Abstract
AIM: To investigate the relationship between the mast cell density (MCD) and the context of clinicopathological parameters and expression of p185, estrogen receptor (ER), and proliferating cell nuclear antigen (PCNA) in gastric carcinoma.
METHODS: Mast cell, p185, ER, and PCNA were detected using immunohistochemical S-P labeling method. Mast cell was counted in tissue of gastric carcinoma and regional lymph nodes respectively, and involved lymph nodes (ILN) were examined as usual.
RESULTS: MCD was significantly related to both age and depth of penetration (χ2 = 4.688,P < 0.05 for age and χ2 = 9.350, P < 0.01 for depth of penetration) between MCD > 21/0.03 mm2 and MCD ≤ 21/0.03 mm2 in 100 patients; MCD in 1-6 ILN group patients was significantly higher than that in 7-15 ILN or > 15 ILN group patients (u = 6.881, 8.055, P < 0.01); There were significant differences intergroup in positive expression rate of p185, ER and PCNA between MCD > 21/ 0.03 mm2 and MCD ≤ 21/0.03 mm2 in 100 patients.
CONCLUSION: Mast cell may have effect on inhibiting invasive growth of tumor, especially in the aged patients; The number of mast cells, in certain degree, may predicate the number of involved lymph nodes, which is valuable for assessment of prognosis; MCD was related to the expression of p185, ER, and PCNA in gastric carcinoma. It suggests that mast cell accumulation may inhibit the proliferation and the dissemination of the gastric carcinoma.
INTRODUCTION
Recently, many studies have reported on the association of mast cell with various tumors[1-9]. In several malignancies, mast cell has been found to correlate with growth, penetration and prognosis of tumor[10-13]. Therefore, our study was undertaken to investigate the relationship between the mast cell density (MCD) and the context of clinicopathological parameters and expression of p185, estrogen receptor (ER), and proliferating cell nuclear antigen (PCNA) in gastric carcinoma.
MATERIALS AND METHODS
Materials
The specimens of gastric carcinoma, histologically confirmed, were surgically obtained from 421 patients. The patients had undergone curative tumor resection at our hospital between 1984 and 1998. And only 100 patients were chosen at random in our study. Among 100 patients, 41 patients had lymph node metastases. All resected tissue specimens were fixed in formalin, embedded in paraffin, and cut into 3-4 μm serial sections. 459 lymph nodes were collected from 41 patients (range, 8-26 per patient).
Methods
Mast cell, p185, ER, and PCNA were detected using immuno-histochemical method (agents from Maixin-Bio Corp. Fuzhou, China).The count of mast cells in the tissue of gastric carcinoma was as described by Takanami et al[10]. A grid (0.15 mm by 0.2 mm) which was defined an area of 0.03 mm2 per field was used for to count mast cells. Similarly, that of mast cells in regional lymph nodes was described by Bowers et al[14].A grid which defined an area of mm2 per field was used. ILN was examined using routine pathological method. The results were expressed as the means ± SD. Statistical analyses were performed using the Chi-square and u test. A P value less than or equal to 0.05 was considered significant.
RESULTS
Table 1 showed the clincopathologic parameters for two groups (high MCD group, MCD was more than 21/0.03 mm2, and low MCD group, MCD was equal to 21/0.03 mm2 or less). There were no significant differences between two groups regarding both degree of differentiation and largest dimension of tumor. However, MCD was significantly related to both age and depth of penetration (P < 0.05 for age and P < 0.01 for depth of penetration) (Figure 1).
Table 1.
Correlation between MCD and Clinicopathologic find-ing of gastric carcinoma (n = 100)
| Variable | MCD > 21/0.03 mm2 | MCD ≤ 21/0.03 mm2 | P Value |
| Age (yrs) | |||
| < 60 | 16 | 25 | < 0.05 |
| ≥ 60 | 36 | 23 | |
| Degree of differentiation | |||
| Well | 18 | 14 | > 0.05 |
| Moderately | 23 | 21 | |
| Poorly | 11 | 13 | |
| Largest dimension of tumor ( in mm) | |||
| ≤ 30 | 30 | 36 | > 0.05 |
| > 30 | 22 | 12 | |
| Depth of penetration | |||
| Involved serosa | 21 | 34 | < 0.01 |
| Not involved serosa | 31 | 14 |
Figure 1.
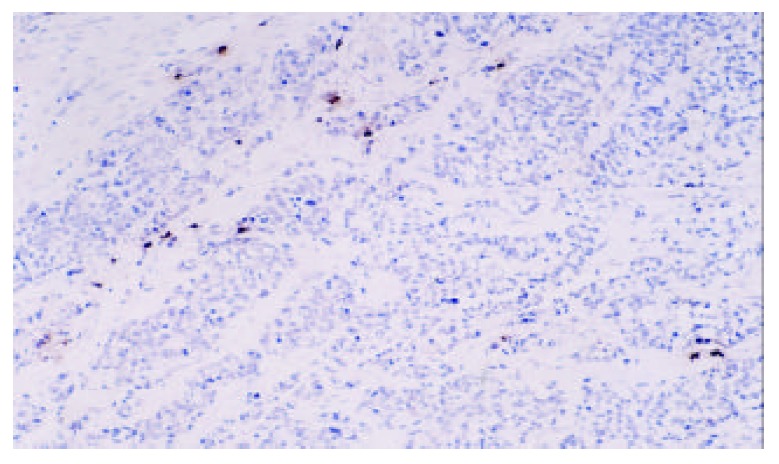
Mast cells of gastric carcinoma (Tryptase labeling, DAB staining, original magnification, × 100)
Table 2 showed correlation between MCD and cancerous metastases in regional lymph nodes. MCD in 1-6 ILN group patients (n = 21) was 12 ± 3.11, 7-15 ILN (n = 14), 6 ± 2.06, > 15 ILN (n = 6), 5 ± 1.33, respectively. MCD in 1-6 ILN group patients was significantly higher than that in 7-15 ILN or > 15 ILN group patients (P < 0.01), but, MCD in 7-15 ILN group patients was not significantly higher than that in > 15 ILN group patients (P > 0.05) (Figure 2).
Table 2.
Correlation between MCD and cancerous metastases in regional lymph nodes (n = 41)
| Variable | n | MCD (mean ± SD, /mm2) |
| 1-6 ILN | 21 | 12 ± 3.11 |
| 7-15 ILN | 14 | 6 ± 2.06 |
| > 15 ILN | 6 | 5 ± 1.33 |
ILN: involved lymph nodes
Figure 2.
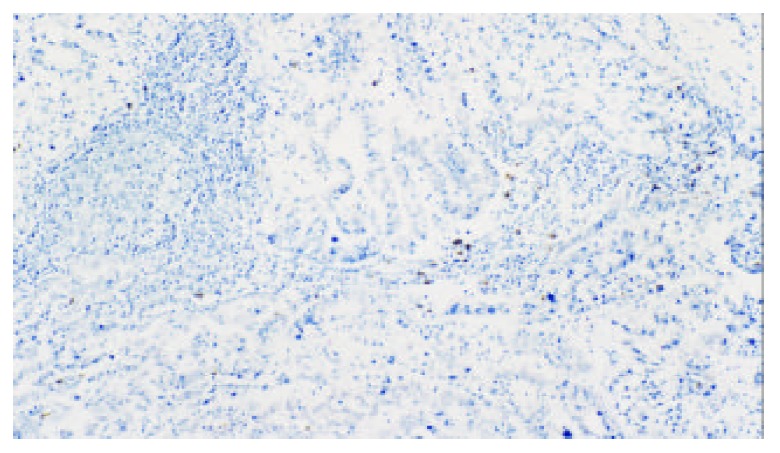
Mast cells of regional lymph nodes of gastric carci-noma (Tryptase labeling, original magnification, × 100)
There were significant differences intergroup in positive expression rate of p185, ER and PCNA between high MCD group (the MCD was more than 21/0.03 mm2) and low MCD group (the MCD was equal to 21/0.03 mm2 or less) (P < 0.01). As was shown in Table 3 (Figures 3, 4 and 5).
Table 3.
Correlation between MCD and the positive expression rate of p185, ER, and PCNA in the gastric carcinoma tissues
| MCD | n |
Positive expression rate (%) |
||
| p185 | ER | PCNA | ||
| > 21/0.03 mm2 | 52 | 22 (42.31) | 11 (21.15) | 12 (23.08) |
| ≤ 21/0.03 mm2 | 48 | 31 (59.62) | 28 (53.84) | 27 (51.92) |
| P Value | < 0.01 | < 0.001 | < 0.01 | |
Figure 3.
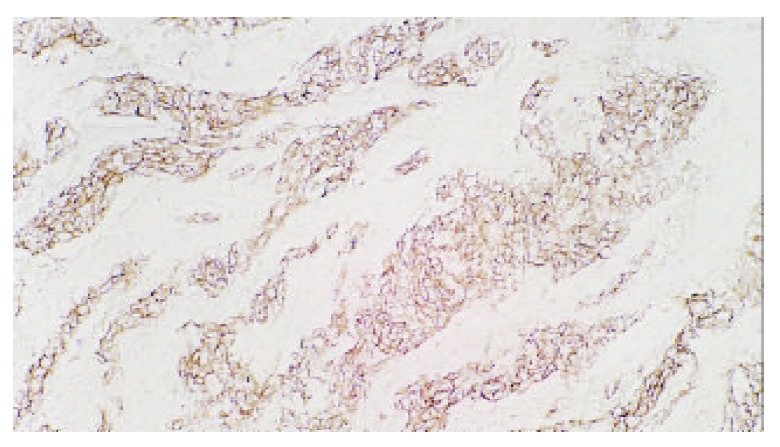
p185 of gastric carcinoma (Original magnification, × 100)
Figure 4.
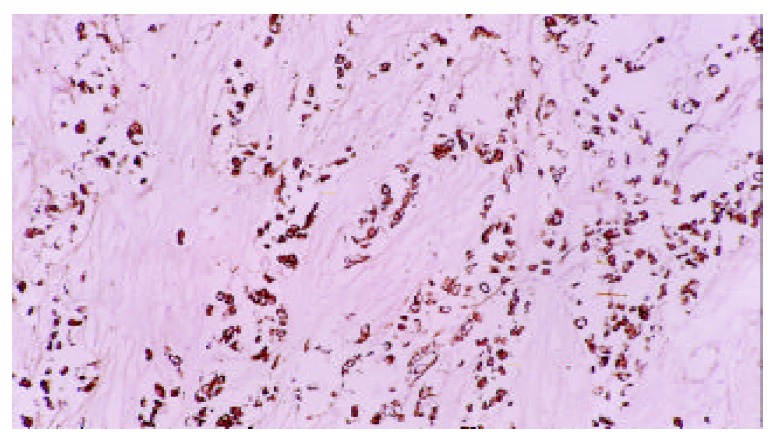
Estrogen receptor of gastric carcinoma (Original magnification, × 100)
Figure 5.
PCNA of gastric carcinoma (Original magnification, × 400)
DISCUSSION
Numerous studies have shown that mast cell plays an important role in tumor growth. Dabbou et al[15,16] found that mast cells were accumulated around the periphery of the invasive and metastatic rat mammary adenocarcinoma, and they believed that interactions between mast cell and tumor cell were important for the growth and invasive properties of the tumor. Nakamura et al[17] found the mechanism of mast cell accumulation at sites of tumors was that tumor cells could produce a factor which might not be the already known mast cell growth factors. Wang et al[18] found that the anti-tumor effect of mast cell might be related to releasing of tumor necrosis factor (TNF) and non-TNF cytotoxicity. Our results revealed there were no significant differences intergroup regarding both degree of differentiation and largest dimension of tumor between MCD > 21/0.03 mm2 and MCD ≤ 21/0.03 mm2. MCD was significantly related to both age and depth of penetration (P < 0.05 for age and P < 0.01 for depth of penetration).
The data indicate the lymph node status is associated with the patients’ prognoses in many malignancies, and may be useful in assessing the outcome of this disease[19-28]. The treatment regimen was depended on not only whether involved lymph node was present or not, but also the number of involved lymph node[20,29]. Maurel et al[30] had examined 7.7 ± 0.2 lymph nodes per specimen in 851 patients with resected colorectal carcinoma, the results strongly pointed out that at least eight lymph nodes must be examined and they called the number of eight as a “golden number”. At least eight lymph nodes per specimen were examined in our study as done by Maurel et al[30]. Roder et al[20] followed up 477 patients with gastric carcinoma of lymph node metastasis, and they found that 5-year survival rates were as follows (1) 1-6 involved lymph nodes: 45.5%; (2) 7-15 involved lymph nodes:29.7%; (3) > 15 involved lymph nodes: 10.4%. There was a highly significant difference in survival (P < 0.0001). Our results indicated that MCD in 1-6 ILN group patients was 12 ± 3.11, 7-15 ILN, 6 ± 2.06, > 15 ILN, 5 ± 1.33, respectively. MCD in 1-6 ILN group patients was significantly higher than that in 7-15 ILN or > 15 ILN group patients (P < 0.01), but, MCD in 7-15 ILN group patients was not significantly higher than that in > 15 ILN group patients (P > 0.05). In other words, the higher MCD was in regional lymph nodes, the lower number of ILN of gastric cancerous metastases was. It demonstrates that the number of mast cells, in certain degree, may predicate the number of involved lymph nodes of gastric cancerous metastases. Therefore, MCD is a valuable parameter for assessing the prognosis of patients with gastric carcinoma.
The C-erbB-2 was first identified in ethylnitosourea-induced rat neuroblastoma. It encodes a 185 kilodalton (kDa) glycoprotein. It was reported recently that overexpression and gene amplification of C-erb B-2 were frequently observed in the intestinal type gastric adenocarcinoma, and it was a prognostic indicator in tumor[31-33]. ER is commonly known to be present in the cell of the breast and endometrium, but have also been identified in diverse normal and neoplastic nonreproductive tissues. The expression of ER has been considered to be a favorable prognostic factor in breast carcinoma and endometrial carcinoma but a poor prognostic factor in gastric carcinoma[34]. PCNA is an auxiliary protein of DNA polymerase delta which plays a major role in synthesizing DNA and is expressed in the nuclei, particularly in the late phase of G1 and S. Therefore PCNA is a useful marker for proliferative activity[35-37]. Our results showed there were significant differences intergroup in the positive expression rate of p185, ER and PCNA between MCD > 21/0.03 mm2 and MCD ≤ 21/0.03 mm2. It suggests that mast cell accumulation may inhibit the proliferation and the dissemination of gastric carcinoma. This finding may provide a molecular foundation of the further study on relation between mast cell and gastric carcinoma.
Footnotes
Edited by Liu HX
References
- 1.Tomita M, Matsuzaki Y, Edagawa M, Shimizu T, Hara M, Sekiya R, Onitsuka T. Association of mast cells with tumor angiogenesis in esophageal squamous cell carcinoma. Dis Esophagus. 2001;14:135–138. doi: 10.1046/j.1442-2050.2001.00171.x. [DOI] [PubMed] [Google Scholar]
- 2.Tomita M, Matsuzaki Y, Onitsuka T. Effect of mast cells on tumor angiogenesis in lung cancer. Ann Thorac Surg. 2000;69:1686–1690. doi: 10.1016/s0003-4975(00)01160-7. [DOI] [PubMed] [Google Scholar]
- 3.Erkiliç S, Erbağci Z. The significance of mast cells associated with basal cell carcinoma. J Dermatol. 2001;28:312–315. doi: 10.1111/j.1346-8138.2001.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 4.Terada T, Matsunaga Y. Increased mast cells in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Hepatol. 2000;33:961–966. doi: 10.1016/s0168-8278(00)80129-4. [DOI] [PubMed] [Google Scholar]
- 5.Wilkins BS, Buchan SL, Webster J, Jones DB. Tryptase-positive mast cells accompany lymphocytic as well as lymphoplasmacytic lymphoma infiltrates in bone marrow trephine biopsies. Histopathology. 2001;39:150–155. doi: 10.1046/j.1365-2559.2001.01173.x. [DOI] [PubMed] [Google Scholar]
- 6.Hart PH, Grimbaldeston MA, Finlay-Jones JJ. Sunlight, immunosuppression and skin cancer: role of histamine and mast cells. Clin Exp Pharmacol Physiol. 2001;28:1–8. doi: 10.1046/j.1440-1681.2001.03392.x. [DOI] [PubMed] [Google Scholar]
- 7.Terada T, Matsunaga Y. Increased mast cells in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Hepatol. 2000;33:961–966. doi: 10.1016/s0168-8278(00)80129-4. [DOI] [PubMed] [Google Scholar]
- 8.Sawatsubashi M, Yamada T, Fukushima N, Mizokami H, Tokunaga O, Shin T. Association of vascular endothelial growth factor and mast cells with angiogenesis in laryngeal squamous cell carcinoma. Virchows Arch. 2000;436:243–248. doi: 10.1007/s004280050037. [DOI] [PubMed] [Google Scholar]
- 9.Johansson S, Landström M, Bjermer L, Henriksson R. Effects of tobacco smoke on tumor growth and radiation response of dunning R3327 prostate adenocarcinoma in rats. Prostate. 2000;42:253–259. doi: 10.1002/(sici)1097-0045(20000301)42:4<253::aid-pros2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Takanami I, Takeuchi K, Naruke M. Mast cell density is associated with angiogenesis and poor prognosis in pulmonary adenocarcinoma. Cancer. 2000;88:2686–2692. [PubMed] [Google Scholar]
- 11.Imada A, Shijubo N, Kojima H, Abe S. Mast cells correlate with angiogenesis and poor outcome in stage I lung adenocarcinoma. Eur Respir J. 2000;15:1087–1093. doi: 10.1034/j.1399-3003.2000.01517.x. [DOI] [PubMed] [Google Scholar]
- 12.Tomita M, Matsuzaki Y, Onitsuka T. Correlation between mast cells and survival rates in patients with pulmonary adenocarcinoma. Lung Cancer. 1999;26:103–108. doi: 10.1016/s0169-5002(99)00076-8. [DOI] [PubMed] [Google Scholar]
- 13.Elpek GO, Gelen T, Aksoy NH, Erdoğan A, Dertsiz L, Demircan A, Keleş N. The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J Clin Pathol. 2001;54:940–944. doi: 10.1136/jcp.54.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowers HM, Mahapatro RC, Kennedy JW. Numbers of mast cells in the axillary lymph nodes of breast cancer patients. Cancer. 1979;43:568–573. doi: 10.1002/1097-0142(197902)43:2<568::aid-cncr2820430225>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Dabbous MK, Haney L, Nicolson GL, Eckley D, Woolley DE. Mast cell modulation of tumour cell proliferation in rat mammary adenocarcinoma 13762NF. Br J Cancer. 1991;63:873–878. doi: 10.1038/bjc.1991.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hultsch T, Brand P, Lohmann S, Saloga J, Kincaid RL, Knop J. Direct evidence that FK506 inhibition of FcepsilonRI-mediated exocytosis from RBL mast cells involves calcineurin. Arch Dermatol Res. 1998;290:258–263. doi: 10.1007/s004030050301. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura K, Tanaka T, Morita E, Kameyoshi Y, Yamamoto S. Enhancement of fibroblast-dependent mast cell growth in mice by a conditioned medium of keratinocyte-derived squamous cell carcinoma cells. Arch Dermatol Res. 1994;287:91–96. doi: 10.1007/BF00370725. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Ruan Y, Wu Z. [Studies of mast cell-mediated cytotoxicity to hepatoma cells in vitro] Zhonghua Zhongliu Zazhi. 1996;18:276–278. [PubMed] [Google Scholar]
- 19.Xu Y, Guo Z. [The number of lymph node with metastases influences survival in patients with cancer of the thoracic esophagus] Zhonghua Zhongliu Zazhi. 2000;22:244–246. [PubMed] [Google Scholar]
- 20.Roder JD, Böttcher K, Busch R, Wittekind C, Hermanek P, Siewert JR. Classification of regional lymph node metastasis from gastric carcinoma. German Gastric Cancer Study Group. Cancer. 1998;82:621–631. [PubMed] [Google Scholar]
- 21.Lanza G, Gafà R, Decarli N. [Pathological factors involved in lymph node status determination in colorectal carcinoma: analysis of 166 cases with long-term follow-up] Pathologica. 2001;93:631–639. [PubMed] [Google Scholar]
- 22.Nakajima Y, Nagai K, Miyake S, Ohashi K, Kawano T, Iwai T. Evaluation of an indicator for lymph node metastasis of esophageal squamous cell carcinoma invading the submucosal layer. Jpn J Cancer Res. 2002;93:305–312. doi: 10.1111/j.1349-7006.2002.tb02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong JH, Steinemann S, Tom P, Morita S, Tauchi-Nishi P. Volume of lymphatic metastases does not independently influence prognosis in colorectal cancer. J Clin Oncol. 2002;20:1506–1511. doi: 10.1200/JCO.2002.20.6.1506. [DOI] [PubMed] [Google Scholar]
- 24.Rouzier R, Extra JM, Klijanienko J, Falcou MC, Asselain B, Vincent-Salomon A, Vielh P, Bourstyn E. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002;20:1304–1310. doi: 10.1200/JCO.2002.20.5.1304. [DOI] [PubMed] [Google Scholar]
- 25.Katai H, Maruyama K, Sasako M, Sano T. Incidence of nodal metastasis around the superior border of the pancreas based on number of metastatic perigastric nodes. Gastric Cancer. 1998;1:115–117. doi: 10.1007/s101200050004. [DOI] [PubMed] [Google Scholar]
- 26.Ichikura T, Morita D, Uchida T, Okura E, Majima T, Ogawa T, Mochizuki H. Sentinel node concept in gastric carcinoma. World J Surg. 2002;26:318–322. doi: 10.1007/s00268-001-0226-x. [DOI] [PubMed] [Google Scholar]
- 27.Lau WK, Blute ML, Bostwick DG, Weaver AL, Sebo TJ, Zincke H. Prognostic factors for survival of patients with pathological Gleason score 7 prostate cancer: differences in outcome between primary Gleason grades 3 and 4. J Urol. 2001;166:1692–1697. [PubMed] [Google Scholar]
- 28.Sasatomi E, Finkelstein SD, Woods JD, Bakker A, Swalsky PA, Luketich JD, Fernando HC, Yousem SA. Comparison of accumulated allele loss between primary tumor and lymph node metastasis in stage II non-small cell lung carcinoma: implications for the timing of lymph node metastasis and prognostic value. Cancer Res. 2002;62:2681–2689. [PubMed] [Google Scholar]
- 29.Niemann TH, Yilmaz AG, Marsh WL, Lucas JG. A half a node or a whole node: a comparison of methods for submitting lymph nodes. Am J Clin Pathol. 1998;109:571–576. doi: 10.1093/ajcp/109.5.571. [DOI] [PubMed] [Google Scholar]
- 30.Maurel J, Launoy G, Grosclaude P, Gignoux M, Arveux P, Mathieu-Daudé H, Raverdy N, Faivre J. Lymph node harvest reporting in patients with carcinoma of the large bowel: a French population-based study. Cancer. 1998;82:1482–1486. [PubMed] [Google Scholar]
- 31.Dursun A, Poyraz A, Celik B, Akyol G. Expression of c-erbB-2 oncoprotein in gastric carcinoma: correlation with histopathologic characteristics and analysis of Ki-67. Pathol Oncol Res. 1999;5:104–106. doi: 10.1053/paor.1999.0171. [DOI] [PubMed] [Google Scholar]
- 32.Aoyagi K, Kohfuji K, Yano S, Murakami N, Miyagi M, Takeda J, Shirouzu K. Evaluation of the epidermal growth factor receptor (EGFR) and c-erbB-2 in superspreading-type and penetrating-type gastric carcinoma. Kurume Med J. 2001;48:197–200. doi: 10.2739/kurumemedj.48.197. [DOI] [PubMed] [Google Scholar]
- 33.Oshima CT, Lanzoni VP, Iriya K, Forones NM. C-erbB-2 oncoprotein in gastric carcinoma: correlation with clinical stage and prognosis. Int J Biol Markers. 2001;16:250–254. doi: 10.1177/172460080101600405. [DOI] [PubMed] [Google Scholar]
- 34.Xin Y, Li XL, Wang YP, Zhang SM, Zheng HC, Wu DY, Zhang YC. Relationship between phenotypes of cell-function differentiation and pathobiological behavior of gastric carcinomas. World J Gastroenterol. 2001;7:53–59. doi: 10.3748/wjg.v7.i1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noda H, Maehara Y, Irie K, Kakeji Y, Yonemura T, Sugimachi K. Increased proliferative activity caused by loss of p21(WAF1/CIP1) expression and its clinical significance in patients with early-stage gastric carcinoma. Cancer. 2002;94:2107–2112. doi: 10.1002/cncr.10417. [DOI] [PubMed] [Google Scholar]
- 36.Tao K, Chen D, Tian Y, Lu X, Yang X. The relationship between apoptosis and the expression of proliferating cell nuclear antigen and the clinical stages in gastric carcinoma. J Tongji Med Univ. 2000;20:222–224. doi: 10.1007/BF02886997. [DOI] [PubMed] [Google Scholar]
- 37.Konno S, Takebayashi Y, Aiba M, Akiyama S, Ogawa K. Clinicopathological and prognostic significance of thymidine phosphorylase and proliferating cell nuclear antigen in gastric carcinoma. Cancer Lett. 2001;166:103–111. doi: 10.1016/s0304-3835(01)00432-3. [DOI] [PubMed] [Google Scholar]


