Abstract
AIM: To investigate the correlation between subcellular daunorubicin distribution and the multidrug resistance phenotype in drug-resistant cell line SMMC-7721/R.
METHODS: The multidrug resistant cell line SMMC-7721/R, a human hepatocellular carcinoma cell line, was established. Antisense oligonucleotides (AS-ODN) were used to obtain different multidrug resistance phenotypes by inhibiting the expression of mdr1 gene and/or multidrug resistance-related protein gene (mrp) using Lipofectamine as delivery agent. Expression of mdr1 and mrp genes was evaluated by RT-PCR and Western blotting. Intracellular daunorubicin (DNR) concentration was measured by flow cytometry. Subcellular DNR distribution was analyzed by confocal laser scanning microscopy. Adriamycin (ADM) and DNR sensitivity was examined by MTT method.
RESULTS: Low level expression of mdr1 and mrp mRNAs and no expression of P-Glycoprotein (P-gp) and multidrug resistance-related protein (P190) were detected in parental sensitive cells SMMC-7721/S, but over-expression of these two genes was observed in drug-resistant cell SMMC-7721/R. The expression of mdr1 and mrp genes in SMMC-7721/R cells was down-regulated to the level in the SMMC-7721/S cells by AS-ODN. Intracellular DNR concentration in SMMC-7721/S cells was 10 times higher than that in SMMC-7721/R cells. In SMMC7721/S cells intracellular DNR distributed evenly in the nucleus and cytoplasm, while in SMMC-7721/R cells DNR distributed in a punctate pattern in the cytoplasm and was reduced in the nucleus. DNR concentration in SMMC-7721/R cells co-transfected with AS-ODNs targeting to mdr1 and mrp mRNAs recovered to 25 percent of that in SMMC7721/S cells. Intracellular DNR distribution pattern in drug-resistant cells treated by AS-ODN was similar to drug-sensitive cell, and the cells resistance index (RI) to DNR and ADM decreased at most from 88.0 and 116.0 to 4.0 and 2.3, respectively. Co-Transfection of two AS-ODNs showed a stronger synergistic effect than separate transfection.
CONCLUSIONS: P-gp and P190 are two members mediating MDR in cell line SMMC7721/R. Intracellular drug concentration increase and subcellular distribution change are two important factors in multidrug resistance (MDR) formation. The second factor, drugs transport by P-gp and P190 from cell nucleus to organell in cytoplasm, may play a more important role.
INTRODUCTION
Multidrug resistance (MDR) remains a significant obstacle for cancer chemotherapy. The MDR observed in many cell lines is most commonly accompanied with overexpression of one or both of the members of the ATP-binding cassette superfamily of transport proteins, P-glycorprotein (P-gp) and multidrug resistance-related protein (Mrp, P190)[1-7]. P-gp or P190 acts as an energy-dependent outward transport pump, removing drugs from the cytoplasm and from the plasma membrane, thereby decreasing intracellular drug accumulation[8-12].
Human hepatocellular carcinoma drug-resistant cell line SMMC7721/R showed a strong multidrug resistance to DNR and other anthracycline, and overexpression of P-gp and P190 was observed in this cell line. Previous studies suggested that subcellular drug distribution contributing to cells drug resistance may be mostly mediated by P-gp and/or P190 in many other cell lines[13-20]. But there is no direct evidence suggesting the role of these two pump proteins in MDR of SMMC7721/R. In order to understand MDR phenotype and mechanism in SMMC7721/R, based on previous studies of antisense technology related to mdr1 gene and mrp gene, we used laser scanning confocal microscopy to evaluate the intracellular distribution of DNR and then explored the correlation of intracellular drug (DNR) transportation and distribution with multidrug resistance phenotype.
MATERIALS AND METHODS
Cell lines and culture conditions
Human hepatocellular carcinoma cell line SMMC-7721 was provided by Cancer Research Institution, West China Hospital of Sichuan University. Drug-resistance cell line was established by the stepwise selection with increasing concentration of ADM as previously described[21]. The ADM gradually increased from 0.005 μmg/mL to 0.1 μmg/mL.
Cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum in a 5% CO2 atmosphere at 37 °C.
Materials
Phosphorothioate antisense oligonucleotides (AS-ODN): targeting to mdr1 start codon region (AS-ODN/mdr1): 5’-CCATCCCGACCTCGCGCTCC-3’[22], targeting to mrp coding region (AS-ODN/mrp): 5’-TGCTGTTCGTGCCCCCGCCG-3’[23]. Control oligonucleotide (AS-ODN/nonsense) was a 20-mer nonsense phosphorothioate oligonucleotide. All oligonucleotides were synthesized by Life Technologies Inc, USA. Lipofectamine, TRIzol, RT-PCR kit, and primers were also purchased from Life Technologies Inc. Lumi-LightPLUS Western Blotting Kit was purchased from Boehringer Mannheim, German. Antibodies against mdr1/P-gp and mrp/P190 were from Santa Cruz, USA. DNR was purchased from Minalo Inc, Italy.
Treatment of cells with AS-ODNs
The experimental protocols were similar to those previously described[22,24]. Briefly, cells (5 × 105) were seeded in a 25 mL flask at 1 × 105 cells/mL and grown to 75% confluence. Cells were transfected with 1.5 nmol of AS-ODN with 50 mL of Lipofectamine. Cells were harvested at different times after transfection for analysis.
Detection of drug sensitivity of cells by MTT[25,26]
Cells were exposed to drug at 37 °C for 2 h, then were washed and seeded (50000 cells/mL) in 96-well microplates for 72 h. MTT (20 μL, 2.5 mg/mL) was added to each well for 3 h. Medium was discarded and 150 μL of DMSO were added to each well. Optical densities were measured at 490 nm (A490). The tumor cells living ratio (TCL) was determined according to the formula: TCL = A490experient/A490control × 100%. The 50% inhibitory concentration (IC50) was calculated according to concentration-TCL curve. Resistance index (RI) was calculated using the formula: RI = IC50 drug resisant cells/IC50 parent cells.
Measurement of mdr1 and mrp mRNAs by RT-PCR
Primers mrp: 5'-TGAAGGACTTCGTGTCAGCC-3' 5'-GTCCATGATGGTGTTGAGCC-3' mdr1: 5'-GGCTCCGATACATGGTTTTCC-3' 3'-TTCAGTGCGATCTTCCCAGC-5'. β2-microglobulin (β2M): 5'-ACCCCCACTGAAAAAGATGA-3' 5'-ATCTTCAAACCTCCATGATG-3'.
β2M expression was used as control for the amount of RNA used. Total RNA from cells was extracted using TRIzol. The effect of AS-ODN was studied after 24 h pre-incubation with AS-ODN and Lipofectamine. Mrp, mdr1, and β2M RNA transcripts were detected using RT-PCR as described before[27,28]. An aliquot of each reaction mixture was then analyzed by electrophoresis on 2% agarose gel. Densitometry was performed using UVP gel image analysis system (BIO-RAD, USA) and the ratio between the target and control PCR products was determined by dividing the densitometric volume of the target band by that of the control band.
Measurement of cell expression of P-gp and P190 with western blotting
The expression of the two proteins was detected according to the manufacturer's instructions of Lumi-LightPLUS Western Blotting Kit. The concentrations of the primary antibodies against P-gp and P190 were 1 μg/mL and 2 μg/mL, respectively. The concentrations of the secondary antibodies were both 0.4 μg/mL.
Observation of intracellular DNR distribution by confocal laser scanning microscopy (CLSM)
The experimental procedures were similar to those previously described[29-31]. Cells (1 × 105) were seeded to 960 mm2 petri dish with a slide inside and incubated in a 5% CO2 atmosphere at 37 °C. After cells had reached 75% confluence, normal medium was replaced with serum-free RPMI 1640 medium and cells were incubated with DNR at 2 μg/mL for 1 h. The effect of AS-ODN was studied after 72 h pre-incubation with AS-ODN and Lipofectamine. After two washes with PBS and addition of drug free medium cells grown on slides were examined with CLSM (MRC-1024ES, BIO-RAD Inc., USA). Cover-slips were mounted on slides, supported and sealed on lacquer tiers to prevent compression and drying out. Intracellular drug fluorescence was observed using the 488 nm laser line for excitation and the filter that allows measurement of emitted light above 515 nm.
Detection of intracellular DNR concentration by flow cytometry (FCM)
Cells (1 × 105) were seeded into 25 mL flask and grown to 95% confluence. They were then dissociated with pancreatin and suspended in serum-free medium. DNR was added to a final concentration of 2 μg/mL and incubated for 1 h at 37 °C. After two washes with PBS each sample was divided into 3 tubes to be analyzed by FCM[32] (Elite ESP, Coulter Inc., USA). Results were expressed as the ratio of fluorescence intensity values between each experimental sample and control sample of SMMC7721/R cells without treatment.
RESULTS
Drug sensitivity
As shown in Table 1, the parental sensitive cells SMMC7721/S were highly sensitive to DNR and ADM. However, the drug resistant cells SMMC7721/R were 88-fold resistant to DNR and 116-fold to ADM, respectively, when compared with SMMC7721/S cells. Resistance index (RI) to DNR and ADM in SMMC7721/R cells co-transfected of AS-ODN/mdr1 (0.5 μmol/L) and AS-ODN/mrp (0.5 μmol/L) decreased from 88 and 116 to 4 and 2.3, respectively. Treatment with separate AS-ODN/mdr1 or AS-ODN/mrp showed a lesser reversal effect on MDR than co-transfection of these two AS-ODNs. Nonsense oligonucleotides did not affect the drug resistance in SMMC7721/R cells.
Table 1.
Effect of AS-ODN on drug sensitivity of cells (¯x ± s)
| Cells |
IC50 (mg/L) |
RI |
||
| DNR | ADM | DNR | ADM | |
| SMMC-7721/S | 0.003 ± 0.0006 | 0.004 ± 0.0008 | 1.0 | |
| SMMC-7721/R | 0.264 ± 0.0094 | 0.463 ± 0.0254 | 88.0 | 116.0 |
| AS-ODN/mdrr1 | 0.094 ± 0.0065 | 0.079 ± 0.0014 | 31.4 | 19.8 |
| AS-ODN/mrp | 0.072 ± 0.0002 | 0.097 ± 0.0009 | 24.0 | 24.3 |
| SMMC-7721/R+ | 0.012 ± 0.0011 | 0.009 ± 0.0007 | 4.00 | 2.3 |
| AS-ODN/mdr1 + mrp | ||||
| SMMC-7721/R+ | 0.245 ± 0.0110 | 0.451 ± 0.0187 | 81.7 | 112.8 |
| AS-ODN/nonsense | ||||
Values (IC50) represent the mean ± standard deviation of at least three experiments and value (RI) represent the mean of three experiments. All values were calculated as described in MATERIALS AND METHODS. The concentration of each AS-ODN is 0.5 μmol/L and the treatment time is 72 h.
Expression of mdr1 geng and mrp gene
As shown by RT-PCR, The amplification products of mrp, mdr1, and β2M were 256 bp, 168 bp, and 120 bp, respectively. Over-expression of mrp and mdr1 mRNAs were detected in SMMC7721/R, but low level mRNA expression in SMMC7721/S was observed. The mRNA expression in SMMC7721/R cells treated with AS-ODN decreased to the level of SMMC7721/S (Figure 1).
Figure 1.
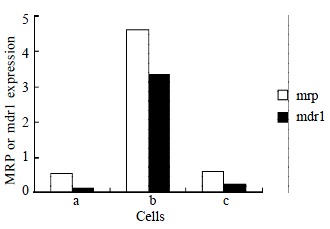
Quantification of PCR. The ratio between the mdr1 or mrp and β2M gene is expressed as described in MATERIALS AND METHODS. (a. parental cell-SMMC7721/S; b. SMMC7721/R; c. SMMC7721/R incubated with 0.5 μmol/L AS-ODN for 24 h)
No P-gp and P190 were detected in parental cells SMMC7721/S. Over-expression of P-gp and P190 was observed in drug resistant cells SMMC7721/R. Treatment of SMMC7721/R with AS-ODNs inhibited the expression of P-gp and P190 (Figure 2).
Figure 2.
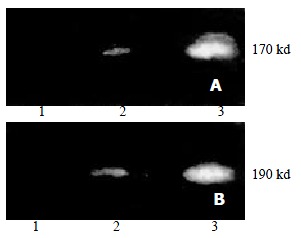
Expression of P-gp and P190 analyzed with Wstern blot. (a) immunoblotted with anit-P-gp antibody; (b) immunoblotted with anti-P190 antibody. 1. parental cell-SMMC7721/S; 2. SMMC7721/R treated by AS-ODN (0.5 μmol/L, 72 h); 3. SMMC7721/R.
Intracellular accumulation of DNR
Intracellular concentration of drugs in drug-resistant cells reduced to 10 percent of that in parental cells SMMC7721/S. Co-transfection of AS-ODN/mdr1+mrp up-regulated intracellular drug concentration to 4 times of that in SMMC7721/R cells. The up-regulation effect of separate transfection of AS-ODN/mdr1 or AS-ODN/mrp was much less than co-transfection (Figure 3).
Figure 3.
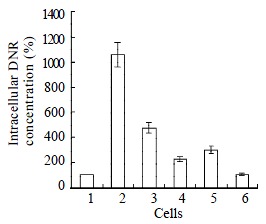
Intracellular DNR concentrations in cells treated with antisense oligonucleotids. The concentration of each AS-ODN is 0.5 mmol/L and the treatment time is 72 h. Data are the mean ± standard deviation of three independent experiments. (1. SMMC7721/R; 2. SMMC7721/S; 3. SMMC7721/R treated with AS-ODN/mdr1+mrp; 4. SMMC7721/R treated with AS-ODN/mdr1; 5. SMMC7721/R treated by AS-ODN/mrp; 6. SMMC7721/R treated with AS-ODN/nonsense.)
Subcellular distribution of DNR
In parental cells SMMC7721/S DNR fluorescence mainly located in the nucleus and was diffusely present in the cytoplasm (Figure 4). In drug-resistant cells SMMC7721/R, DNR fluorescence retained in the perinuclear zone and in peripheral punctate vesicles, with little or no drug in the nuclear zone (Figure 5).
Figure 4.
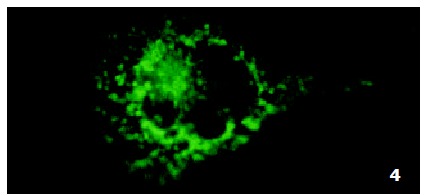
Intracellular DNR distribution in parental sensitive cells SMMC7721/S.
Figure 5.
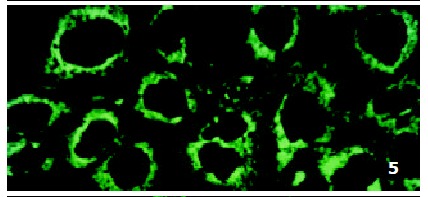
Intracellular DNR distribution in drug-resistant cells SMMC7721/R.
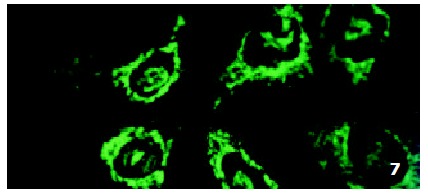
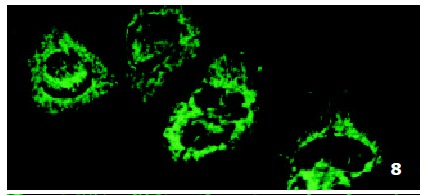
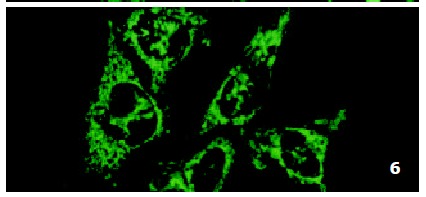
In SMMC7721/R cells incubated separately with AS-ODN/mrp or AS-ODN/mdr1 for 72 h, the subcellular distribution of DNR was similar to parental cells SMMC7721/S, with less intranuclear fluorescence intensity than in SMMC7721/S cells. Cells transfected separately with AS-ODN/mrp or AS-ODN/mdr1 did not show any difference in subcellular DNR distribution (Figure 6 and Figure 7). Co-transfection of AS-ODN/mdr1 and AS-ODN/mrp caused more drugs accumulation in nuclear than that in cells incubated separately with either AS-ODN (Figure 8). Nonsense oligonucleotides did not affect the drug subcellular distribution pattern in drug-resistant cells SMMC7721/R (Figure 9).
Figure 6.
Intracellular DNR distribution in drug-resistant cells SMMC7721/R pre-treated with AS-ODN/mdr1 (0.5 μmol/L) for 72 h.
Figure 7.
Intracellular DNR distribution in drug-resistant cells SMMC7721/R treated with AS-ODN/mrp (0.5 μmol/L) for 72 h.
Figure 8.
Intracellular DNR distribution in drug-resistant cells SMMC7721/R pre-treated with AS-ODN/mdr1 and AS-ODN/mrp. Each AS-ODN concentration is 0.5 μmol/L and the treatment time is 72 h.
Figure 9.
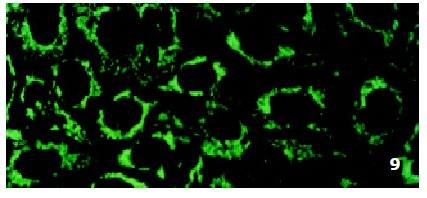
Intracellular DNR distribution in drug-resistant cells SMMC7721/R treated with nonsense oligonucleotides (0.5 μmol/L) for 72 h.
DISCUSSION
It has been reported that parental SMMC7721 cells have no or low level expression of mdri/P-gp and mrp/P190. However, over-expression of P-gp and P190 was observed in drug-resistant cells SMMC7721/R, which was confirmed in our study.
In order to obtain cell line with different multidrug resistance phenotypes, we made use of the specificity of antisense oligonucleotides in down-regulating gene expression to specifically block mdr1/P-gp or mrp/P190 function. Antisense technology is increasingly becoming a reliable tool for manipulation of gene expression and rapidly moving into the therapeutic arena[33-47]. Down-regulation of expression of mdr1 mRNA/P-gp and mrp mRNA/P190 by AS-ODN was observed and accompanied with recovery of drug sensitivity in SMMC7721/R cells. Drug-resistant SMMC7721/R cells co-transfected with AS-ODN/mrp and AS-ODN/mdr1 showed 50-fold drug sensitivity to ADM, when compared with drug-resistant cells SMMC7721/R.
The fluorescence emitted by anthracycline DNR, a substrate of P-gp and P190, can be detected by FCM and CSLM[14,17,31,48,51]. There is a good correlation between intracellular drug concentration and cells drug sensitivity[8,16,49,50,52,53]. In our studies, FCM data enforced the opinion that reduction of intracellular drug concentration results in increase of drug resistance in cells.
Intracellular DNR concentration in cell line SMMC7721/R decreased to 10 percent of that in SMMC7721/S and meanwhile resistance to ADM and DNR increased by 116 times and 88 times respectively than that of SMMC7721/S. Co-transfection of AS-ODN/mrp and AS-ODN/mdr1 only restored intracellular DNR concentration to 40 percent of that of SMMC7721/S. Inconsistently, the drug resistance to DNR was reduced from 88-fold to 4-fold and to ADM from 116-fold to 2.3-fold when compared with SMMC7721/S. These data indicate that some other factors must play a more important role in MDR mechanism besides the reduction of intracellular drug accumulation. The mechanism focused in recently is modified drug localization[13,51,54]. We used CSLM technology to explore this interesting point.
In our studies, we observed that in cell line SMMC7721/S DNR fluorescence distributed evenly in the nucleus and cytoplasm, while in cell line SMMC7721/R DNR distributed in a punctate pattern in the cytoplasm and was reduced in the nucleus. Transfection of AS-ODN changed the the subcellular DNR distribution pattern in SMMC7721/S cells to that in SMMC7721/R cells. This observation indicates that P-gp or P190 not only pumps DNR out of cells, but also transports DNR from nuclear to cytoplasm and into some organelles such as Golgi apparatus. As P-gp or P190 probably locates in cell membrane, nuclear membrane, Golgi apparatus, or endoplasmic reticulum[13,54-59], the actions of these two proteins may cause reduction of intracellular and intranuclear drug concentration and drug accumulation in some organelles. The combined effect may prevent the targeting of the drugs to nucleus, which reduces cell death even if the total amount of drugs inside the cells was not dropped significantly. Golgi apparatus is widely believed to be the organelle which holds the drugs[54,59]. Previous studies found that there were some differences in subcellular drug distribution pattern between different cell lines in which MDR phenotype was mediated by P-gp and by P190[49]. However, we did not observe such phenomenon in the present study.
Our studies suggest that after the potent inhibition of P-gp or P190 expression by AS-ODN intracellular drug concentration was increased and subcellular drug distribution changed, which leads to the reversal of multidrug resistance in cell line SMMC7721/R. Therefore, we believe that over-expression of P-gp and/or P190 is an important mechanism in mediating MDR in cell line SMMC7721/R.
Although co-transfection of AS-ODNs targeting the two genes didn't enhance the inhibitory effect on expression of P-gp or P190 when compared with separate transfection of either AS-ODN, the synergistic effects of the two AS-ODNs on reduction of intracellular or intranuclear drugs and on recovery of cell drug sensitivity were much more prominent than separate transfection. This finding indicates that MDR in SMMC7721/R is mediated at least by both P-gp and P190. The combination of MDR reversal methods against these proteins is effective in drug-resistant cells.
Footnotes
Edited by Bo XN
Supported by the grant from National Nature Science Foundation of China, No.39770723
Author contributions: All authors contributed equally to the work.
References
- 1.van Brussel JP, van Steenbrugge GJ, Romijn JC, Schröder FH, Mickisch GH. Chemosensitivity of prostate cancer cell lines and expression of multidrug resistance-related proteins. Eur J Cancer. 1999;35:664–671. doi: 10.1016/s0959-8049(98)00435-3. [DOI] [PubMed] [Google Scholar]
- 2.Liu ZM, Shou NH. Expression significance of mdr1 gene in gastric carcinoma tissue. Shijie Huaren Xiaohua Zazhi. 1999;7:145–146. [Google Scholar]
- 3.Ning XX, Wu KC, Shi YQ, Wang X, Zhao YQ, Fan DM. Construction and expression of gastric cancer MG7 mimic epitope fused to heat shock protein 70. Shijie Huaren Xiaohua Zazhi. 2001;9:892–896. [Google Scholar]
- 4.Leith CP, Kopecky KJ, Chen IM, Eijdems L, Slovak ML, McConnell TS, Head DR, Weick J, Grever MR, Appelbaum FR, et al. Frequency and clinical significance of the expression of the multidrug resistance proteins MDR1/P-glycoprotein, MRP1, and LRP in acute myeloid leukemia: a Southwest Oncology Group Study. Blood. 1999;94:1086–1099. [PubMed] [Google Scholar]
- 5.Zhang LJ, Chen KN, Xu GW, Xing HP, Shi XT. Congenital expression of mdr-1 gene in tissues of carcinoma and its relation with pathomorphology and prognosis. World J Gastroenterol. 1999;5:53–56. doi: 10.3748/wjg.v5.i1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin F, Shi YQ, Zhao WP, Xiao B, Miao JY, Fan DM. Suppression of P-gp induced multiple drug resistance in a drug resistant gastric cancer cell line by overexpression of Fas. World J Gastroenterol. 2000;6:664–670. doi: 10.3748/wjg.v6.i5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu B, Staren E, Iwamura T, Appert H, Howard J. Effects of Taxotere on invasive potential and multidrug resistance phenotype in pancreatic carcinoma cell line SUIT-2. World J Gastroenterol. 2001;7:143–148. doi: 10.3748/wjg.v7.i1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferlini C, Distefano M, Pignatelli F, Lin S, Riva A, Bombardelli E, Mancuso S, Ojima I, Scambia G. Antitumour activity of novel taxanes that act at the same time as cytotoxic agents and P-glycoprotein inhibitors. Br J Cancer. 2000;83:1762–1768. doi: 10.1054/bjoc.2000.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wada H, Saikawa Y, Niida Y, Nishimura R, Noguchi T, Matsukawa H, Ichihara T, Koizumi S. Selectively induced high MRP gene expression in multidrug-resistant human HL60 leukemia cells. Exp Hematol. 1999;27:99–109. doi: 10.1016/s0301-472x(98)00027-7. [DOI] [PubMed] [Google Scholar]
- 10.Benderra Z, Morjani H, Trussardi A, Manfait M. Characterization of H+-ATPase-dependent activity of multidrug resistance-associated protein in homoharringtonine-resistant human leukemic K562 cells•L. eukemia. 1998;12:1539–1544. doi: 10.1038/sj.leu.2401166. [DOI] [PubMed] [Google Scholar]
- 11.Tkaczyk-Gobis K, Tarasiuk J, Seksek O, Stefanska B, Borowski E, Garnier-Suillerot A. Transport of new non-cross-resistant antitumor compounds of the benzoperimidine family in multidrug resistant cells. Eur J Pharmacol. 2001;413:131–141. doi: 10.1016/s0014-2999(01)00728-2. [DOI] [PubMed] [Google Scholar]
- 12.Heijn M, Hooijberg JH, Scheffer GL, Szabó G, Westerhoff HV, Lankelma J. Anthracyclines modulate multidrug resistance protein (MRP) mediated organic anion transport. Biochim Biophys Acta. 1997;1326:12–22. doi: 10.1016/s0005-2736(97)00003-5. [DOI] [PubMed] [Google Scholar]
- 13.Larsen AK, Escargueil AE, Skladanowski A. Resistance mechanisms associated with altered intracellular distribution of anticancer agents. Pharmacol Ther. 2000;85:217–229. doi: 10.1016/s0163-7258(99)00073-x. [DOI] [PubMed] [Google Scholar]
- 14.Borg AG, Burgess R, Green LM, Scheper RJ, Liu Yin JA. P-glycoprotein and multidrug resistance-associated protein, but not lung resistance protein, lower the intracellular daunorubicin accumulation in acute myeloid leukaemic cells. Br J Haematol. 2000;108:48–54. doi: 10.1046/j.1365-2141.2000.01793.x. [DOI] [PubMed] [Google Scholar]
- 15.Okumura H, Chen ZS, Sakou M, Sumizawa T, Furukawa T, Komatsu M, Ikeda R, Suzuki H, Hirota K, Aikou T, et al. Reversal of P-glycoprotein and multidrug-resistance protein-mediated drug resistance in KB cells by 5-O-benzoylated taxinine K. Mol Pharmacol. 2000;58:1563–1569. doi: 10.1124/mol.58.6.1563. [DOI] [PubMed] [Google Scholar]
- 16.Manciu L, Chang X, Riordan JR, Buyse F, Ruysschaert JM. Nucleotide-induced conformational changes in the human multidrug resistance protein MRP1 are related to the capacity of chemotherapeutic drugs to accumulate or not in resistant cells. FEBS Lett. 2001;493:31–35. doi: 10.1016/s0014-5793(01)02270-0. [DOI] [PubMed] [Google Scholar]
- 17.Gong Y, Wang Y, Chen F, Han J, Miao J, Shao N, Fang Z, Ou Yang R. Identification of the subcellular localization of daunorubicin in multidrug-resistant K562 cell line. Leuk Res. 2000;24:769–774. doi: 10.1016/s0145-2126(00)00048-5. [DOI] [PubMed] [Google Scholar]
- 18.Chou KM, Paul Krapcho A, Hacker MP. Impact of the basic amine on the biological activity and intracellular distribution of an aza-anthrapyrazole: BBR 3422. Biochem Pharmacol. 2001;62:1337–1343. doi: 10.1016/s0006-2952(01)00797-3. [DOI] [PubMed] [Google Scholar]
- 19.Mankhetkorn S, Teodori E, Garnier-Suillerot A. Partial inhibition of the P-glycoprotein-mediated transport of anthracyclines in viable resistant K562 cells after irradiation in the presence of a verapamil analogue. Chem Biol Interact. 1999;121:125–140. doi: 10.1016/s0009-2797(99)00095-2. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch-Ernst KI, Ziemann C, Rustenbeck I, Kahl GF. Inhibitors of mdr1-dependent transport activity delay accumulation of the mdr1 substrate rhodamine 123 in primary rat hepatocyte cultures. Toxicology. 2001;167:47–57. doi: 10.1016/s0300-483x(01)00457-7. [DOI] [PubMed] [Google Scholar]
- 21.Yu LF, Zhang YP, Qiao MM, Wu YL. Establishment and characterization of vincristine-resistant MKN28/VCR, MKN45/VCR of human gastric cancer cell lines. Shijie Huaren Xiaohua Zazhi. 2001;9:297–301. [Google Scholar]
- 22.Alahari SK, DeLong R, Fisher MH, Dean NM, Viliet P, Juliano RL. Novel chemically modified oligonucleotides provide potent inhibition of P-glycoprotein expression. J Pharmacol Exp Ther. 1998;286:419–428. [PubMed] [Google Scholar]
- 23.Stewart AJ, Canitrot Y, Baracchini E, Dean NM, Deeley RG, Cole SP. Reduction of expression of the multidrug resistance protein (MRP) in human tumor cells by antisense phosphorothioate oligonucleotides. Biochem Pharmacol. 1996;51:461–469. doi: 10.1016/0006-2952(95)02220-1. [DOI] [PubMed] [Google Scholar]
- 24.Motomura S, Motoji T, Takanashi M, Wang YH, Shiozaki H, Sugawara I, Aikawa E, Tomida A, Tsuruo T, Kanda N, et al. Inhibition of P-glycoprotein and recovery of drug sensitivity of human acute leukemic blast cells by multidrug resistance gene (mdr1) antisense oligonucleotides. Blood. 1998;91:3163–3171. [PubMed] [Google Scholar]
- 25.Cao WX, Ou JM, Fei XF, Zhu ZG, Yin HR, Yan M, Lin YZ. Methionine-dependence and combination chemotherapy on human gastric cancer cells in vitro. World J Gastroenterol. 2002;8:230–232. doi: 10.3748/wjg.v8.i2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu B, Staren E, Iwamura T, Appert H, Howard J. Taxotere resistance in SUIT Taxotere resistance in pancreatic carcinoma cell line SUIT 2 and its sublines. World J Gastroenterol. 2001;7:855–859. doi: 10.3748/wjg.v7.i6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen WX, Li YM, Yu CH, Cai WM, Zheng M, Chen F. Quantitative analysis of transforming growth factor beta 1 mRNA in patients with alcoholic liver disease. World J Gastroenterol. 2002;8:379–381. doi: 10.3748/wjg.v8.i2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Li XH, Peng Y. Liver sinusoidal endothelial cell injury by neutrophils in rats with acute obstructive cholangitis. World J Gastroenterol. 2002;8:342–345. doi: 10.3748/wjg.v8.i2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen ZY, Shen WY, Chen MH, Shen J, Cai WJ, Yi Z. Nitric oxide and calcium ions in apoptotic esophageal carcinoma cells induced by arsenite. World J Gastroenterol. 2002;8:40–43. doi: 10.3748/wjg.v8.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang JP, Duan GR, Zhao YL, Du DW. Effect of P16 gene expression in growth of human hepatic carcinoma cell line 7721 with confocal microscopic analysis. Shijie Huaren Xiaohua Zazhi. 2000;8:767–770. [Google Scholar]
- 31.Cheng SD, Wu YL, Zhang YP, Qiao MM, Guo QS. Abnormal drug accumulation in multidrug resistant gastric carcinoma cells. Shijie Huaren Xiaohua Zazhi. 2001;9:131–134. [Google Scholar]
- 32.Gao F, Yi J, Shi GY, Li H, Shi XG, Tang XM. The sensitivity of digestive tract tumor cells to As2O3 is associated with the inherent cellular level of reactive oxygen species. World J Gastroenterol. 2002;8:36–39. doi: 10.3748/wjg.v8.i1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agrawal S. Importance of nucleotide sequence and chemical modifications of antisense oligonucleotides. Biochim Biophys Acta. 1999;1489:53–68. doi: 10.1016/s0167-4781(99)00141-4. [DOI] [PubMed] [Google Scholar]
- 34.Gleave ME, Miyake H, Zellweger T, Chi K, July L, Nelson C, Rennie P. Use of antisense oligonucleotides targeting the antiapoptotic gene, clusterin/testosterone-repressed prostate message 2, to enhance androgen sensitivity and chemosensitivity in prostate cancer. Urology. 2001;58:39–49. doi: 10.1016/s0090-4295(01)01241-9. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Chen L, Walker V, Jacob TJ. Antisense to MDR1 mRNA reduces P-glycoprotein expression, swelling-activated C1- current and volume regulation in bovine ciliary epithelial cells. J Physiol. 1998;511(Pt 1):33–44. doi: 10.1111/j.1469-7793.1998.033bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nie QH, Cheng YQ, Xie YM, Zhou YX, Cao YZ. Inhibiting effect of antisense oligonucleotides phosphorthioate on gene expression of TIMP-1 in rat liver fibrosis. World J Gastroenterol. 2001;7:363–369. doi: 10.3748/wjg.v7.i3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal N, Gewirtz AM. Oligonucleotide therapeutics for hematologic disorders. Biochim Biophys Acta. 1999;1489:85–96. doi: 10.1016/s0167-4781(99)00142-6. [DOI] [PubMed] [Google Scholar]
- 38.Giraud-Panis M, Leng M. Transplatin-modified oligonucleotides as modulators of gene expression. Pharmacol Ther. 2000;85:175–181. doi: 10.1016/s0163-7258(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 39.Wang XW, Yuan JH, Zhang RG, Guo LX, Xie Y, Xie H. Antihepatoma effect of alpha-fetoprotein antisense phosphorothioate oligodeoxyribonucleotides in vitro and in mice. World J Gastroenterol. 2001;7:345–351. doi: 10.3748/wjg.v7.i3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang YC, Li Y, Qian GX. Reduction of tumorigenicity of SMMC-7721 hepatoma cells by vascular endothelial growth factor antisense gene therapy. World J Gastroenterol. 2001;7:22–27. doi: 10.3748/wjg.v7.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Y, Zhou J, Wu JS, Dou KF. Inhibitory effects of EGFR antisense oligodeox ynucleotide in human colorectal cancer cell line. World J Gastroenterol. 2000;6:747–749. doi: 10.3748/wjg.v6.i5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Li SN, Wang XN. CEA and AFP expression in human hepatoma cells transfected with antisense IGF-I gene. World J Gastroenterol. 1998;4:30–32. doi: 10.3748/wjg.v4.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhoug S, Wen SM, Zhang DF, Wang QL, Wang SQ, Ren H. Sequencing of PCR amplified HBV DNA pre-c and c regions in the 2.2.15 cells and antiviral action by targeted antisense oligonucleotide directed against sequence. World J Gastroenterol. 1998;4:434–436. doi: 10.3748/wjg.v4.i5.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang FX, Zhang XY, Fan DM, Deng ZY, Yan Y, Wu HP, Fan JJ. Antisense telomerase RNA induced human gastric cancer cell apoptosis. World J Gastroenterol. 2000;6:430–432. doi: 10.3748/wjg.v6.i3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu DH, Zhang XY, Fan DM, Huang YX, Zhang JS, Huang WQ, Zhang YQ, Huang QS, Ma WY, Chai YB, et al. Expression of vascular endothelial growth factor and its role in oncogenesis of human gastric carcinoma. World J Gastroenterol. 2001;7:500–505. doi: 10.3748/wjg.v7.i4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu ZP, Wang YJ, Li JG, Zhou YA. VEGF165 antisense RNA suppresses oncogenic properties of human esophageal squamous cell carcinoma. World J Gastroenterol. 2002;8:44–48. doi: 10.3748/wjg.v8.i1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao B, Shi YQ, Zhao YQ, You H, Wang ZY, Liu XL, Yin F, Qiao TD, Fan DM. Transduction of Fas gene or Bcl-2 antisense RNA sensitizes cultured drug resistant gastric cancer cells to chemotherapeutic drugs. World J Gastroenterol. 1998;4:421–425. doi: 10.3748/wjg.v4.i5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrao P, Sincock P, Cole S, Ashman L. Intracellular P-gp contributes to functional drug efflux and resistance in acute myeloid leukaemia. Leuk Res. 2001;25:395–405. doi: 10.1016/s0145-2126(00)00156-9. [DOI] [PubMed] [Google Scholar]
- 49.Gong YP, Liu T, Jia YQ, Qin L, Deng CQ, Yang RY. Comparison of Pgp- and MRP-mediated multidrug resistance in leukemia cell lines. Int J Hematol. 2002;75:154–160. doi: 10.1007/BF02982020. [DOI] [PubMed] [Google Scholar]
- 50.Benderra Z, Trussardi A, Morjani H, Villa AM, Doglia SM, Manfait M. Regulation of cellular glutathione modulates nuclear accumulation of daunorubicin in human MCF7 cells overexpressing multidrug resistance associated protein. Eur J Cancer. 2000;36:428–434. doi: 10.1016/s0959-8049(99)00288-9. [DOI] [PubMed] [Google Scholar]
- 51.Hayes JH, Soroka CJ, Rios-Velez L, Boyer JL. Hepatic sequestration and modulation of the canalicular transport of the organic cation, daunorubicin, in the Rat. Hepatology. 1999;29:483–493. doi: 10.1002/hep.510290216. [DOI] [PubMed] [Google Scholar]
- 52.Chou TC, Depew KM, Zheng YH, Safer ML, Chan D, Helfrich B, Zatorska D, Zatorski A, Bornmann W, Danishefsky SJ. Reversal of anticancer multidrug resistance by the ardeemins. Proc Natl Acad Sci USA. 1998;95:8369–8374. doi: 10.1073/pnas.95.14.8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Courtois A, Payen L, Vernhet L, de Vries EG, Guillouzo A, Fardel O. Inhibition of multidrug resistance-associated protein (MRP) activity by rifampicin in human multidrug-resistant lung tumor cells. Cancer Lett. 1999;139:97–104. doi: 10.1016/s0304-3835(99)00024-5. [DOI] [PubMed] [Google Scholar]
- 54.Belhoussine R, Morjani H, Millot JM, Sharonov S, Manfait M. Confocal scanning microspectrofluorometry reveals specific anthracyline accumulation in cytoplasmic organelles of multidrug-resistant cancer cells. J Histochem Cytochem. 1998;46:1369–1376. doi: 10.1177/002215549804601205. [DOI] [PubMed] [Google Scholar]
- 55.Zhang JT. Determinant of the extracellular location of the N-terminus of human multidrug-resistance-associated protein. Biochem J. 2000;348 Pt 3:597–606. [PMC free article] [PubMed] [Google Scholar]
- 56.Laupèze B, Amiot L, Bertho N, Grosset JM, Lehne G, Fauchet R, Fardel O. Differential expression of the efflux pumps P-glycoprotein and multidrug resistance-associated protein in human monocyte-derived dendritic cells. Hum Immunol. 2001;62:1073–1080. doi: 10.1016/s0198-8859(01)00307-x. [DOI] [PubMed] [Google Scholar]
- 57.Meschini S, Calcabrini A, Monti E, Del Bufalo D, Stringaro A, Dolfini E, Arancia G. Intracellular P-glycoprotein expression is associated with the intrinsic multidrug resistance phenotype in human colon adenocarcinoma cells. Int J Cancer. 2000;87:615–628. [PubMed] [Google Scholar]
- 58.Demeule M, Jodoin J, Gingras D, Béliveau R. P-glycoprotein is localized in caveolae in resistant cells and in brain capillaries. FEBS Lett. 2000;466:219–224. doi: 10.1016/s0014-5793(00)01087-5. [DOI] [PubMed] [Google Scholar]
- 59.Kipp H, Arias IM. Newly synthesized canalicular ABC transporters are directly targeted from the Golgi to the hepatocyte apical domain in rat liver. J Biol Chem. 2000;275:15917–15925. doi: 10.1074/jbc.M909875199. [DOI] [PubMed] [Google Scholar]


