Abstract
AIM: To explore the role of focal adhesion kinase (FAK) in the apoptosis in culture-activated rat hepatic stellate cells (HSCs) using a specific anti-FAK antibody.
METHODS: Rat HSCs were prepared from Wistar rats by in situ perfusion of collagenase and pronase and single-step density Nycodenze gradient. Culture-activated HSCs were serum-starved and treated with the anti-FAK antibodies for 24, 48 or 72 h. The apoptosis of HSC was detected by DNA-fragment assay, flow cytometry and caspase-3 activity determination. The expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) mRNA was assessed by reverse transcription polymerase chain reaction (RT-PCR).
RESULTS: The experiment showed that anti-FAK antibodies induced apoptosis of culture-activated rat HSCs. This phenomenon displayed the classical features of apoptotic cell death (DNA fragmentation, cell cycle analysis) after treated with 30 mg·L-1 FAK antibody for 72 h, and accompanied by a significant increase of caspase-3 activity (1208 ± 76) vs (309 ± 28) nmol·min-1·g-1, t = 208.5, P < 0.05. Meanwhile, treatment with the FAK antibody in HSCs could markedly decrease the TIMP-1 mRNA expression (0.07 ± 0.01 vs 0.38 ± 0.03, t = 2.72, P < 0.05).
CONCLUSION: FAK plays an important role in the survival of HSCs and the specific anti-FAK antibody could induce the apoptosis in rat HSCs.
INTRODUCTION
Focal adhesion kinase (FAK) is a non-receptor tyrosine ubiquitously expressed in cells. It was initially shown to be the initiator of focal adhesion formation in adherent cells, after its binding to integrins which induce its autophosphorylation[1]. However, it can also be activated by a great variety of other stimuli being able to act on different intracellular signaling, and neuropeptides[2-4]. Its autophosphorylation is followed by a submembranous localization which is crucial for the biological roles of FAK, including cell spreading, migration, proliferation, survival and prevention of apoptosis[5-7]. Proteolytic cleavage of FAK by caspase-3 has been reported during growth factor deprivation-induced apoptosis in human umbilical vein endothelial cells[8], which implies an association between FAK and apoptosis[9,10]. The pathologic basis of hepatic cirrhosis is fibrosis and hepatic stellate cells (HSC) are presently regarded as one of the key cell types involved in the progression of liver fibrosis[11-13]. The perpetuation of HSC activation leads to an increased number of collagen-producing cells and finally to the accumulation of extracellular matrix (ECM)[14-16]. Therefore, the strategy for terminating the proliferation of activated HSC by apoptosis might be an exciting therapy for patients with chronic liver injury and fibrosis[17-19].
FAK has also been shown to play an important role in the HSC activation[20]. PLC γ recruitment by FAK during HSC adhesion is an important process implicating a link between integrin and PDGF-mediated signal pathways to regulate HSC adhesion and mobility[21]. An adherence dependent pp125FAK-paxillin signaling pathway in fibroblasts inhibited damage-induced apoptosis[22]. Thus, we hypothesized that the modulation of biological roles of FAK by a neutralizing anti-FAK antibody might stop the fibroproliferative response and induce apoptosis in HSC.
MATERIALS AND METHODS
Materials
Male Wistar rats were obtained from the Experimental Animal Center of West China Medical Center of Sichuan University (West China University of Medical Sciences, Chengdu, Sichuan). Dulbecco's modified medium (DMEM), Trypsin-EDTA and new born calf serum (CS) were from GibcoBRL (Maryland, USA). Pronase, Collagenase B and DNAase I were from Roche Molecular Biochemicals, (Mannhein, Germany). Nycodenz was from Sigma (ST. Louis, USA). Antibodies to Desmin, α-smooth muscle actin (α-SMA) were obtained from Dako (Glostrup, Denmark). Affinity-purified polyclonal antibody to FAK (epitope mapping at the carboxy terminus of focal adhesion kinase) were purchased from Santa Cruz (Santa Cruz, USA). The caspase-3 cellular activity assay kit was purchased from CalBiochem-Novabiochem Corporation (San Diego, USA).
Methods
HSC isolation and apoptosis induction HSCs were isolated from male Wistar rats by in situ pronase-collagenase perfusion and single-step Nycodenz gradient[23]. The cells were seeded at a density of 1.5 × 105/cm2 on glass coverslips in 6-well culture plate or 100-mm dishes (Falcon) and maintained in DMEM containing 200 mL·L-1 heat-inactivated new-born calf serum. The purity of HSC preparations was assessed by intrinsic vitamin A autofluorescence and immuocytochemistry with antibody against desmin. The viability of the cells was evaluated by the Trypan blue dye exclusion test. The purity and viability of the primary cells exceeded 90% and 95%, respectively. Therefore, HSC cultured on uncoated plastic dishes spontaneously acquired an activated phenotype, characterized by expression of α-SMA and by loss of vitamin A droplets[24,25]. After reaching confluency (about 10-14 d after plating), activated HSC were detached by incubation with trypsin, and split in a 1:2 ratio. Experiments were performed on cells between the second and 5th passages using 3 independent cell lines, and the purity of activated HSC exceeded 98%. HSC (5 × 106) were plated in uncoated plastic dishes for 4 h and the medium was changed to serum-free DMEM for 24 h to synchronize the HSC in the G1 phase of the cell cycle[26]. The antibodies against FAK was filer-sterilized and added to the serum-free DMEM medium containing 1 g·L-1 bovine serum albumin (the final concentration of anti-FAK antibodies was 30 mg·L-1). The analysis of apoptosis was carried out after 24-72 h of incubation with the antibodies. The serum-free DMEM medium containing the antibodies was changed every 24 h.
Analysis of DNA fragmentation HSC from the anti-FAK antibodies treated was pooled for DNA fragmentation analysis. A DNA fragmentation assay was performed as described previously[27]. In brief, HSC was gently lysed for 30 min at 48 °C in a buffer containing 5 mmol·L-1 Tris buffer (pH7.4), 20 mmol·L-1 EDTA, and 5 mL·L-1 Triton X-100. After centrifugation at 15000 r·min-1 for 15 min, supernatants containing soluble fragmented DNA were collected and treated with RNAase (20 mg·L-1), followed by proteinase K (20 mg·L-1) digestion. DNA fragments were precipitated in 990 mL·L-1 ethanol. Samples were then electrophoresed on a 20 g·L-1 agarose gel, visualized with 1 g·L-1 ethidium bromide and photographed under short-wave ultraviolet light.
Flow cytometry Cell viability was determined using trypan blue dye exclusion, and the existence of apoptotic cells was confirmed as well by the appearance of a sub-G0/G1 peak fraction in the cell cycle analysis[28]. For the cell cycle analysis, ethanol-fixed cells were stained with propidium iodide (50 mg·L-1) in the presence of RNase A (100 mg·L-1), and then analyzed using the fluorescence-activated cell sorter (FACS, Coulter, EPICS ELITE ESP model), with a cell cycle analysis program.
Cellular caspase-3 activity determination The cellular caspase-3 activity assay from Calbiochem-Novabiochem Corporation measures the colorimetric reaction of the cleavage of the amino acid motif DEVD, thereby releasing the chromophorep-nitroanilide[29]. Following phosphate-buffered saline washing, cell lysate was prepared according to the manufactures’ instructions. The level of caspase-3 enzymatic activity on the cell lysate is directly proportional to the color reaction that was quantitated spectrophotometrically at a wavelength of 405 nm, using a microplate reader for 96 wells (Bio Rad, model 550). And the total protein content of each cell lysate was determined by the Coomassie dye binding assay (Bradford method). Data were corrected for background (no substrate or no cell lysate) and caspase-3 activities were expressed as nmol·min-1·g-1 of protein.
TIMP-1 mRNA detection by RT-PCR The total RNA was isolated from HSC using Trizol reagent (Life Technologies, Inc, USA), precipitated in ethanol and resuspended in sterile RNAase-free water for storage at -70 °C, as described previously[30]. One-step reverse transcription-polymerase chain reaction (RT-PCR) was performed according to the method of the supplier (TitanTM one tube RT-PCR kit, Roche Molecular Biochemicals). Primers for rat tissue inhibitors metalloproteinase-1 (TIMP-1) and GAPDH were designed using the Primer3 program from Whitehead Institute for Biomedical Research (Cambridge, MA, USA)[31], synthesized and purified by HPLC in Gibco BRL Custom Primers (HongKong). Primer sequences were as follows: TIMP-1 sense, 5'-GACCTGGTCATAAGGGCTAAA-3'; antisense, 5'-GCCCGTGATGAGAAACTCTTCACT-3'; GAPDH sense, 5'-ACCACAGTCCATGCCATCAC-3'; antisense, 5'-TCCACCACCCTGTTGCTGTA-3', and the expected size was 216 bp and 452 bp respectively. One microgram RNA was added to each reaction and the RT-PCR was performed in the following steps: reverse-transcription was performed at 50 °C for 30 min; and amplification was performed in a thermal controller (model PTC-100, MJ Research, Watertown, USA) for 35 cycles (denaturation at 94 °C for 1 min, annealing at 56 °C for 1 min and extension at 72 °C for 1 min), and 10 min at 72 °C for final extension after the last cycle. Five μL of the PCR products was analyzed by 20 g·L-1 agarose gel electrophoresis with TAE buffer at 80 V for 40 min, visualized with ethidium bromide and photographed under UV light. The semi-quantitative analysis was performed. TIMP-1/GAPDH quotient is the indication of TIMP-1. Experiments were performed at least three times with similar results.
Statistical analysis
Results of cell cycle analysis were expressed as percentage of total examined cells and statistical analysis was performed by χ² test. Other results were expressed as ¯x ± s. Differences between means were analyzed with Student t test for paired samples. A value of P < 0.05 was considered statistically significant.
RESULTS
DNA fragmentation assay
We investigated the role of FAK in the survival of HSC to rescue cells from apoptosis. An antibody to FAK that could inhibit it activation was used to test this hypothesis. This antibody binds to the COOH-terminal region of FAK, which contains the targeting sequence that is required for efficient recruitment of FAK to the focal adhesion[22,32,33]. HSC was treated with 30 mg·L-1 anti-FAK antibodies in DMEM without serum. Genomic DNA fragment analysis performed 48-72 h after treatment demonstrating an oligonucleosomal DNA ladder for the treated cells, and the cells after treatment for 24 h showed minimal DNA ladder whereas the control cells in DMEM without the antibodies displayed no DNA degradation (Figure 1).
Figure 1.
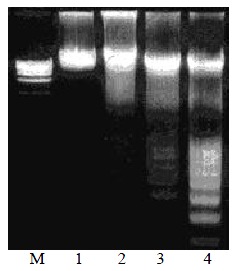
Oligonucleosomal genomic DNA fragmentation. Lane M: DNA marker of PBR322; Lane 1: Control HSCs; Lane 2, 3, 4: HSC treated with anti-FAK antibodies for 24, 48 or 72 h.
Flow cytometry
A predominant sub-G1 population (39.8%) characteristic of apoptosis was observed in anti-FAK antibodies treated HSCs for 72 h by propidium iodide staining and flow cytometric analysis, while the control cells only had a (5.2%) sub-G1 population (Figure 2). There was significant difference between these two groups (39.8% vs 5.2%, χ² = 1716.4, P < 0.001). And a significant sub-G1 population (16.5%) was also observed in anti-FAK antibodies treated HSCs for 48 h, while the control cells only had a (3.1%) sub-G1 population (16.5% vs 3.1%, χ² = 507.8, P < 0.001). However, there was no significant difference between the 24-hour treatment and controls (3.1% vs 2.7%, χ² = 1.4, P > 0.05).
Figure 2.
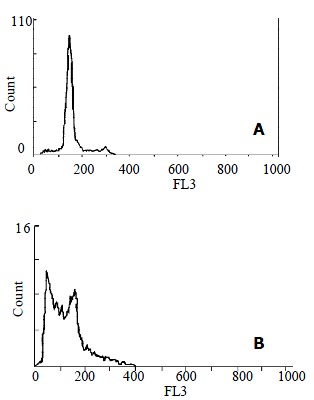
Flow cytometric analysis of HSCs. A: Control HSC; B: HSC treated with anti-FAK antibodies for 72 h showed the presence of a hypodiploid (sub-G1) fraction, indicating DNA degradation.
Cellular caspase-3 activity
Anti-FAK antibodies -induced apoptosis was accompanied by a significant time-dependent increase of caspase-3 activity (Table 1).
Table 1.
Caspase-3 activation in HSCs by anti-FAK antibodies (n = 6)
| Groups | Caspase-3 activity (nmole·min-1·g-1) |
| Control (24 h) | 36.5 ± 12.6 |
| Treatment (24 h) | 41.9 ± 15.3 |
| Control (48 h) | 110.7 ± 18.6 |
| Treatment (48 h) | 233.5 ± 25.9a |
| Control (72 h) | 36.5 ± 12.6 |
| Treatment (72 h) | 1208.5 ± 76.4a |
P < 0.05, vs control (233.5 ± 25.9 vs 110.7 ± 18.6, t = 33.9, P < 0.05; 1208.5 ± 76.4 vs 36.5 ± 2.6, t = 208.5, P < 0.05)
Effect of anti-FAK antibody on the expression of TIMP-1 in HSCs
To evaluate whether the anti-FAK antibody affects the expression of TIMP-1 in HSCs, RT-PCR analysis was performed to detect the gene expression lever of TIMP-1 after the treatment with anti-FAK antibodies in HSC. The mRNA expression levels of cells that treated with the antibodies for 72 h was remarkably decreased as against that of the controls (0.07 ± 0.01 vs 0.38 ± 0.03, P < 0.05, Figure 3). But there was no significant difference between the cells treated with the antibodies for 48 h or 24 h and their controls (P > 0.05).
Figure 3.
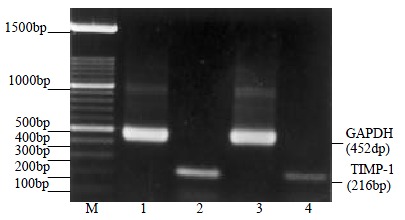
Electrophoresis analysis of RT-PCR product. Lane M: 100 bp DNA ladder; Lane 1, 2: amplified from cDNA from the control HSCs; Lane 3, 4: amplified from cDNA from HSCs treated with anti-FAK antibodies for 72 h. The positive bands of 452 bp and 216 bp represented GAPDH and TIMP-1 respectively. Lane 4 showed decreased expression of TIMP-1 in HSC compared with lane 2.
DISCUSSION
Liver fibrosis is characterized by an accumulation of extracellular matrix protein that impairs normal function with severity. It represents the common end point of the majority of chronic liver injuries. Ultimately, it results in distortion of the liver architecture (cirrhosis) which is associated with disturbance of liver function and significant morbidity and mortality[34-37]. At the cellular level there is now a wealth of evidence indicating that HSC represents the pivot of the fibrotic process. In the injured liver and during culture, quiescent HSCs transform from a retonoid rich pericyte-like cell to a myofibroblast-like cells (MFB). This so-called "activation" is associated with a loss of vitamin A droplets, increased proliferation, and sensitivity towards endothelins, increased production of ECM proteins, in addition to multiple alterations in gene expression[11-15]. Activation and transformation of HSCs into MFB may be viewed as a "wound-healing response" however, little is known about the termination of this process, while HSC is abundant in the diseased liver tissue during fibrogenesis, and resolution of liver fibrogenesis is associated with reduced number of HSC. In the recovery from liver injury, apoptositic HSC was detected in parallel to a reduction in the total number of HSC within the liver tissue and an essential element of this recovery process is apoptosis of activated HSC[17-19]. Apoptosis (or programmed cell death) is the controlled mechanism by which cells are eliminated from tissue without eliciting an inflammatory response. Apoptosis of HSC may therefore play a central role in the resolution of fibrosis by eliminating the source of both the neomatrix and the metalloproteinase (collagenase) inhibitors and thereby facilitating net matrix degradation. Therefore, promoting HSC apoptosis may be a viable method to facilitate matrix degradation in fibrotic liver, thereby manipulating the fibrotic process. An understanding of the control of HSC apoptosis is important precisely because regulating this process may provide a novel therapeutic approach to the treatment of advanced hepatic fibrosis[18-20].
Anchorage of cells to the ECM is mediated by integrins[38], which not only mediated cell adhesion but also initiate intracellular signal transduction[39]. A family of nonreceptor tyrosine kinases, composed of FAK, proline-rich tyrosine kinase (PYK-2), and integrin-linked kinase complex with the intracellular domain of integrins, leading to activation of various signaling pathways subsequent to integrin stimulation. Since the initial discovery and characterization of FAK, a number of different functions have been proposed for this unusual tyrosine kinase. FAK plays a pivotal role in transducing survival signals mediated by engagement if integrins with the ECM, enabling the cell to enter the cell cycle, thereby preventing apoptosis[1,40]. In rat HSCs, a soluble RGD peptide that blocks attachment to fibronectin and vitronectin triggered apoptosis in the serum-free condition, suggesting that integrin-mediated events can regulate death decisions in HSC[27]. Furthermore, the authors[27] reported that RGD peptides reduce the phosphorylation of FAK in HSC. Therefore in the current study we have described the role of FAK in the apoptosis in HSC.
Recently, a potential role for FAK in the suppression of apoptosis has been suggested in different cell types[7,41]. This study showed that anti-FAK antibodies induced apoptosis of culture-activated rat HSCs. This phenomenon displayed the classical features of apoptotic cell death (DNA fragmentation, cell cycle analysis), and accompanied by a significant increase of caspase-3 activity. Caspases, a family of the interleukin-1β converting enzyme (ICE) family of cysteine proteases, are key intracellular mediators of apoptosis[42,43]. Caspase-3, also known as CPP32, Yama or apopain, is one of the principle caspases found in apoptotic cells[44]. Our observations suggest that induction of apoptosis in HSCs by anti-FAK antibodies may through the caspase-3 activation.
The data presented in this paper demonstrate that treatment of HSC with anti-FAK antibodies decreased the expression of TIMP-1 mRNA. Matrix metalloproteinases (MMP) and their specific inhibitors (TIMP) are thought to play an essential role in liver injury associated with tissue remodeling[45-47], and it was reported that antisense oligonucleotides directed to TIMP-1 had some anti-hepatic fibrosis effect in the experimental immune hepatic rat models[48]. MMP or their inhibitors (TIMP) have been suggested to regulate apoptosis, and TIMP-1 inhibits cell death induced by hydrogen peroxide, adriamycin, or X-ray radiation[49]. It has recently shown that recovery from established experimental fibrosis can occur through the apoptosis of HSCs and is associated with reductions in liver collagen and expression of the TIMP-1 and TIMP-2[47]. Furthermore, FAK signaling pathway plays a pivotal role in the secretion of MMPs[50]. Therefore, the anti-FAK antibody regulated apoptotic pathway in HSCs might be triggered by interference with the TIMP-1 function.
In conclusion, integrin-ECM interactions influence apoptosis in rat HSC, and FAK is required for transducing survival signals from ECM in HSC. Our experiment provided a link between FAK and caspase-3, the expression of TIMP-1 and HSC survival. Thus the regulation of apoptosis may be very important in HSC biology.
Footnotes
Edited by Ma JY
Supported by the National Natural Science Foundation of China, No.39800054
References
- 1.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 2.Ben Mahdi MH, Andrieu V, Pasquier C. Focal adhesion kinase regulation by oxidative stress in different cell types. IUBMB Life. 2000;50:291–299. doi: 10.1080/713803721. [DOI] [PubMed] [Google Scholar]
- 3.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 4.Monteiro HP, Gruia-Gray J, Peranovich TM, de Oliveira LC, Stern A. Nitric oxide stimulates tyrosine phosphorylation of focal adhesion kinase, Src kinase, and mitogen-activated protein kinases in murine fibroblasts. Free Radic Biol Med. 2000;28:174–182. doi: 10.1016/s0891-5849(99)00233-6. [DOI] [PubMed] [Google Scholar]
- 5.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 6.Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–5613. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- 7.Gauthier R, Harnois C, Drolet JF, Reed JC, Vézina A, Vachon PH. Human intestinal epithelial cell survival: differentiation state-specific control mechanisms. Am J Physiol Cell Physiol. 2001;280:C1540–C1554. doi: 10.1152/ajpcell.2001.280.6.C1540. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Ghazaleh R, Kabir J, Jia H, Lobo M, Zachary I. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem J. 2001;360:255–264. doi: 10.1042/0264-6021:3600255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Water B, Nagelkerke JF, Stevens JL. Dephosphorylation of focal adhesion kinase (FAK) and loss of focal contacts precede caspase-mediated cleavage of FAK during apoptosis in renal epithelial cells. J Biol Chem. 1999;274:13328–13337. doi: 10.1074/jbc.274.19.13328. [DOI] [PubMed] [Google Scholar]
- 10.Sonoda Y, Matsumoto Y, Funakoshi M, Yamamoto D, Hanks SK, Kasahara T. Anti-apoptotic role of focal adhesion kinase (FAK). Induction of inhibitor-of-apoptosis proteins and apoptosis suppression by the overexpression of FAK in a human leukemic cell line, HL-60. J Biol Chem. 2000;275:16309–16315. doi: 10.1074/jbc.275.21.16309. [DOI] [PubMed] [Google Scholar]
- 11.Albanis E, Friedman SL. Hepatic fibrosis. Pathogenesis and principles of therapy. Clin Liver Dis. 2001;5:315–334, v-vi. doi: 10.1016/s1089-3261(05)70168-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhu YH, Hu DR, Nie QH, Liu GD, Tan ZX. Study on activation and c-fos, c-jun expression of in vitro cultured human hepatic stellate cells. Shijie Huaren Xiaohua Zazhi. 2000;8:299–302. [Google Scholar]
- 13.Eng FJ, Friedman SL. Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Am J Physiol Gastrointest Liver Physiol. 2000;279:G7–G11. doi: 10.1152/ajpgi.2000.279.1.G7. [DOI] [PubMed] [Google Scholar]
- 14.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 15.Huang GC, Zhang JS. Intercellular signal transduction of activated hepatic stellate cells. Shijie Huaren Xiaohua Zazhi. 2001;9:1056–1060. [Google Scholar]
- 16.Du WD, Zhang YE, Zhai WR, Zhou XM. Dynamic changes of type I, III and IV collagen synthesis and distribution of collagen-producing cells in carbon tetrachloride-induced rat liver fibrosis. World J Gastroenterol. 1999;5:397–403. doi: 10.3748/wjg.v5.i5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright MC, Issa R, Smart DE, Trim N, Murray GI, Primrose JN, Arthur MJ, Iredale JP, Mann DA. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology. 2001;121:685–698. doi: 10.1053/gast.2001.27188. [DOI] [PubMed] [Google Scholar]
- 18.Kato R, Kamiya S, Ueki M, Yajima H, Ishii T, Nakamura H, Katayama T, Fukai F. The fibronectin-derived antiadhesive peptides suppress the myofibroblastic conversion of rat hepatic stellate cells. Exp Cell Res. 2001;265:54–63. doi: 10.1006/excr.2001.5179. [DOI] [PubMed] [Google Scholar]
- 19.Issa R, Williams E, Trim N, Kendall T, Arthur MJ, Reichen J, Benyon RC, Iredale JP. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut. 2001;48:548–557. doi: 10.1136/gut.48.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Britton RS, Bacon BR. Intracellular signaling pathways in stellate cell activation. Alcohol Clin Exp Res. 1999;23:922–925. [PubMed] [Google Scholar]
- 21.Carloni V, Pinzani M, Giusti S, Romanelli RG, Parola M, Bellomo G, Failli P, Hamilton AD, Sebti SM, Laffi G, et al. Tyrosine phosphorylation of focal adhesion kinase by PDGF is dependent on ras in human hepatic stellate cells. Hepatology. 2000;31:131–140. doi: 10.1002/hep.510310121. [DOI] [PubMed] [Google Scholar]
- 22.Harrington EO, Smeglin A, Newton J, Ballard G, Rounds S. Protein tyrosine phosphatase-dependent proteolysis of focal adhesion complexes in endothelial cell apoptosis. Am J Physiol Lung Cell Mol Physiol. 2001;280:L342–L353. doi: 10.1152/ajplung.2001.280.2.L342. [DOI] [PubMed] [Google Scholar]
- 23.Tang YW, Yao XX. Regulating effect of HCC cells on the activation of stellate cells. Shijie Huaren Xiaohua Zazhi. 2001;9:202–204. [Google Scholar]
- 24.Huang GC, Zhang JS, Zhang YE. Effects of retinoic acid on proliferation, phenotype and expression of cyclin-dependent kinase inhibitors in TGF-beta1-stimulated rat hepatic stellate cells. World J Gastroenterol. 2000;6:819–823. doi: 10.3748/wjg.v6.i6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JY, Zhang QS, Guo JS, Hu MY. Effects of glycyrrhetinic acid on collagen metabolism of hepatic stellate cells at different stages of liver fibrosis in rats. World J Gastroenterol. 2001;7:115–119. doi: 10.3748/wjg.v7.i1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis PK, Ho A, Dowdy SF. Biological methods for cell-cycle synchronization of mammalian cells. Biotechniques. 2001;30:1322–136, 1328, 1322-136. doi: 10.2144/01306rv01. [DOI] [PubMed] [Google Scholar]
- 27.Iwamoto H, Sakai H, Tada S, Nakamuta M, Nawata H. Induction of apoptosis in rat hepatic stellate cells by disruption of integrin-mediated cell adhesion. J Lab Clin Med. 1999;134:83–89. doi: 10.1016/s0022-2143(99)90057-4. [DOI] [PubMed] [Google Scholar]
- 28.Guo YQ, Zhu ZH, Li JF. Flow cytometric analysis of apoptosis and proliferation in gastric cancer and precancerous lesion. Shijie Huaren Xiaohua Zazhi. 2000;8:983–987. [Google Scholar]
- 29.Fischer R, Schmitt M, Bode JG, Häussinger D. Expression of the peripheral-type benzodiazepine receptor and apoptosis induction in hepatic stellate cells. Gastroenterology. 2001;120:1212–1226. doi: 10.1053/gast.2001.23260. [DOI] [PubMed] [Google Scholar]
- 30.Jiang YF, Yang ZH, Hu JQ. Recurrence or metastasis of HCC: predictors, early detection and experimental antiangiogenic therapy. World J Gastroenterol. 2000;6:61–65. doi: 10.3748/wjg.v6.i1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 32.Hauck CR, Sieg DJ, Hsia DA, Loftus JC, Gaarde WA, Monia BP, Schlaepfer DD. Inhibition of focal adhesion kinase expression or activity disrupts epidermal growth factor-stimulated signaling promoting the migration of invasive human carcinoma cells. Cancer Res. 2001;61:7079–7090. [PubMed] [Google Scholar]
- 33.Nolan K, Lacoste J, Parsons JT. Regulated expression of focal adhesion kinase-related nonkinase, the autonomously expressed C-terminal domain of focal adhesion kinase. Mol Cell Biol. 1999;19:6120–6129. doi: 10.1128/mcb.19.9.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001;21:437–451. doi: 10.1055/s-2001-17558. [DOI] [PubMed] [Google Scholar]
- 35.Cheng ML, Wu YY, Huang KF, Luo TY, Ding YS, Lu YY, Liu RC, Wu J. Clinical study on the treatment of liver fibrosis due to hepatitis B by IFN-alpha (1) and traditional medicine preparation. World J Gastroenterol. 1999;5:267–269. doi: 10.3748/wjg.v5.i3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang FS, Wu ZZ. Current situation in studies of gene therapy for liver cirrhosis and liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:371–373. [Google Scholar]
- 37.Yao XX. Diagnosis and treatment of liver fibrosis. Shijie Huaren Xiaohua Zazhi. 2000;8:681–689. [Google Scholar]
- 38.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 39.van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 40.Oktay M, Wary KK, Dans M, Birge RB, Giancotti FG. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J Cell Biol. 1999;145:1461–1469. doi: 10.1083/jcb.145.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu LH, Yang X, Bradham CA, Brenner DA, Baldwin AS, Craven RJ, Cance WG. The focal adhesion kinase suppresses transformation-associated, anchorage-independent apoptosis in human breast cancer cells. Involvement of death receptor-related signaling pathways. J Biol Chem. 2000;275:30597–30604. doi: 10.1074/jbc.M910027199. [DOI] [PubMed] [Google Scholar]
- 42.Leist M, Jäättelä M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki A, Shiraki K. Tumor cell "dead or alive": caspase and survivin regulate cell death, cell cycle and cell survival. Histol Histopathol. 2001;16:583–593. doi: 10.14670/HH-16.583. [DOI] [PubMed] [Google Scholar]
- 44.Springer JE, Nottingham SA, McEwen ML, Azbill RD, Jin Y. Caspase-3 apoptotic signaling following injury to the central nervous system. Clin Chem Lab Med. 2001;39:299–307. doi: 10.1515/CCLM.2001.046. [DOI] [PubMed] [Google Scholar]
- 45.McCrudden R, Iredale JP. Liver fibrosis, the hepatic stellate cell and tissue inhibitors of metalloproteinases. Histol Histopathol. 2000;15:1159–1168. doi: 10.14670/HH-15.1159. [DOI] [PubMed] [Google Scholar]
- 46.Knittel T, Mehde M, Grundmann A, Saile B, Scharf JG, Ramadori G. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol. 2000;113:443–453. doi: 10.1007/s004180000150. [DOI] [PubMed] [Google Scholar]
- 47.Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245–G249. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- 48.Nie QH, Cheng YQ, Xie YM, Zhou YX, Cao YZ. Inhibiting effect of antisense oligonucleotides phosphorthioate on gene expression of TIMP-1 in rat liver fibrosis. World J Gastroenterol. 2001;7:363–369. doi: 10.3748/wjg.v7.i3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li G, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 inhibits apoptosis of human breast epithelial cells. Cancer Res. 1999;59:6267–6275. [PubMed] [Google Scholar]
- 50.Sein TT, Thant AA, Hiraiwa Y, Amin AR, Sohara Y, Liu Y, Matsuda S, Yamamoto T, Hamaguchi M. A role for FAK in the Concanavalin A-dependent secretion of matrix metalloproteinase-2 and -9. Oncogene. 2000;19:5539–5542. doi: 10.1038/sj.onc.1203932. [DOI] [PubMed] [Google Scholar]


