Abstract
AIM: Regional chemotherapy using hepatic artery catheters is a good method of treating patients with colorectal cancer liver metastases. We investigated the survival of patients with liver metastases from colorectal cancer using 5-fluorouracil (5-FU) and mitomycin C Cthrough implantable hepatic arterial infusion port.
METHODS: Seventy-five patients with inoperable liver metastases from colorectal cancer were included between March, 1992 and November, 2001. We placed implantable hepatic arterial catheter (HAC) port by laparotomy.5-FU, 1000 mg/m2/d continuous infusion for five days every four weeks, was delivered in the hepatic arterial catheter through the port. Mitomycin C, 30 mg/m2/d infusion in the first day every cycle through the port. Response to the treatment was evaluated by serial determinations of plasma CEA and imaging techniques consisting of computerized tomography and sonography of liver.
RESULTS: Sixty-eight were performed hepatic artery chemotherapy and fifty-six were followed up among seventy-five HAC patients. Twenty-six patients (46.4%) have responded and 4 complete remission were achieved. Eight patients (14.3%) had stable liver metastases. Twenty-two patients (39.3%) were progressed with increased tumor size and number. Twenty-nine patients (51.8%) had a decreased serum CEA level, while 10 patients (17.9%) were stable and 17 patients (30.4%) had an increased serum CEA level. There were no operative death in this series. Complications, which occurred in 18 patients (32.1%), were as followed: hepatic artery thrombosis in 11, Upper gastric and intestinal bleeding in 3, liver abscess in 1, pocket infection in 1, cholangitis in 1, and hepatic artery pseudo-aneurysm in one patient.
CONCLUSION: Combined infusion of 5-FU and mitomycin C by hepatic artery catheter port is an effective treatment for liver metastases from colorectal cancer. The high response and lower complication rates prove the adjuvant treatment of colorectal cancer with this treatment.
INTRODUCTION
Colorectal cancer is one of the leading causes of cancer-related mortality in China[1-29]. Approximately half of the patients undergoing apparently curative resection will die within 5 years because of recurrent disease, mostly with liver metastases[30]. Liver was the only site affected in 50% of these patients. Synchronous hepatic metastases are detected in 20% of patients under bowel resection, while metachronous disease occurs in another 30%[31-33]. However about 5%-20% of patients with colorectal hepatic metastases undergo resection with curative intention, resulting in a five year survival of 20%-40%. The one-year and three-year survivals of untreated patients with liver metastases are 31% and 2.6%, respectively[34-36]. Most liver metastases were unresectable because of the number, size, position of tumors and general conditions unsuitable for liver resection.
Unfortunately, the results of conventional systemic chemotherapy have been disappointing. Single-agent 5-flourouracil (5-FU), which has been used for many years to treat metastatic colorectal cancer, has a response rate of approximately less than 20%. The addition of mitomycin C produces higher response rates with a trend towards increase survival and probably similar results can be achieved with high dose 5-FU alone[37,38].
As most drugs have a steep dose-dependent curve, it is a basic pharmacokinetic principle that if one can increase drug delivery to tumors then increased response rates can be achieved. An alternative approach to the therapy of liver metastases is therefore to deliver the drug intra-arterially[39,40]. Hepatic artery catheter chemotherapy is a therapeutic possibility for unresectable liver metastases for many years. The rational for Hepatic artery catheter chemotherapy is based on the fact that liver metastases over 1 cm derive most their blood supply from hepatic artery. The other rational is the high first pass hepatic extraction of the drug used for this approach. Both factors make high local drug concentrations with reduced systemic toxicity and allow treatment with relatively high dosages compared to intravenous treatment[41,42].
We report our experience of an intra-arterial combined use of 5-FU and mitomycin C in patients with unresectable colorectal liver metastases. The aim of this approach was firstly achieve high response with combination of drugs.
MATERIALS AND METHODS
Patients
Seventy-five patients (43 male, 32 female) with a median age of 58.3 years (range 31-76) with multifocal colorectal metastases confined to the liver and not suitable to surgical resection and other regional ablation which include radio frequence ablation, ethanol injection, cryotherapy and laser ablation, were included in the study between January 1992 and November 2001. Seventeen patients had synchronous and 58 patients had metachronous liver metastases. Preoperative assessment included computerized tomography (CT) scan of abdomen and pelvis, either radiographic or CT examination of the chest and colonscopy of bowel to exclude extrahepatic disease. Some patients were undergone Positron Emission Tomography (PET) recently. Selective superior mesenteric angiography was performed before surgery to define hepatic arterial anatomy in some patients. Histological confirmation of the presence of liver metastases was obtained by ultrasound guided fine needle biopsy or fine needle biopsy in laparotomy before implantation of hepatic artery catheter port. Blood test including hematology, liver function, renal function and CEA were performed before operation.
Hepatic artery catheter port
Patients underwent a laparotomy and the hepatic artery catheter (HAC) was positioned in the ligated gastroduodenal artery with the catheter tip located at the junction of the gastroduodenal and common hepatic artery, thereby gaining access to the hepatic arterial flow. The other end was connected to a subcutaneous infusion port placed over the left coastal margin. A cholecystectomy was routinely performed to prevent potential chemical cholecystitis during chemotherapy. Various surgical maneuvers were used in patients with aberrant hepatic arterial anatomy, which include position the catheter in other artery and bypass of hepatic artery with artificial vessels. Adequate perfusion of the liver was confirmed at the time of operation with a test of blue dye. The port was flushed with heparinized saline (1000 μ/mL).
Chemotherapy
All patients received continuously hepatic arterial 5-FU and mitomycin C perfusion using an ambulatory pump. Patients received 1000 mg/m2/d 5-FU in 12 h for five days every 4 wk. Thirty mg/m2 mitomycin C was perfused in 2 h after the use of 5-FU in first day every cycle. Ten microgram of dexamethasone was given before perfusion everyday. Cimetidine and Losec were prescribed as prophylaxis against gastroduodenal ulceration. Haemological and biochemical toxicity were assessed every 2 wk and graded according to WHO toxicity criteria. In patients with significant side effects, the subsequent dose was delayed until recovery.
Study parameters
Evaluation of the HAC were performed every two cycles by CT scan or ultrasound of liver and serum CEA, while a chest film or CT and blood test were also made. Completed response was defined as the disappearance of all tumor. Partial response was defined as a > 50% decrease of the lesion size and number. Progressive response was defined as a > 25% increase in any measurable lesion or the appearance of a new lesion. Stable response was defined as < 25% increase and < 50% decrease of the lesion. The decisions of further treatment were made after 6 cycles intra-arterial chemotherapy. Patients who progressed were offered alternative treatment. Patient whose catheters were thrombosis or unusable because other complications but had complete, partial and stable response, were commenced on an intravenous chemotherapy using 5-FU and mitomycin C.
Statistical analysis
Time to progression and overall survival were estimated by Kaplan-Meier survival curves using SPSS. Seven patients, which were not regularly performed chemotherapy due to the toxicity or rejected chemotherapy, were excluded from the study. Twelve patients were not follow up in time and accurately were not included in this study.
RESULTS
Treatment and survival
Of the 75 patients, four patients rejected intra-arterial 5-FU and mitomycin C chemotherapy. Three patients would not continue the chemotherapy because of the severe toxicity of cytotoxic drugs, although many procedures were performed. During 68 patients who continued at least 6 cycles of chemotherapy, twelve patients were not completely and accurately followed up. So, only fifty-six patients were treated and studied. The median number of cycles received were 10 (range 6-25). Median follow up were 21 mo (range 8-37 mo). Forty-one patients have died till the close day of follow up. Predicted median survival from the time of catheter insertion was 15 mo (Figure 1).
Figure 1.
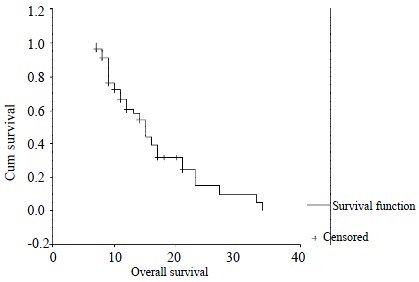
Kaplan-Meier survival curve. Median survival 15 mo.
Response
Response is expression as best response during the course of intra-arterial treatment. Twenty-six patients (46.4%) were responded, of whom, 4 had complete response and 22 had partial response. Eight patients (14.3%) were stable, whereas 22 patients (39.3%) were progressed. Twenty-nine patients (51.8%) had a decreased serum CEA level, while 10 patients (17.9%) were stable and 17 patients (30.4%) had an increased serum CEA level. There were no operative death in this series.
Complications
Hepatic artery thrombosis (in 11 patients, 19.8%) is the complications most frequently observed in patients with HAC. The presenting symptoms of this complications were abdominal pain and obstruction of the catheter. It was confirmed by angiography in 6 patients and by Doppler ultrasound examination in 5 patients. Upper gastric and intestinal bleeding were present in 3 patients, which due to perforation of gastric and duodenal ulceration at surgery in 2 patients and duodenal ulceration bleeding at gastroscopy in 1 patient. There were all performed before 1995 when we did not use blue dye. There were one patient presented with high fever after intra-arterial chemotherapy. She was diagnosed liver abscess by CT scan of liver and cured by ultrasound guide drainage. One patients which has a pocket infection has healed after intravenous antibiotics and drainage. One patients with cholangitis has to move the catheter and port. There are still one bleeding ulcer due to hepatic artery pseudo-aneurysm at surgery.
DISCUSSION
When colorectal cancer metastasises to liver, the prognosis becomes very awful. More than fifty percent colorectal cancer liver metastases patients will died in six months. For this reason, various treatment have been attempted, which include HAC, radio frequence ablation[44], cryotherapy[45], ethanol injection[46], systemic chemotherapy[47] and radiotherapy[48]. As most cytotoxic drugs have a steep dose-dependence response curve: the higher the concentration of the drug, the higher the antitumor effects[39]. Hepatic artery catheter chemotherapy emerged in this condition. Besides this, metastatic tumors in the liver derived their blood supply mainly from the hepatic artery (90%-95%). HAC can directly perfuse the chemotherapy to liver metastases from colorectal cancer. The drugs will result in a prolonged exposure of high concentration to the tumors, while reducing the systemic side effects following the metabolism of the drug in the liver. HAC were undertaken more than 30 years for these reasons[49-51].
There are some studies compared intra-arterial chemotherapy with conventional systemic chemotherapy showed consistently higher response rates in patients receiving intra-arterial chemotherapy[49]. In the United Kingdom, Patients were randomized to receive intra-arterial through a totally implantable infusion device; in the latter group 20% patients were given systemic chemotherapy. Survival was significantly longer in the intra-arterial group (median survival 405 days compared with 226 days). The intra-arterial group also had a better quality of life than those received systemic chemotherapy[52]. Other study showed that the response rate was 43% in the intra-arterial group compared with 9% in the systemic chemotherapy group. Furthermore, the intra-arterial group showed a significant increase in survival of one year (64% compared with 44%) and two years (23% compared with 13%). Other studies also showed a higher response rates in intra-arterial chemotherapy than that in systemic chemotherapy[53].
In all studies, 5-FU and FUDR were chosen for the arterial route of administration. As 84%-99% of FUDR is extracted by the liver on first pass, it seemed logical to use FUDR to achieve the dual objective of high levels within the tumor and low plasma levels, thereby increasing the probability of the tumor's response while minimizing the systemic toxicity[54]. But 55% of patients using FUDR in the UK and French studies developed extra-hepatic progression, suggesting that these patients may have had occult extra-hepatic disease at the time of entry into the trial or during the intra-arterial chemotherapy. The lower plasma level of drugs have been misplaced. But, 5-FU which has a lower hepatic extraction rather than FUDR, allowed the drug to "spill over" into the systemic circulation[53]. Mitomycin C is also a valuable drug for the systemic chemotherapy in colorectal cancer. In this way, we hoped to maximize the response rates within the liver but also to suppress the development of extrahepatic metastases by combined use of 5-FU and mitomycin C. It seemed likely that both of these objectives could be achieved with a response rate of 46.4%. At the same time, systemic toxicity was relatively mild and no chemical hepatitis or biliary sclerosis developed as that in FUDR.
Imaging and CEA are good markers in follow up of patients. Serum CEA determinations were the most sensitive indicator of tumor regression or recurrence and proceed changes in the imaging test by at least 1-2 mo. Angiography CT (CTA) seems to be the most sensitive to change in tumor size and find new metastases. Sonography is the cheapest way to follow up.
Although there are some complications in HAC, it is still a feasible method for colorectal cancer liver metastases. Most complications was related to the technique of surgery and care of patients[55]. Meticulous attention to ligation of all vessels that would cause gastroduodenal misperfusion can eliminate gastritis, duodenitis, and gastroduodenal ulceration related to HAC chemotherapy[56]. The intraoperative blue injection are necessary to confirm total hepatic perfusion and to rule out any gastroduodenal misperfusion. Gastroscopy investigation of the gastroduodenal perfusion of blue injected through the port is very important before initiating HAC chemotherapy. Displacement of the catheter tip into the hepatic artery often leads to thrombosis of the artery. Dismal migration of the catheter into the gastroduodenal artery may cause vessel or catheter occlusion, hemorrhage, aneurysm, and rupture of the gastroduodenal artery[57]. These complications were all emerged in the early stage of this procedure when we did not have so much experience to avoid these. Liver abscess and infection of port were due to unstrictly sterilize procedure which can be avoided at all.
In conclusion, HAC 5-FU and mitomycin C chemotherapy is better than systemic chemotherapy and other combination of drugs in colorectal cancer liver metastases, which were not suitable for surgery, cryotherapy, radio frequence ablation and other adjunctive therapy, although there are still 39.3% patients progression and some complications, some of them were dangerous.
Footnotes
Edited by Yan T
Supported by Youth Natural Scientific Foundation of Heilongjiang Province and Harbin
References
- 1.Xiong B, Gong LL, Zhang F, Hu MB, Yuan HY. TGF beta1 expression and angiogenesis in colorectal cancer tissue. World J Gastroenterol. 2002;8:496–498. doi: 10.3748/wjg.v8.i3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng S, Liu XY, Ding KF, Wang LB, Qiu PL, Ding XF, Shen YZ, Shen GF, Sun QR, Li WD, et al. Reduction of the incidence and mortality of rectal cancer by polypectomy: a prospective cohort study in Haining County. World J Gastroenterol. 2002;8:488–492. doi: 10.3748/wjg.v8.i3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan J, Zhang ZQ, Zhu C, Wang MW, Zhao DH, Fu YH, Zhang JP, Wang YH, Wu BY. Colonoscopic screening and follow-up for colorectal cancer in the elderly. World J Gastroenterol. 2002;8:267–269. doi: 10.3748/wjg.v8.i2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao B, Wang ZJ, Xu YF, Wan YL, Li P, Huang YT. Report of 16 kindreds and one kindred with hMLH1 germline mutation. World J Gastroenterol. 2002;8:263–266. doi: 10.3748/wjg.v8.i2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu BP, Xiao B, Wan TM, Zhang YL, Zhang ZS, Zhou DY, Lai ZS, Gao CF. Construction and selection of the natural immune Fab antibody phage display library from patients with colorectal cancer. World J Gastroenterol. 2001;7:811–815. doi: 10.3748/wjg.v7.i6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Q, Sun MH, Lu HF, Zhang TM, Mo SJ, Xu Y, Cai SJ, Zhu XZ, Shi DR. Clinicopathological and molecular genetic analysis of 4 typical Chinese HNPCC families. World J Gastroenterol. 2001;7:805–810. doi: 10.3748/wjg.v7.i6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu JW, Yu BM, Ji YB, Zheng MH, Li DH. Upregulation of vascular endothelial growth factor by hydrogen peroxide in human colon cancer. World J Gastroenterol. 2002;8:153–157. doi: 10.3748/wjg.v8.i1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun K, Jin BQ, Feng Q, Zhu Y, Yang K, Liu XS, Dong BQ. Identification of CD226 ligand on colo205 cell surface. World J Gastroenterol. 2002;8:108–113. doi: 10.3748/wjg.v8.i1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang YL, Zhang ZS, Wu BP, Zhou DY. Early diagnosis for colorectal cancer in China. World J Gastroenterol. 2002;8:21–25. doi: 10.3748/wjg.v8.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo MJ, Lai MD. Identification of differentially expressed genes in normal mucosa, adenoma and adenocarcinoma of colon by SSH. World J Gastroenterol. 2001;7:726–731. doi: 10.3748/wjg.v7.i5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yi J, Wang ZW, Cang H, Chen YY, Zhao R, Yu BM, Tang XM. p16 gene methylation in colorectal cancers associated with Duke's staging. World J Gastroenterol. 2001;7:722–725. doi: 10.3748/wjg.v7.i5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makin GB, Breen DJ, Monson JR. The impact of new technology on surgery for colorectal cancer. World J Gastroenterol. 2001;7:612–621. doi: 10.3748/wjg.v7.i5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XG, Song JD, Wang YQ. Differential expression of a novel colorectal cancer differentiation-related gene in colorectal cancer. World J Gastroenterol. 2001;7:551–554. doi: 10.3748/wjg.v7.i4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang LP, Liang K, Shen Y, Yin WB, Hans G, Zeng YJ. Neutron-induced apoptosis of HR8348 cells in vitro. World J Gastroenterol. 2001;7:435–439. doi: 10.3748/wjg.v7.i3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng CX, Zhan WH, Zhao JZ, Zheng D, Wang DP, He YL, Zheng ZQ. The prognostic value of preoperative serum levels of CEA, CA19-9 and CA72-4 in patients with colorectal cancer. World J Gastroenterol. 2001;7:431–434. doi: 10.3748/wjg.v7.i3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li XW, Ding YQ, Cai JJ, Yang SQ, An LB, Qiao DF. Studies on mechanism of Sialy Lewis-X antigen in liver metastases of human colorectal carcinoma. World J Gastroenterol. 2001;7:425–430. doi: 10.3748/wjg.v7.i3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui JH, Krueger U, Henne-Bruns D, Kremer B, Kalthoff H. Orthotopic transplantation model of human gastrointestinal cancer and detection of micrometastases. World J Gastroenterol. 2001;7:381–386. doi: 10.3748/wjg.v7.i3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan P, Sun MH, Zhang JS, Zhu XZ, Shi DR. APC and K-ras gene mutation in aberrant crypt foci of human colon. World J Gastroenterol. 2001;7:352–356. doi: 10.3748/wjg.v7.i3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng YC, Zhen YS, Zheng S, Xue YC. Activity of boanmycin against colorectal cancer. World J Gastroenterol. 2001;7:93–97. doi: 10.3748/wjg.v7.i1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng ZH, Xing TH, Qiu GQ, Tang HM. Relationship between Fas/FasL expression and apoptosis of colon adenocarcinoma cell lines. World J Gastroenterol. 2001;7:88–92. doi: 10.3748/wjg.v7.i1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu BP, Zhang YL, Zhou DY, Gao CF, Lai ZS. Microsatellite instability, MMR gene expression and proliferation kinetics in colorectal cancer with famillial predisposition. World J Gastroenterol. 2000;6:902–905. doi: 10.3748/wjg.v6.i6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Y, Zhou J, Wu JS, Dou KF. Inhibitory effects of EGFR antisense oligodeox ynucleotide in human colorectal cancer cell line. World J Gastroenterol. 2000;6:747–749. doi: 10.3748/wjg.v6.i5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia XD, Han C. Chemoprevention of tea on colorectal cancer induced by dimethylhydrazine in Wistar rats. World J Gastroenterol. 2000;6:699–703. doi: 10.3748/wjg.v6.i5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo WJ, Zhou GD, Wu HJ, Liu YQ, Wu RG, Zhang WD. Ultrastructural localization of glutathione S-transferase-pi in human colorectal cancer cells. World J Gastroenterol. 2000;6:454–455. doi: 10.3748/wjg.v6.i3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YX, Ruan CP, Li L, Shi JH, Kong XT. Clinical significance of changes of perioperative T cell and expression of its activatedantigen in colorectal cancer patients. World J Gastroenterol. 1999;5:181–182. doi: 10.3748/wjg.v5.i2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu JY, Wang S, Zhu JG, Zhou GH, Sun QB. Expression of B7 costimulation molecules by colorectal cancer cells reducestumorigenicity and induces anti-tumor immunity. World J Gastroenterol. 1999;5:147–151. doi: 10.3748/wjg.v5.i2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan W, Lin DX, Xiao Y, Kadlubar F, Chen JS. Chemoprevention of 2-amino-1-methyl-6-phenyli-midazo 4,5-b pyridine-induced carcinogen-DNA adducts by Chinese cabbage in rats. World J Gastroenterol. 1999;5:138–142. doi: 10.3748/wjg.v5.i2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu QZ, Tuo CW, Wang B, Wu BQ, Zhang YH. Liver metastasis models of human colorectal carcinoma established in nude mice by orthotopic transplantation and their biologic characteristic. World J Gastroenterol. 1998;4:409–411. doi: 10.3748/wjg.v4.i5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu JY, Su JZ, Pi ZM, Zhu JG, Zhou GH, Sun QB. Radioimmunoimaging of colorectal cancer using (99m)Tc labeled monoclonal antibody. World J Gastroenterol. 1998;4:303–306. doi: 10.3748/wjg.v4.i4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor I, Gillams AR. Colorectal liver metastases: alternatives to resection. J R Soc Med. 2000;93:576–579. doi: 10.1177/014107680009301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bleiberg H, Hendlisz A. Advanced colorectal cancer treatment in Europe: what have we achieved? Anticancer Drugs. 2002;13:461–471. doi: 10.1097/00001813-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Lujan HJ, Plasencia G, Jacobs M, Viamonte M, Hartmann RF. Long-term survival after laparoscopic colon resection for cancer: complete five-year follow-up. Dis Colon Rectum. 2002;45:491–501. doi: 10.1007/s10350-004-6227-8. [DOI] [PubMed] [Google Scholar]
- 33.Jass JR, Young J, Leggett BA. Evolution of colorectal cancer: change of pace and change of direction. J Gastroenterol Hepatol. 2002;17:17–26. doi: 10.1046/j.1440-1746.2002.02635.x. [DOI] [PubMed] [Google Scholar]
- 34.Wudel LJ, Chapman WC, Shyr Y, Davidson M, Jeyakumar A, Rogers SO, Allos T, Stain SC. Disparate outcomes in patients with colorectal cancer: effect of race on long-term survival. Arch Surg. 2002;137:550–554; discussion 550-554;. doi: 10.1001/archsurg.137.5.550. [DOI] [PubMed] [Google Scholar]
- 35.Dizon DS, Kemeny NE. Intrahepatic arterial infusion of chemotherapy: clinical results. Semin Oncol. 2002;29:126–135. doi: 10.1053/sonc.2002.31680. [DOI] [PubMed] [Google Scholar]
- 36.Ponz D. Prevention and chemoprevention of colorectal neoplasms. Dig Liver Dis. 2002;34:59–69. doi: 10.1016/s1590-8658(02)80061-5. [DOI] [PubMed] [Google Scholar]
- 37.Kemeny NE, Ron IG. Hepatic arterial chemotherapy in metastatic colorectal patients. Semin Oncol. 1999;26:524–535. [PubMed] [Google Scholar]
- 38.McCarthy M. Arterial chemotherapy improves survival after hepatic metastases. Lancet. 1999;353:1771. doi: 10.1016/S0140-6736(05)75874-8. [DOI] [PubMed] [Google Scholar]
- 39.Ensminger WD. Intrahepatic arterial infusion of chemotherapy: pharmacologic principles. Semin Oncol. 2002;29:119–125. doi: 10.1053/sonc.2002.31679. [DOI] [PubMed] [Google Scholar]
- 40.van Riel JM, van Groeningen CJ, Giaccone G, Pinedo HM. Hepatic arterial chemotherapy for colorectal cancer metastatic to the liver. Oncology. 2000;59:89–97. doi: 10.1159/000012142. [DOI] [PubMed] [Google Scholar]
- 41.Aldrighetti L, Arru M, Angeli E, Venturini M, Salvioni M, Ronzoni M, Caterini R, Ferla G. Percutaneous vs. surgical placement of hepatic artery indwelling catheters for regional chemotherapy. Hepatogastroenterology. 2002;49:513–517. [PubMed] [Google Scholar]
- 42.Howell JD, Warren HW, Anderson JH, Kerr DJ, McArdle CS. Intra-arterial 5-fluorouracil and intravenous folinic acid in the treatment of liver metastases from colorectal cancer. Eur J Surg. 1999;165:652–658. doi: 10.1080/11024159950189708. [DOI] [PubMed] [Google Scholar]
- 43.Aldrighetti L, Arru M, Ronzoni M, Salvioni M, Villa E, Ferla G. Extrahepatic biliary stenoses after hepatic arterial infusion (HAI) of floxuridine (FUdR) for liver metastases from colorectal cancer. Hepatogastroenterology. 2001;48:1302–1307. [PubMed] [Google Scholar]
- 44.Liu LX, Jiang HC, Piao DX. Radiofrequence ablation of liver cancers. World J Gastroenterol. 2002;8:393–399. doi: 10.3748/wjg.v8.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sotsky TK, Ravikumar TS. Cryotherapy in the treatment of liver metastases from colorectal cancer. Semin Oncol. 2002;29:183–191. doi: 10.1053/sonc.2002.31674. [DOI] [PubMed] [Google Scholar]
- 46.Livraghi T. Guidelines for treatment of liver cancer. Eur J Ultrasound. 2001;13:167–176. doi: 10.1016/s0929-8266(01)00129-x. [DOI] [PubMed] [Google Scholar]
- 47.Biasco G, Gallerani E. Treatment of liver metastases from colorectal cancer: what is the best approach today? Dig Liver Dis. 2001;33:438–444. doi: 10.1016/s1590-8658(01)80018-9. [DOI] [PubMed] [Google Scholar]
- 48.Malik U, Mohiuddin M. External-beam radiotherapy in the management of liver metastases. Semin Oncol. 2002;29:196–201. doi: 10.1053/sonc.2002.31675. [DOI] [PubMed] [Google Scholar]
- 49.Lygidakis NJ, Sgourakis G, Dedemadi G, Safioleus MC, Nestoridis J. Regional chemoimmunotherapy for nonresectable metastatic liver disease of colorectal origin. A prospective randomized study. Hepatogastroenterology. 2001;48:1085–1087. [PubMed] [Google Scholar]
- 50.Zanon C, Grosso M, Clara R, Alabiso O, Chiappino I, Miraglia S, Martinotti R, Bortolini M, Rizzo M, Gazzera C. Combined regional and systemic chemotherapy by a mini-invasive approach for the treatment of colorectal liver metastases. Am J Clin Oncol. 2001;24:354–359. doi: 10.1097/00000421-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 51.van Riel JM, van Groeningen CJ, Albers SH, Cazemier M, Meijer S, Bleichrodt R, van den Berg FG, Pinedo HM, Giaccone G. Hepatic arterial 5-fluorouracil in patients with liver metastases of colorectal cancer: single-centre experience in 145 patients. Ann Oncol. 2000;11:1563–1570. doi: 10.1023/a:1008369520179. [DOI] [PubMed] [Google Scholar]
- 52.Allen-Mersh TG, Earlam S, Fordy C, Abrams K, Houghton J. Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases. Lancet. 1994;344:1255–1260. doi: 10.1016/s0140-6736(94)90750-1. [DOI] [PubMed] [Google Scholar]
- 53.Howell JD, McArdle CS, Kerr DJ, Buckles J, Ledermann JA, Taylor I, Gallagher HJ, Budden J. A phase II study of regional 2-weekly 5-fluorouracil infusion with intravenous folinic acid in the treatment of colorectal liver metastases. Br J Cancer. 1997;76:1390–1393. doi: 10.1038/bjc.1997.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pelosi E, Bar F, Battista S, Bellò M, Bucchi MC, Alabiso O, Molino G, Bisi G. Hepatic arterial infusion chemotherapy for unresectable confined liver metastases: prediction of systemic toxicity with the application of a scintigraphic and pharmacokinetic approach. Cancer Chemother Pharmacol. 1999;44:505–510. doi: 10.1007/s002800051125. [DOI] [PubMed] [Google Scholar]
- 55.Kemeny N, Fata F. Hepatic-arterial chemotherapy. Lancet Oncol. 2001;2:418–428. doi: 10.1016/S1470-2045(00)00419-8. [DOI] [PubMed] [Google Scholar]
- 56.Fiorentini G, De Giorgi U, Giovanis P, Guadagni S, Cantore M, Marangolo M. Intra-arterial hepatic chemotherapy (IAHC) for liver metastases from colorectal cancer: need of guidelines for catheter positioning, port management, and anti-coagulant therapy. Ann Oncol. 2001;12:1023. doi: 10.1023/a:1011119714461. [DOI] [PubMed] [Google Scholar]
- 57.Eid A, Reissman P, Zamir G, Pikarsky AJ. Reconstruction of replaced right hepatic artery, to implant a single-catheter port for intra-arterial hepatic chemotherapy. Am Surg. 1998;64:261–262. [PubMed] [Google Scholar]


