Abstract
AIM: To investigate the resistance mechanism of 5-fluorouracil (5-FU) in Bel7402/5-FU cells which was established in our lab by in vitro continuous stepwise exposure of human hepatocellular carcinoma (HCC) cell line Bel7402 to 5-FU.
METHODS: The expression of multidrug resistance-associated protein (MRP) and thymidylate synthase (TS) in Bel7402 cells was detected by immonocytochemistry. The fluorescein (FLU) accumulation, an index of MRP functional activity, was determined by flow cytometry. The distribution of FLU was observed by confocal laser scanning microscope. The spectrofluorometry was used to show the intracelluar content of glutathione (GSH). Cell growth inhibition was determined by MTT assay. The activity of glutathione S-transferases (GSTs) was determined by spectrophotometry.
RESULTS: A higher expression of MRP in the Bel7402/5-FU cells was observed by using monoclonal mouse anti-MRP antibody, MRPr-1, in comparison with Bel7402 cells. Bel7402/5-FU cells also showed a significant decrease of FLU accumulation. FLU mainly accumulated in the nucleus with a high nuclear/cytoplasmic ratio in Bel7402 cells, whereas there was no difference of FLU accumulation between the nucleus and cytoplasm in Bel7402/5-FU cells. The intracellular GSH content in Bel7402/5-FU cells was almost 3 folds higher than that in Bel7402 cells. Addition of D, L-buthione-S, R-sulfoximine (BSO) dose-dependently reduced the GSH content in Bel7402/ 5-FU cells, however, only a weak enhancement on the cytotoxicity of 5-FU and doxorubicin (Dox) to Bel7402/5-FU cells was observed. Bel7402/5-FU cells also exhibited 29.1% higher total GSTs activity than Bel7402 cells. Immunocytochemical staining by using anti-TS monoclonal antibody TS 106 showed that the level of TS in Bel7402/5-FU cells elevated markedly as compared with Bel7402 cells.
CONCLUSION: The continuous exposure of Bel7402 cells to 5-FU led to overexpression of TS and MRP, as well as increased intracellular GSH content and total GST activity.
INTRODUCTION
It is well known that HCC is one of the malignant tumors with poor chemosensitivity to anticancer agents. To date, the fluoropyrimidine fluorouracil, such as 5-FU, is still the first choice drug in the treatment of HCC[1-3]. However, its usage is limited because of the rapid development of acquired resistance. So far, there is no paper dealing with the establishment of acquired resistance of 5-FU in HCC and its active mechanism of resistance in the literature. Then we established a 160-fold 5-FU resistant cell line named Bel7402/ 5-FU from the parental HCC Bel7402 cell line through about 6 months of continuous 5-FU selection. In addition to resistance to 5-FU, the Bel7402/5-FU cells also showed 3-4-fold cross-resistance to Dox and cytarabine, but no cross-resistance to vincristine, taxol, cisplatin and hydroxycamptothecine. Our prior study revealed that Bel7402 and Bel7402/5-FU cells showed no difference in P-glycoprotein (P-gp) levels.
Nishiyama[4] showed that the increased expression of MRP was closely related to 5-FU resistance in gastrointestinal cancer cell lines. MRP is also a member of the ABC superfamily of membrane transport proteins, which has certain sequence homology with P-gp. The drug-resistance pattern conferred by MRP is similar but not identical to that of P-gp[5,6]. It is believed that MRP prefers to transport anionic drugs and neutral drugs conjugated to acidic ligands, such as GSH, glucuronate, or sulfate[7,8]. GSH has been suggested as an important component of MRP-mediated MDR and drug transport[9]. GSH can combine with anticancer drugs to form less toxic and more water soluble GSH conjugates, which can be exported from cells by MRP[10,11]. The conjugation reaction is catalyzed by GSTs. The elevation of cellular GSH and the elevated expression and activity of GSTs are associated with the development of drug-resistance phenotype of tumor cells to alkylating compounds, including melphalan, cyclophosphamide, chlorambucil, nitrogen mustard, doxorubicin[12-14].
TS catalyses the reductive methylation of deoxyuridine monophosphate to deoxythymidine monophosphate using 5, 10-methylene-tetrahydrofolate as a methyl donor co-substrate. This reaction provides the only de novo source of dTMP and is an essential step in DNA biosynthesis. TS is a critical target for fluoropyrimidine, including 5-FU. In vitro and in vivo studies have reported that increased TS protein levels and gene amplification and/or decreased inhibition of TS activity may be associated with the resistance to 5-FU[15,16].
The purpose of the present study was to determine whether the change of MRP, GSH/GST and TS contributed to the 5-FU resistance observed in Bel7402/5-FU cells.
MATERIALS AND METHODS
Materials
Drugs and reagents 5-FU was purchased from Xudong Haipu Pharmaceutical Inc. (Shanghai, China). 1-chloro-2,4-dinitrobenzene (CDNB) and O-phthalaldyhyde (OPT), GSH and 3-4,5-dimethyl-thiazol-2,5-diphenyl tetrazolium bromide (MTT) was obtained from Sigma Chemical Co. (St. Louis, MO). Monoclonal mouse anti-MRP, MRP-r1 and FITC-labeled goat anti-mouse IgG were obtained from Zhong Shan Biotechnology Co., LTD (Beijing, China). Thymidylate synthase Ab-1 TS 106 was obtained from Neomarker (Fremont, CA). Fluorescein (FLU) was obtained from Amresco (Solon, Ohio).
Cell lines The parental human hepatocelluar carcinoma Bel7402 cells and the 5-FU selected drug-resistant Bel7402/5-FU cells were grown in RPIM1640 (GIBCO) media containing 10% newborn calf serum, 100 unit/mL penicillin and 100 μg/mL streptomycin. Cells were kept at 37 °C in a 5% CO2-95% air atmosphere, and passaged by 0.25% trypsin plus 0.02% EDTA treatment. Bel7402/5-FU cells were routinely maintained in the presence of 10 μg/mL 5-FU. Cells were however cultured in the absence of 5-FU for at least 3 days before their use for experiments.
Methods
Immunofluorescence assay of MRP expression Bel7402 and Bel7402/5-FU cells were seeded on to sterilized slides. After 24hr of incubation, cells were washed twice with cold PBS and fixed with 70% methanol at -20 °C for 10 min. All subsequent procedures were done at room temperature. All slides were washed once with blocking solution (1% bovine serum albumin/5% normal goat serum/PBS), incubated in blocking solution with 0.1% Tween 20 for 30 min, followed by incubation with MRPr1 which was diluted 1:30 in blocking solution with 0.1% Tween 20 for 1 h. Then slides were washed once with blocking solution and incubated with FITC-conjugated goat anti-mouse IgG which was diluted 1:50 in blocking solution at room temperature for 1 hour. Finally, Slides were examined under confocal laser scanning microscope (BIO– RAD MRC1024) for fluorescence intensities[17].
FLU accumulation Assessment of MRP functional activity was carried out by measuring the intracellular accumulation of FLU[18]. The confluent Bel7402 and Bel7402/5-FU monolayers were preincubated for 30 min at 37 °C in the culture medium, then exposed to 100 μmol·L-1 FLU for 3hr at 37 °C. After washing for three times with ice-cold PBS, cells were rapidly collected and intracellular fluorescence of FLU was immediately measured with flow cytometer (Coulter, EPICS XL).
Cellular FLU distribution Bel7402 or Bel7402/5-FU cells were plated onto 6-multiwell plate (Coster) for examining FLU distribution by using confocal laser scanning microscopy. The cells were exposed to 100 μmol·L-1 FLU for 3hr at 37 °C. After this incubation period, the loading solutions were removed and cells were washed for three times with ice-cold PBS containing 1% bovine serum albumin and examined under confocal laser scanning microscope (MERIDIANTM Ultima 212)[19].
Measurement of cellular GSH content Bel7402/5-FU cells incubated with or without different concentrations of BSO for 24 hrs and Bel7402 cells were lysed by addition of 0.2% Triton X-100 in PBS. Afterwards, 20% HPO3 was added to precipitate proteins. The cells were centrifuged at 4 °C at 10000 r.p.m. for 10 min and the supernatant fraction was used for the measurement of GSH[20]. Briefly, to 0.4 mL of the supernatant, 0.1 mL OPT (containing 0.1 mg OPT) and 2.5 mL PBS (0.1 mol·L-1) -EDTA (5 mmol·L-1) buffer (pH 8.0) were added. After thorough mixing and incubation at room temperature for 15 min, the solution was transferred to a quartz cuvette. Its fluorescence intensity at 420 nm was determined with the excitation at 350 nm by a fluorescence spectrophotometer (HITACHI, MPF-4).
Chemosensitivity testing The effect of BSO on the cytotoxicity of Dox and 5-FU to Bel7402/5-FU cells was assayed by a MTT method[21]. Briefly, Bel7402/5-FU cells were plated in 96-well plates at 3000 cells per well. After 24 hr, various concentrations of the test drugs were added in quadruplicate samples for each concentration. The cells were exposed to drugs for continuous 3 days. Then MTT (0.5 mg·mL-1) was added and the plates were incubated at 37 °C in 5% CO2 for 4hr before 150 μL 100% dimethyl sulfoxide was added to each well. The optical density of each well was determined by a microplate reader (Bio-Rad Model 450) at a wavelength of 570 nm.
Total GST activity Bel7402 and Bel7402/5-FU cells were washed with PBS and resuspended in 200 μL TMS solution (50 mmol·L-1 Tris, pH 7.5, 3 mmol·L-1 MgCl2, 200 mmol·L-1 sucrose). After sonication at 4 °C (60sec), they were centrifuged at 10000 rpm for 25 min. To 10 μL cell supernatant, 2.9 mL sodium phosphate buffer (0.1 mol·L-1, pH 6.5) and 0.1 mL reduced GSH were added. The reaction was carried out at room temperature by the addition of 30 mM CDNB (in DMSO). Formation of S-2,4 dinitophenyl glutathione was monitored spectrophotometrically by the increase in absorbance at 340 nm (ε 340 = 9.6 mM-1·CM- 1). GST activity was expressed as nmol·min-1·mg-1 protein in the cells[22].
TS expression Bel7402 and Bel7402/5FU cells in the exponential phase of growth were seeded onto sterile slides and incubated at 37 °C in 5% CO2 for 24 hr. After washing with cold PBS, cells were fixed in cold acetone for 10 min. The cells were subsequently rinsed in PBS and incubate with normal goat serum (10% in PBS) at room temperature for 30 min to reduce nonspecific binding. Cells were then incubated with TS 106 (1:50 dilution) at 37 °C for 2 hr. After being washed with PBS, all slides were incubated with FITC-labeled goat anti-mouse IgG at 37 °C for 1 hr. All slides were washed with PBS again and examined under confocal laser scanning microscope (BIO-RAD MRC1024)[23].
RESULTS
Expression of MRP
Fluorescence immunostaining signal was weakly detectable in Bel7402 cells and a denser staining was found in plasma membrane of Bel7402/5-FU cells. The resistant Bel7402/5-FU cells (Figure 1B) showed an enhanced immunoreactivity to MRP compared to that of sensitive Bel7402 cells (Figure 1A), which indicated that the resistant cells contained elevated level of MRP.
Figure 1.
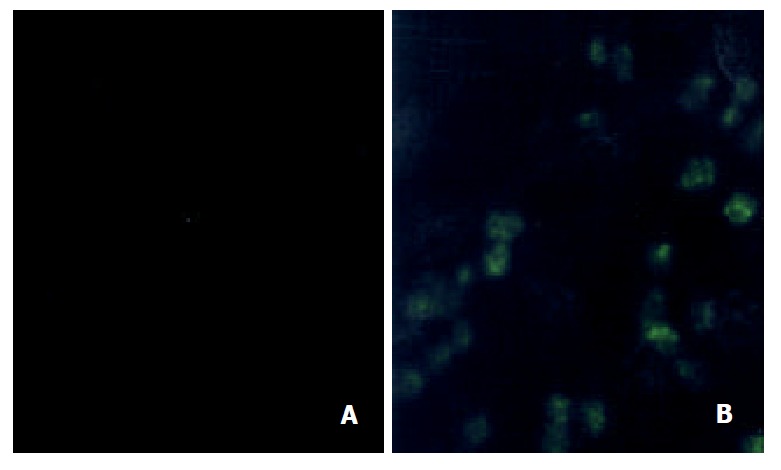
Immunocytochemical detection of MRP in Bel7402 and its 5-FU resistant cells in cytoprep slides. (A) Bel7402 cells; (B) Bel7402/5-FU cells. The cells were fixed in 70% methanol, and incubated with anti-MRP monoclonal antibody MRPr-1 for 1 hr at room temperature. FITC-conjugated goat anti-mouse IgG was added to examine the fluorescence staining ofMRP under confocal laser scanning microscope.
FLU accumulation as an index of MRP functional activity
The previous result showed that Bel7402 and Bel7402/5-FU cells differed in MRP expression, then we performed the functional MRP assay to demonstrate if the established difference in the levels of MRP in the two cells were indicative for differences in the MRP activity. FLU is a well-recognized substrate of MRP but not of P-gp. The use of FLU and FLU analogs as probes for MRP functional activity has been reported by Huai-Yun et al[24]. The results of flow cytometry showed that the accumulation of FLU in Bel7402 cells was almost two timed higher than that in Bel7402/5-FU cells (Figure 2). In other word, MRP functional activity in the resistant Bel7402/5-FU cells was higher than that in the parental cells.
Figure 2.
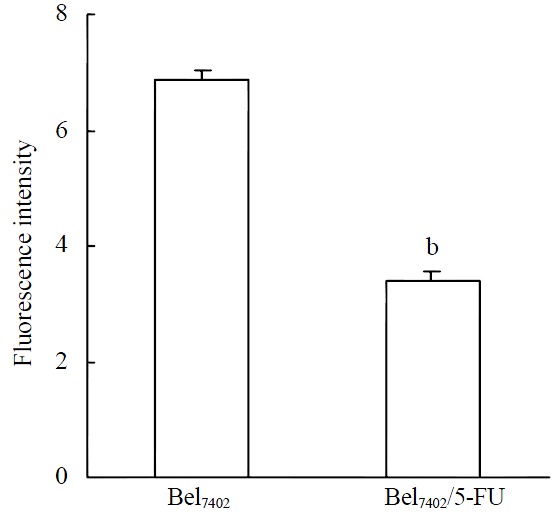
FLU accumulation in Bel7402 and Bel7402/5-FU cells. The cells were incubated with 100 μmol·L-1 FLU for 3hr. After washing, the cells were trypsinized and intracellular fluorescence of FLU was determined with a flow cytometer. Data were pooled from three independent experiments. bP < 0.001 vs Bel7402 cells
FLU redistribution
In drug-selected MRP-overexpressing cells, alterations in drug localization have been observed in addition to decreased accumulation[25]. The intracellular distribution of FLU was directly examined by confocal laser scanning microscopy (Figure 3). When exposed to FLU for 3 hours, besides the difference in FLU accumulation between the two cell lines, different localization of FLU in both cell lines was also observed. FLU mainly accumulated in the nucleus with a high nuclear/cytoplasmic ratio in Bel7402 cells (Figure 3A), whereas there was no difference of location between nucleus and cytoplasm in Bel7402/5-FU cells (Figure 3B).
Figure 3.
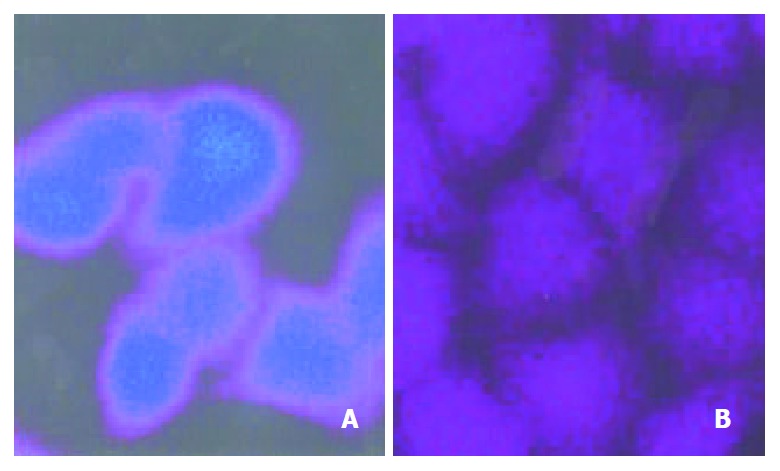
FLU distribution in Bel7402 and Bel7402/5-FU cells. The cells were incubated with 100 μmol·L-1 FLU for 3 hr. Then the intracellular distribution of FLU was revealed by confocal la-ser scanning microscopy. (A) Bel7402 cells; (B) Bel7402/5-FU cells.
GSH levels and depletion of GSH by BSO
Since GSH implicates in the mechanism of MRP-mediated drug resistance, the intracellular GSH content was determined in Bel7402 and Bel7402/5-FU cells. As shown in Table 1, the GSH content in Bel7402/5-FU cells increased almost two folds in comparison with that in Bel7402 cells. When Bel7402/5-FU cells were incubated with BSO, an inhibitor of GSH biosynthesis, at 5, 50 and 100 μmol·L-1 for 24 hours, the GSH content in the cells decreased in a dose-dependent manner.
Table 1.
GSH content and GSTs activity in Bel7402 and Bel7402/5-FU cells and the effect of BSO on the intracellular GSH content in Bel7402/5-FU cells
| BSO (μmol·L-1) |
Subline |
||
| Bel7402/5-FU | Bel7402 | ||
| GSH content (mg/mg protein) | - | 0.952 ± 0.257 | 0.322 ± 0.196b |
| 5 | 0.520 ± 0.107 | ||
| 50 | 0.427 ± 0.200a | ||
| 100 | 0.403 ± 0.135a | ||
| GSTs activity (nmol/min/mg protein) | - | 19.1 ± 1.9 | 14.8 ± 1.2c |
GSH content was determined in Bel7402/5-FU cells treated with or without BSO for 24 hr and Bel7402 cells. GSTs activity was assayed by the method of Ghalia et al[22] using 30 mmol·L-1 GSH and 30 mmol·L-1 CDNB as substrates. Results are presented as mean ± SD from three independent experiments, each per-formed in triplicates. Differences for significance were tested by using a Student’s t-test:
P < 0.05,
P < 0.01 vs Bel7402/5-FU cells without the addition of BSO;
P < 0.05 vs the GSTs activity in Bel7402/5-FU cells
Effect of BSO on the resistance of Bel7402/5-FU cells
Sublethal concentrations of BSO (5, 50, 100 μmol·L-1) were added to test the role of GSH in the sensitivity of Bel7402/5-FU cells to 5-FU and Dox. As a result, the addition of BSO caused a slight left shift in the dose-effect pattern of 5-FU (Figure 4A) and Dox (Figure 4B) in Bel7402/5-FU cells, indicating that the inhibition of GSH biosynthesis by BSO only had a weak reversal activity on the resistance of Bel7402/5-FU cells.
Figure 4.
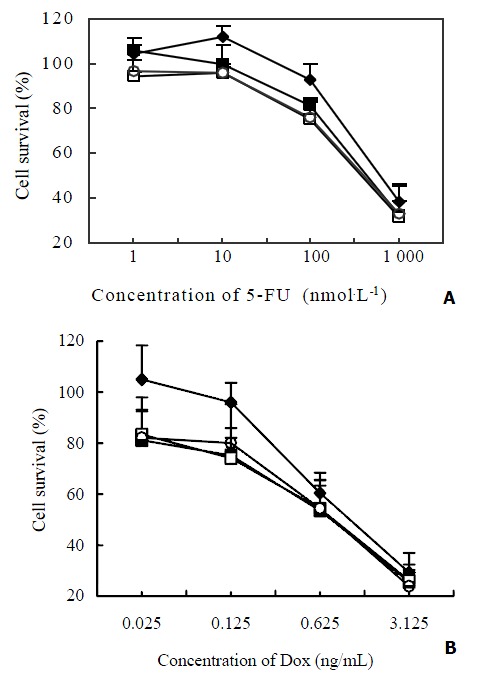
Effect of BSO on the cytotoxicity of 5-FU and Dox to Bel7402/5-FU cells. The cells were incubated with different con-centrations of 5-FU (Figure 4A) or Dox (Figure 4B) in the ab-sence (◆) or presence of BSO at the concentration of 5 μmol·L-1 (□), 50 μmol·L-1 (○) and 100 μmol·L-1 (■) respectively for 72 hr. Cell survival was determined by MTT assay. The values represent the means ± SD of three experiments.
GST enzymatic activity
GSTs catalyze the coupling reaction of intracellular electrophiles with GSH. Elevation of GSTs particularly GSTp is associated with an acquired resistance of cells to certain anticancer drugs. Table 1 showed the total GST activity in Bel7402/5-FU cells were 29.1% higher than that in Bel7402 cells. The difference between both cell lines was significant (P < 0.05).
TS levels
In order to determine whether there is difference of TS expression in Bel7402 and Bel7402/5-FU cell lines, an immunocytochemical analysis was performed by using MAb TS 106. As shown in Figure 5, the pattern of TS 106 reactivity was a dark granular appearance and diffusely spread throughout the cytoplasm in Bel7402/5-FU cells. The cytochemical staining intensity of TS 106 in Bel7402/5-FU cells (Figure 5B) markedly increased as compared with the parental Bel7402 cells (Figure 5A).
Figure 5.
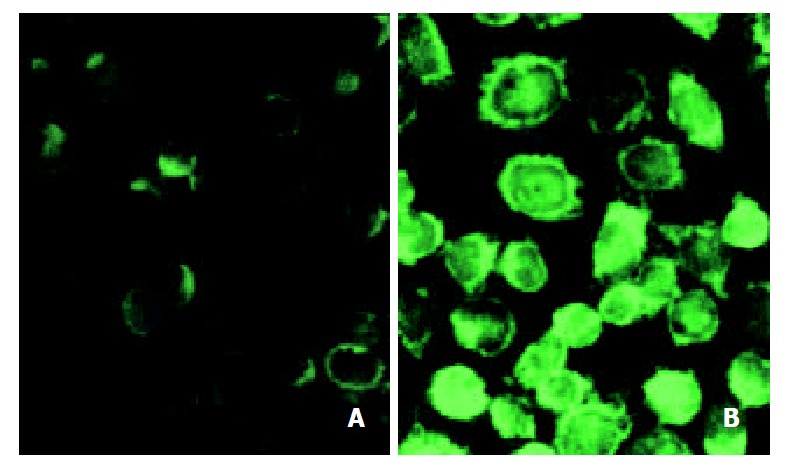
Immunocytochemical analysis of TS from parental and 5-FU resistant Bel7402 cells. The cytoprep slides were made as described in “Methods”. The slides were fixed in acetone, incubated with TS 106 and stained by using FITC-conjugated goat anti-mouse IgG. TS expression was determined by con-focal laser scanning microscopy. (A) Bel7402 cells; (B) Bel7402/ 5-FU cells.
DISCUSSION
In vitro continuous stepwise exposure of HCC cell line Bel7402 to 5-FU for 6 months resulted in a 160 fold resistant subline Bel7402/5-FU in our laboratory. The established resistance to 5-FU only slightly decreased or remained unchanged without continuous exposure to 5-FU for one month. Verapamil, a classical MDR reversal agent, significantly reversed the resistance to VCR, taxol and Dox with similar fold reversal in Bel7402 and Bel7402/5-FU cell lines, but it only had little effect on the chemosensitivity to 5-FU and hydroxycamptothecine in both cells. In our previous study on the required resistant mechanism of Bel7402/5-FU cells to 5-FU, we found that there was no difference in the expression level of the mdr-1 product P-gp between the two cells by immunocytochemical staining and Western-blotting analysis.
Up-regulation of MRP protein was observed by using the monoclonal antibody against MRP, MRPr-1 in this study. It was reported that[26] the gene expression of MRP correlated well with 5-FU resistance in 7 human gastrointestinal cancer cell lines. In some multidrug resistance cancer cells without alterations in levels of MRP, no resistance to 5-FU was found[27,28]. MRP has been postulated to cause multidrug resistance by exporting the anticancer drugs out of the cells, then the accumulation of drugs in cells decreased. Unlike P-gp, MRP can be localized in both the plasma membrane and membranes of intracellular organelles, including the endoplasmic reticulum and Golgi apparatus, thus cause intracellular or cytoplasmic sequestration of drug and prevent pharmaceuticals from reaching their cellular targets[25,29]. Anticancer drugs such as anthracyclines localize to the nucleus and cytoplasm with a high nuclear/cytoplasmic ratio in sensitive cancer cells. In contrast, anthracyclines accumulate mainly in the perinuclear region or in cytoplasmic vesicles in drug-selected MRP-overexpressing cells, and much less in the nucleus. In our experiments, the functional activity of MRP was investigated by flow cytometry using FLU accumulation as a probe. The accumulation of FLU in Bel7402/5-FU cells was only about half of the amount of FLU in the parental Bel7402 cells, suggesting an increased activity of MRP in the resistant Bel7402/5-FU cells. Furthermore, decreased nuclear/cytoplasmic ratio of FLU fluorescence was observed in Bel7402/5-FU cells as compared to that in the parental Bel7402 cells. All of these observations indicated that the decreased accumulation of FLU and the alteration in FLU distribution in Bel7402/5-FU cells may stem from up-regulated MRP.
MRP has been suggested to be the GS-X pump for multivalent organic anions such as cysteinyl leukotrienes, GSH disulfide and various GSH S-conjugates[8,30]. Intracellular GSH content is closely related to MRP-mediated multidrug resistance, since GSH interacts directly with MRP and this interaction causes a change in MRP structure that facilitates the binding and/or transport of anticancer drugs. Alternatively, GSH and anticancer drugs may spontaneously form a complex that behaves as a MRP substrate[31,32]. Using selective GSH modulators to decrease cellular GSH levels can sensitize tumor cells to the killing effects of chemotherapeutics[33]. BSO is a specific inhibitor of the rate-limiting enzyme of the GSH biosynthetic pathway, γ-glutamylcysteine synthetase. Treatment of either drug-selected or transfected resistant cells with BSO markedly depletes GSH and restores sensitivity to anticancer drugs that are transported by MRP[34-36]. In the present report, the resistant Bel7402/5-FU cells exhibited a significantly higher intracelluar GSH content and higher overall GST activity than 5-FU sensitive Bel7402 cells. Addition of BSO dose-dependently reduced the GSH content in Bel7402/5-FU cells. However, only a weak enhancement in the cytotoxicity of 5-FU and Dox to Bel7402/5-FU cells by BSO was observed in growth inhibition assay. Moreover, BSO restores sensitivity to Dox more effectively than to 5-FU. Till now there is no direct evidence that 5-FU is a substrate of GSTs or MRP. It appeared that the elevated levels of the GSH/GST system and higher expression of MRP did not to play an important role in the 5-FU resistance development. These alterations maybe associated with the cross-resistance of Dox and cytarabine in the Bel7402/5-FU cells.
5-FU is widely used in the treatment of breast, gastrointestinal, and head and neck cancers. The induction of TS following short-term 5-FU exposure is a generally observed phenomenon in malignant breast and colon cells[37]. A number of clinical evaluations referred to the relationship of TS expression in tumors to clinical response and survival of cancer patients receiving 5-FU-based chemotherapy[38,39]. Fukushima et al[40] established a human tumor sub-line resistant to 5-FU by using colorectal xenografts in nude mice and an increase in TS activity was observed in this resistant cell. According to our study, the 5-FU resistant HCC cells exhibited the elevated levels of TS protein by immunocytochemical detection, which is a sensitive and quantitative method of detecting human TS by monoantibody TS 106 by a direct comparison of Western blotting and biochemical TS assay[22]. Although it has been demonstrated a close correlation between TS protein levels and sensitivity to 5-FU in clinic, several other mechanisms of acquired resistance to 5-FU have been attributed to TS, such as gene amplification, increased dUMP levels, decreased FdUMP accumulation, decreased stability of ternary complex, depletion of intracellular folates and so on[41]. Only TS protein expression was investigated in this research. Further investigations should be performed to determine whether other altered effects on TS occur in this Bel7402/5-FU cells.
In summary, our results clearly showed that TS expression increased remarkably in 5-FU resistant Bel7402/5-FU cells. In addition, the expression of MRP as well as GSTs activity and GSH content also increased. The overexpression of TS may be the main cause for the resistance development of 5-FU. The changes in MRP and GSH/GST system seemed to result in the cross-resistance of Dox in Bel7402/5-FU cells.
Footnotes
Edited by Bo XN
References
- 1.Tono T, Hasuike Y, Ohzato H, Takatsuka Y, Kikkawa N. Limited but definite efficacy of prophylactic hepatic arterial infusion chemotherapy after curative resection of colorectal liver metastases: A randomized study. Cancer. 2000;88:1549–1556. [PubMed] [Google Scholar]
- 2.Guo WJ, Yu EX. Evaluation of combined therapy with chemoembolization and irradiation for large hepatocellular carcinoma. Br J Radiol. 2000;73:1091–1097. doi: 10.1259/bjr.73.874.11271902. [DOI] [PubMed] [Google Scholar]
- 3.Aguayo A, Patt YZ. Nonsurgical treatment of hepatocellular carcinoma. Semin Oncol. 2001;28:503–513. doi: 10.1016/s0093-7754(01)90143-5. [DOI] [PubMed] [Google Scholar]
- 4.Nishiyama M, Yamamoto W, Park JS, Okamoto R, Hanaoka H, Takano H, Saito N, Matsukawa M, Shirasaka T, Kurihara M. Low-dose cisplatin and 5-fluorouracil in combination can repress increased gene expression of cellular resistance determinants to themselves. Clin Cancer Res. 1999;5:2620–2628. [PubMed] [Google Scholar]
- 5.Kuwano M, Toh S, Uchiumi T, Takano H, Kohno K, Wada M. Multidrug resistance-associated protein subfamily transporters and drug resistance. Anticancer Drug Des. 1999;14:123–131. [PubMed] [Google Scholar]
- 6.van Zuylen L, Nooter K, Sparreboom A, Verweij J. Development of multidrug-resistance convertors: sense or nonsense. Invest New Drugs. 2000;18:205–220. doi: 10.1023/a:1006487003814. [DOI] [PubMed] [Google Scholar]
- 7.Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 8.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 9.Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11:265–283. doi: 10.1016/s0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 10.Rappa G, Finch RA, Sartorelli AC, Lorico A. New insights into the biology and pharmacology of the multidrug resistance protein (MRP) from gene knockout models. Biochem Pharmacol. 1999;58:557–562. doi: 10.1016/s0006-2952(99)00074-x. [DOI] [PubMed] [Google Scholar]
- 11.Keppler D. Export pumps for glutathione S-conjugates. Free Radic Biol Med. 1999;27:985–991. doi: 10.1016/s0891-5849(99)00171-9. [DOI] [PubMed] [Google Scholar]
- 12.Akiyama S, Chen ZS, Sumizawa T, Furukawa T. Resistance to cisplatin. Anticancer Drug Des. 1999;14:143–151. [PubMed] [Google Scholar]
- 13.Panasci L, Paiement JP, Christodoulopoulos G, Belenkov A, Malapetsa A, Aloyz R. Chlorambucil drug resistance in chronic lymphocytic leukemia: the emerging role of DNA repair. Clin Cancer Res. 2001;7:454–461. [PubMed] [Google Scholar]
- 14.Bredel M. Anticancer drug resistance in primary human brain tumors. Brain Res Brain Res Rev. 2001;35:161–204. doi: 10.1016/s0165-0173(01)00045-5. [DOI] [PubMed] [Google Scholar]
- 15.Mirjolet JF, Barberi-Heyob M, Merlin JL, Marchal S, Etienne MC, Milano G, Bey P. Thymidylate synthase expression and activity: relation to S-phase parameters and 5-fluorouracil sensitivity. Br J Cancer. 1998;78:62–68. doi: 10.1038/bjc.1998.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Triest B, Pinedo HM, van Hensbergen Y, Smid K, Telleman F, Schoenmakers PS, van der Wilt CL, van Laar JA, Noordhuis P, Jansen G, et al. Thymidylate synthase level as the main predictive parameter for sensitivity to 5-fluorouracil, but not for folate-based thymidylate synthase inhibitors, in 13 nonselected colon cancer cell lines. Clin Cancer Res. 1999;5:643–654. [PubMed] [Google Scholar]
- 17.Wada H, Saikawa Y, Niida Y, Nishimura R, Noguchi T, Matsukawa H, Ichihara T, Koizumi S. Selectively induced high MRP gene expression in multidrug-resistant human HL60 leukemia cells. Exp Hematol. 1999;27:99–109. doi: 10.1016/s0301-472x(98)00027-7. [DOI] [PubMed] [Google Scholar]
- 18.Declèves X, Regina A, Laplanche JL, Roux F, Boval B, Launay JM, Scherrmann JM. Functional expression of P-glycoprotein and multidrug resistance-associated protein (Mrp1) in primary cultures of rat astrocytes. J Neurosci Res. 2000;60:594–601. doi: 10.1002/(SICI)1097-4547(20000601)60:5<594::AID-JNR4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Miller DW, Batrakova EV, Kabanov AV. Inhibition of multidrug resistance-associated protein (MRP) functional activity with pluronic block copolymers. Pharm Res. 1999;16:396–401. doi: 10.1023/a:1018873702411. [DOI] [PubMed] [Google Scholar]
- 20.Rappa G, Lorico A, Flavell RA, Sartorelli AC. Evidence that the multidrug resistance protein (MRP) functions as a co-transporter of glutathione and natural product toxins. Cancer Res. 1997;57:5232–5237. [PubMed] [Google Scholar]
- 21.Benderra Z, Trussardi A, Morjani H, Villa AM, Doglia SM, Manfait M. Regulation of cellular glutathione modulates nuclear accumulation of daunorubicin in human MCF7 cells overexpressing multidrug resistance associated protein. Eur J Cancer. 2000;36:428–434. doi: 10.1016/s0959-8049(99)00288-9. [DOI] [PubMed] [Google Scholar]
- 22.Ghalia AA, Rabboh NA, el Shalakani A, Seada L, Khalifa A. Estimation of glutathione S-transferase and its Pi isoenzyme in tumor tissues and sera of patients with ovarian cancer. Anticancer Res. 2000;20:1229–1235. [PubMed] [Google Scholar]
- 23.Johnston PG, Liang CM, Henry S, Chabner BA, Allegra CJ. Production and characterization of monoclonal antibodies that localize human thymidylate synthase in the cytoplasm of human cells and tissue. Cancer Res. 1991;51:6668–6676. [PubMed] [Google Scholar]
- 24.Huai-Yun H, Secrest DT, Mark KS, Carney D, Brandquist C, Elmquist WF, Miller DW. Expression of multidrug resistance-associated protein (MRP) in brain microvessel endothelial cells. Biochem Biophys Res Commun. 1998;243:816–820. doi: 10.1006/bbrc.1997.8132. [DOI] [PubMed] [Google Scholar]
- 25.Ross DD. Novel mechanisms of drug resistance in leukemia. Leukemia. 2000;14:467–473. doi: 10.1038/sj.leu.2401694. [DOI] [PubMed] [Google Scholar]
- 26.Kirihara Y, Yamamoto W, Toge T, Nishiyama M. Dihydropyrimidine dehydrogenase, multidrug resistance-associated protein, and thymidylate synthase gene expression levels can predict 5-fluorouracil resistance in human gastrointestinal cancer cells. Int J Oncol. 1999;14:551–556. doi: 10.3892/ijo.14.3.551. [DOI] [PubMed] [Google Scholar]
- 27.Iida N, Takara K, Ohmoto N, Nakamura T, Kimura T, Wada A, Hirai M, Sakaeda T, Okumura K. Reversal effects of antifungal drugs on multidrug resistance in MDR1-overexpressing HeLa cells. Biol Pharm Bull. 2001;24:1032–1036. doi: 10.1248/bpb.24.1032. [DOI] [PubMed] [Google Scholar]
- 28.Liu B, Staren ED, Iwamura T, Appert HE, Howard JM. Mechanisms of taxotere-related drug resistance in pancreatic carcinoma. J Surg Res. 2001;99:179–186. doi: 10.1006/jsre.2001.6126. [DOI] [PubMed] [Google Scholar]
- 29.Aszalos A, Ross DD. Biochemical and clinical aspects of efflux pump related resistance to anti-cancer drugs. Anticancer Res. 1998;18:2937–2944. [PubMed] [Google Scholar]
- 30.Bradshaw DM, Arceci RJ. Clinical relevance of transmembrane drug efflux as a mechanism of multidrug resistance. J Clin Oncol. 1998;16:3674–3690. doi: 10.1200/JCO.1998.16.11.3674. [DOI] [PubMed] [Google Scholar]
- 31.Loe DW, Deeley RG, Cole SP. Characterization of vincristine transport by the M(r) 190,000 multidrug resistance protein (MRP): evidence for cotransport with reduced glutathione. Cancer Res. 1998;58:5130–5136. [PubMed] [Google Scholar]
- 32.Salerno M, Garnier-Suillerot A. Kinetics of glutathione and daunorubicin efflux from multidrug resistance protein overexpressing small-cell lung cancer cells. Eur J Pharmacol. 2001;421:1–9. doi: 10.1016/s0014-2999(01)00992-x. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Carystinos GD, Batist G. Potential for selective modulation of glutathione in cancer chemotherapy. Chem Biol Interact. 1998;111-112:263–275. doi: 10.1016/s0009-2797(97)00166-x. [DOI] [PubMed] [Google Scholar]
- 34.Cole SP, Deeley RG. Multidrug resistance mediated by the ATP-binding cassette transporter protein MRP. Bioessays. 1998;20:931–940. doi: 10.1002/(SICI)1521-1878(199811)20:11<931::AID-BIES8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 35.Benderra Z, Trussardi A, Morjani H, Villa AM, Doglia SM, Manfait M. Regulation of cellular glutathione modulates nuclear accumulation of daunorubicin in human MCF7 cells overexpressing multidrug resistance associated protein. Eur J Cancer. 2000;36:428–434. doi: 10.1016/s0959-8049(99)00288-9. [DOI] [PubMed] [Google Scholar]
- 36.Lewandowicz GM, Britt P, Elgie AW, Williamson CJ, Coley HM, Hall AG, Sargent JM. Cellular glutathione content, in vitro chemoresponse, and the effect of BSO modulation in samples derived from patients with advanced ovarian cancer. Gynecol Oncol. 2002;85:298–304. doi: 10.1006/gyno.2002.6617. [DOI] [PubMed] [Google Scholar]
- 37.Parr AL, Drake JC, Gress RE, Schwartz G, Steinberg SM, Allegra CJ. 5-fluorouracil-mediated thymidylate synthase induction in malignant and nonmalignant human cells. Biochem Pharmacol. 1998;56:231–235. doi: 10.1016/s0006-2952(98)00152-x. [DOI] [PubMed] [Google Scholar]
- 38.Kuniyasu T, Nakamura T, Tabuchi Y, Kuroda Y. Immunohistochemical evaluation of thymidylate synthase in gastric carcinoma using a new polyclonal antibody: the clinical role of thymidylate synthase as a prognostic indicator and its therapeutic usefulness. Cancer. 1998;83:1300–1306. doi: 10.1002/(sici)1097-0142(19981001)83:7<1300::aid-cncr5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 39.Yeh KH, Shun CT, Chen CL, Lin JT, Lee WJ, Lee PH, Chen YC, Cheng AL. High expression of thymidylate synthase is associated with the drug resistance of gastric carcinoma to high dose 5-fluorouracil-based systemic chemotherapy. Cancer. 1998;82:1626–1631. doi: 10.1002/(sici)1097-0142(19980501)82:9<1626::aid-cncr5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Fukushima M, Fujioka A, Uchida J, Nakagawa F, Takechi T. Thymidylate synthase (TS) and ribonucleotide reductase (RNR) may be involved in acquired resistance to 5-fluorouracil (5-FU) in human cancer xenografts in vivo. Eur J Cancer. 2001;37:1681–1687. doi: 10.1016/s0959-8049(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 41.Peters GJ, Backus HH, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J, Calvert AH, Marsh S, et al. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta. 2002;1587:194–205. doi: 10.1016/s0925-4439(02)00082-0. [DOI] [PubMed] [Google Scholar]


