prostate tissue in dogs. Acad Radiol. 1995;2:61–65. doi: 10.1016/s1076-6332(05)80248-x. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg SN, Gazelle GS, Dawson SL, Rittman WJ, Mueller PR, Rosenthal DI. Tissue ablation with radiofrequency: effect of probe size, gauge, duration, and temperature on lesion volume. Acad Radiol. 1995;2:399–404. doi: 10.1016/s1076-6332(05)80342-3. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg SN, Gazelle GS, Halpern EF, Rittman WJ, Mueller PR, Rosenthal DI. Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad Radiol. 1996;3:212–218. doi: 10.1016/s1076-6332(96)80443-0. [DOI] [PubMed] [Google Scholar]
- 36.Goldberg SN, Gazelle GS, Dawson SL, Rittman WJ, Mueller PR, Rosenthal DI. Tissue ablation with radiofrequency using multiprobe arrays. Acad Radiol. 1995;2:670–674. [PubMed] [Google Scholar]
- 37.Miao Y, Ni Y, Mulier S, Wang K, Hoey MF, Mulier P, Penninckx F, Yu J, De Scheerder I, Baert AL, et al. Ex vivo experiment on radiofrequency liver ablation with saline infusion through a screw-tip cannulated electrode. J Surg Res. 1997;71:19–24. doi: 10.1006/jsre.1997.5133. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg SN, Solbiati L, Hahn PF, Cosman E, Conrad JE, Fogle R, Gazelle GS. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology. 1998;209:371–379. doi: 10.1148/radiology.209.2.9807561. [DOI] [PubMed] [Google Scholar]
- 39.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 40.Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–337. doi: 10.1148/radiol.2232010775. [DOI] [PubMed] [Google Scholar]
- 41.Heisterkamp J, van Hillegersberg R, Ijzermans JN. Interstitial laser coagulation for hepatic tumours. Br J Surg. 1999;86:293–304. doi: 10.1046/j.1365-2168.1999.01059.x. [DOI] [PubMed] [Google Scholar]
- 42.Bilchik AJ, Wood TF, Allegra D, Tsioulias GJ, Chung M, Rose DM, Ramming KP, Morton DL. Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms: a proposed algorithm. Arch Surg. 2000;135:657–662; discussion 662-664. doi: 10.1001/archsurg.135.6.657. [DOI] [PubMed] [Google Scholar]
- 43.Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearson AS, Izzo F, Fleming RY, Ellis LM, Delrio P, Roh MS, Granchi J, Curley SA. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg. 1999;178:592–599. doi: 10.1016/s0002-9610(99)00234-2. [DOI] [PubMed] [Google Scholar]
- 45.Scudamore CH, Lee SI, Patterson EJ, Buczkowski AK, July LV, Chung SW, Buckley AR, Ho SG, Owen DA. Radiofrequency ablation followed by resection of malignant liver tumors. Am J Surg. 1999;177:411–417. doi: 10.1016/s0002-9610(99)00068-9. [DOI] [PubMed] [Google Scholar]
- 46.Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchianò A, Fornari F, Quaretti P, Tolla GD, Ambrosi C, Mazzaferro V, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119–126. doi: 10.1148/radiology.217.1.r00se02119. [DOI] [PubMed] [Google Scholar]
- 47.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 48.Wong SL, Edwards MJ, Chao C, Simpson D, McMasters KM. Radiofrequency ablation for unresectable hepatic tumors. Am J Surg. 2001;182:552–557. doi: 10.1016/s0002-9610(01)00813-3. [DOI] [PubMed] [Google Scholar]


