Abstract
AIM: To evaluate the possibility of the induction of anti-tumor immune response by transfecting the colorectal cancer cells with chemokine MCP-3 gene.
METHODS: Mouse MCP-3 gene was transduced into mouse colorectal cancer cells CMT93 by using of Liposome. G418-resistant clones were selected and the MCP-3 mRNA expression was detected by RT-PCR. The chemotactic activity of MCP-3 in the cell culture supernatant was detected by Chemotaxis assay. The tumorigenicity of wild type CMT93 and CMT93 gene transfectants were detected by in vivo experiments. The immune cell infiltrations in tumor tissue and tumor metastasis were detected histopathologically.
RESULTS: MCP-3 mRNA expression was detected by RT-PCR in gene-transfected cells (CMT93/MCP-3), but not in control groups. And MCP-3 secreted in the cell culture supernatant possessed chemotatic activity. The results from in vivo experiments showed that the tumorigenicity of CMT93/MCP-3 had not decreased, but the tumors derived from CMT93/MCP-3 cells grew more slowly than those from CMT93 cells (1.021 ± 0.253) cm2 vs (1.769 ± 0.371) cm2, P < 0.05) or CMT93/mock cells (1.021 ± 0.253) cm2 vs (1.680 ± 0.643) cm2, P < 0.05). Histophathological results showed few immune cells infiltrating in the tumor tissue derived from the controls. In the tumor tissue derived from CMT93/MCP-3, infiltrating immune cells increased. In addition, no tumor metastasis was found in all mice inoculated with CMT93/ MCP-3 tumor cells. But all mice had tumor metastasis in CMT93 controls and 4 in 5 mice had tumor metastasis in CMT93/mock controls.
CONCLUSION: The results suggested that the transfection of chemokine MCP-3 gene could promote the induction of anti-colorectal cancer immunity, but the tumor growth could not be inhibited completely by merely MCP-3 gene transfection.
INTRODUCTION
Chemokines, a kind of polypeptide with low molecular weight, can activate and chemoattract immune cells and play a pivotal role in host defense and in immunological conditions such as autoimmune reaction and malignant tumors[1]. Chemokines such as monocyte chemotactic protein-1 (MCP-1) is produced by a variety of tumors such as ovarian carcinoma[2] Kaposi’s sarcoma[3], cervical carcinoma[4], and melanoma[5]. There are evidences suggesting that chemokines play an important role in immune cells infiltrating in tumor tissue. Chemokine gene transferred into tumor cells elicits anti-tumor effect such as reducing tumorigenicity and inhibiting tumor growth[1].
MCP-3 is a CC chemokine identified from osteosarcoma supernatant[6]. By binding to CCR1, CCR2, and CCR3[7-11], MCP-3 acts on a lot of immune cells. Tumor gene therapy with MCP-3 had been reported in p815 mastocytoma mouse model[12] and human cervical carcinoma xenografts[13]. But the anti-tumor effect by MCP-3 gene transfer in colorectal cancer model has not been determined. In our study, we found that after the transfecting of CMT93 colorectal cancer cell with MCP-3 gene, tumor growth was retarded and tumor metastasis was inhibited completely by promoting immune cells infiltration in tumor tissue.
MATERIALS AND METHODS
Materials
Male or female C57BL/6 mice, 6-8 weeks old, were purchased from Shanghai Experimental Animal Center of Chinese Academy of Sciences. CMT93 is a mouse colon cancer cell line from an induced carcinoma of mouse rectum[14], which was purchased from ATCC in America. Plasmid pCMV/MCP3 containing mouse MCP3 gene and plasmid pCDNA/YT4 containing mouse CCR1 (C-C chemokine receptor 1) gene were presented kindly by Dr. Ji Ming Wang in NCI in America. MCP-3 RT-PCR primers were presented kindly by Dr. Ji Ming Wang as well.
Transfection of tumor cells with MCP-3 gene
According to the LipofectAMINE (Gibco) kit protocol. Briefly, (1)2 μg plasmid DNA and 15 μL LipofectAMINE are added to 200 μL DMEM without FCS, mix gently, and incubate at 37 °C for 30 min. (2) Wash the CMT93 cells in 6 well plate with FCS-free DMEM. (3) Add 0.8 mL of serum-free DMEM to the tube containing the DNA and lipofectAMINE complexes, mix gently and overlay the rinsed cells. (4) Incubate the cells with complexes for 5 h at 37 °C in CO2 incubator. (5) Add 1mL of DMEM containing 200 mL·L-1 serum, incubate overnight. (6) Replace the medium with fresh DMEM. (7) Digest the cells and plate them to the 100 mL bottles, incubate 24 h. (8) Replace the medium with fresh DMEM containing 800 μg/mL G418 and select the positive clones.
RNA preparation and reverse transcription polymerase chain reaction (RT-PCR)
The Total RNA from cells was extracted with RNeasy Mini kit (QIAGEN) according to the manufacture’s instructions. Total of 0.5 μg RNA was used for RT-PCR. For mouse MCP-3, sense primer 5’-TCTGTGCCTGCTGCTCATAG-3’ (nucleotide 73-92) and antisense primer 5’-CTTTGGAGTTGGGGTTTTCA-3’ (nucleotide 321-340) were used to yield a 268-base pair product. For mouse β-actin, sense primer 5’-TGTGATGGTGGGAATGGGTCGG-3’ and antisense primer 5’-TTTGATGTCACGCACGATTTCC3’ were purchased from STRAATAGENE to amplify a 514-base pair fragment. RT-PCR was performed with high fidelity ProSTRARTM HF single-tube RT-PCR system (STRATAGEBE), consisting of a 15-min reverse transcription at 37 °C, 1 min of inactivation of Moloney murine leukemia virus reverse transcriptase at 95 °C, 40 cycles of denaturing at 95 °C (30 s), annealing at 60 °C (30 s), and extension at 68 °C (2 min), as well as a final extension for 10 min at 68 °C.
Chemotaxsis assay
Chemotaxis assays were performed using 48-well chemotaxis charmbers (Neuro probe, Cabin John, MD, USA). As described previously[15]. First 27-29 μL cell culture supernatants from CMT93 and its gene transfectants were placed in the wells of the lower compartment of the chamber. 50 μL cells (1 × 106/mL in binding medium, BM: RPMI1640 containing 10 mL·L-1 BSA, 25 mmol·L-1 HEPES) were plated in the wells of the upper compartment. The upper and lower compartments were separated by a polycarbonate filter (Osmonics, Livermore, CA, USA; 10 μm pore-size) which was pre-coated with 50 μg·mL-1 collagen type I (Collaborative Biomedical Products, Bedford, MA, USA). After incubation at 37 °C for 5 h, the filter was removed, stained, and the cells migrated across the filter were counted under light microscope after coding the samples. The results were expressed as chemotaxis index (CI), which represents the fold increase in the number of migrated cells in response to chemoattractants over the spontaneous cell migration in response to control medium.
Tumorigenicity
As previously reported[16], Balb/c mice were inoculated subcutaneously on the back with 5 × 106 CMT93/MCP-3 cells (8 mice), or CMT93/mock cells (7 mice) or CMT93 cells (5 mice). Tumor sizes were assessed for every 5 days by measuring perpendicular diameters with caliper (0.002 cm). The tumor sizes from each groups were expressed as mean square (long diameter × short diameter). On day 14, one mice in each groups was selected randomly and sacrificed for histologic evaluation. The other mice were observed and the tumor metastasis was measured.
Histologic evaluation
Tissues were removed from the site of tumor cell inoculation 14 days after injection, or from the drainage lymph node of the site of tumor cell inoculation. Then fixed in 100 mL·L-1 formalin, blocked in paraffin, sectioned at 4-6 um, and stained with hematoxylin and eosin. The infiltrating leukocytes in tumor tissue were observed and evaluated, and the tumor metastasis in the drainage lymph node was determined.
RESULTS
RT-PCR
After gene transfection and the following G418-resistant clone selection, we detected MCP-3 mRNA expression in MCP-3 gene transfected CMT93 cells by RT-PCR using one-step kit. In contrast, the wild type CMT93 cells and the mock transfected CMT93 cells did not express MCP-3 (Figure 1).
Figure 1.
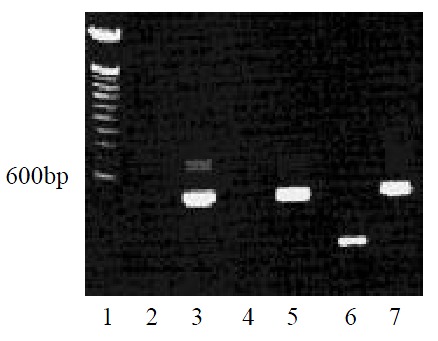
MCP-3 mRNA expression of CMT93 or its gene transfectants (RT-PCR). Lane 1: 100bp DNA ladder marker; Lane 2:CMT93+Primer MCP-3; Lane 3: CMT93 + Primer β-actin; Lane 4: CMT93/Mock + Primer MCP-3; Lane 5: CMT93/Mock + Primer β-actin; Lane 6: CMT93/MCP-3 + Primer MCP-3; Lane 7: CMT93/MCP-3 + Primer β-actin.
Chemotaxsis
After the detection of MCP-3 mRNA expression, we tested the biological function of MCP-3 secreted in the culture supernatant. Chemotaxis analyses revealed that the RBL/2H3 cells transfected with CCR1, which is one of the receptors of MCP-3, migrated when supernatants from MCP-3 transfected CMT93 (CMT93/ MCP-3) cells were present in the lower wells of the chemotaxis chamber (Figure 2, 3D). No increased cell migration occurred when supernatants from mock transfected CMT93 (CMT93/ mock) or wild type CMT93 cells were present in the lower wells of the chemotaxis chamber (Figure 2, Figure 3C, 3B). These results showed that not only the transfected MCP-3 gene expressed but also the expressed protein possessed biological function.
Figure 2.
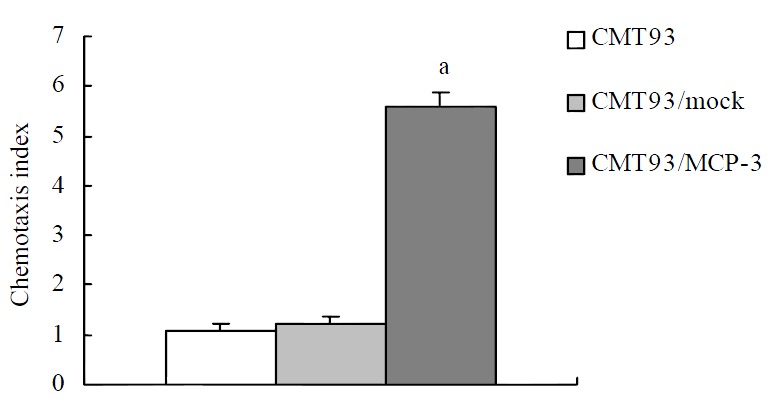
Chemotactic activity of MCP-3 in the supernatant from CMT93 cells or its gene transfectants. P < 0.05 vs CMT93 or CMT93/mock group.
Figure 3.
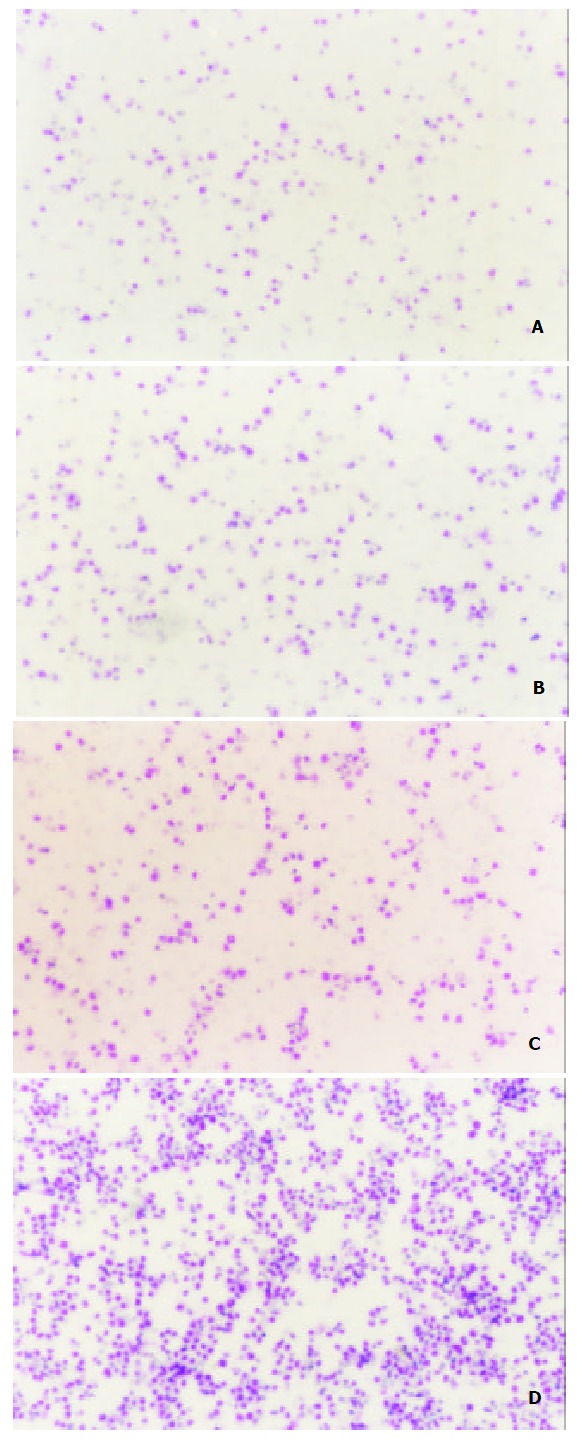
Chemotactic activity of MCP-3 in supernatant from CMT93 or its transfectants (Wright staining showed the chemoattracted target cells × 25). A: medium; B: CMT93 supernatant. The chemoattracted cells were not increased significantly; C: CMT93/Mock supernatant. The chemoattracted cells were not increased significantly; D: CMT93/MCP-3 supernatant. The chemoattracted cells were increased significantly.
MCP-3 retards tumor growth
To examine whether MCP-3 secretion retard tumor growth, we injected MCP-3 transfectants subcutaneously into Balb/c mice to test their tumorigenicity. As showed in Figure 4, all mice had tumor growth on day 5 after tumor cell inoculation. But tumors from CMT93/MCP-3 grew more slowly than that from mock transfectants or wild type CMT93 (P < 0.05, Table 1). These results suggested though MCP-3 secretion could not inhibit tumor formation completely; it retarded tumor growth.
Figure 4.
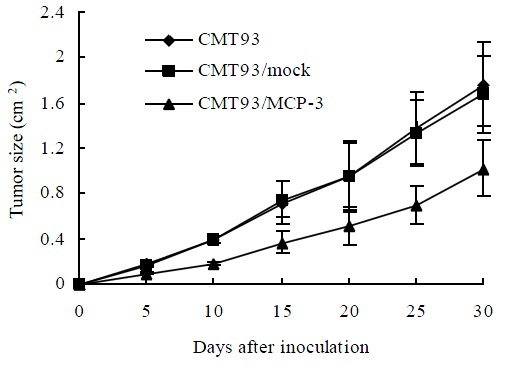
The tumor growth curves of CMT93 or its gene transfectants
Table 1.
Tumor size in the mice inoculated with CMT93 or its gene transfectants (cm2, -x ± s)
| Days after tumor injection |
Tumor size in the mice inoculated with |
||
| CMT93 | CMT93/mock | CMT93/MCP-3 | |
| 5 | 0.181 ± 0.020 | 0.170 ± 0.012 | 0.097 ± 0.012a |
| 10 | 0.398 ± 0.030 | 0.397 ± 0.025 | 0.186 ± 0.019a |
| 15 | 0.720 ± 0.185 | 0.751 ± 0.157 | 0.372 ± 0.104a |
| 20 | 0.950 ± 0.303 | 0.954 ± 0.309 | 0.416 ± 0.167a |
| 25 | 1.385 ± 0.222 | 1.335 ± 0.292 | 0.699 ± 0.170a |
| 30 | 1.769 ± 0.371 | 1.680 ± 0.643 | 1.021 ± 0.253a |
P < 0.05 vs CMT93 or CMT93/mock group
Immune cells infiltration
MCP-3 gene transfer profoundly altered leukocyte recruitment in tumors grown in syngeneic Balb/c mice. On day 14 after tumor inoculation, all the mice showed tumor growths of about (0.6-0.8) × (0.6-0.8) cm2 in size, which allowed a histologic study. Histologic results showed that tumor derived from MCP-3 gene transfected CMT93 cells contained much more infiltrating leukocytes, but tumor derived from the wild type CMT93 cells contained less leukocytes (Figure 5). Infiltrating leukocytes were located in the cancer nests mainly. The tumors derived from mock transfectants contained less leukocytes as well (data not shown). These results suggested that the effect of MCP-3 gene transfer in retarding tumor growth maybe related to the infiltration of leukocytes which were chemoattracted by MCP-3.
Figure 5.
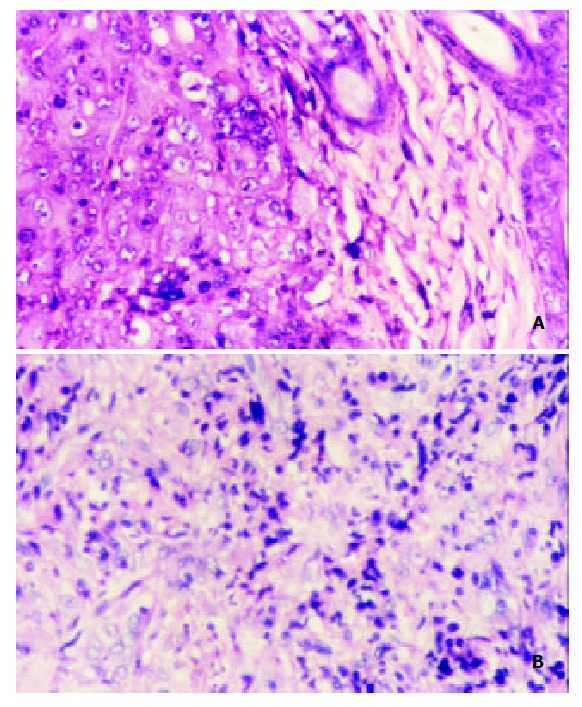
Histopathology of the tumors derived from CMT93 or CMT93/MCP3 (HE staining × 200). A: tumor derived from CMT93 cells; few infiltrated immune cells were found; B:tu-mor derived from CMT93/MCP-3 cells, the infiltrated immune cells were increased.
Tumor metastasis
On day 30, tumors in all 4 mice inoculated with CMT93 cells into the backs metastasized, two to inguinal drainage lymph node, other two to cervical drainage lymph node. Of tumors in 5 mice inoculated with CMT93/mock cells in the backs, 4 metastasized to inguinal drainage lymph node, and the other one did not metastasize. Tumors in all 7 mice inoculated with CMT93/MCP-3 did not metastasize. Histological study showed a metastatic lymph node (Figure 6A). In another metastasis lesion, tumor cells went out of the lymph node to invade the muscle tissue (Figure 6B). The percentage of tumor metastasis of MCP-3 gene transfectants was significantly lower than that of wild type or mock transfected CMT93 tumor cells (Table 2, P < 0.05).
Figure 6.
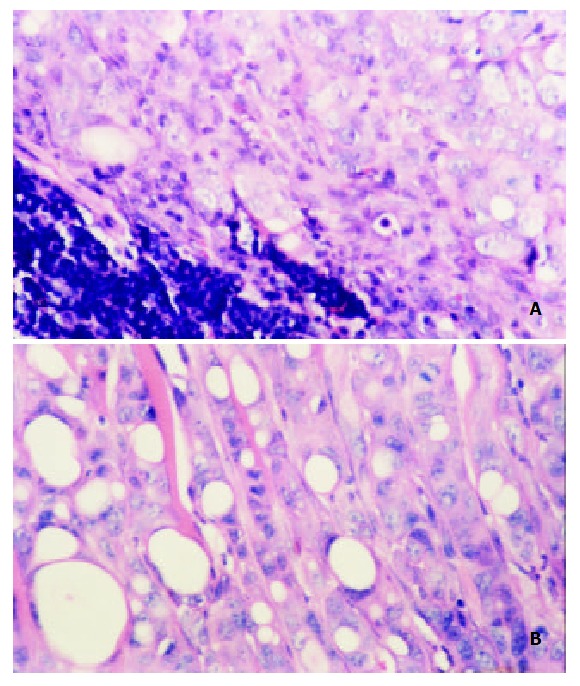
Histopathology of tumor derived from CMT93 me-tastasized to the local drainage lymph node(HE staining × 200). A: up-left is the lymph tissue,down-right is the tumor tissue; B: tumor infiltrated to the muscle tissue.
Table 2.
Tumor metastasis in mice inoculated with CMT93 or its gene transfectants
| Miceinoculated with | No. metastasized | No. not metastasized | Percentage(%) |
| CMT93 | 4 | 0 | 100 |
| CMT93/Mock | 4 | 1 | 80 |
| CMT93/MCP-3 | 0 | 7 | 0ab |
P < 0.001 vs CMT93 group;
P < 0.05 vs CMT93/Mock group
DISCUSSION
Chemokines characterized by their ability to induce directional migration and activation of leukocytes comprise the superfamily of polypeptides which is the largest family of cytokines. Produced by a variety of cell types including hematopoietic and non-hematopoietic origin, chemokines regulate leukocyte adhesion, trafficking, homing and angiogenesis, and contribute to lymphopoiesis and hematopoiesis. In vivo studies using neutralizing antibodies, antagonists, or by deletion of chemokines and their receptors have revealed that chemokines and their receptors play a pivotal role in host defense and in immunological conditions[1].
The fact that chemokines attract different leukocyte populations and promote their functions has prompted a number of studies on the effect of chemokine gene transduction into tumor cells. Not only CC chemokines such as MCP-1 (monocyte chemotactic protein-1)[17,18], MCP-3 (monocyte chemotactic protein-3)[12,13], MIP-1alpha (macrophage inflammatory protein-1alpha)[19], MIP-1beta (macrophage inflammatory protein-1beta)[20], SLC (secondary lymphoid tissue chemokine)[21], MIP-3alpha (macrophage inflammatory protein 3alpha)[22], TCA3(T-cell activation gene 3)[23], RANTES(regulated on activation normally T cell expressed and secreted)[24], but also CXC chemokines including ELR- (angiostatic) chemokines such as IP10 (interferon-inducible protein 10)[25-28], MIG (monokine induced by interferon gamma)[29,30], PF4 (platelet factor 4)[31], and ELR+ (angiogenic) chemokines such as IL-8 (interleukin-8)[32-33] had been selected for tumor gene immunotherapy. In addition, C chemokine lymphotactin[34-36] had been reported in tumor gene therapy as well. Divergent results were obtained depending on the tumor models and whether human or murine chemokine genes were used.
MCP-3 had a wide spectrum of action on different immune cells, including monocytes[10], T cells[37,38], NK cells[39], basophils[40], eosinophils[40], and neutrophils[10]. In addition, MCP-3 was active on DC as well[41-43]. In P815 mastocytoma mouse model, MCP-3 gene transfer reduced tumorigenicity by augmenting immune cell infiltration in tumor tissue and dendritic cells accumulation in peritumorous tissue[12]. MCP-3 gene transfer also induced leukocyte infiltration and reduces growth of human cervical carcinoma cell xenografts[13].
In our study, MCP-3 gene was transduced into mouse colorectal cancer CMT93 tumor cells to determine the anti-tumor activity of MCP-3 in colorectal cancer model. Though tumorigenicity of CMT93 was not reduced, and the tumors can’t be rejected completely after MCP-3 gene transfer, but the tumors grew more slowly than those of controls. And MCP-3 gene transfer inhibited tumor metastasis thoroughly. In the tumor tissue from MCP-3 gene transfectants, infiltrated immune cells increased. We speculate that the anti-tumor immunity elicited by MCP-3 gene transfer is type I T cell-dependent just as reported[12]. We found the humoral response against CMT93 tumor cells in the serum from CMT93/MCP-3 primed mouse did not increase (data not shown), and few neutrophils was found in tumor tissue. These results reversely suggested a type I T cell-dependent response was contributed to the anti-tumor immunity in MCP-3 gene transfer. Further study is needed to prove whether dendritic cells contribute to the anti-tumor immunity elicited by MCP-3 gene transfer or not.
Footnotes
Edited by Lu HM
References
- 1.Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998;220:1–17. doi: 10.1016/s0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 2.Negus RP, Stamp GW, Relf MG, Burke F, Malik ST, Bernasconi S, Allavena P, Sozzani S, Mantovani A, Balkwill FR. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest. 1995;95:2391–2396. doi: 10.1172/JCI117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sciacca FL, Stürzl M, Bussolino F, Sironi M, Brandstetter H, Zietz C, Zhou D, Matteucci C, Peri G, Sozzani S. Expression of adhesion molecules, platelet-activating factor, and chemokines by Kaposi's sarcoma cells. J Immunol. 1994;153:4816–4825. [PubMed] [Google Scholar]
- 4.Riethdorf L, Riethdorf S, Gützlaff K, Prall F, Löning T. Differential expression of the monocyte chemoattractant protein-1 gene in human papillomavirus-16-infected squamous intraepithelial lesions and squamous cell carcinomas of the cervix uteri. Am J Pathol. 1996;149:1469–1476. [PMC free article] [PubMed] [Google Scholar]
- 5.Graves DT, Barnhill R, Galanopoulos T, Antoniades HN. Expression of monocyte chemotactic protein-1 in human melanoma in vivo. Am J Pathol. 1992;140:9–14. [PMC free article] [PubMed] [Google Scholar]
- 6.Van Damme J, Proost P, Lenaerts JP, Opdenakker G. Structural and functional identification of two human, tumor-derived monocyte chemotactic proteins (MCP-2 and MCP-3) belonging to the chemokine family. J Exp Med. 1992;176:59–65. doi: 10.1084/jem.176.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sozzani S, Zhou D, Locati M, Rieppi M, Proost P, Magazin M, Vita N, van Damme J, Mantovani A. Receptors and transduction pathways for monocyte chemotactic protein-2 and monocyte chemotactic protein-3. Similarities and differences with MCP-1. J Immunol. 1994;152:3615–3622. [PubMed] [Google Scholar]
- 8.Neote K, DiGregorio D, Mak JY, Horuk R, Schall TJ. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell. 1993;72:415–425. doi: 10.1016/0092-8674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- 9.Gao JL, Kuhns DB, Tiffany HL, McDermott D, Li X, Francke U, Murphy PM. Structure and functional expression of the human macrophage inflammatory protein 1 alpha/RANTES receptor. J Exp Med. 1993;177:1421–1427. doi: 10.1084/jem.177.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu LL, McVicar DW, Ben-Baruch A, Kuhns DB, Johnston J, Oppenheim JJ, Wang JM. Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors: binding and signaling of MCP3 through shared as well as unique receptors on monocytes and neutrophils. Eur J Immunol. 1995;25:2612–2617. doi: 10.1002/eji.1830250931. [DOI] [PubMed] [Google Scholar]
- 11.Ponath PD, Qin S, Post TW, Wang J, Wu L, Gerard NP, Newman W, Gerard C, Mackay CR. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fioretti F, Fradelizi D, Stoppacciaro A, Ramponi S, Ruco L, Minty A, Sozzani S, Garlanda C, Vecchi A, Mantovani A. Reduced tumorigenicity and augmented leukocyte infiltration after monocyte chemotactic protein-3 (MCP-3) gene transfer: perivascular accumulation of dendritic cells in peritumoral tissue and neutrophil recruitment within the tumor. J Immunol. 1998;161:342–346. [PubMed] [Google Scholar]
- 13.Wetzel K, Menten P, Opdënakker G, Van Damme J, Gröne HJ, Giese N, Vecchi A, Sozzani S, Cornelis JJ, Rommelaere J, et al. Transduction of human MCP-3 by a parvoviral vector induces leukocyte infiltration and reduces growth of human cervical carcinoma cell xenografts. J Gene Med. 2001;3:326–337. doi: 10.1002/jgm.191. [DOI] [PubMed] [Google Scholar]
- 14.Franks LM, Hemmings VJ. A cell line from an induced carcinoma of mouse rectum. J Pathol. 1978;124:35–38. doi: 10.1002/path.1711240108. [DOI] [PubMed] [Google Scholar]
- 15.Deng X, Ueda H, Su SB, Gong W, Dunlop NM, Gao JL, Murphy PM, Wang JM. A synthetic peptide derived from human immunodeficiency virus type 1 gp120 downregulates the expression and function of chemokine receptors CCR5 and CXCR4 in monocytes by activating the 7-transmembrane G-protein-coupled receptor FPRL1/LXA4R. Blood. 1999;94:1165–1173. [PubMed] [Google Scholar]
- 16.Hu JY, Wang S, Zhu JG, Zhou GH, Sun QB. Expression of B7 costimulation molecules by colorectal cancer cells reducestumorigenicity and induces anti-tumor immunity. World J Gastroenterol. 1999;5:147–151. doi: 10.3748/wjg.v5.i2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai Y, Kaneko S, Nakamoto Y, Kagaya T, Mukaida N, Kobayashi K. Enhanced anti-tumor effects of herpes simplex virus thymidine kinase/ganciclovir system by codelivering monocyte chemoattractant protein-1 in hepatocellular carcinoma. Cancer Gene Ther. 2001;8:695–704. doi: 10.1038/sj.cgt.7700360. [DOI] [PubMed] [Google Scholar]
- 18.Nakashima E, Kubota Y, Matsushita R, Ozaki E, Ichimura F, Kawahara S, Nakanishi I, Kuno K, Matsushima K. Synergistic antitumor interaction of human monocyte chemotactant protein-1 gene transfer and modulator for tumor-infiltrating macrophages. Pharm Res. 1998;15:685–689. doi: 10.1023/a:1011906600304. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima E, Oya A, Kubota Y, Kanada N, Matsushita R, Takeda K, Ichimura F, Kuno K, Mukaida N, Hirose K, et al. A candidate for cancer gene therapy: MIP-1 alpha gene transfer to an adenocarcinoma cell line reduced tumorigenicity and induced protective immunity in immunocompetent mice. Pharm Res. 1996;13:1896–1901. doi: 10.1023/a:1016057830271. [DOI] [PubMed] [Google Scholar]
- 20.Miyata T, Yamamoto S, Sakamoto K, Morishita R, Kaneda Y. Novel immunotherapy for peritoneal dissemination of murine colon cancer with macrophage inflammatory protein-1beta mediated by a tumor-specific vector, HVJ cationic liposomes. Cancer Gene Ther. 2001;8:852–860. doi: 10.1038/sj.cgt.7700384. [DOI] [PubMed] [Google Scholar]
- 21.Kirk CJ, Hartigan-O'Connor D, Nickoloff BJ, Chamberlain JS, Giedlin M, Aukerman L, Mule JJ. T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: augmentation of dendritic cell-based immunotherapy. Cancer Res. 2001;61:2062–2070. [PubMed] [Google Scholar]
- 22.Fushimi T, Kojima A, Moore MA, Crystal RG. Macrophage inflammatory protein 3alpha transgene attracts dendritic cells to established murine tumors and suppresses tumor growth. J Clin Invest. 2000;105:1383–1393. doi: 10.1172/JCI7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laning J, Kawasaki H, Tanaka E, Luo Y, Dorf ME. Inhibition of in vivo tumor growth by the beta chemokine, TCA3. J Immunol. 1994;153:4625–4635. [PubMed] [Google Scholar]
- 24.Mulé JJ, Custer M, Averbook B, Yang JC, Weber JS, Goeddel DV, Rosenberg SA, Schall TJ. RANTES secretion by gene-modified tumor cells results in loss of tumorigenicity in vivo: role of immune cell subpopulations. Hum Gene Ther. 1996;7:1545–1553. doi: 10.1089/hum.1996.7.13-1545. [DOI] [PubMed] [Google Scholar]
- 25.Feldman AL, Friedl J, Lans TE, Libutti SK, Lorang D, Miller MS, Turner EM, Hewitt SM, Alexander HR. Retroviral gene transfer of interferon-inducible protein 10 inhibits growth of human melanoma xenografts. Int J Cancer. 2002;99:149–153. doi: 10.1002/ijc.10292. [DOI] [PubMed] [Google Scholar]
- 26.Palmer K, Hitt M, Emtage PC, Gyorffy S, Gauldie J. Combined CXC chemokine and interleukin-12 gene transfer enhances antitumor immunity. Gene Ther. 2001;8:282–290. doi: 10.1038/sj.gt.3301386. [DOI] [PubMed] [Google Scholar]
- 27.Narvaiza I, Mazzolini G, Barajas M, Duarte M, Zaratiegui M, Qian C, Melero I, Prieto J. Intratumoral coinjection of two adenoviruses, one encoding the chemokine IFN-gamma-inducible protein-10 and another encoding IL-12, results in marked antitumoral synergy. J Immunol. 2000;164:3112–3122. doi: 10.4049/jimmunol.164.6.3112. [DOI] [PubMed] [Google Scholar]
- 28.Luster AD, Leder P. IP-10, a -C-X-C- chemokine, elicits a potent thymus-dependent antitumor response in vivo. J Exp Med. 1993;178:1057–1065. doi: 10.1084/jem.178.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruehlmann JM, Xiang R, Niethammer AG, Ba Y, Pertl U, Dolman CS, Gillies SD, Reisfeld RA. MIG (CXCL9) chemokine gene therapy combines with antibody-cytokine fusion protein to suppress growth and dissemination of murine colon carcinoma. Cancer Res. 2001;61:8498–8503. [PubMed] [Google Scholar]
- 30.Addison CL, Arenberg DA, Morris SB, Xue YY, Burdick MD, Mulligan MS, Iannettoni MD, Strieter RM. The CXC chemokine, monokine induced by interferon-gamma, inhibits non-small cell lung carcinoma tumor growth and metastasis. Hum Gene Ther. 2000;11:247–261. doi: 10.1089/10430340050015996. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka T, Manome Y, Wen P, Kufe DW, Fine HA. Viral vector-mediated transduction of a modified platelet factor 4 cDNA inhibits angiogenesis and tumor growth. Nat Med. 1997;3:437–442. doi: 10.1038/nm0497-437. [DOI] [PubMed] [Google Scholar]
- 32.Yamanaka R, Tanaka R, Yoshida S, Saitoh T, Fujita K. Growth inhibition of human glioma cells modulated by retrovirus gene transfection with antisense IL-8. J Neurooncol. 1995;25:59–65. doi: 10.1007/BF01054723. [DOI] [PubMed] [Google Scholar]
- 33.Hirose K, Hakozaki M, Nyunoya Y, Kobayashi Y, Matsushita K, Takenouchi T, Mikata A, Mukaida N, Matsushima K. Chemokine gene transfection into tumour cells reduced tumorigenicity in nude mice in association with neutrophilic infiltration. Br J Cancer. 1995;72:708–714. doi: 10.1038/bjc.1995.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia DJ, Zhang WP, Zheng S, Wang J, Pan JP, Wang Q, Zhang LH, Hamada H, Cao X. Lymphotactin cotransfection enhances the therapeutic efficacy of dendritic cells genetically modified with melanoma antigen gp100. Gene Ther. 2002;9:592–601. doi: 10.1038/sj.gt.3301694. [DOI] [PubMed] [Google Scholar]
- 35.Emtage PC, Wan Y, Hitt M, Graham FL, Muller WJ, Zlotnik A, Gauldie J. Adenoviral vectors expressing lymphotactin and interleukin 2 or lymphotactin and interleukin 12 synergize to facilitate tumor regression in murine breast cancer models. Hum Gene Ther. 1999;10:697–709. doi: 10.1089/10430349950018463. [DOI] [PubMed] [Google Scholar]
- 36.Huang H, Li F, Gordon JR, Xiang J. Synergistic enhancement of antitumor immunity with adoptively transferred tumor-specific CD4+ and CD8+ T cells and intratumoral lymphotactin transgene expression. Cancer Res. 2002;62:2043–2051. [PubMed] [Google Scholar]
- 37.Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Monocyte chemotactic proteins MCP-1, MCP-2, and MCP-3 are major attractants for human CD4+ and CD8+ T lymphocytes. FASEB J. 1994;8:1055–1060. doi: 10.1096/fasebj.8.13.7926371. [DOI] [PubMed] [Google Scholar]
- 38.Taub DD, Proost P, Murphy WJ, Anver M, Longo DL, van Damme J, Oppenheim JJ. Monocyte chemotactic protein-1 (MCP-1), -2, and -3 are chemotactic for human T lymphocytes. J Clin Invest. 1995;95:1370–1376. doi: 10.1172/JCI117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allavena P, Bianchi G, Zhou D, van Damme J, Jílek P, Sozzani S, Mantovani A. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. Eur J Immunol. 1994;24:3233–3236. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- 40.Dahinden CA, Geiser T, Brunner T, von Tscharner V, Caput D, Ferrara P, Minty A, Baggiolini M. Monocyte chemotactic protein 3 is a most effective basophil- and eosinophil-activating chemokine. J Exp Med. 1994;179:751–756. doi: 10.1084/jem.179.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sozzani S, Sallusto F, Luini W, Zhou D, Piemonti L, Allavena P, Van Damme J, Valitutti S, Lanzavecchia A, Mantovani A. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J Immunol. 1995;155:3292–3295. [PubMed] [Google Scholar]
- 42.Vecchi A, Massimiliano L, Ramponi S, Luini W, Bernasconi S, Bonecchi R, Allavena P, Parmentier M, Mantovani A, Sozzani S. Differential responsiveness to constitutive vs. inducible chemokines of immature and mature mouse dendritic cells. J Leukoc Biol. 1999;66:489–494. doi: 10.1002/jlb.66.3.489. [DOI] [PubMed] [Google Scholar]
- 43.Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol. 1996;60:365–371. doi: 10.1002/jlb.60.3.365. [DOI] [PubMed] [Google Scholar]


