Abstract
AIM: To investigate the expression of p28/gankyrin gene and its role in the carcinogenetic process of human hepatocellular carcinoma (HCC).
METHODS: 64 specimens of HCC and para-carcinoma tissues, 22 specimens of non-tumor liver tissues (7 normal, 15 cirrhosis), 10 specimens of normal human tissues and 5 hepatoma cell lines were studied for the expression of p28/gankyrin by Northern blot. The expression of p28/gankyrin protein was detected immunohistochemically by using the specific polyclonal antibody.
RESULTS: Northern blot analysis indicated that the expression of p28/gankyrin mRNA was intensively distributed in brain and heart, weakly in lung, spleen and muscle, undetectable in digestive system including liver, pancreas, stomach, small and large intestines. p28/gankyrin mRNA was absent in normal liver, weakly detected in liver cirrhosis and in 18 of 64 para-carcinoma liver tissues. In contrast, the expression of p28/gankyrin mRNA was intensively detected in all 5 hepatoma cell lines tested, markedly increased in 57 of 64 and moderately increased in 5 of 64 HCC samples. In comparison with liver cirrhosis and para-carcinoma liver tissues, the average expression of p28/gankyrin mRNA in HCC was increased 3.6- (2.901 ± 0.507 vs 0.805 ± 0.252, P < 0.05) and 5.2-fold (2.901 ± 0.507 vs 0.557 ± 0.203, P < 0.01), respectively. In addition, p28/gankyrin mRNA expression level was higher in HCC with portal vein tumor thrombus and microscopic hepatic vein involvement (P = 0.021 and P = 0.047, respectively). The overexpression of p28/gankyrin protein in HCC was targeted in hepatic tumor cells, not in bile duct cells and other interstitial cells.
CONCLUSION: Overexpression of p28/gankyrin in HCC plays an important role and contributes to the metastasis potential in the process of carcinogenesis. p28/gankyrin may become a specific biological tissue marker for the pathological diagnosis of HCC.
INTRODUCTION
Human primary hepatocellular carcinoma (HCC) is one of the most common types of malignant cancer in Asia and Africa where hepatitis virus infection and exposure to specific liver carcinogens are prevalent[1-4]. HCC has ranked second in cancer mortality in China since the 1990s and is increasing in frequency among males in many countries[5,6]. Although the major viral and environmental risk factors for HCC development have been unraveled[7,8], the oncogenic pathways leading to malignant transformation of liver cells have long remained obscure[9]. It has been widely reported that some tumor suppressor genes such as p53 and p16INK4A play a vital role in the development of HCC[10-13], while few oncogenes that control growth behavior or metastatic potential of HCC have been underscored.
p28 gene was initially cloned in human cDNA library by comparing the amino-acid sequence of a subunit isolated from the purified bovine erythrocyte PA700 complex with protein structures in databases of Homo-Protein cDNA Bank[14-16]. p28 protein is one of the non-ATPase subunits of PA700 (also called 19 S complex), a 700 kDa multisubunit regulatory complex of the human 26 S proteasome[17]. Intriguingly, p28 protein contained six conserved motifs known as 'ankyrin repeats's[18-20], implying that this subunit may contribute to interaction of the 26 S proteasome with other proteins. Recently, another gene named 'gankyrin' was cloned by cDNA subtractive hybridization in HCC and its sequence is identical to p28 gene[21]. Because of the putative proteasome connection, the oncogenic effect of p28/gankyrin in HCC may be associated with the ubiquitin-proteasome pathway[22-24]. So far, the mechanism of up-regulation of p28/gankyrin in HCC is still unknown.
In order to elucidate the role of p28/gankyrin in carcinogenesis of HCC and its correlation with clinical parameters, the following study was carried out.
MATERIALS AND METHODS
Sample collection and processing
All 64 HCC specimens and their para-carcinoma tissues (more than 2 cm away from the focus), were sampled from 64 patients who had undergone curative hepatectomy (58 men and 6 women; mean age 46.4 ± 10.5 years). Patients who had received radiotherapy or chemotherapy before hepatectomy were excluded. Non-tumor liver tissues were obtained from 22 patients who had received hepatic hemangiomatomy. Ten different types of human normal tissues were from 2 men of accidental deaths. They were all cases from 1999 to 2000 in Eastern Hepatobiliary Surgery Hospital in Shanghai, China. Informed consent was obtained from all patients for subsequent use of their resected tissues. These specimens were immediately dissected into small pieces under aseptic condition within half an hour, quickly-frozen and preserved in liquid nitrogen before subsequent procedures. The specimens used for immunohistochemistry (IHC) were routinely processed, formalin-fixed and paraffin-embedded, at least 2 serial paraffin sections of 4 mm - 6 mm thick were made, one for hematoxylin and eosin (HE) staining and the other for p28/gankyrin protein detection.
Cells lines
A series of cell lines (ATCC, Rockville, MD) were investigated in this study, including HepG2 (ATCC HB-8065), HuH-7, SK-Hep-1 (ATCC HTB-52), Chang liver (ATCC CCL-13), and a human fetal hepatocyte cell line WRL 68 (ATCC CL-48). They were maintained, as specified by the suppliers, in Dulbecco's modified Eagle medium or other recommended mediums supplemented with 10% fetal bovine serum at 37 °C in a humidified atmosphere of 5% CO2 in air.
Northern blot analysis of p28/gankyrin transcript
Preparation and labeling of the probe Polymerase chain reaction (PCR) of a human fetal liver cDNA library (provided by Max-Planck Institute) was performed in a final volume of 50 mL containing all four dNTPs (each at 200 mmol/L), 1.25 mmol/L MgCl2, 2.5 units of Taq (TaKaRa Biotech, Dalian, China) and each primer at 0.5 mmol/L. The following temperature program was used: 1 cycle at 94 °C for 5 min, 35 cycles at 94 °C for 40 s, 52 °C for 30 s and 72 °C for 55 s, followed by a final extension at 72 °C for 8 min. Primers used for amplification were human p28/gankyrin sense primer corresponding to nucleotides 2-19 (5'-GCGGATCCAGTAGTTGCTGGGACAGC-3', and antisense primer complementary to nucleotides 830-847 (5'-GCGAATTCGGAACAAGAGTCAACATG- 3' with the BamH I and EcoR I restriction sites at their 5' strand ends respectively. The PCR product was cloned into pcDNA3.1 vector (Invitrogen, Groningen, Netherlands) to generate the clone full. hup28. pcDNA3.1, which was confirmed by sequencing with an automatic DNA sequencer (ABI model 3700). The purified PCR product was labeled with α-32P-dATP by a random primer labeling method as described[25] with Prime-α-Gene Labeling System (Promega, Madison, WI).
RNA extraction and preparation of the hybridization membrane Samples subjected to RNA analysis were isolated from surgical specimens of HCC and the para-carcinoma liver tissues of 64 patients and from a series of non-tumor liver samples, normal human tissues and hepatoma cell lines by the acid guanidinium thiocyanate-phenol-chloroform extraction method as previously described[26]. Total RNA (40 mg) was denatured and separated by electrophoresis in a 1.0% agarose gel containing 2.2 mol/L formaldehyde and then transferred to nitrocellulose membranes (BA85, Schleicher Schuell, Germany). The membranes were dried in a vacuum drying over at 80 °C for 2 h and sealed in a plastic bag for use.
Northern blot analysis Hybridization of the membranes was performed by using the labeled p28/gankyrin cDNA as the probe at 42 °C for 20 h in a solution containing 50% formamide, 5 × SSC, 0.1% SDS and 5 × Denhardt’s after the membranes had been pre-hybridized in the same solution with 0.1 mg/mL salmon sperm DNA at 42 °C for 4 h. After this hybridization, the membranes were rinsed in stringent conditions (65 °C for 30 min in a washing buffer of 0.1 × SSC and 0.1% SDS) and then exposed to Kodak X-ray film at -80 °C for 14 d. The expression level of mRNA was normalized with ethidium bromide-stained 18 s rRNA as internal standard and analyzed by Phosphor Imager (FLA 2000, Fujifilm, Japan).
Immunohistochemical analysis of P28/Gankyrin protein
Preparation of the polyclonal antibody A polyclonal antibody against p28/gankyrin protein (226 amino acid) was prepared. Briefly, a 683-bp fragment from nucleotide 2 to 684 (amino acid 1-221) was generated by PCR and subcloned into the fusion expression vector pPROEX-HTb (Gibco/BRL Life Technologies, NY) using BamH I and Xho I. The expression vector was then transformed into competent Escherichia coli DH5α for induction of 6 × histidine-fused protein with 0.6 mmol/L isopropyl-1-thio-b-D-galactopyranoside (IPTG) at 37 °C for 3 h. The cells were harvested and lysed with ice-cold TS buffer containing 50 mmol/L Tri-HCl (pH8.0), 150 mmol/L NaCl, 1 mg/mL lysozyme, 5 mmol/L EDTA, 0.5 mmol/L phenylmethylsulfonyl fluoride, 0.2 mg/L aprotinin and 1% Triton X-100. The lysates were purified by affinity chromatography with Ni-NTa agarose column (Qiagen, Germany) and identified by electrophoresis on a 10% SDS-PAGE gel. The purified fusion protein emulsified with Freund's adjuvant (Gibco/BRL Life Technologies, NY) was used to immunize two New Zealand rabbits (male, 2.5-3.0 kg) for preparation of the polyclonal antibody. Antibody valence was determined by double immunodiffusion test on 1% agar gel. For the specificity, affinity chromatography was applied to purify the antibody from the immune sera by an antigen column.
Immunohistochemical staining p28/gankyrin protein was detected in HCCs, para-carcinoma liver tissues and SK-Hep1 cell line by using peroxidase-labeled secondary antibody two-step IHC technique with the DAKO EnVision system (DAKO Corporation, carpinteria, USA). All paraffin embedded samples were deparaffined and rehydrated, and the SK-Hep1 cells cultured on coverslips were fixed in 4% polyformalin for 30 min, then pretreated with citrate buffer (0.01 mol/L citric acid: pH6.0) for 15 min at 100 °C in a microwave oven. After being treated with 0.3% H2O2 for 10 min to block the endogenous peroxidase, the sections were incubated with 10% fetal calf serum for 30 min to reduce nonspecific binding, and then the primary p28/gankyrin antibody was applied to the sections at 4 °C overnight. The sections were subsequently incubated with horseradish peroxidase (HR) -labeled goat anti-rabbit immunoglobulin for 30 min, followed by incubation with prepared liquid 3,3'-diaminobenzidine (DA) + substrate- chromogen solution for 10 min at room temperature. Finally, the sections were counterstained with hematoxylin. The negative controls were conducted by substituting rabbit normal serum for the primary antibody to verify the possibility of false-positive responses from the secondary antibody.
Clinical data and histopathological parameters
The following variables were evaluated: age, sex, HBsAg, accompanying cirrhosis, serum α-fetoprotein (AFP) level, tumor size, satellite nodules, formation of tumor capsule, fibrous capsular infiltration, portal vein tumor thrombus, microscopic invasion of the tumor into hepatic vein, and histological grade of HCC according to Edmonson and Steiner's classification[27].
Statistical analysis
Data of p28/gankyrin mRNA levels were presented as means ± SD. Significance of differences was assessed by analysis of variance (ANOVA) or Student's t-test. p values of ≤ 0.05 were considered statistically significant.
RESULTS
Clinical profiles and histopathologic examination
64 patients with primary HCC were recruited in this study, including 58 males and 6 females with ages ranging from 24 to 74 years (mean, 46.4 ± 10.5 years). Serum anti-hepatitis B virus was detected in 45 (70.3%) of 64 patients and positive anti-hepatitis C virus in serum was found in 6 (18.8%) of 32 patients examined. Fifty (78.1%) patients showed increased serum AFP level and levels above 1000 mg/L were observed in 29 of them. Histopathologically, 47 of 64 HCCs and 15 of 22 non-tumor liver tissues displayed cirrhosis. Among the 46 patients with fibrocapsule formation around HCC, 21 had broken through and 19 of them had microscopic hepatic vein involvement. Portal vein tumor thrombus was found in 18 (28.1%) patients and 32 (50.0%) patients had satellite nodules in primary HCC. According to Edmonson and Steiner's classification, 20 (31.3%) and 44 (68.7%) patients were classified to grade I-II group and grade III-IV group, respectively.
Tissue distribution of p28/gankyrin mRNA
Northern blot analysis showed that p28/gankyrin was expressed as one single transcript of 1.5 kb, corresponding well with the size of the cloned cDNA (Figure 1 A). p28/gankyrin was strongly expressed in brain and heart, whereas weaker expression was found in lung and spleen. Faint signals were discerned in muscle, but no signals were observed in digestive system including liver, pancreas, stomach, small and large intestine.
Figure 1.
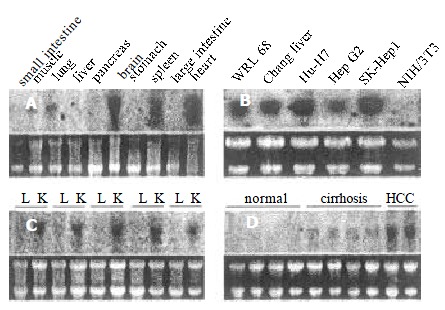
Northern blot of p28/gankyrin in a series of human normal tissues, hepatoma cell lines, HCC and non-tumor liver tissues. (A) p28/gankyrin expression in ten different normal adult human tissues. (B) p28/gankyrin expression in five hepatoma cell lines and NIH/3T3 cell. (C) p28/gankyrin expression in HCC and para-carcinoma liver tissues. L indicates the para-carcinoma liver tissues, and K indicates the HCC tissues. (D) Comparison of p28/gankyrin expression in normal human liver tissues, liver cirrhosis and HCC tissues. Each bottom panel showed equal amount of total RNA loading as indicated in 28 s and 18 s rRNA.
p28/gankyrin expression in hepatoma cell lines
In the 5 human hepatoma cell lines, p28/gankyrin mRNA was detected as the expected size with various expression levels (Figure 1 B). It was expressed strongly in SK-Hep1, weakly in HepG2 and no expression has been found in NIH/3T3 cell line.
p28/gankyrin expression in HCC, para-carcinoma and non-tumor liver tissues
p28/gankyrin mRNA expression levels were higher in the 64 HCC samples than in the para-carcinoma tissues (Figure 1 C). p28/gankyrin mRNA levels were markedly increased in 57 of 64 (89.1%), moderately increased in 5 of 64 (7.8%) HCC samples. In the remaining two HCC samples, p28/gankyrin mRNA was expressed at the same level as the para-carcinoma liver tissues. In contrast, p28/gankyrin mRNA expression was weakly detected in 18 of 64 (28%) and was below the level of detection in 46 of 64 (72%) para-carcinoma liver samples. In 22 non-tumor liver samples, p28/gankyirn was absent in 7 normal livers and weakly present in 15 liver cirrhosis tissues (Figure 1D). Densitometric analysis of all northern blots indicated that compared with liver cirrhosis and para-carcinoma liver tissues, the average expression of p28/gankyrin mRNA in HCC was increased by 3.6- (2.901 ± 0.507 vs 0.805 ± 0.252, P < 0.05) and 5.2-fold (2.901 ± 0.507 vs 0.557 ± 0.203, P < 0.01), respectively (Figure 2A).
Figure 2.
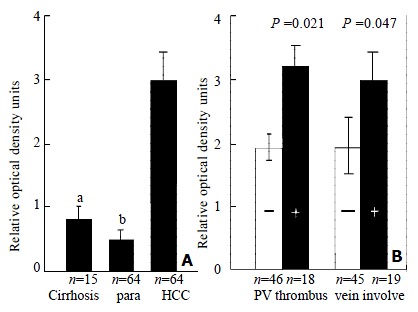
Densitometric analysis. The ratio of the optical density of p28/gankyrin mRNA to the corresponding 18 S rRNA signals was calculated and expressed as means ± SD. (A) Comparison of p28/gankyrin expression in human liver cirrhosis, para-carcinoma liver tissues and HCCs. aThe expression in HCCs was significantly higher than that in liver cirrhosis (P < 0.05). bThe expression in HCCs was significantly higher than that in para-carcinoma liver tissues (P < 0.01). (B) Clinicopathological significance of p28/gankyrin expression in HCCs. p28/gankyrin mRNA levels were significantly higher (P = 0.021) in portal vein thrombus group than in non-thrombus group, higher (P = 0.047) in microscopic hepatic vein involvement group than in non-hepatic vein involvement group.
Correlation of p28/gankyrin mRNA expression with clinicopathological parameters
Comparison of clinical profiles of HCC patients with p28/gankyrin mRNA expression was summarized in Table 1. From these results, age, sex, HBsAg, and accompanying cirrhosis were not significantly correlated with the expression levels of p28/gankyrin (P > 0.05). Relationships of p28/gankyrin mRNA expression with histopathological parameters were listed in Table 2. These results showed that there were no significant correlation between p28/gankyrin mRNA expression and various parameters, including serum AFP level, tumor size, satellite nodules, formation of tumor capsule, fibrous capsular infiltration, and histological grade of HCC. However, it was noted that p28/gankyrin mRNA levels (Figure 2B) were significantly higher (P = 0.021) in portal vein thrombus group (n = 18) than in non-thrombus group (n = 46), higher (P = 0.047) in microscopic hepatic vein involvement group (n = 19) than in non-hepatic vein involvement group (n = 45). This fact implies that tumors with high levels of p28/gankyrin mRNA expression tend to be more metastatic than those with low levels.
Table 1.
Clinical profiles of patients with HCC: Comparison with p28 mRNA expression
| Variables | n | mRNA levela | Df | p-valueb |
| Age (years) | 62 | 0.231 | ||
| < 40 | 18 | 2.910 ± 0.695 | ||
| 40-60 | 35 | 2.790 ± 0.367 | ||
| > 60 | 11 | 1.673 ± 0.546 | ||
| Sex | 62 | 0.401 | ||
| Male | 58 | 2.734 ± 0.422 | ||
| Female | 6 | 2.650 ± 0.238 | ||
| HBsAg | 62 | 0.269 | ||
| Positive | 45 | 2.919 ± 0.426 | ||
| Negative | 19 | 2.317 ± 0.362 | ||
| Cirrhosis | 62 | 0.180 | ||
| Absent | 17 | 3.157 ± 0.885 | ||
| Present | 47 | 2.631 ± 0.301 |
Abbreviations: HBsAg, hepatitis B surface antigen.
Results were expressed as the mean ± standard deviation.
p-value was based on the analysis of variance (ANOVA) or Student's t-test, variances were adjusted by Bartlett's method.
Table 2.
Clinicopathological features of patients with HCC: Comparison with p28 mRNA expression
| Variables | n | mRNA levela | Df | p-valueb |
| Serum AFP (ng/mL) | 62 | 0.330 | ||
| < 25 | 14 | 2.439 ± 0.362 | ||
| 25-1000 | 21 | 2.469 ± 0.273 | ||
| > 1000 | 29 | 2.821 ± 0.389 | ||
| Tumor size (cm) | 62 | 0.124 | ||
| < 5 | 21 | 2.393 ± 0.206 | ||
| 5-10 | 27 | 2.852 ± 0.468 | ||
| > 10 | 16 | 3.001 ± 0.652 | ||
| Capsule formation | 62 | 0.242 | ||
| Positive | 46 | 2.661 ± 0.421 | ||
| Negative | 18 | 3.016 ± 0.460 | ||
| Fibrous capsular infiltration | 44 | 0.240 | ||
| Positive | 21 | 2.833 ± 0.636 | ||
| Negative | 25 | 2.513 ± 0.226 | ||
| Hepatic vein involvement | 62 | 0.047 | ||
| Positive | 19 | 3.726 ± 0.306 | ||
| Negative | 45 | 2.442 ± 0.532 | ||
| Satellite nodules | 62 | 0.243 | ||
| Absent | 32 | 2.467 ± 0.209 | ||
| Present | 32 | 2.931 ± 0.554 | ||
| Portal vein thrombus | 62 | 0.021 | ||
| Absent | 46 | 2.390 ± 0.230 | ||
| Present | 18 | 3.971 ± 0.384 | ||
| Histological gradec | 62 | 0.466 | ||
| I-II | 20 | 2.809 ± 0.538 | ||
| III-IV | 44 | 2.859 ± 0.655 |
Abbreviations: serum AFP, serum alpha-fetoprotein.
Results were expressed as the mean ± standard deviation.
p-value was based on the analysis of variance (ANOVA) or Student's t-test, variances were adjusted by Bartlett'smethod. cAccording to Edmonson and Steiner's classification.
Immunohistochemical examination
Immunohistochemistry was performed in HCCs and corresponding para-carcinoma liver tissues to determine the exact cellular site of p28/gankyrin protein. p28/gankyrin was moderately to intensely present in the cytoplasm of most hepatocytes in HCC tissues, while nuclear immunostaining was also occasionally observed (Figure 3A). In para-carcinoma liver tissues, p28/gankyrin was weakly present in the cytoplasm of hepatocytes (Figure 3B). The histospecific expression of p28/gankyrin was absent in the bile duct cells, blood vein endometrial cells and other interstitial cells in liver tissues (Figure 3C). In SK-Hep1 cell line immunostaining, p28/gankyrin was mainly present in the cytoplasm, while occasionally in the nucleus (Figure 3D).
Figure 3.
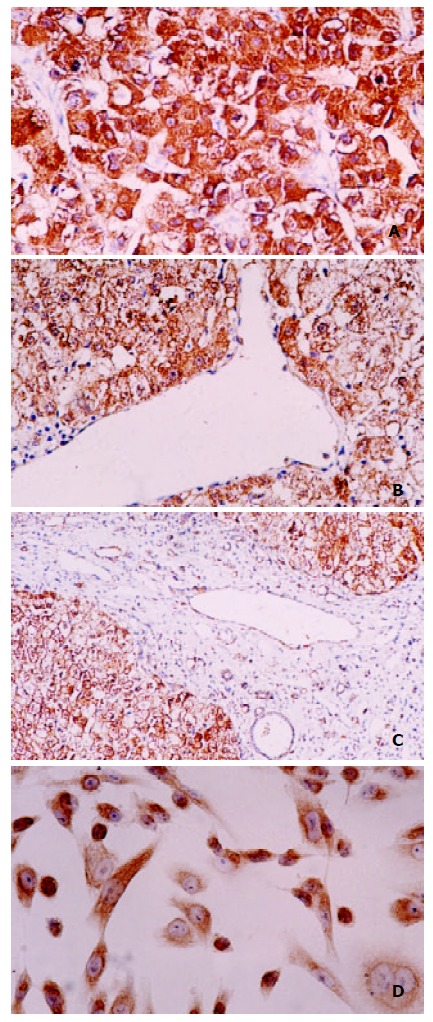
Immunohistochemical staining of P28/Gankyrin in HCC, para-carcinoma liver tissue and SK-Hep1 cell line. (A) p28/gankyrin was moderately to intensely present in the cytoplasm of most hepatocytes in HCC tissue, and occasionally present in the nucleus of hepatocytes; (B) In contrast, p28/gankyrin was weakly present in the cytoplasm of hepatocytes in para-carcinoma liver tissue; (C) p28/gankyrin protein signals were specifically present in the cytoplasm of hepatocytes, while absent in bile duct cells, blood vein endometrial cells and other interstitial cells; (D) In SK-Hep1 cells, p28/gankyrin was mostly located in cytoplasm and occasionally in the nucleus.
DISCUSSION
p28/gankyrin gene has been identified recently as an oncogene expressed in HCC and its up-regulation is not related to the grade or stage of the tumors[21]. However, the above mentioned study did not address the potential importance of normal or cirrhosis liver, in the context of high p28/gankyrin expression observed in HCC, nor did it investigate p28/gankyrin mRNA expression with respect to histopathological parameters of HCC. In the present study, p28/gankyrin mRNA expression was examined in the normal liver, liver cirrhosis, and HCC. p28/gankyrin mRNA was absent in normal liver samples, weakly expressed in liver cirrhosis samples and intensively expressed in 62 of 64 (96.8%) HCC samples compared with the para-carcinoma liver tissues by northern blot analysis. These findings indicate that p28/gankyrin is a useful biological marker of HCC and plays an important role in the process of liver carcinogenesis.
Interestingly, p28/gankyrin mRNA levels were significantly higher in HCCs with portal vein tumor thrombosis than in those without. Microscopic hepatic vein involvement is also an important correlated parameter with regard to p28/gankyirn mRNA expression. It is well known that hepatocellular carcinoma has a strong propensity to invade vessel and duct systems[28-30]. Rapid invasion and metastasis of HCC seriously hold back the clinical therapy and also significantly affect the prognosis of patients with HCC, as well as HCC recurrence after surgery[31-33]. According to our study, p28/gankyrin may play a significant role in the promotion of metastatic potential in HCC. Though the detailed mechanism of the promotion role of p28/gankyrin in HCC metastasis is currently under investigation, more valuable clinical management of HCC can be applied by the evaluation of p28/gankryin expression combined with other effective markers.
In the present study, p28/gankyrin was highly expressed in human brain and heart, weakly expressed in lung, spleen and muscle, suggesting that p28/gankyrin has a physiological role in these tissues. However, p28/gankyrin mRNA was not detected in human digestive system including liver, pancreas, stomach, small and large intestines. This result indicates that p28/gankyrin expression may be developmentally heterogeneous. Up-regulation of p28/gankyrin mRNA in liver where it is normally absent correlates significantly with the occurrence of HCC, which is like the alteration of serum AFP levels in patients with HCC[34,35]. This incident implies that to understand the mechanism of liver carcinogenesis promoted by p28/gankryin, the involvement of p28/gankryin in liver development process should be considered.
p28/gankyrin was strongly expressed in SK-Hep1 cells, while weakly in HepG2 cells. It should be noted that SK-Hep1, not HepG2, has the tumorigenic potential when inoculated in nude mice[36]. Thus, the higher expression of p28/gankyrin in SK-Hep1, to some extent, contributes to the cell tumorigenesis. This conclusion coincides well with our experiment that p28/gankyrin caused cell transformation after being transfected into NIH/3T3 cells (data not shown).
By using our specific polyclonal antibody against p28/gankyrin protein, the cellular localization of p28/gankyrin expression in HCC was determined. p28/gankyrin protein was mainly localized in the cytoplasm of hepatocytes, from weakly in hepatocytes of para-carcinoma cirrhosis liver to intensely in hepatocytes of HCC. Occasionally, the nuclear localization of p28/gankyrin in malignant hepatocytes of HCC was also observed. It is interesting that p28/gankyrin protein can not be detected in bile duct cells, blood vein endometrial cells and other interstitial cells in liver tissues. The prospective application of p28/gankyrin in clinical pathological diagnosis as a specific tissue marker should be investigated further by collecting more examination data and statistical analysis. In conclusion, p28/gankyrin mRNA is markedly overexpressed in HCC compared with para-carcinoma liver, normal liver or liver cirrhosis. In addition, in HCC, p28/gankyrin mRNA expression correlates significantly with metastasis potential of this cancer. The p28/gankyrinprotein is particularly highly expressed in the hepatocytes of HCC. These findings suggest that p28/gankyrin plays an important role in the carcinogenesis of HCC by influencing tumor metastasis behavior and that it may serve as a new specific tissue marker for the pathological diagnosis of HCC.
Footnotes
Edited by Pang LH
Supported by the Chinese National Distinguished Young Scholar Awards, No.39825114, Chinese National Key Project of Basic Research, No. G1998051210 and the Key Project of the Chinese National Natural Science Foundation, No.39830080.
References
- 1.Teo EK, Fock KM. Hepatocellular carcinoma: an Asian perspective. Dig Dis. 2001;19:263–268. doi: 10.1159/000050692. [DOI] [PubMed] [Google Scholar]
- 2.Yu MC, Yuan JM, Govindarajan S, Ross RK. Epidemiology of hepatocellular carcinoma. Can J Gastroenterol. 2000;14:703–709. doi: 10.1155/2000/371801. [DOI] [PubMed] [Google Scholar]
- 3.Guo SP, Wang WL, Zhai YQ, Zhao YL. Expression of nuclear factor-kappa B in hepatocellular carcinoma and its relation with the X protein of hepatitis B virus. World J Gastroenterol. 2001;7:340–344. doi: 10.3748/wjg.v7.i3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sylla A, Diallo MS, Castegnaro J, Wild CP. Interactions between hepatitis B virus infection and exposure to aflatoxins in the development of hepatocellular carcinoma: a molecular epidemiological approach. Mutat Res. 1999;428:187–196. doi: 10.1016/s1383-5742(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 5.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shea KA, Fleming LE, Wilkinson JD, Wohler-Torres B, McKinnon JA. Hepatocellular carcinoma incidence in Florida. Ethnic and racial distribution. Cancer. 2001;91:1046–1051. [PubMed] [Google Scholar]
- 7.Yu MW, Chiu YH, Yang SY, Santella RM, Chern HD, Liaw YF, Chen CJ. Cytochrome P450 1A1 genetic polymorphisms and risk of hepatocellular carcinoma among chronic hepatitis B carriers. Br J Cancer. 1999;80:598–603. doi: 10.1038/sj.bjc.6690397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zondervan PE, Wink J, Alers JC, IJzermans JN, Schalm SW, de Man RA, van Dekken H. Molecular cytogenetic evaluation of virus-associated and non-viral hepatocellular carcinoma: analysis of 26 carcinomas and 12 concurrent dysplasias. J Pathol. 2000;192:207–215. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH690>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Feitelson MA, Sun B, Satiroglu Tufan NL, Liu J, Pan J, Lian Z. Genetic mechanisms of hepatocarcinogenesis. Oncogene. 2002;21:2593–2604. doi: 10.1038/sj.onc.1205434. [DOI] [PubMed] [Google Scholar]
- 10.Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991;350:427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Wang Y, Zhou Q, Gui SY, Li X. The point mutation of p53 gene exon7 in hepatocellular carcinoma from Anhui Province, a non HCC prevalent area in China. World J Gastroenterol. 2002;8:480–482. doi: 10.3748/wjg.v8.i3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liew CT, Li HM, Lo KW, Leow CK, Chan JY, Hin LY, Lau WY, Lai PB, Lim BK, Huang J, et al. High frequency of p16INK4A gene alterations in hepatocellular carcinoma. Oncogene. 1999;18:789–795. doi: 10.1038/sj.onc.1202359. [DOI] [PubMed] [Google Scholar]
- 13.Jin M, Piao Z, Kim NG, Park C, Shin EC, Park JH, Jung HJ, Kim CG, Kim H. p16 is a major inactivation target in hepatocellular carcinoma. Cancer. 2000;89:60–68. doi: 10.1002/1097-0142(20000701)89:1<60::aid-cncr9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.DeMartino GN, Moomaw CR, Zagnitko OP, Proske RJ, Chu-Ping M, Afendis SJ, Swaffield JC, Slaughter CA. PA700, an ATP-dependent activator of the 20 S proteasome, is an ATPase containing multiple members of a nucleotide-binding protein family. J Biol Chem. 1994;269:20878–20884. [PubMed] [Google Scholar]
- 15.Kato S, Sekine S, Oh SW, Kim NS, Umezawa Y, Abe N, Yokoyama-Kobayashi M, Aoki T. Construction of a human full-length cDNA bank. Gene. 1994;150:243–250. doi: 10.1016/0378-1119(94)90433-2. [DOI] [PubMed] [Google Scholar]
- 16.Hori T, Kato S, Saeki M, DeMartino GN, Slaughter CA, Takeuchi J, Toh-e A, Tanaka K. cDNA cloning and functional analysis of p28 (Nas6p) and p40.5 (Nas7p), two novel regulatory subunits of the 26S proteasome. Gene. 1998;216:113–122. doi: 10.1016/s0378-1119(98)00309-6. [DOI] [PubMed] [Google Scholar]
- 17.Baumeister W, Walz J, Zühl F, Seemüller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–380. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- 18.Michaely P, Bennett V. The ANK repeat: a ubiquitous motif involved in macromolecular recognition. Trends Cell Biol. 1992;2:127–129. doi: 10.1016/0962-8924(92)90084-z. [DOI] [PubMed] [Google Scholar]
- 19.Bork P. Hundreds of ankyrin-like repeats in functionally diverse proteins: mobile modules that cross phyla horizontally? Proteins. 1993;17:363–374. doi: 10.1002/prot.340170405. [DOI] [PubMed] [Google Scholar]
- 20.Venkataramani R, Swaminathan K, Marmorstein R. Crystal structure of the CDK4/6 inhibitory protein p18INK4c provides insights into ankyrin-like repeat structure/function and tumor-derived p16INK4 mutations. Nat Struct Biol. 1998;5:74–81. doi: 10.1038/nsb0198-74. [DOI] [PubMed] [Google Scholar]
- 21.Higashitsuji H, Itoh K, Nagao T, Dawson S, Nonoguchi K, Kido T, Mayer RJ, Arii S, Fujita J. Reduced stability of retinoblastoma protein by gankyrin, an oncogenic ankyrin-repeat protein overexpressed in hepatomas. Nat Med. 2000;6:96–99. doi: 10.1038/71600. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Sampath A, Raychaudhuri P, Bagchi S. Both Rb and E7 are regulated by the ubiquitin proteasome pathway in HPV-containing cervical tumor cells. Oncogene. 2001;20:4740–4749. doi: 10.1038/sj.onc.1204655. [DOI] [PubMed] [Google Scholar]
- 23.Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spataro V, Norbury C, Harris AL. The ubiquitin-proteasome pathway in cancer. Br J Cancer. 1998;77:448–455. doi: 10.1038/bjc.1998.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. In: A laboratory manual., editor. 2nd editors. New York: Cold Spring Harbor Laboratory Press; 1989. pp. 502–506. [Google Scholar]
- 26.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 27.EDMONDSON HA, STEINER PE. Primary carcinoma of the liver: a study of 100 cases among 48, 900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Yuki K, Hirohashi S, Sakamoto M, Kanai T, Shimosato Y. Growth and spread of hepatocellular carcinoma. A review of 240 consecutive autopsy cases. Cancer. 1990;66:2174–2179. doi: 10.1002/1097-0142(19901115)66:10<2174::aid-cncr2820661022>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 29.Toyosaka A, Okamoto E, Mitsunobu M, Oriyama T, Nakao N, Miura K. Intrahepatic metastases in hepatocellular carcinoma: evidence for spread via the portal vein as an efferent vessel. Am J Gastroenterol. 1996;91:1610–1615. [PubMed] [Google Scholar]
- 30.Ikeda Y, Matsumata T, Adachi E, Hayashi H, Takenaka K, Sugimachi K. Hepatocellular carcinoma of the intrabiliary growth type. Int Surg. 1997;82:76–78. [PubMed] [Google Scholar]
- 31.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamanaka J, Yamanaka N, Nakasho K, Tanaka T, Ando T, Yasui C, Kuroda N, Takata M, Maeda S, Matsushita K, et al. Clinicopathologic analysis of stage II-III hepatocellular carcinoma showing early massive recurrence after liver resection. J Gastroenterol Hepatol. 2000;15:1192–1198. doi: 10.1046/j.1440-1746.2000.02323.x. [DOI] [PubMed] [Google Scholar]
- 33.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. [PubMed] [Google Scholar]
- 34.Izumi R, Shimizu K, Kiriyama M, Hashimoto T, Urade M, Yagi M, Mizukami Y, Nonomura A, Miyazaki I. Alpha-fetoprotein production by hepatocellular carcinoma is prognostic of poor patient survival. J Surg Oncol. 1992;49:151–155. doi: 10.1002/jso.2930490305. [DOI] [PubMed] [Google Scholar]
- 35.Nomura F, Ohnishi K, Tanabe Y. Clinical features and prognosis of hepatocellular carcinoma with reference to serum alpha-fetoprotein levels. Analysis of 606 patients. Cancer. 1989;64:1700–1707. doi: 10.1002/1097-0142(19891015)64:8<1700::aid-cncr2820640824>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 36.Marra CA, de Alaniz MJ. Incorporation and metabolic conversion of saturated and unsaturated fatty acids in SK-Hep1 human hepatoma cells in culture. Mol Cell Biochem. 1992;117:107–118. doi: 10.1007/BF00230749. [DOI] [PubMed] [Google Scholar]


