Abstract
AIM: To investigate the prevalence of hepatitis G virus (HGV) infection and to analyse the homology of different HGV strains in Southern China.
METHODS: A total of 1993 sera from different groups in Guangdong, Hong Kong, and Yunnan were detected by reverse transcription polymerase chain reaction (RT-PCR). The nucleotide sequences of 5’untranslated region (5’UTR) derived from 20 strains and NS5 region from 3 strains were determined.
RESULTS: The positive rate of HGV RNA was 0.89% in community population, 2.57% in blood donors, 17.86% in intravenous drug abusers, 14.13% in patients with hemodialysis, 13.66% in those with hepatocellular carcinoma, 25.30% in non A-E hepatitis, 7.22% in hepatitis B, 12.73% in hepatitis C, 41.67% in patients received bone marrow transplantation, respectively. The homology was 90.40%-100% in 5’UTR among different strains, while that of NS5 region was 93.3%-94% in nucleotide sequence, and 97%-99.2% in amino acid sequence.
CONCLUSION: These results showed that there was a high incidence of HGV infection in patients from Southern China, being treated for bone marrow transplantation, hepatocellular carcinoma and those on haemodialysis. Furthermore, there was also a high frequency of co-infection of HGV with HBV, HCV, non A-E viral hepatitis and that among intravenous drug abusers. The study also showed that sequence variation in different strains was associated with geographical factors but there was no significant difference in 5’UTR in circulating viruses between different patient groups. Finally, by sequential analysis of viral species present in individual patients over a three months period there was no evidence of sequence variation in the 5' UTR.
INTRODUCTION
Recently, a novel RNA virus of the Flaviviridae family has been identified by two groups of researchers working independently and designated as GBV-C or HGV[1,2]. These viruses are now considered to be different isolates of the same virus. The predominant route of transmission of HGV appears to be parenteral by contaminated blood and blood products although other routes, such as vertical transmission or through saliva may also exist[3-14]. HGV contains a positive-sense, single stranded RNA genome approximately 9.4 kb in length that encodes a single long open reading frame (ORF) coding for two putative envelope proteins (E1 and E2), and several nonstructural proteins (NS1-NS5) (Figure 1). The coding region is flanked by a long 5’-untranslated region (5’UTR) and 3’-untranslated region (3’UTR). The putative core protein which has been described in related viruses such as HCV appears to be truncated or even absent in different isolates of HGV[15]. 1%-3% of healthy blood donors are infected with HGV in the USA and in Europe and HGV infection was found to be common in subjects with various forms of chronic liver disease[16,17]. It has been reported that HGV can be associated with either acute or persistent infection[18,19]. Some studies suggested the possibility of a link between fulminant hepatitis and HGV infection[20-22]. However, the clinical implications of HGV infection have not been clearly determined since the vast majority of infected individuals do not show liver injury[23-28]. In this report, HGV prevalence in Southern China was investigated using an RT-PCR-based survey of different patient populations. Furthermore, sequence analysis of some PCR products from the 5’UTR and NS5A regions allowed homology comparisons to be carried out for epidemiological analysis.
Figure 1.

Structure of HGV genome
MATERIALS AND METHODS
Subjects
From 1994 to 1997, 1993 serum samples from 1991 subjects were obtained from Guangdong province, Yunnan province, and Hong Kong Special Administrative Region in Southern China. These serum samples were stored at -70 °C. Hepatitis C virus infection was confirmed by RT-PCR and enzyme-linked immunosorbent assay (ELISA, second-generation). Commercially available ELISAs were used for immunoglobulin M (IgM) antibodies to hepatitis A virus, for hepatitis B surface antigen (HBsAg) and antibodies to hepatitis B core antigen (HBcAb), hepatitis D virus and hepatitis E virus. Patients with hepatocellular carcinoma were histologically confirmed.
In 2 patients serial serum samples were collected 3 months after the first sample. In addition, 22 different samples derived from 20 subjects were analysed by nucleotide sequencing of either PCR fragments from the 5’UTR (19 patients), the NS5A region (2 patients) or both (1patient) (Table 1).
Table 1.
Samples for sequencing
| Sample | Sex | Age (yrs) | Origin | Diagnosis | Regions for sequencing |
| GD1 | F | 58 | Guangdong | non A-E hepatitis | 5’UTR |
| GD3027 | M | 47 | Guangdong | hepatitis B | 5’UTR, NS5A |
| GD3040 | M | 76 | Guangdong | hepatitis B | 5’UTR |
| GD3064 | F | 20 | Guangdong | non A-E hepatitis | 5’UTR |
| GDCA | M | 26 | Guangdong | hepatocellular carcinoma | 5’UTR |
| YN1 | M | 26 | Yunnan | intravenous drug user | 5’UTR |
| YN2 | M | 38 | Yunnan | intravenous drug user | 5’UTR |
| YN3 | M | 45 | Yunnan | intravenous drug user | 5’UTR |
| HKC9 | F | 42 | Hong Kong | hepatitis C | 5’UTR |
| HKC16 | M | 38 | Hong Kong | hepatitis C | 5’UTR |
| HK8 | M | 56 | Hong Kong | recipient of bone marrow | 5’UTR |
| HK9 | M | 42 | Hong Kong | recipient of bone marrow | 5’UTR |
| HK10 | M | 38 | Hong Kong | recipient of bone marrow | 5’UTR |
| HK11 | M | 42 | Hong Kong | recipient of bone marrow | 5’UTR |
| HK12 | M | 42 | Hong Kong | recipient of bone marrow | 5’UTR |
| HK24 | F | 42 | Hong Kong | recipient of bone marrow | 5’UTR |
| HK80 | M | 40 | Hong Kong | recipient of bone marrow | 5’UTR |
| HK108 | M | 18 | Hong Kong | recipient of bone marrow | 5’UTR |
| HK116 | M | 42 | Hong Kong | recipient of bone marrow | 5’UTR |
| HK120 | M | 40 | Hong Kong | recipient of bone marrow | 5’UTR |
| A132 | M | 20 | Hong Kong | blood donor | NS5A |
| A711 | M | 25 | Hong Kong | blood donor | NS5A |
HK9 and HK12 were derived from the same patient after a 3-month interval; HK11 and HK116 were also from a patient after a 3-month interval
Detection of HGV RNA by reverse transcriptase-polymerase chain reaction
RNA was extracted from 100 μL of serum using a modification of the enzyme digestion and heat-denaturation method. The serum was added to 10 μL enzyme digestive mixture (Tris 10 mM pH 7.8, EDTA 5 mM, proteinase K 300 μg/mL) at 55 °C for 30 min and then 98 °C for 15 min. After centrifugation, 5 μL of supernatant were used for the synthesis of cDNA. The synthesis was performed at 42 °C for 45 min with 2.5U AMV reverse transcriptase (Promega) in a 10 μL reaction mixture containing 1 × buffer,0.25 mM dNTPs,8U RNAsin (Promega) and 100 ng specific antisense external primer (G2 or 36) (Table 2).
Table 2.
Primers used in RT-PCR
| Primer | Polarity | Position | Nucleotide sequence |
| G1 | + | 117-136 | 5’ ATGCGTGATGACAGGGTTGG 3’ |
| G2 | - | 451-471 | 5’ TAGGTGGCCCCATGCATTTCC 3’ |
| G3 | + | 161-180 | 5’ GGTAGCCACTATAGGTGGGT 3’ |
| G4 | - | 379-398 | 5’ CACTGGTCCTTGTCAACTCG 3’ |
| 33 | + | 6672-6697 | 5’ GTTGAATTCGCGATGGAGCGCTACAC 3’ |
| 34 | - | 7267-7292 | 5’ CTGGGATCCGTATCATGTATGGTTCT 3’ |
| 35 | + | 6573-6592 | 5’ TCGATTGCTGTAGCTGAGCC 3’ |
| 36 | - | 7327-7346 | 5’ GGTAAGTTCATTGCCCACCA 3’ |
The numbering is identical to that of the PNF2161 strain of HGV[1].
PCR amplification was performed using primers specific for 5’UTR and NS5A region (Table 2). The first-round PCR amplification of the cDNA was carried out in 20 μL of reaction mixture containing 5 μL of cDNA product, 1 × PCR buffer (Promega), 1.5 mM MgCl2, 100 pmoles of each sense and antisense external primers (G1 and G2, or 35 and 36), 20 μM each dNTP and 2U Taq DNA polymerase (Promega). PCR was performed for 30 cycles with the following reaction cycle: 94 °C for 40 secs, 55 °C for 40secs, 72 °C for 60secs (2 min for NS5A amplification). 5 μL of the first PCR product were subjected to a second amplification for 35 cycles under the same condition as for the first PCR, using sense and antisense inner primers (G3 and G4, or 33 and 34). The amplified products were visualized by 2% agarose gel electrophoresis and ethidium bromide staining.
Cloning of HGV NS5A PCR fragments
The PCR products of HGV NS5A were purified by phenol-chloroform extraction, precipitated by ethanol, dissolved in water and digested with EcoRI and BamHI (Boehringer Mannheim). The fragments were recovered from low melting point agarose gels and cloned into vector pUC19. Resulting recombinants were identified by enzyme-digestion and the same NS5A-specific second PCR procedure as that described above.
Sequence determination and homology analysis
The 238-base pair amplification products of HGV 5’UTR were directly sequenced by HGV 5’UTR-specific inner primers (G3 and G4), while the NS5A clones were sequenced using pUC19-specific primers hybridizing to sequences flanking the cloned fragment. The sequencing was performed using a double-strand DNA cycle sequencing system by the dye termination method in a ABI 310 automated DNA sequencer (Applied Biosystems Inc.). Nucleotides obtained and putative amino acid sequences were then compared with each other and with the published HGV prototype sequences by program DNASIS, program PROSIS and program Lasergene.
RESULTS
Prevalence of HGV in Southern China
Statistical analyses were performed using Fisher’s exact tests and a significance level was set at P = 0.05. The prevalence of HGV infection from different groups in Southern China was shown in Table 3.
Table 3.
Prevalence of HGV infection from different groups in Southern China
| Group | cases | HGV RNA Positive cases(%) |
| Bone marrow transplantation | 108 | 45(41.67) |
| Haemodialysis | 92 | 13(14.13) |
| Intravenous drug users | 84 | 15(17.86) |
| Hepatocellular carcinoma | 161 | 22(13.66) |
| Hepatitis B | 263 | 19(7.22) |
| Hepatitis C | 55 | 7(12.73) |
| Hepatitis B + Hepatitis A | 16 | 1(6.25) |
| Hepatitis B + Hepatitis C | 6 | 1(16.67) |
| Hepatitis B + Hepatitis E | 26 | 3(11.54) |
| Hepatitis E | 29 | 2(6.90) |
| Non A-E hepatitis | 83 | 21(25.30) |
| Blood donors | 506 | 13(2.57) |
| General population | 562 | 5(0.89) |
| Total | 1991 |
The infectious rate of HGV in general population from Southern China is 0.89%. The following groups were highly significantly different from both the blood donors and general population group: recipient of bone marrow transplantation, haemodialysis patients, intravenous drug abusers, patients with hepatocellular carcinoma, non A-E hepatitis, hepatitis B (P < 0.0001), and hepatitis C (P < 0.002). Patients with bone marrow transplantation had the highest carrier rate of 41%, which was significantly higher than the other groups (P < 0.05). The following groups were not significantly different from the blood donors or general population group: hepatitis B + A, hepatitis B + C, hepatitis B+E, hepatitis E (P > 0.05). There was no significant difference between the blood donor and general population groups (P = 0.054).
Nucleotide sequence of the 5’UTR from 20 HGV isolates
An alignment of the nucleotide sequence of the 5’UTR from the 20 HGV isolates we studied being presented in Figure 2. Sequence analysis demonstrated that the 5’UTR was highly conserved and the homology varied between 90.4% and 100% among different isolates from Southern China. We found that the 5’UTR of HGV consisted of highly conserved domains interspersed between variable domains. The most variable domain spans 38 nucleotides (positions 187 to 222). Molecular evolutionary phylogenetic tree was constructed to clarify the relationship among different HGV strains, using Clustal method with Weighted residue weight table.
Figure 2.

Alignment of 5’UTR cDNA sequences of 20 HGV isolates from Southern China. GD stands for Guangdong, YN for Yunnan, HK for Hong Kong. C964 was derived from Northern China, PNF2161 and R10291 from United States[1], GBV-C from West Africa[2]. Identical nucleotides are shown as dots. The numbering is according to Linnen et al[1].
Some highly conserved regions were observed, including No.223 to No.298, No.327 to No.345, No.349 to No.372, but relative diversity regions were also observed, such as the regions from No.187 to No.222, No.299 to No.306. Some isolates had 100% identical sequence in 5’UTR, such as GD3040, GD3064 from Guangdong and HKC16, HK9, HK12, HK108 from Hong Kong.
Geographical variability was also demonstrated: greater homology was seen in strains obtained from Southern China, the homology was from 95% to 100% among Guangdong strains, 93% to 100% within Hong Kong strains, 94% to 100% between Guangdong and Hong Kong strains. While a lower level of homology compared to the strain from Northern China, which was 90% to 94% between Southern China and Northern China strains, with even lower homology when compared with those from other countries, which was 85% to 92% between Southern China and the reported prototype strains from USA and West Africa.
We also studied the HGV 5’UTR sequence in the same patients over a period of 3 months and observed no mutation, as shown in Figure 2. HK9 and HK12 were from one patient, HK11 and HK116 from another one.
Nucleotide sequence of partial NS5A region from 3 HGV isolates
Similar to 5’UTR, 621 base pair of NS5A region from 3 HGV isolates in Southern China are well conserved with the homology of 93.3%-94.0% (Figure 3). While a lower homology was seen when compared to those in USA and West Africa, which was from 87.3% to 93.5%. Different nucleotides were randomly distributed throughout the sequences and most mutations were silent, with the result of deduced amino acid homology of 97.9%-98.9% (Figure 4). The study also suggests that the variation may be associated with geographical factor.
Figure 3.

Comparison of NS5 cDNA sequence of HGV A132 (HongKong strain) with the corresponding sequences from GD3027 (Guangdong strain), A711 (Hong Kong strain) and the reported isolates (C964, PNF2161, R10291, GBV-C). Dots indicate identity with sequence of HGV A132.
Figure 4.
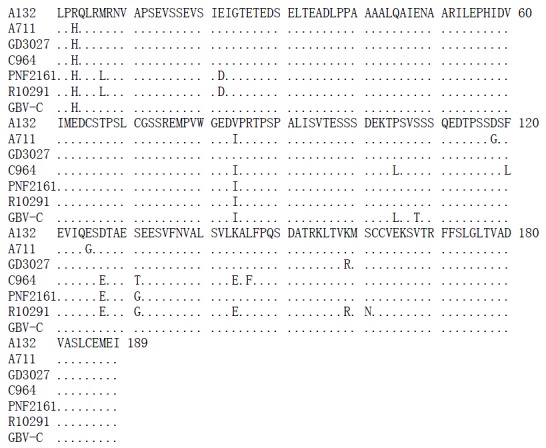
Comparison of deduced amino acid sequence of HGV A132 NS5 with the corresponding sequences from A711, GD3027 strains and C964, PNF2161, R10291, GBV-C isolates.
DISCUSSION
We studied 1993 sera from 1991 patients with various forms of viral hepatitis, hepatocellular carcinoma, intravenous drug abusers, recipients of bone-marrow transplantation, blood donors and general population from 3 different regions in Southern China, including Guangdong, Hong Kong and Yunnan. HGV RNA was detected in as high as 41.67% (45/108), 14.13% (13/92), 17.86% (15/84), 13.66% (22/161), 12.73% (7/55), 25.30% (21/83) of patients with bone-marrow transplantation, hemodialysis, intravenous drug abusers, hepatocellular carcinoma, hepatitis C and non A-E hepatitis, respectively. It is noteworthy that nearly 42% of recipients of bone-marrow transplantation were HGV RNA positive. This is understandable, because these patients have usually received many transfusions or blood products from a large number of donors. This also demonstrated the fact, that HGV was transmissible via blood transfusion[29].
13 (2.57%) out of 506 blood donors were positive for HGV RNA, the difference of the positive rate from the general population (0.89%) was statistically not significant. In addition, HGV RNA was found to be 7.22% (19/263) among patients with hepatitis B, 6.90% (2/29) among patients with hepatitis E, 6.25% (1/16) among patients with coinfection of hepatitis B and hepatitis A, 16.67% (1/6) among coinfection of hepatitis B and hepatitis C, and 11.54% (3/26) among coinfection of hepatitis B and hepatitis E. Using Fisher’s exact tests to work out the exact P-values in situations where the number of positive cases was less than 5, the P-values obtained were dependent on the magnitude of the difference in prevalence of HGV infection between those groups, but also on the total number of cases in each group. Hence, although the prevalence of HGV infection was 16.7% in hepatitis B+C group, we were not able to show it was different from either blood donors or the general population because the number was small (only 6 cases) in hepatitis B + C group. Same situations occurred in groups of hepatitis B + A, hepatitis B+E, and hepatitis E. However, the difference between hepatitis B and the general population was significant even though the prevalence of HGV infection was only 7.22% because of large number of cases (263 cases) in hepatitis B group.
In this study we determined the nucleotide sequence of the 5’UTR spanned 238 nucleotides with positions 161 to 398 from 20 HGV isolates in Southern China. The data confirmed that the 5’UTR of the HGV genome was well conserved among HGV isolates from Southern China, with homology more than 90.4%, even 100% identical sequence in some strains, such as GD3040, GD3064, HKC16, HK9 and HK108. Phylogenetic analysis here supported the conclusion that sequence variation in different HGV strains was associated with geographical factors because greater homology was seen between the Southern China strains, while a lower homology when compared to the strains from Northern China, even lower homology was observed in comparison with those from other countries[30,31].
Partial sequences of NS5A gene region flanking positions 6672 to 7292 from 3 HGV strains were also determined. Nucleotide variations from each other were seen at 6.3% to 7.1% scattered along the genome and most of them had not caused change in amino acid, which implied that this region might have a biological role.
Sequencing samples for 5’UTR were derived from different patient groups, including non A-E hepatitis, hepatitis B, hepatitis C, hepatocellular carcinoma, intravenous drug abusers, recipient of bone marrow transplantation and blood donor. However, no apparent variation in 5’UTR was found between different groups in Southern China, which suggested that the heterogeneity among different HGV isolates was not related to patient groups in the same geographical area.
HK9 and HK12 were from one patient over a period of three months, HK11 and HK116 from another patient in the same interval. The follow-up study of HGV 5’UTR sequence changes in the two patients with bone-marrow transplantation from Hong Kong suggested that the 5’UTR was not susceptible to mutations as frequently observed in other RNA viruses during a 3-month interval. However, this is only within a relatively short period and whether it has the potential to mutate over a longer period remains to be seen.
Footnotes
Supported by the National Natural Science Foundation of China, NO. 39600130 and the grant from the Department of Health of Guangdong Province.
Edited by Wu XN
References
- 1.Linnen J, Wages J, Zhang-Keck ZY, Fry KE, Krawczynski KZ, Alter H, Koonin E, Gallagher M, Alter M, Hadziyannis S, et al. Molecular cloning and disease association of hepatitis G virus: a transfusion-transmissible agent. Science. 1996;271:505–508. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- 2.Leary TP, Muerhoff AS, Simons JN, Pilot-Matias TJ, Erker JC, Chalmers ML, Schlauder GG, Dawson GJ, Desai SM, Mushahwar IK. Sequence and genomic organization of GBV-C: a novel member of the flaviviridae associated with human non-A-E hepatitis. J Med Virol. 1996;48:60–67. doi: 10.1002/(SICI)1096-9071(199601)48:1<60::AID-JMV10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 3.Lefrère JJ, Roudot-Thoraval F, Morand-Joubert L, Brossard Y, Parnet-Mathieu F, Mariotti M, Agis F, Rouet G, Lerable J, Lefèvre G, et al. Prevalence of GB virus type C/hepatitis G virus RNA and of anti-E2 in individuals at high or low risk for blood-borne or sexually transmitted viruses: evidence of sexual and parenteral transmission. Transfusion. 1999;39:83–94. doi: 10.1046/j.1537-2995.1999.39199116899.x. [DOI] [PubMed] [Google Scholar]
- 4.Lefrère JJ, Sender A, Mercier B, Mariotti M, Pernot F, Soulié JC, Malvoisin A, Berry M, Gabai A, Lattes F, et al. High rate of GB virus type C/HGV transmission from mother to infant: possible implications for the prevalence of infection in blood donors. Transfusion. 2000;40:602–607. doi: 10.1046/j.1537-2995.2000.40050602.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen M, Sönnerborg A, Johansson B, Sällberg M. Detection of hepatitis G virus (GB virus C) RNA in human saliva. J Clin Microbiol. 1997;35:973–975. doi: 10.1128/jcm.35.4.973-975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seemayer CA, Viazov S, Philipp T, Roggendorf M. Detection of GBV-C/HGV RNA in saliva and serum, but not in urine of infected patients. Infection. 1998;26:39–41. doi: 10.1007/BF02768751. [DOI] [PubMed] [Google Scholar]
- 7.Ohto H, Ujiie N, Sato A, Okamoto H, Mayumi M. Mother-to-infant transmission of GB virus type C/HGV. Transfusion. 2000;40:725–730. doi: 10.1046/j.1537-2995.2000.40060725.x. [DOI] [PubMed] [Google Scholar]
- 8.Fischler B, Lara C, Chen M, Sönnerborg A, Nemeth A, Sällberg M. Genetic evidence for mother-to-infant transmission of hepatitis G virus. J Infect Dis. 1997;176:281–285. doi: 10.1086/517267. [DOI] [PubMed] [Google Scholar]
- 9.Tian DY, Yang DF, Xia NS, Zhang ZG, Lei HB, Huang YC. The serological prevalence and risk factor analysis of hepatitis G virus infection in Hubei Province of China. World J Gastroenterol. 2000;6:585–587. doi: 10.3748/wjg.v6.i4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong RX, Luo HT, Zhang RX, Li GR, Lu L. Investigation on infection of hepatitis G virus in 105 cases of drug abusers. World J Gastroenterol. 2000;6(Suppl 3):63. [Google Scholar]
- 11.Bourlet T, Berthelot P, Grattard F, Genin C, Lucht FR, Pozzetto B. Detection of GB virus C/hepatitis G virus in semen and saliva of HIV type-1 infected men. Clin Microbiol Infect. 2002;8:352–357. doi: 10.1046/j.1469-0691.2002.00425.x. [DOI] [PubMed] [Google Scholar]
- 12.Frey SE, Homan SM, Sokol-Anderson M, Cayco MT, Cortorreal P, Musial CE, Di Bisceglie A. Evidence for probable sexual transmission of the hepatitis g virus. Clin Infect Dis. 2002;34:1033–1038. doi: 10.1086/339206. [DOI] [PubMed] [Google Scholar]
- 13.Clevenberg P, Durant J, Halfon P, Tran A, Manos T, Rahelinirina V, Yang G, Benzaken S, Ouzan D, Rampal P, et al. High prevalence of GB virus C/hepatitis G virus infection in different risk groups of HIV-infected patients. Clin Microbiol Infect. 1998;4:644–647. doi: 10.1111/j.1469-0691.1998.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 14.Eugenia QR, Ana QR, Carmen M. Investigation of saliva, faeces, urine or semen samples for the presence of GBV-C RNA. Eur J Epidemiol. 2001;17:271–274. doi: 10.1023/a:1017916506897. [DOI] [PubMed] [Google Scholar]
- 15.Katayama K, Kageyama T, Fukushi S, Hoshino FB, Kurihara C, Ishiyama N, Okamura H, Oya A. Full-length GBV-C/HGV genomes from nine Japanese isolates: characterization by comparative analyses. Arch Virol. 1998;143:1063–1075. doi: 10.1007/s007050050356. [DOI] [PubMed] [Google Scholar]
- 16.Marrone A, Shih JW, Nakatsuji Y, Alter HJ, Lau D, Vergalla J, Hoofnagle JH. Serum hepatitis G virus RNA in patients with chronic viral hepatitis. Am J Gastroenterol. 1997;92:1992–1996. [PubMed] [Google Scholar]
- 17.Zhong RX, Luo HT, Zhang RX, Li GR, Lu L. Investigation on infection of hepatitis G virus in 105 cases of drug abusers. World J Gastroenterol. 2000;6(Suppl 3):63. [Google Scholar]
- 18.Yashina TL, Favorov MO, Khudyakov YE, Fields HA, Znoiko OO, Shkurko TV, Bonafonte T, Sevall JS, Agopian MS, Peter JB. Detection of hepatitis G virus (HGV) RNA: clinical characteristics of acute HGV infection. J Infect Dis. 1997;175:1302–1307. doi: 10.1086/516460. [DOI] [PubMed] [Google Scholar]
- 19.Ling BH, Zhuang H, Cui YH, An WF, Li ZJ, Wang SP, Zhu WF. A cross-sectional study on HGV infection in a rural population. World J Gastroenterol. 1998;4:489–492. doi: 10.3748/wjg.v4.i6.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moaven LD, Locarnini SA, Bowden DS, Kim JP, Breschkin A, McCaw R, Yun A, Wages J, Jones B, Angus P. Hepatitis G virus and fulminant hepatic failure: evidence for transfusion-related infection. J Hepatol. 1997;27:613–619. doi: 10.1016/s0168-8278(97)80077-3. [DOI] [PubMed] [Google Scholar]
- 21.Sheng L, Soumillion A, Beckers N, Wu CG, Verslype C, Nevens F, Pirenne J, Aerts R, Kosala H, Fevery J, et al. Hepatitis G virus infection in acute fulminant hepatitis: prevalence of HGV infection and sequence analysis of a specific viral strain. J Viral Hepat. 1998;5:301–306. doi: 10.1046/j.1365-2893.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- 22.Anastassopoulou CG, Delladetsima JK, Anagnostopoulos G, Katsoulidou A, Papachristopoulos A, Tassopoulos NC, Theodoridou M, Hatzakis A. Fulminant hepatic failure in a pediatric patient with active GB virus C (GBV-C)/hepatitis G virus (HGV) infection. Hepatol Res. 2002;23:85–89. doi: 10.1016/s1386-6346(01)00166-8. [DOI] [PubMed] [Google Scholar]
- 23.Kao J, Chen D. GB virus-C/hepatitis G virus infection in Taiwan: a virus that fails to cause a disease. J Biomed Sci. 1999;6:220–225. doi: 10.1007/BF02253563. [DOI] [PubMed] [Google Scholar]
- 24.Yu JG, Hou XR, Pan W, Zhang GS, Zhou XM. PCR detection of hepatitis G virus RNA in sera and liver tissues from patients with chronic hepatitis C. Shijie Huaren Xiaohua Zazhi. 1998;6:580. [Google Scholar]
- 25.Zhao XP, Yang DL, Wang BJ, Yang Y, Shen HX, Peng ZH, Hao LJ. Immunohistochemical study of HGV expression in liver of patients with hepatitis G. Shijie Huaren Xiaohua Zazhi. 1998;6:586. [Google Scholar]
- 26.Xu JZ, Yang ZG, Le MZ, Wang MR, He CL, Sui YH. A study on pathogenicity of hepatitis G virus. World J Gastroenterol. 2001;7:547–550. doi: 10.3748/wjg.v7.i4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reshetnyak VI, Sharafanova TI, Ilchenko LU, Golovanova EV, Poroshenko GG. Peripheral blood lymphocytes DNA in patients with chronic liver diseases. World J Gastroenterol. 2001;7:235–237. doi: 10.3748/wjg.v7.i2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren H, Zhu FL, Zhu SY, Song Y, Qi ZT. Immunogenicity of HGV NS5 protein expressed from Sf9 insect cells. World J Gastroenterol. 2001;7:98–101. doi: 10.3748/wjg.v7.i1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan J, Chen LL, Luo YH, Mao YF, He M. High frequencies of HGV and TTV infections in blood donors in Hangzhou. World J Gastroenterol. 2001;7:637–641. doi: 10.3748/wjg.v7.i5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang XT, Zhuang H, Song HB, Li HM, Zhang HY, Yu Y. Partial sequencing of 5' noncoding region of 7 HGV strains isolated from different areas of China. World J Gastroenterol. 1999;5:432–434. doi: 10.3748/wjg.v5.i5.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling B, Zhuang H, Wang L, Zhao H, Zhang S. [Analysis of hepatitis G virus genotypes in some areas of China] Zhonghua Yufang Yixue Zazhi. 2000;34:354–357. [PubMed] [Google Scholar]


