Abstract
AIM: To compare intensity-modulated radiotherapy (IMRT) with conformal radiotherapy (CRT) by investigating the dose profiles of primary tumors, electively treated regions, and the doses to organs at risk.
METHODS: CRT and IMRT plans were designed for five patients with upper esophageal carcinoma. For each patient, target volumes for primary lesions (67.2 Gy) and electively treated regions (50.4 Gy) were predefined. An experienced planner manually designed one CRT plan. Four IMRT plans were generated with the same dose-volume constraints, but with different beam arrangements. Indices including dose distributions, dose volume histograms (DVHs) and conformity index were compared.
RESULTS: The plans with three intensity-modulated beams were discarded because the doses to spinal cord were lager than the tolerable dose 45Gy, and the dose on areas near the skin was up to 50Gy. When the number of intensity beams increased to five, IMRT plans were better than CRT plans in terms of the dose conformity and homogeneity of targets and the dose to OARs. The dose distributions changed little when the beam number increased from five to seven and nine.
CONCLUSION: IMRT is superior to CRT for the treatment of upper esophageal carcinoma with simultaneous integrated boost (SIB). Five equispaced coplanar intensity-modulated beams can produce desirable dose distributions. The primary tumor can get higher equivalent dose by SIB technique. The SIB-IMRT technique shortens the total treatment time, and is an easier, more efficient, and perhaps a less error-prone way in delivering IMRT.
INTRODUCTION
Esophageal carcinoma is one of the most common cancers in china. Surgery and radiotherapy have always been the main treatment methods[1-4]. Chen et al[5] reported that the 5-year survival rate by radiation alone was comparable to that by surgery for patients with operable upper third lesions. Therefore, for diseases located in the upper esophageal region, including cervical and upper thoracic esophagus, radiotherapy is an efficient treatment selection. To give a higher radiation dose of between 60 Gy and 70 Gy to primary tumors and approximately 45 Gy to 50 Gy to electively irradiated lymph nodal regions is necessary for tumor local control. Spinal cord restricts the dose escalation of tumor and may affect the outcome of radiotherapy with conventional techniques, because it is close to cervical and upper thoracic esophagus and its endurance dose is less than 45 Gy. Lung is another dose limit factor in radiotherapy of esophageal cancer. Conformal radiotherapy (CRT) can reduce the irradiation volume of lung. But intensity-modulated radiotherapy (IMRT) is capable of producing more highly conformal dose distribution to a target and steeper dose gradients around the target edges than CRT. This capability makes it possible to give a high dose to the target volume while sparing adjacent normal tissues. Studies have shown benefits of IMRT in the treatment of head-and-neck[6-13] and other cancers[14-20].
The conventional technique for esophageal cancer is to use initial anterior-posterior large field arrangement followed by multi-field technique to boost the primary tumor with field size reduction. The large-field plan and boost plan are created independently. This makes it difficult to determine how the two plans affect each other. Tissues irradiated during the large-field phase receive unwanted additional dose during the boost phase from the beams irradiating the gross tumor. If simultaneous integrated boost (SIB) approach is applied, different dose requirements to the primary tumor and the elective regions can receive different doses within one fraction, and only one plan is needed for the entire course of treatment. Therefore, SIB-IMRT technique can overcome the drawbacks of conventional technique. Further more, it may shorten the treatment course by integrating the boost. The advantages of SIB-IMRT have been demonstrated for head-and-neck and prostate cancers[21-24].
The purpose of our study was to evaluate SIB-IMRT technique for upper esophageal carcinoma and to compare the effect of SIB-IMRT with SIB-CRT. We analyzed the dose distributions of primary tumor and electively treated regions and the doses to lung and spinal cord, and investigated the influence of the number of intensity-modulated beams to the dose distributions.
MATERIALS AND METHODS
Patients were immobilized in supine position. Planning CT scans were performed at 5 mm slice thickness using a dedicated helical CT scanner (Siemens, Somatom Plus 4). The entire lungs were scanned for further plan evaluation. CT images were transferred to the inverse treatment planning system (MDS Nordion, Helax-TMS 6.1) through network (Siemens, Lantis). Two target volumes, CTV1 and CTV2, were outlined on each set of the CT images. CTV1 included the gross tumor volume (GTV) with a margin of 3 cm in superior and inferior directions and 0.5 cm to 1.5 cm in other directions. CTV2 included correlated lymphatic drainage regions and extended to cricothyroid membrane. Margins of 0.5 cm were added to the CTVs in all directions to generate the planning target volumes (PTVs). The length of PTV1s ranged from 10.5 cm to 14.5 cm, and that of PTV2s ranged from 13 cm to 17 cm. The volume of PTV1s ranged from 167 cm3 to 251 cm3, and that of PTV2s ranged from 234 cm3 to 590 cm3 (note: PTV1 was surrounded by PTV2. The volume of PTV2 did not include the volume of corresponding PTV1). The patients were numbered in increasing orders with the size of PTV1. The spinal cord and lung were also contoured on the images.
The goal of the treatment was to deliver a prescribed dose of 67.2 Gy to at least 95% of PTV1 in 2.4 Gy fractions, and 50.4 Gy to at least 95% of PTV2 in 1.8 Gy fractions. The maximum dose to the spinal cord was 45 Gy. For the lungs, V20Gy (the volume of the lung received more than 20 Gy) should be less than 25% of lung volume and V30Gy (the volume of the lung received more than 30 Gy) should be less than 20% of lung.
One CRT plan and four IMRT plans were designed for each patient. Beam energy was 6MV X-ray. An experienced planner designed the beam arrangements of CRT plan by using trial and error method. The beam number and directions were manually adjusted to avoid the spinal cord and spare lung, and the beam weights were selected in order to maximize PTVs dose homogeneity. The beam number, directions, wedges and shape were different for these five patients because these parameters were set according to the position and shape of the targets. For example, the CRT plan of patient 1 used eight conformal beams from seven directions (i.e. 0o, 45o, 80o, 150o, 225o, 280o and 300o), and that of patient 3 used six conformal beams from five directions (i.e. 0o, 70o, 150o, 210o and 305o). Two beams, the large one covering PTV2, and the small one covering PTV1 could be in one beam direction. The beam arrangements of IMRT plans were same for these five patients by using three, five, seven and nine equispaced non-opposed coplanar beams in 360o beginning with 0o, respectively. The gantry angles for each beam arrangement are listed in Table 1. The beams would be delivered in a “step and shoot” mode with multileaf collimators. Delivery sequences were generated under the condition that the number of segments should be no more than 15 for each beam and the intensity levels were 10. The same dose-volume constraints were used for all the plannings during inverse optimization.
Table 1.
Beam arrangement of IMRT plans
| Number of beams | Gantry angles |
| 3 | 0o, 120o, 240o |
| 5 | 0o, 72 o, 144 o, 216o, 288o |
| 7 | 0o, 52 o, 103 o, 154 o, 206 o, 257o, 308o |
| 9 | 0o, 40 o, 80 o, 120 o, 160 o, 200 o, 240 o, 280o, 320o |
For the purpose of comparison, all plans were normalized to make 95% of PTV1 receive the prescribed dose of 67.2 Gy. The following parameters of these plans in each patient were compared, isodose distributions, DVHs, conformity indices (CI), mean dose, standard deviation (SD) and D95 (lowest dose encompassing 95% of the target) for PTVs, maximum dose for spinal cord, V20Gy, V30Gy and mean dose for lungs.
The equation for calculating conformity index is as follows[9,25]:
Math 1 (1)
Math 1.
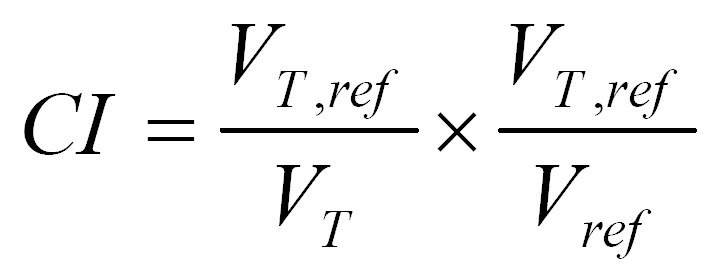
Math(A1).
where VT is the target volume, VT,ref is the target volume covered by the reference isodose line, Vref is the total volume covered by the reference isodose line. The value of CI is between zero and one. A CI of one represents the ideal situation that the target volume coincides exactly with the treatment volume, a CI of zero represents a plan in which there is no overlap between the two volumes. The reference dose was 67.2 Gy for PTV1 and 50.4 Gy for PTV2.
RESULTS
Isodose distributions
Since the plans of these patients had similar results of dose distributions in terms of target volume coverage and OARs sparing, only the isodose distributions and DVHs of patient 3 having the median PTV1 were presented. Figure 1 shows the isodose distributions on axial images for CRT and IMRT plans. PTV1 and PTV2 are shown as white lines. PTV1 was inside PTV2. The isodose lines were displayed on an absolute dose scale, the isodose levels of 67.2 Gy, 50.4 Gy, and 20 Gy were shown. All plans showed similar prescribed dose coverages of PTV1 and PTV2. However, the dose distributions outside the targets were different. For the IMRT plan with three beams, high isodose lines covered more normal tissues, the cervical spinal cord received more than 45 Gy. Some areas near the skin received a dose as high as 50 Gy. The dose to spinal cord exceeded the endurance dose for four of the five patients (i.e. patient 2 to patient 5). Therefore, three beam IMRT plan was unacceptable. For the CRT plan, high isodose lines covered more normal tissues, indicating that the dose distributions of the 5, 7, and 9 beam IMRT plans were more conformal than CRT plan.
Figure 1.
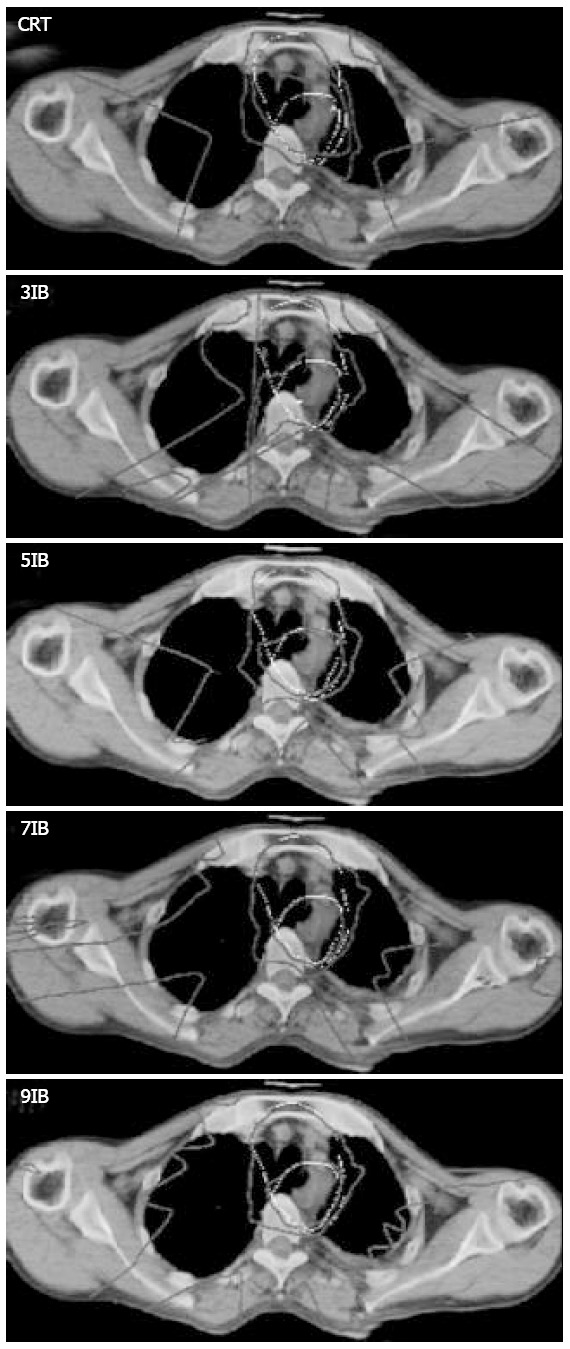
Isodose distributions on axial images for CRT and IMRT plans of patient 3. The white lines represent PTV1 and PTV2. PTV1 was inside PTV2. The isodose levels of 67.2 Gy, 50.4 Gy and 20 Gy were shown. IB stands for intensity-modulated beam.
Targets
DVHs of CRT and IMRT plans for PTV1 and PTV2 of patient 3 are shown in Figures 2A and 2B. The mean results for these five patients are listed in Table 2. Three beam IMRT plan was presented to show the influence of the number of intensity-modulated beams. The targets’ dose homogeneity and conformity of CRT plan were much worse than those of IMRT plans with the beam number no less than five. DVHs were similar to IMRT plans, and the indices did not show any obvious difference as the beam number increased from five to seven and nine. The conformity was improved as the number of intensity-modulated beams increased, but the improvement was marginal when beam number was over five.
Figure 2.
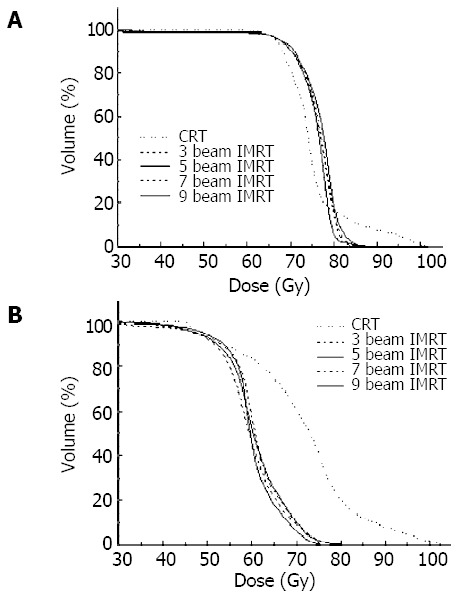
DVHs of PTVs for CRT and IMRT plans of patient 3. (A), PTV1; (B), PTV2.
Table 2.
Results for CRT and IMRT in five upper esophageal cancer patients(mean±SD)
| Stucture | Parameter | CRT | 3F IMRT | 5F IMRT | 7F IMRT | 9F IMRT |
| PTV1 | Mean dose (Gy) | 73.0 ± 3.0 | 74.9 ± 3.8 | 74.2 ± 2.3 | 73.9 ± 2.0 | 74.1 ± 1.5 |
| SD (%) | 6.3 ± 3.7 | 6.7 ± 2.7 | 6.0 ± 2.0 | 5.7 ± 2.1 | 6.0 ± 1.7 | |
| D95 (Gy) | 67.2 ± 0.0 | 67.2 ± 0.0 | 67.2 ± 0.0 | 67.2 ± 0.0 | 67.2 ± 0.0 | |
| CI | 0.47 ± 0.16 | 0.59 ± 0.18 | 0.70 ± 0.04 | 0.74 ± 0.06 | 0.75 ± 0.07 | |
| PTV2 | Mean dose (Gy) | 65.3 ± 4.4 | 60.0 ± 2.3 | 59.3 ± 1.4 | 58.4 ± 1.8 | 58.4 ± 2.0 |
| SD (%) | 11.6 ± 3.8 | 9.2 ± 1.8 | 8.8 ± 1.5 | 8.5 ± 1.9 | 8.6 ± 1.8 | |
| D95 (Gy) | 52.4 ± 3.3 | 51.2 ± 2.8 | 50.8 ± 3.4 | 50.5 ± 3.3 | 51.2 ± 2.9 | |
| CI | 0.52 ± 0.08 | 0.46 ± 0.11 | 0.64 ± 0.06 | 0.67 ± 0.07 | 0.68 ± 0.08 | |
| Lung | Mean dose (Gy) | 12.4 ± 1.7 | 11.1 ± 1.2 | 10.8 ± 1.3 | 10.7 ± 1.3 | 10.9 ± 1.2 |
| V20 (%) | 24.7 ± 2.8 | 22.1 ± 1.7 | 22.4 ± 0.7 | 23.2 ± 1.6 | 23.8 ± 1.7 | |
| V30 (%) | 18.6 ± 2.9 | 15.1 ± 1.6 | 15.0 ± 1.9 | 13.5 ± 2.2 | 13.5 ± 3.2 | |
| Spinal cord | Maximum dose (Gy) | 40.9 ± 2.7 | 56.9 ± 7.2 | 43.9 ± 1.0 | 43.3 ± 0.8 | 42.0 ± 1.5 |
OARs
The mean results of OARs for these five patients are listed in Table 2. The three beam IMRT plans were unacceptable as their doses to spinal cord were lager than the tolerable dose 45 Gy. The results in five, seven, and nine beam IMRT plans were similar and better than those of CRT plan in terms of sparing lung. The mean doses of lung for the five, seven, and nine beam IMRT plans were almost identical.
DISCUSSION
The cervical and upper thoracic esophageal regions are characterized by variation of body thickness, the distance of esophagus to the body surface, and the closeness of the target to the spinal cord. Acceptable SIB-IMRT plans were superior to SIB-CRT plans in treating tumors in these regions. The dose homogeneity of PTV2 improved, the volume of higher dose outside the primary tumor decreased, the dose conformity improved. The difference between SIB-CRT plans and SIB-IMRT plans was due to that SIB-CRT did not compensate for the variations of body thickness and depth of esophagus. It was difficult to protect the spinal cord while keeping the dose uniformity in the target volume with SIB-CRT plans. In addition, the benefit of CRT plan depended greatly upon the planner’s experiences, and many trials were required to figure out the beam directions and weights. On the other hand, the planner only arranged the beam directions (Table 1) and set the dose-volume constraints in designing IMRT plan. The other work was done by the inverse treatment planning system.
Therefore, it took less time to design an IMRT plan than to adjust a CRT plan.
The ideal number of beams in an IMRT plan has not been decided. Generally, a larger number of beams would provide more parameters to adjust and, therefore a greater flexibility to achieve a desired dose distribution. However, the more the number of beams was used, the more the effort was required for planning, quality assurance, dosimetric verification and treatment. Practically, it was desirable to reduce the number of beams to as few as possible without compromising the quality of the treatment. Soderstrom and Brahme[26,27] concluded that fewer intensity-modulated beams were needed than uniform beams to achieve the same or even better results, perhaps as few as three beams were sufficient in most cases provided beam angles were optimized. Mohan and Ling[27,28] believed that the ideal minimum number of beams would depend upon a variety of geometrical and biological factors and the desired target dose level to achieve an adequate local control. Studies[8,11,22,29] showed that the beams less than ten were enough for most clinical requirements. Thus, the number of beams used in our study was less than 10. Pirzkall et al[30] concluded that the ideal number of beams was influenced by the photon energy for deeply seated targets.
From the results of this study, the plan with three equispaced coplanar intensity-modulated beams could not meet the requirement of OARs and its high dose conformity was worse than those with more beams. It might be due to the fact that the beam directions were not optimized. Five equispaced coplanar intensity-modulated beams were sufficient to get an adequate high dose coverage and a dose homogeneity for both targets of upper esophageal cancer. There was no obvious improvement in target dose homogeneity, conformity and the dose to OARs with more than five beams. Therefore we believed that for SIB-IMRT of upper esophageal carcinoma, five beams were sufficient to deliver a satisfactory dose distribution and further increase of the number of beams would complicate the treatment without significant improvement of dose distribution.
In addition, concomitant boost treatment may offer some radiobiological advantage in terms of a lower dose per fraction to normal tissues while delivering a higher dose per fraction to the targets. In this study, the dose per fraction to PTV1 and PTV2 was 2.4 Gy and 1.8 Gy, respectively. To compare with the conventional fractionation (2 Gy/f), we calculated the normalized total dose (NTD)[22,23] that was the biological equivalent dose given in 2 Gy/f by linear-quadratic (LQ) model[31]. The NTD for PTV1 and PTV2 was about 70 Gy and 49 Gy, respectively. Therefore, the primary tumor could get a higher dose with SIB treatment while the elective regions had an adequate dose. It was beneficial to tumor local control.
In conclusion, for SIB treatment of upper esophageal carcinoma, IMRT is better than CRT in terms of the target volume coverage, OARs sparing and time cost in treatment planning process. Five equispaced coplanar intensity-modulated beams produce desirable dose distributions. The SIB-IMRT technique not only shortens the total treatment time but also is an easier, more efficient, and perhaps a less error-prone way of delivering IMRT. Primary tumor can get a higher equivalent dose by SIB. The effect of SIB-IMRT is currently under clinical trial in our hospital.
Footnotes
Edited by Ren SY and Wang XL Proofread by Xu FM
References
- 1.Xiao ZF. Esophageal carcinoma. In: Yin WB, Gu XZ, eds. 3rded. Radiation oncology. Bejing: Peking union medical college press. 2002:598–622. [Google Scholar]
- 2.Wang SJ, Wen DG, Zhang J, Man X, Liu H. Intensify standardized therapy for esophageal and stomach cancer in tumor hospitals. World J Gastroenterol. 2001;7:80–82. doi: 10.3748/wjg.v7.i1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao ZF, Yang ZY, Zhou ZM, Yin WB, Gu XZ. Radiotherapy of double primary esophageal carcinoma. World J Gastroenterol. 2000;6:145–146. doi: 10.3748/wjg.v6.i1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen DF. External radiotherapy in combination with brachytherapy in the treatment of 121 cases of esophageal cancer. Shijie Huaren Xiaohua Zazhi. 1998;6:127. [Google Scholar]
- 5.Chen DF, Yang ZY, Yin WB. Radiotherapy of 180 cases of oper-able esophageal carcinoma. China Natl J New Gastroenterol. 1997;3:123–126. doi: 10.3748/wjg.v3.i2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang D, Xia P, Akazawa P, Akazawa C, Quivey JM, Verhey LJ, Kaplan M, Lee N. Comparison of treatment plans using inten-sity-modulated radiotherapy and three-dimensional conformal radiotherapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:158–168. doi: 10.1016/s0360-3016(03)00080-4. [DOI] [PubMed] [Google Scholar]
- 7.Lee N, Xia P, Quivey JM, Sultanem K, Poon I, Akazawa C, Akazawa P, Weinberg V, Fu KK. Intensity-modulated radio-therapy in the treatment of nasopharyngeal carcinoma: an up-date of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53:12–22. doi: 10.1016/s0360-3016(02)02724-4. [DOI] [PubMed] [Google Scholar]
- 8.Hsiung CY, Yorke ED, Chui CS, Hunt MA, Ling CC, Huang EY, Wang CJ, Chen HC, Yeh SA, Hsu HC, et al. Intensity-modulated radiotherapy versus conventional three-dimensional conformal radiotherapy for boost or salvage treatment of na-sopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2002;53:638–647. doi: 10.1016/s0360-3016(02)02760-8. [DOI] [PubMed] [Google Scholar]
- 9.Bragg CM, Conway J, Robinson MH. The role of intensity-modu-lated radiotherapy in the treatment of parotid tumors. Int J Radiat Oncol Biol Phys. 2002;52:729–738. doi: 10.1016/s0360-3016(01)02660-8. [DOI] [PubMed] [Google Scholar]
- 10.Adams EJ, Nutting CM, Convery DJ, Cosgrove VP, Henk JM, Dearnaley DP, Webb S. Potential role of intensity-modulated ra-diotherapy in the treatment of tumors of the maxillary sinus. Int J Radiat Oncol Biol Phys. 2001;51:579–588. doi: 10.1016/s0360-3016(01)01655-8. [DOI] [PubMed] [Google Scholar]
- 11.Hunt MA, Zelefsky MJ, Wolden S, Chui CS, LoSasso T, Rosenzweig K, Chong L, Spirou SV, Fromme L, Lumley M, et al. Treatment planning and delivery of intensity-modulated radiotherapy for primary nasopharynx cancer. Int J Radiat Oncol Biol Phys. 2001;49:623–632. doi: 10.1016/s0360-3016(00)01389-4. [DOI] [PubMed] [Google Scholar]
- 12.Xia P, Fu KK, Wong GW, Akazawa C, Verhey LJ. Comparison of treatment plans involving intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2000;48:329–337. doi: 10.1016/s0360-3016(00)00585-x. [DOI] [PubMed] [Google Scholar]
- 13.Posner MD, Quivey JM, Akazawa PF, Xia P, Akazawa C, Verhey LJ. Dose optimization for the treatment of anaplastic thyroid carcinoma: a comparison of treatment planning techniques. Int J Radiat Oncol Biol Phys. 2000;48:475–483. doi: 10.1016/s0360-3016(00)00621-0. [DOI] [PubMed] [Google Scholar]
- 14.De Meerleer GO, Vakaet LA, De Gersem WR, De Wagter C, De Naeyer B, De Neve W. Radiotherapy of prostate cancer with or without intensity modulated beams: a planning comparison. Int J Radiat Oncol Biol Phys. 2000;47:639–648. doi: 10.1016/s0360-3016(00)00419-3. [DOI] [PubMed] [Google Scholar]
- 15.Zelefsky MJ, Fuks Z, Happersett L, Lee HJ, Ling CC, Burman CM, Hunt M, Wolfe T, Venkatraman ES, Jackson A, et al. Clinical experience with intensity modulated ra-diation therapy (IMRT) in prostate cancer. Radiother Oncol. 2000;55:241–249. doi: 10.1016/s0167-8140(99)00100-0. [DOI] [PubMed] [Google Scholar]
- 16.Ling CC, Burman C, Chui CS, Kutcher GJ, Leibel SA, LoSasso T, Mohan R, Bortfeld T, Reinstein L, Spirou S, et al. Conformal radiation treatment of prostate cancer using inversely-planned intensity-modulated photon beams produced with dynamic multileaf collimation. Int J Radiat Oncol Biol Phys. 1996;35:721–730. doi: 10.1016/0360-3016(96)00174-5. [DOI] [PubMed] [Google Scholar]
- 17.Pirzkall A, Carol M, Lohr F, Hoss A, Wannenmacher M, Debus J. Comparison of intensity-modulated radiotherapy with conven-tional conformal radiotherapy for complex-shaped tumors. Int J Radiat Oncol Biol Phys. 2000;48:1371–1380. doi: 10.1016/s0360-3016(00)00772-0. [DOI] [PubMed] [Google Scholar]
- 18.Evans PM, Donovan EM, Partridge M, Childs PJ, Convery DJ, Eagle S, Hansen VN, Suter BL, Yarnold JR. The delivery of inten-sity modulated radiotherapy to the breast using multiple static Fields. Radiother Oncol. 2000;57:79–89. doi: 10.1016/s0167-8140(00)00263-2. [DOI] [PubMed] [Google Scholar]
- 19.Hong L, Hunt M, Chui C, Spirou S, Forster K, Lee H, Yahalom J, Kutcher GJ, McCormick B. Intensity-modulated tangential beam irradiation of the intact breast. Int J Radiat Oncol Biol Phys. 1999;44:1155–1164. doi: 10.1016/s0360-3016(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 20.Cardinale RM, Benedict SH, Wu Q, Zwicker RD, Gaballa HE, Mohan R. A comparison of three stereotactic radiotherapy techniques: ARCS vs. noncoplanar fixed field fields vs. intensity modulation. Int J Radiat Oncol Biol Phys. 1998;42:431–436. doi: 10.1016/s0360-3016(98)00206-5. [DOI] [PubMed] [Google Scholar]
- 21.Bos LJ, Damen EMF, de Boer RW, Mijnheer BJ, McShan DL, Fraass BA, Kessler ML, Lebeaque JV. Reduction of rectal dose by integration of the boost in the large-field treatment plan for prostate irradiation. Int J Radiat Oncol Biol Phys. 2002;52:254–265. doi: 10.1016/s0360-3016(01)02676-1. [DOI] [PubMed] [Google Scholar]
- 22.Wu Q, Manning M, Schmidt-Ullrich R, Mohan R. The potential for sparing of parotids and escalation of biologically effective dose with intensity-modulated radiation treatments of head and neck cancers: A treatment design study. Int J Radiat Oncol Biol Phys. 2000;46:195–205. doi: 10.1016/s0360-3016(99)00304-1. [DOI] [PubMed] [Google Scholar]
- 23.Mohan R, Wu Q, Manning M, Schmidt-Ullrich R. Radiobiologi-cal considerations in the design of fractionation strategies for intensity modulated radiation therapy of head and neck cancers. Int J Radiat Oncol Biol Phys. 2000;46:619–630. doi: 10.1016/s0360-3016(99)00438-1. [DOI] [PubMed] [Google Scholar]
- 24.Butler EB, Teh BS, Grant WH 3rd, Uhl BM, Kuppersmith RB, Chiu JK, Donovan DT, Woo SY. SMART (simultaneous modu-lated accelerated radiation therapy) boost: a new accelerated frac-tionation schedule for the treatment of head and neck cancer with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 1999;45:21–32. doi: 10.1016/s0360-3016(99)00101-7. [DOI] [PubMed] [Google Scholar]
- 25.van’t Riet A, MaK ACA, Moerland MA, Elders LH, van der Zee W. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys. 1997;37:731–736. doi: 10.1016/s0360-3016(96)00601-3. [DOI] [PubMed] [Google Scholar]
- 26.Söderström S, Brahme A. Which is the most suitable number of photon beam portals in coplanar radiation therapy? Int J Radiat Oncol Biol Phys. 1995;33:151–159. doi: 10.1016/0360-3016(95)00113-D. [DOI] [PubMed] [Google Scholar]
- 27.Söderström S, Brahme A. Small is beautiful-and often enough: In response to the editorial by Mohan and Ling. Int J Radiat Oncol Biol Phys. 1996;34:757–759. doi: 10.1016/0360-3016(96)84800-0. [DOI] [PubMed] [Google Scholar]
- 28.Mohan R, Ling CC. When becometh less more? (editorial) Int J Radiat Oncol Biol Phys. 1995;33:235–237. doi: 10.1016/0360-3016(95)02020-C. [DOI] [PubMed] [Google Scholar]
- 29.Stein J, Mohan R, Wang XH, Bortfeld T, Wu Q, Preiser K, Ling CC, Schlegel W. Number and orientations of beams in intensity-modulated radiation treatments. Med Phys. 1997;24:149–160. doi: 10.1118/1.597923. [DOI] [PubMed] [Google Scholar]
- 30.Pirzkall A, Carol MP, Pickett B, Xia P, Roach M 3rd, Verhey LJ. The effect of beam energy and number of fields on photon-based IMRT for deep-seated targets. Int J Radiat Oncol Biol Phys. 2002;53:434–442. doi: 10.1016/s0360-3016(02)02750-5. [DOI] [PubMed] [Google Scholar]
- 31.Tai P, Van Dyk J, Yu E, Battista J, Schmid M, Stitt L, Tonita J, Coad T. Radiation treatment for cervical esophagus: patterns of practice study in Canada, 1996. Int J Radiat Oncol Biol Phys. 2000;47:703–712. doi: 10.1016/s0360-3016(00)00484-3. [DOI] [PubMed] [Google Scholar]


