Abstract
AIM: rAAV mediated endostatin gene therapy has been examined as a new method for treating cancer. However, a sustained and high protein delivery is required to achieve the desired therapeutic effects. We evaluated the impact of topoisomerase inhibitors in rAAV delivered endostatin gene therapy in a liver tumor model.
METHODS: rAAV containing endostatin expression cassettes were transduced into hepatoma cell lines. To test whether the topoisomerase inhibitor pretreatment increased the expression of endostatin, Western blotting and ELISA were performed. The biologic activity of endostatin was confirmed by endothelial cell proliferation and tube formation assays. The anti-tumor effects of the rAAV-endostatin vector combined with a topoisomerase inhibitor, etoposide, were evaluated in a mouse liver tumor model.
RESULTS: Topoisomerase inhibitors, including camptothecin and etoposide, were found to increase the endostatin expression level in vitro. The over-expressed endostatin, as a result of pretreatment with a topoisomerase inhibitor, was also biologically active. In animal experiments, the combined therapy of topoisomerase inhibitor, etoposide with the rAAV-endostatin vector had the best tumor-suppressive effect and tumor foci were barely observed in livers of the treated mice. Pretreatment with an etoposide increased the level of endostatin in the liver and serum of rAAV-endostatin treated mice. Finally, the mice treated with rAAV-endostatin in combination with etoposide showed the longest survival among the experimental models.
CONCLUSION: rAAV delivered endostatin gene therapy in combination with a topoisomerase inhibitor pretreatment is an effective modality for anticancer gene therapy.
INTRODUCTION
Antiangiogenic therapy for cancer has emerged as an exciting new therapeutic modality because tumors are angiogenesis-dependent during growth and metastasis[1-6]. One of the most potent endogenous angiogenic inhibitors, endostatin, has been reported to inhibit endothelial proliferation and regression of solid tumors[7-10]. Although endostatin induces and sustaines the dormancy of tumor growth, large quantities of proteins are needed for prolonged periods[9]. Moreover, besides being difficulty to be purified, endostatin has a short half-life in vivo. In order to circumvent the obstacle presented by the pharmacokinetics of endostatin, delivery of the gene cassettes encoding endostatin has been attempted[11-19].
Recombinant adeno-associated virus (rAAV) vector is a good candidate for antiangiogenesis-based cancer gene therapy[20]. rAAV vector is derived from a nonpathogenic parvovirus that is capable of integrating into the host DNA, which allows the long-term expression. In addition, removal of the viral coding sequences minimizes immunogenicity. The rAAV vector has a broad host tropism and transducts in dividing and non-dividing cells. The liver is an important target for gene therapy, because of its large size, its protein synthesizing capacity and because it is easily accessible to vectors. Although the rAAV is a promising vector for liver-directed gene therapy, its potential for therapeutic use has been limited due to its inefficient transduction into the liver[21,22]. In order to achieve high serum levels of endostatin with a stable expression, the transduction of non-dividing cell populations is essential in liver-directed gene therapy. Some topoisomerase inhibitors, such as etoposide or camptothecin, increase the transduction efficiency of the rAAV in non-dividing cells as well as in dividing cells[23-25].
Therefore, this study investigated the potential of a rAAV vector-mediated endostatin gene therapy in combination with topoisomerase inhibitor in a liver tumor model. This paper demonstrates that a topoisomerase inhibitor in a rAAV delivered endostatin gene therapy enhances the antiangiogenic effects, and that this method has the potential to be used as a new strategy for cancer gene therapy.
MATERIALS AND METHODS
Cells culture
Hepa1c1c7 mouse hepatoma cell line (ATCC CRL 2026), S-180 murine sarcoma cell line (ATCC CCL-8) and 293-EBNA cells (transformed human embryonic kidney, ATCC R620-07) were grown in DMEM (Gibco BRL, Grand Island, NY) with 100 mL/L heat inactivated (30 min at 56 °C) fetal bovine serum, 2 mmol/L L-glutamine, 100 units/mL penicillin, and 100 mg/mL streptomycin at 37 °C in 50 mL/LCO2. The 293-EBNA cell line was maintained in medium containing geneticin (G418, 250 µg/mL; Gibco BRL). Human umbilical vein endothelial cells (HUVEC) were isolated from the human umbilical vein (Institutional review board approved protocol with informed consent) using a collagenase type I (Sigma, St. Louis, MO) perfusion. The cells were then grown on gelatin-coated tissue culture plates in M199 medium (Sigma) supplemented with 100 ng/mL heparin (Gibco BRL), 200 mL/L FBS, 100 mg/mL streptomycin, 100 U/mL penicillin, 3 ng/mL bFGF (Upstate, Waltham, MA).
rAAV vector construction and production
pEndoSTHB vector was kindly provided by Dr K.K. Tanabe (Harvard Medical School, Boston, MA), which contained murine endostatin cDNA downstream of murine Ig κ-chain signal peptide and upstream of a c-myc epitope[26]. This plasmid was modified by site-directed mutagenesis (oligonucleotide primer 1: ACC-TCT-TTC-TCC-AAG-TAA-TGA-CTC-CAG-TGT-GGT-GGA, oligonucleotide primer 2: TCC-ACC-ACA-CTG-GAG-TCA-TTA-CTT-GGA-GAA-AGA-GGT), which mutated the sequence upstream of the c-myc epitope into stop codons to remove c-myc tag[27]. AAV-helper free system (Stratagene, La Jolla, CA) was used to produce rAAV. SalI – XhoI fragment from modified pEndoSTHB was subcloned into the pCMV-MCS vector (Stratagene). Once this expression construct was verified, the NotI fragment, containing the expression cassette of endostatin, was cloned into the pAAV-LacZ viral expression vector (Stratagene). The parental vector rAAV-Lac Z was used as control. pAAV-endostatin containing the cytomegalovirus (CMV) promoter with a murine Ig κ-chain signal peptide was flanked by cDNA of murine endostatin. The rAAV vectors were produced using a standard triple-plasmid transfection method, and purified by a heparin sulfate column separation[28]. Briefly, the recombinant expression plasmid was co-transfected into 293-EBNA cells with pHelper (Stratagene) and pAAV-RC (Stratagene), which supply all the trans-acting factors required for AAV replication and packaging in 293-EBNA cells. rAAV stocks were subjected to 3 rounds of freezing and thawing. After cell debris was removed by centrifugation, the stocks were filtered using a low protein binding 5 µm syringe filter (Millipore, Bedford, MA), followed by a 0.8 µm syringe filter and subsequently by heparin agarose column (Sigma) separation. The viruses were finally concentrated in a millipore concentrator (100-ku cut off) and titrated by taking the average of three quantitative real time PCR using a LightCycler-FastStart DNA Master SYBR Green system (Roche Molecular Biochemicals, Mannheimn, Bermany) (forward primer: GGC-TAG-CCA-CCA-TGG-AGA-CAG-ACA, reverse primer: ACA-CTG-GAG-TCA-TTA-CTT-GGA-GAA, 10 min pre-incubation at 94 °C followed by 50 cycles at 94 °C for 15 s, at 60 °C for 5 s and at 72 °C for 10 s in a 7 700 Q-PCR machine, Applied Biosystems, Foster City, California).
Treatment with topoisomerase inhibitors
Stock solutions of etoposide (10 mmol/L) (Laboratories Lilly France, Fegersheim, France) and camptothecin (10 mmol/L) (Yakult Honsha, Tokyo, Japan) were stored at -20 °C and diluted into HBSS (Hanks’ balanced salt solution, Gibco BRL) for use in experiments. Hepa1c1c7 cells were pretreated with either 3 μmol/L of etoposide or 10 μmol/L of camptothecin for 6 h, and then washed twice with L-DMEM (20 g/LFBS, 2 mmol/L L-glutamine in DMEM) prior to the rAAV addition. The vector was then added for transduction, and the plates were swirled gently at 30 min intervals during an incubation of 2 h. H-DMEM (180 g/LFBS, 2 mmol/L L-glutamine) was then added to each plate, and incubation was continued for 48 h at 37 °C.
Western blot analysis
Western blot was performed on the protein from conditioned medium of rAAV transduced Hepa1c1c7 cells. The conditioned medium was concentrated in a Microcon YM-10 (millipore) and subjected to electrophoresis under reducing conditions on a 40-120 g/L NuPAGE gel (Invitrogen, San Diego, CA). The proteins obtained were transferred onto a nitrocellulose membrane (Invitrogen) and incubated overnight in 50 g/L nonfat milk in PBS at 4 °C. After three 10-min washes in 19 g/L nonfat milk, 1 g/L Tween 20 in PBS, the membranes were incubated in monoclonal goat anti-mouse endostatin antibodies (R&D Systems, Minneapolis, MN) diluted 1:500. After washing, the membranes were incubated in horseradish peroxidase-conjugated donkey anti-goat immunoglobulin (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:4 000 and the proteins were detected using an ECL plus kit (Amersham Pharmacia Biotechnology, Uppsala, Sweden).
Endostatin enzyme immunoassay
Endostatin levels in the conditioned medium of cultured cells or in the mouse serum were determined using a mouse endostatin immunoassay kit (Chemicon International, Temecula, CA, United States).
Endothelial cell proliferation assay
HUVECs were plated on 5 × 103 in 96-well gelatin-coated plates and allowed to attach in complete medium for 4 h. The medium was then replaced by the conditioned medium, where the endostatin concentration was measured by an ELISA assay. After 1 h, an equal volume of 2 × complete medium was added, and the number of cells was quantified by a colorimetric MTT assay on the indicated days. The test was performed in triplicate.
Endothelial cell tube formation assay
Twenty-four well plates were coated with 50 μL of Matrigel (BD Biosciences, Bedford, MA) in an ice bath and then incubated at 37 °C for 1 h. HUVECs at a density of 5 × 104 cells in each well were seeded and cultured in the conditioned media. After 18-h incubation, the plates were photographed. All tests were performed in triplicate.
In vivo tumor model
Animal experiments were carried out in accordance with the policies of Animal Research Committee of the Yonsei University College of Medicine. Twenty-five 8-wk-old female ICR mice (Charles River Laboratories, Wilmington, MA) were randomly divided into 5 equal groups, namely no treatment and rAAV-LacZ treatment alone as the control, treated with rAAV-LacZ in combination with etoposide pretreatment, treated with rAAV-endostatin alone, and treated with rAAV-endostatin in combination with etoposide pretreatment. In the pretreatment group, etoposide (40 mg/kg) in 200 μL HBSS was administered 3 times for a week by an intraperitoneal injection beginning 7 d before rAAV injection. Hepatic tumors were induced by directly injecting 5 × 106 S -180 murine sarcoma cells into the liver. Simultaneously, 500 μL of rAAV-mEndostatin (1.5 × 1012 viral particles) was injected into the spleen in the endostatin treatment groups in order to deliver viral particles into the liver. The mice were sacrificed 7 d after tumor cell injections by a halothane overdose to examine hepatic tumors. The tumor volume (TV) was determined using the following formula: TV = (Length × width2)/2. In order to evaluate the long-term survival, the experiment was repeated and the mice were followed up for 2 mo. The survival time was defined from the day of tumor injection to death. The mice that were alive at the end of the follow-up period were estimated as the censored observation.
Localization of endostatin expression in liver
The livers were harvested and 5 μm-thick sections of the formalin-fixed, paraffin-embedded specimens were deparaffinized in xylene and heated in a citrate buffer for 10 min. Endogenous peroxidase activity was blocked by incubation with 10 mL/L H2O2 and 10 g/L Triton X-100. Gout anti-mouse antibodies were applied at a dilution of 1:60 overnight at 4 °C. Biotinylated rabbit anti-gout antibodies (Vector, Burlingame, CA) were then applied for 1 h at room temperature at a 1:200 dilution. After incubation with streptavidin conjugated to horseradish peroxidase, a substrate containing chromogen, 3,3’ diaminobenzidine tetrahydrochloride, was added and the slides were counterstained with hematoxylin. All the slides were air-dried and kept in dark at 4 °C until evaluated.
Microvessel density (MVD) assessment
In order to analyze hepatic tumor microvessels, tissue sections (5 μm) of formalin-fixed, paraffin-embedded specimens were evaluated using rat anti-mouse CD34 antibodies (1:50; RAM34; Pharmingen, San Diego, CA) as the primary antibody and biotinylated rabbit anti-rat antibodies (Vector, Burlingame, CA) as the secondary antibody. After incubation of the tissue with streptavidin conjugated to horseradish peroxidase, the reactions were visualized by a substrate containing chromogen, 3,3’ diaminobenzidine tetrahydrochloride. The slides were counterstained with hematoxylin. At least 5 thin slices were made from each tumor and used for MVD assessment. MVD was estimated by counting the number of CD34-positive vessels in the tumor area, which was representative of the highest MVD at × 200 magnification. The counts were typically made in 3-5 hot spots, and the highest MVD was used to characterize the tumor.
Statistical analysis
The data were expressed as mean ± SD. Student’s t test was used to analyze the statistical differences in endostatin levels in the conditioned medium of cultured cells or in mouse serum, endothelial cell proliferation, tumor size, or microvessel assessment among the groups. The Kaplan and Meier method was used to calculate the survival rate in the in vivo experiment and the survival differences between the groups were evaluated using the log-rank test. A P < 0.05 was considered statistically significant.
RESULTS
Increased in vitro expression of endostatin molecules on rAAV-endostatin transduced hepatoma cells by pretreatment with topoisomerase inhibitors
Hepa1c1c7 mouse hepatoma cells in the 10 cm plate were incubated with either rAAV-endostatin or rAAV-LacZ vectors (1 × 104 viral particles/cell) for 48 h. In the pretreatment group, etoposide (3 µmol/L) or camptothecin (10 µmol/L) was administered 6 h prior to transduction. Conditioned media were concentrated 10 times and used in both Western blotting and ELISA. The in vitro expression of endostatin was detected by Western blot analysis. The recombinant endostatin was visualized in the supernatant of Hepa1c1c7 cells transduced with rAAV-endostatin, but not in the supernatant of Hepa1c1c7 transduced with rAAV-LacZ. The endostatin expression level was enhanced as a result of the pretreatment with either etoposide or camptothecin (Figure 1A). ELISA was performed to quantify the expression level. Hepa1c1c7 cells transduced with rAAV-endostatin expressed endostatin (19.0 ± 3.0 ng/mL) compared with the vector control (0.3 ± 0.3 ng/mL) and mock control (0.3 ± 0.2 ng/mL), and topoisomerase inhibitors enhanced significantly the endostatin expression level (P < 0.05) (Figure 1B). In the range of concentrations used in this study, etoposide or camptothecin had little effect on Hepa1c1c7 cell growth (data not shown). Etoposide increased the endostatin expression level more than camptothecin (43.3 ± 5.1 vs 30.7 ± 5.7 ng/mL, P < 0.05). Consequently, etoposide was chosen as a combination therapy in the in vivo experiment.
Figure 1.
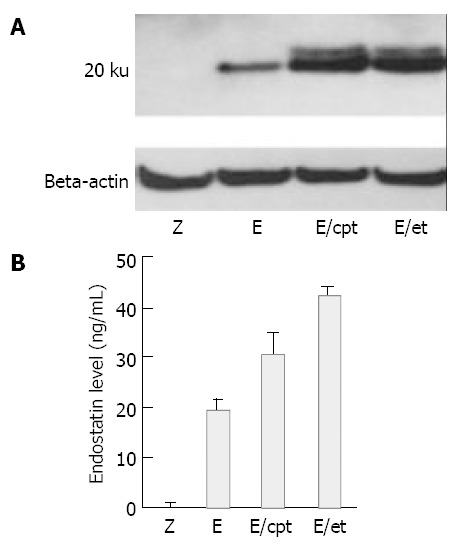
Effects of topoisomerase inhibitors on rAAV mediated endostatin expression level. Hepa1c1c7 mouse hepatoma cells were transduced with 1 × 104 particles/cell of rAAV-endostatin or rAAV-LacZ. In pretreatment group, etoposide (3 µmol/L) or camptothecin (10 µmol/L) was administered 6 h before transduction. Fourty-eight hours later, the expression of endostatin was determined. A: Analysis of protein expression by NuPAGE electrophoresis, B: Concentration of endostatin measured by ELISA (P < 0.05, rAAV-endostatin in combination with pretreatment groups versus other groups). Z: rAAV-LacZ without pretreatment, E: rAAV-endostatin without pretreatment, E/et: rAAV-endostatin pretreated with etoposide, E/cpt: rAAV-endostatin pretreated with camptothecin.
In vitro biological activities of endostatin produced by rAAV vectors and pretreatment with topoisomerase inhibitors
As expected, the conditioned media from Hepa1c1c7 cells transduced with the control rAAV-LacZ vector did not influence either endothelial cell proliferation or tube formation compared to the conditioned media from the non-treated control (data not shown). Etoposide and camptothecin had a minor effect on the growth of HUVECs, and recombinant endostatin actively reduced endothelial cell growth. However, the conditioned media from rAAV-enodstatin combined with etoposide group showed very strong inhibition (Figure 2A). Similarly, the recombinant endostatin suppressed tube formation of endothelial cells, and the rAAV-endostatin combined with etoposide group had the highest effect (Figure 2B).
Figure 2.
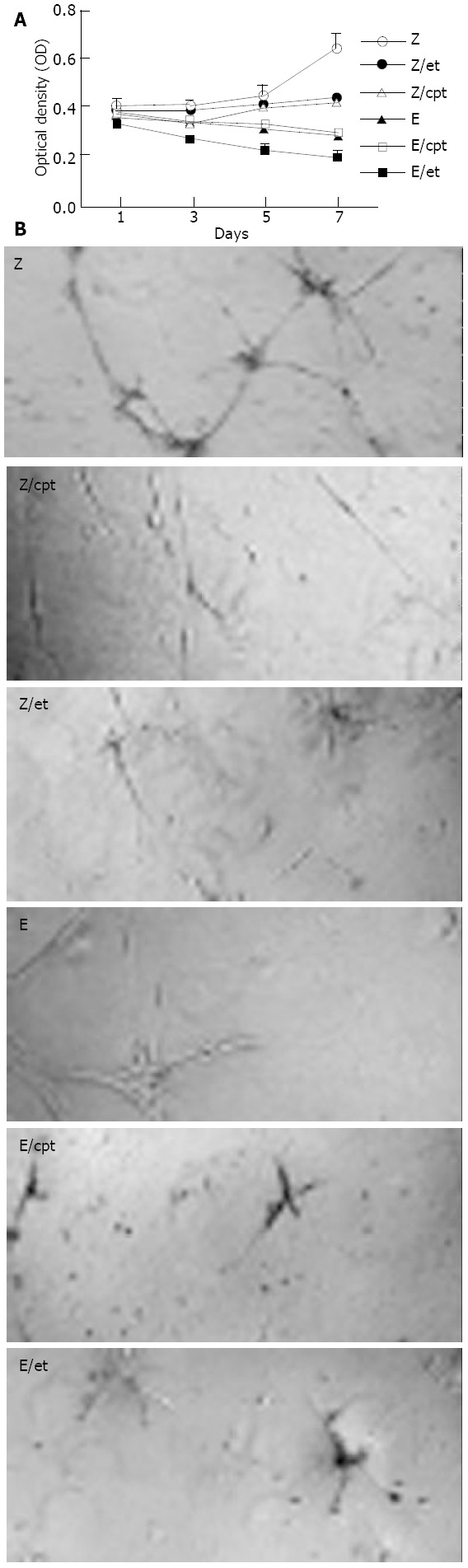
In vitro biological activities of expressed endostatin. A: 5 × 103 HUVECs in a 96-well were cultured in the conditioned media from Hepa1c1c7 mouse hepatoma cells without pre-treatment (Z), those from the cells pretreated with etoposide (Z/et) or camptothecin (Z/cpt), those from the rAAV-endostatin transduced cells without pretreatment (E), those from rAAV-endostatin transduced cells pretreated with etoposide (E/et) or camptothecin (E/cpt). The number of cells was then calculated by a MTT assay. Each value represents mean ± SD of 3 independent experiments (P < 0.05, rAAV-endostatin in combination with pretreatment groups versus other groups). B: Impact on tube formation of endothelial cells. HUVECs were seeded into 24-well plates coated with Matrigel at a density of 5 × 104 cells in each well and cultured in the conditioned media. After 18 h incubation, the level of cell growth and differen-tiation was observed. All tests were performed in triplicate.
Synergic effect of rAAV-endostatin with topoisomerase inhibitors in a mouse liver tumor model
Hepatic tumors were formed by injecting S-180 murine sarcoma cells directly into the predetermined site of the liver, and rAAV-Lac-Z treatment mice had similar hepatic tumors compared to non-treated mice (170.0 ± 38.6 and 159.7 ± 27.7 mm3, respectively). rAAV-LacZ in combination with etoposide and rAAV-endostatin reduced hepatic tumor burden (52.0 ± 9.4 and 9.3 ± 4.5 mm3, respectively) (P < 0.05, treated group vs non-treated or rAAV-Lac-Z treatment group). Interestingly, tumor nodules were barely observed in the rAAV-endostatin plus etoposide group (Figure 3). Serum endostatin was hardly shown in non-treatment group (30.4 ± 20.8 ng/mL), rAAV-LacZ treatment alone (34.2 ± 21.4 ng/mL) and rAAV-LacZ plus etoposide groups (25.8 ± 19.9 ng/mL), although rAAV-endostatin induced detectable serum endostatin level (191.1 ± 54.7 ng/mL, P < 0.05, rAAV-endostatin group vs control groups). In contrast, rAAV-endostatin plus etoposide induced the highest endostatin level (321.5 ± 54.3 ng/mL, P < 0.05, rAAV-endostatin plus etoposide group vs other groups) (Figure 4). The in vivo expression of endostatin was detected immunohistochemically. Staining with anti-endostatin antibodies revealed positive cells in vessels of the liver sections from the rAAV-endostatin treatment group, whereas the control sections were negative, and a dramatic increase was observed in rAAV-endostatin plus etoposide treatment mice (Figure 5). Moreover, endostatin was stained in hepatocytes of rAAV-endostatin plus etoposide treatment mice. The microvessel densities in tumor were estimated by CD34 staining, which were found to be decreased in the rAAV treatment group (19.3 ± 4.5, P < 0.05, rAAV treatment group vs control groups) compared with the mock control (97.7 ± 15.2) and the vector control (105.2 ± 17.6). As expected, the rAAV-endostatin plus etoposide treatment mice had the lowest microvessel density (7.6 ± 1.5) (Table 1). All the non-treated or rAAV-lacZ treated mice died within 30 d. rAAV-LacZ plus etoposide treatment barely affected the survival and rAAV-endostatin extended the survival time. However, the rAAV-endostatin plus etoposide treatment mice had the longest survival (Figure 6). In addition, significant endostatin expressions were detected in the surviving mice of rAAV-endostatin plus etoposide treatment group even after 2 mo (data not shown).
Figure 3.
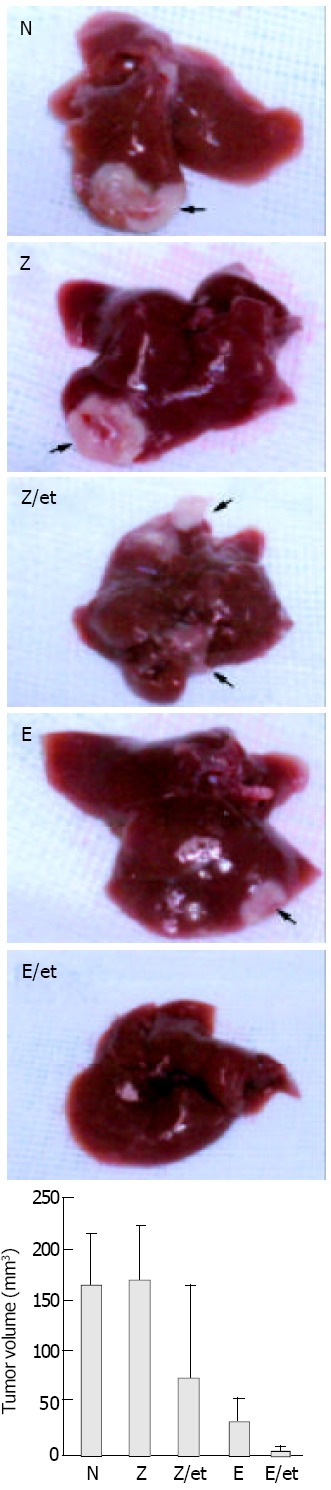
Effect of rAAV-endostatin in combination with etoposide on murine sarcoma bearing mice. Twenty five mice bearing S-180 murine sarcoma cells were randomly divided into 5 groups, namely no treatment, rAAV-LacZ alone, rAAV-LaZ plus etoposide pretreatment, rAAV-endostatin alone, and rAAV-endostatin plus etoposide pretreatment. In the pretreat-ment group, etoposide (40 mg/kg) was administered 3 times for one week by an intraperitoneal injection beginning 7 d prior to rAAV injection, and 1.5 × 1012 viral particles of rAAV- rAAVendostatin vector were injected into the spleen simultaneously with tumor cell inoculation (5 × 106 S-180 cells) into the liver. The tumor volume was determined 7 d after injecting murine sarcoma cells (P < 0.05, rAAV plus etoposide group versus other groups). Tumor volume = (Length × width2)/2.
Figure 4.
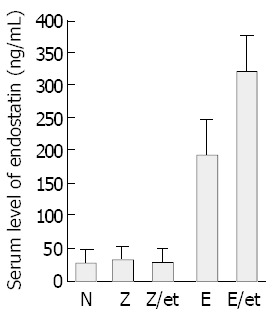
Mouse endostatin levels determined in sera of mice inoculated with murine sarcoma cells. S-180 murine sarcoma cells were inoculated into liver. Seven days later, ELISA determined the endostatin concentration and the results were expressed as mean ± SD of 5 animals (P < 0.05, rAAV-endostatin plus etoposide group versus other groups).
Figure 5.
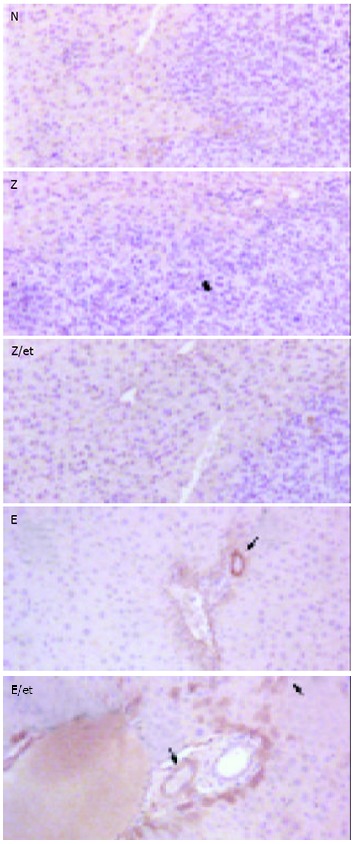
Detection of endostatin in livers of tumor-bearing mice. Hepatic tumors were induced by injecting murine sar-coma cells into the liver. After 7 d, the livers were harvested from the mice of different groups and analyzed by immuno-histochemical staining for endostatin. Livers without treatment, rAAV-LacZ alone and rAAV-LacZ plus etoposide pretreatment did not express endostatin. The vessels of livers treated with rAAV-endostatin were stained with anti-endostatin antibodies and a significant increase was observed in the rAAV-endostatin plus etoposide treatment group (arrow). Endostatin was also detected in hepatocytes of the rAAV-endostatin plus etoposide treatment group (arrow head).
Table 1.
Microvessel assessment of S-180 murine sarcoma tumors
| Group | Microvessel density |
| No treatment | 97.7 ± 15.2 |
| rAAV-LacZ alone | 105.2 ± 17.6 |
| rAAV-LacZ plus etoposide | 52.0 ± 9.4 |
| rAAV-endostatin alone | 19.3 ± 4.5 |
| rAAV-endostatin plus etoposide | 7.6 ± 1.5a |
The microvessel density was measured with a light microscope in the tumor representative of the highest microvessel density at magnification × 200 (“hot spot”).
P < 0.05, rAAV-endostatin plus etoposide group against other groups.
Figure 6.
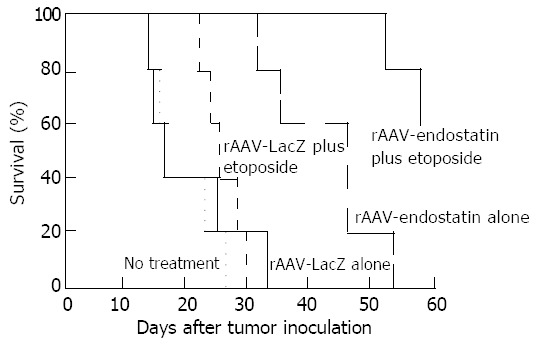
Survival time of sarcoma-bearing mice. Twenty-five mice were randomly divided into 5 groups: no treatment, rAAV-LacZ alone, rAAV-LacZ plus etoposide, rAAV-endostatin alone, or rAAV-endostatin plus etoposide. The tu-mor-bearing mice treated with rAAV-endostatin in combina-tion with etoposide had a significantly longer survival than those in other groups (P < 0.05).
DISCUSSION
The transduction of non-dividing cell populations is an attractive goal of gene therapy, especially in terms of antiangiogenesis against cancer. rAAV vectors could transfer a foreign gene into non-dividing cells, but the gene transfer efficiency was too low[29]. The transduction of non-dividing cells by AAV vectors was increased by DNA-damaging agents, such as γ-and UV-irradiation and cisplatinum, which were toxic to normal cells at the concentrations needed to increase transduction[30]. In contrast, pretreatment with topoisomerase inhibitors increased AAV vector mediated transduction of non-dividing cells with a lower cytotoxicity[23]. It was found in this study that topoisomerase inhibitors in rAAV mediated endostatin gene therapy increased endostatin expression level. Etoposide more effectively enhanced the expression of target molecules than camptothecin. This rAAV-endostatin plus etoposide treatment induced anti-angiogenic effects to a significantly larger extent than rAAV treatment only, and endostatin expression levels were found to correlate well with the antiangiogenic effects.
rAAV with a CMV promoter used in this study, proved stable and strongly expressed endostatin. Etoposide could irreversibly inhibit CMV replication and suppress viral DNA and late viral-protein synthesis[31]. It is unclear whether or not etoposide inhibits the function of CMV promoter. This study did not evaluate the effect of etoposide on CMV promoter. However, topoisomerase inhibitor increased the overall endostatin expression level.
This study examined the in vivo antitumor effects of combined therapy with rAAV-endostatin and etoposide. In this mouse model of a hepatic tumor, etoposide had little antitumor effect, and rAAV-endostatin alone was insufficient to control a hepatic tumor. However, the combined modality significantly enhanced the tumor response. Interestingly, endostatin expression was immunohistochemically detected in hepatocytes and was significantly increased around vessels in the liver of the rAAV-endostatin plus etoposide treatment group compared with those of the rAAV-endostatin alone group. The topoisomerase inhibitor increased the transduction efficiency of AAV in both S-phase and non S-phase cells, and hepatocytes were much more efficiently transduced than other cells[24]. Overall, rAAV-endostatin in combination with etoposide increased the endostatin expression level in hepatocytes of mice, and induced sufficient control in the hepatic tumor model. One potential obstacle to the clinical application of rAAV-mediated anti-angiogenesis gene therapy is that it maintains high levels of the target molecules over a long-term. rAAV vector-mediated cancer gene therapy protocols combined with topoisomerase inhibitor pretreatment might be a solution to this problem.
ACKNOWLEDGMENTS
The authors thank Dr Woo Ik Yang in the Department of Pathology for his technical assistance.
Footnotes
Supported by a faculty research grant of Yonsei University College of Medicine for 2002, No. 2002-06
Edited by Xu JY and Wang XL Proofread by Xu FM
References
- 1.Guo XL, Lin GJ, Zhao H, Gao Y, Qian LP, Xu SR, Fu LN, Xu Q, Wang JJ. Inhibitory effects of docetaxel on expression of VEGF, bFGF and MMPs of LS174T cell. World J Gastroenterol. 2003;9:1995–1998. doi: 10.3748/wjg.v9.i9.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta MK, Qin RY. Mechanism and its regulation of tumor-induced angiogenesis. World J Gastroenterol. 2003;9:1144–1155. doi: 10.3748/wjg.v9.i6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daly ME, Makris A, Reed M, Lewis CE. Hemostatic regulators of tumor angiogenesis: a source of antiangiogenic agents for cancer treatment? J Natl Cancer Inst. 2003;95:1660–1673. doi: 10.1093/jnci/djg101. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Role of angiogenesis in tumour growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Angiogenesis inhibitors: a new class of drugs. Can-cer Biol Ther. 2003;2(4 Suppl):S127–133. [PubMed] [Google Scholar]
- 6.Kiselev SM, Lutsenko SV, Severin SE, Severin ES. Tumor angiogenesis inhibitors. Biochemistry (Mosc) 2003;68:497–513. doi: 10.1023/a:1023984107503. [DOI] [PubMed] [Google Scholar]
- 7.Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Fu GF, Fan YR, Shi CF, Liu XJ, Xu GX, Wang JJ. Potent inhibition of angiogenesis and liver tumor growth by administration of an aerosol containing a transferrin-liposome-endostatin complex. World J Gastroenterol. 2003;9:262–266. doi: 10.3748/wjg.v9.i2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Liu FK, Li X, Li JS, Xu GX. Retrovirus-mediated gene transfer of human endostatin inhibits growth of human liver carcinoma cells SMMC7721 in nude mice. World J Gastroenterol. 2002;8:1045–1049. doi: 10.3748/wjg.v8.i6.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blezinger P, Wang J, Gondo M, Quezada A, Mehrens D, French M, Singhal A, Sullivan S, Rolland A, Ralston R, et al. Systemic inhibition of tumor growth and tumor metastases by intramuscular administration of the endostatin gene. Nat Biotechnol. 1999;17:343–348. doi: 10.1038/7895. [DOI] [PubMed] [Google Scholar]
- 12.Chen QR, Kumar D, Stass SA, Mixson AJ. Liposomes complexed to plasmids encoding angiostatin and endostatin inhibit breast cancer in nude mice. Cancer Res. 1999;59:3308–3312. [PubMed] [Google Scholar]
- 13.Ding I, Sun JZ, Fenton B, Liu WM, Kimsely P, Okunieff P, Min W. Intratumoral administration of endostatin plasmid inhibits vascular growth and perfusion in MCa-4 murine mammary carcinomas. Cancer Res. 2001;61:526–531. [PubMed] [Google Scholar]
- 14.Feldman AL, Alexander HR, Hewitt SM, Lorang D, Thiruvathukal CE, Turner EM, Libutti SK. Effect of retroviral endostatin gene transfer on subcutaneous and intraperitoneal growth of murine tumors. J Natl Cancer Inst. 2001;93:1014–1020. doi: 10.1093/jnci/93.13.1014. [DOI] [PubMed] [Google Scholar]
- 15.Langer JC, Klotman ME, Hanss B, Tulchin N, Bruggeman LA, Klotman PE, Lipkowitz MS. Adeno-associated virus gene transfer into renal cells: potential for in vivo gene delivery. Exp Nephrol. 1998;6:189–194. doi: 10.1159/000020522. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Zhang X, Xia X, Zhou L, Breau R, Suen J, Hanna E. Intramuscular electroporation delivery of IFN-alpha gene therapy for inhibition of tumor growth located at a distant site. Gene Ther. 2001;8:400–407. doi: 10.1038/sj.gt.3301418. [DOI] [PubMed] [Google Scholar]
- 17.Nakashima Y, Yano M, Kobayashi Y, Moriyama S, Sasaki H, Toyama T, Yamashita H, Fukai I, Iwase H, Yamakawa Y, et al. Endostatin gene therapy on murine lung metastases model utilizing cationic vector-mediated intravenous gene delivery. Gene Ther. 2003;10:123–130. doi: 10.1038/sj.gt.3301856. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen JT, Wu P, Clouse ME, Hlatky L, Terwilliger EF. Adeno-associated virus-mediated delivery of antiangiogenic factors as an antitumor strategy. Cancer Res. 1998;58:5673–5677. [PubMed] [Google Scholar]
- 19.Wang X, Liu FK, Li X, Li JS, Xu GX. Inhibitory effect of endostatin expressed by human liver carcinoma SMMC7721 on endothelial cell proliferation in vitro. World J Gastroenterol. 2002;8:253–257. doi: 10.3748/wjg.v8.i2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 21.Xiao W, Berta SC, Lu MM, Moscioni AD, Tazelaar J, Wilson JM. Adeno-associated virus as a vector for liver-directed gene therapy. J Virol. 1998;72:10222–10226. doi: 10.1128/jvi.72.12.10222-10226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.High K. AAV-mediated gene transfer for hemophilia. Genet Med. 2002;4:56S–61S. doi: 10.1097/00125817-200211001-00012. [DOI] [PubMed] [Google Scholar]
- 23.Russell DW, Alexander IE, Miller AD. DNA synthesis and topoisomerase inhibitors increase transduction by adeno-associated virus vectors. Proc Natl Acad Sci USA. 1995;92:5719–5723. doi: 10.1073/pnas.92.12.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koeberl DD, Alexander IE, Halbert CL, Russell DW, Miller AD. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proc Natl Acad Sci USA. 1997;94:1426–1431. doi: 10.1073/pnas.94.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng D, Qian C, Sun Y, Barajas MA, Prieto J. Transduction of hepatocellular carcinoma (HCC) using recombinant adeno-associated virus (rAAV): in vitro and in vivo effects of genotoxic agents. J Hepatol. 2000;32:975–985. doi: 10.1016/s0168-8278(00)80102-6. [DOI] [PubMed] [Google Scholar]
- 26.Yoon SS, Eto H, Lin CM, Nakamura H, Pawlik TM, Song SU, Tanabe KK. Mouse endostatin inhibits the formation of lung and liver metastases. Cancer Res. 1999;59:6251–6256. [PubMed] [Google Scholar]
- 27.Weiner MP, Costa GL, Schoettlin W, Cline J, Mathur E, Bauer JC. Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene. 1994;151:119–123. doi: 10.1016/0378-1119(94)90641-6. [DOI] [PubMed] [Google Scholar]
- 28.Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ, Iwaki Y, Colosi P. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- 29.Russell DW, Miller AD, Alexander IE. Adeno-associated virus vectors preferentially transduce cells in S phase. Proc Natl Acad Sci USA. 1994;91:8915–8919. doi: 10.1073/pnas.91.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexander IE, Russell DW, Miller AD. DNA-damaging agents greatly increase the transduction of nondividing cells by adeno-associated virus vectors. J Virol. 1994;68:8282–8287. doi: 10.1128/jvi.68.12.8282-8287.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang ES, Benson JD, Huong SM, Wilson B, van der Horst C. Irreversible inhibition of human cytomegalovirus replication by topoisomerase II inhibitor, etoposide: a new strategy for the treatment of human cytomegalovirus infection. Antiviral Res. 1992;17:17–32. doi: 10.1016/0166-3542(92)90087-l. [DOI] [PubMed] [Google Scholar]


