Abstract
AIM: To purify and identify heme oxygenase (HO) isomers which exist in rat liver, spleen and brain treated with hematin and phenylhydrazine and in untreated rat liver and to investigate the characteristics of HO isomers, to isolate and confirm the rat HO-1 cDNA that actually encodes HO-1 by expressing cDNA in monkey kidney cells (COS-1 cells), to prepare the rat heme oxygenase-1 (HO-1) mutant and to detect inhibition of HO-1 mutated enzyme.
METHODS: First, rat liver, spleen and brain microsomal fractions were purified by DEAE-Sephacel and hydroxylapatite. The characteristics including activity, immunity and inducibility of two isomers (HO-1 and HO-2), and their apparent molecular weight were measured by detecting enzymatic activities, SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting analysis, respectively. Second, plasmid pcDNA3HO1 containing native rat HO-1 cDNA and pcDNA3HO1D25 carrying mutated rat HO-1 cDNA (His25Ala) were constructed by site-directed mutagenesis. COS-1 cells transfected with pcDNA3HO1 and pcDNA3HO1D25 were collected and disrupted by sonication, the microsomes were prepared by ultracentrifugation. Third, the inhibition of rat HO-1 mutant was analyzed.
RESULTS: Two isomers were purified and identified in treated rat liver, spleen, brain and untreated rat liver. HO-1 was the predominant form with a ratio of 2.0:1 and 3.2:1 of HO-1 and HO-2 in liver and spleen, respectively, but only the activity of HO-2 in the brain and untreated liver could be detected. The apparent molecular weights of HO-1 and HO-2 were about Mr 30000 and Mr 36000 under reducing conditions, respectively. The antiserum against liver HO-2 was employed in Western blotting analysis, the reactivity of HO-1 in the liver was not observed. The plasmid pcDNA3HO1 was highly expressed in endoplasmic reticulum of transfected COS-1 cells. The specific activity was ≈5-fold higher than that of the control. However, the enzyme activity of mutated HO-1 declined. While an equal amount of mutant was added to the enzyme reaction system, the levels of bilirubin decreased 42%.
CONCLUSION: The studies suggest that HO-1 and HO-2 exist in the hematin and phenylhydrazine treated rat liver and spleen, but only HO-2 in the brain and untreated liver. Two constitutive forms are different in molecular weight, inducibility and immunochemical properties. The activity of expressed HO-1 in COS-1 cells is higher than that of purified enzyme from rat spleen tissue. It suggests that this clone has an insert of 1030 base-pairs encodes HO-1. His25Ala mutant reduced the formation of bilirubin and it suggests that the mutant could competely bind the heme with native enzyme.
INTRODUCTION
Cellular levels of heme are regulated by the rates of its synthesis and degradation. Heme catabolism occurs by oxidative cleavage of the -methene bridge of the tetrapyrrole, eventually leading to the formation of equimolar amounts of biliverdin and the release of contained iron atom. Biliverdin is then rapidly reduced to form bilirubin. The heme oxygenase (HO) system controls the rate-limiting step in heme catabolism. Three isomers (HO-1, HO-2 and HO-3) have been identified that catalyze this reaction. HO-1 is a Mr 30000 that is inducible by heme and other numerous stimuli being the predominant form in liver and spleen. HO-2 is a constitutively synthesized Mr 36000 protein that is abundant in brain and testis. HO-3 is a Mr 33000 protein that, so far, has only been expressed constitutively in rat neurones. They are the products of different genes[1-32].
Overexpression of HO-1 gene can lead to hyperbilirubinemia in human with certain hepatic disorder, especially patients in whom bilirubin disposition is impaired for developmental or genetic reasons, i.e., in newborns and in patients with Crigler-Najjar type I syndrome. So, the characterization of the two constitutive forms and the expression of HO-1 gene would have considerable experimental value in elucidating the role of the enzyme in physiological and pathological processes and finally in effective prevention and treatment of hyperbilirubinemia.
The objective of this study was to purify and identify HO isomers (HO-1 and HO-2) in treated rat liver, spleen, brain and untreated rat liver, to construct the expression plasmid pcDNA3HO1 carrying HO-1 cDNA and to confirm that the cDNA actually encodes HO-1 by expressing cDNA in COS-1 cells. On the basis of these findings, we have expected the substitution of histindine residues essential for heme binding would eliminate HO activity, so site-directed mutagenesis was applied to construct mutated rat HO-1 cDNA (Histindine25→Alanine, His25Ala), then the activity of mutant was detected and compared with that of native enzyme. The inhibition of this mutant was also examined by determining the levels of bilirubin in the enzymatic reaction mixture.
MATERIALS AND METHODS
Preparation of tissue
The rats (purchased from Shanghai Institute of Cell Biology, Academia Sinica) were allowed to food and water ad libitum and were injected with 40 μmol/kg hematin (pH 7.4), intraperitoneally; 100 mg/kg phenylhydrazine, intravenously. The animals were killed 20 h after treatment.
Purification and identification of HO-1 and HO-2
All operations were carried out below 4 °C. The rat liver, spleen and brain tissues were added 2 volumes of 0.25 mol/L sucrose solution containing 0.1 mmol/L EDTA. The mixture was homogenized and centrifuged at 9000 g for 5 min. The supernatant fractions were collected and subsequently centrifuged at 150000 g for 1 h. The microsomal pellet was treated according to the method described by Maines et al[30] The solubilized microsomes were diluted with 1 volume of 0.05 mmol/L DTT solution, with pH adjusted to 8 and loaded onto DEAE-Sephacel column (2.5 × 13.6 cm) that was previously equilibrated with 20 mmol/L Tris-HCl buffer (pH 7.5) containing 0.05 mmol/L EDTA, 0.5% TritonX-100, 0.1% sodium cholate and 0.05 mmol/L DTT. The column was eluted with concurrent linear gradients of KCl (0-0.4 mol/L) and TritonX-100 (0.5%-0.9%) prepared with the equilibration buffer. 3-4 mL fractions were collected and analyzed for HO activity.
The pooled DEAE-Sephacel fractions containing the first peak of HO activity and the second peak of HO activity were dialyzed against distilled water for 24 h below 4 °C, and then concentrated and loaded onto a hydroxylapatite column equilibrated with 10 mmol/L potassium phosphated buffer, pH 7.5, respectively. The column was washed with 10 mmol/L potassium phosphate buffer, pH 7.2, containing 0.05 mmol/L EDTA, 0.4% TritonX-100 and 0.1% sodium cholate, followed by the same solution containing 20% glycerol. The column was eluted with a linear gradient of 10-260 mmol/L potassium phosphate in the above buffer. The fractions containing HO activity were collected.
Construction of expression plasmid carrying native rat HO-1 cDNA
The expression vector pcDNA3 was linearized by digestion with BamHI and the single-stranded ends were filled in by treatment with DNA polymerase I (Klenow fragment). The resulting blunt ends were used for ligation with XhoI (-59) / HindIII (971) cDNA fragment isolated from pRHO1, which was provided by Shibahara. Both ends of the fragment were converted to blunt ends before ligation. The plasmid pcDNA3HO1 that encodes native HO-1 was constructed.
Mutagenesis of cDNA for rat HO-1 and construction of pcDNA3HO1D25
The expression vector pcDNA3HO1 was cleaved with XbaI. The region of the template was amplified in polymerase chain reaction. 5’-terminal primers was T7 and 3’-terminal primer was a synthetic oligonucleotide (5’-CTCAGAATTCTCTGCACGGGTGGCCACCTCC-3’). The amplified products were digested by HindIII/EcoRI and the resulting DNA fragments (HindIII→EcoRI, 171 bp) were ligated to pBS KS which had been pretreated with HindIII/EcoRI. The recombinant plasmid (pBS KSHO1-171) was digested with EcoRI and the single stranded ends were filled in by treatment with Klenow frangment followed by dephosphorylation with calf intestinal alkaline phosphatase.
To construct the plasmid pBS KSHO1D25 (4034 bp), the pcDNA3HO1 was digested with EcoRI. The DNA fragments (EcoRI→EcoRI, 932 bp) from pcDNA3HO1 was purified and inserted into EcoRI site of pBS KSHO1-171.
Finally, the vector pBS KSHO1D25 was cut with HindIII and BamHI, and ligated to pcDNA3, which had been also pretreated with Hind III and BamHI, to con stru ct pcDNA3HO1D25 (6525 bp).
Transfection of COS-1 cells
COS-1 cells, which were provided by Shanghai Institute of Cell Biology, Academia Sinica, were maintained in Dulbecco’s modified Eagle’s medium containing 2.5% newborn calf serum. Confluent cells, seeded in 75 mL culture flask, were fed with medium 4 h before addition of plasmid DNA. Plasmid DNA (10 μg per flask) was used to transfect COS-1 cells by the calcium phosphate method. DNA (at 10 μg/mL) was adjusted to 250 mmol/L CaCl2 and added slowly to an equal volume of sterile 2 × HBS with constant agitation. The calcium phosphate-DNA precipitate was allowed to form for 30 min, and 1 mL of precipitate was added to an adequate volume of medium that covered recipient cells. After 4 h of exposure to DNA, the medium was replaced with fresh medium, and the cells were allowed to incubate for an additional 48 h, then cells were collected and stored at-70 °C until assay for HO-1. The cells were disrupted by sonication and pellets (microsomes) were prepared by centrifugation at 105000 g, 0-4 °C, 1 h. The microsomes were suspended in 50 mmol/ L potassium phosphate buffer, pH 7.4/0.1% Triton X-100, subjected to SDS-PAGE to confirm whether the specific band was located in the position of Mr 30000.
Measurement of heme oxygenase isomers and expressed products activity
The activity of HO isomers and expressed products was determined by measuring the bilirubin formation monitored as an absorbance increase at 464 nm. One unit of the enzyme was defined as the amount catalyzing the formation of 1 nmol of bilirubin per 1 h.
SDS-polyacrylamide gel electrophoresis of HO isomers and expressed products
Electrophoretic procedures were performed according to Laemmli’s method in order to identify HO isomers and their molecular weight.
Production of antiserum
The purified rat liver HO-2 fraction mixed with 100 μL of 0.9% NaCl solution and injected intraperitoneally into Kunming mice (10-15 g) purchased from Shanghai Institute of Cell Biology, Academia Sinica. Then these mice were boosted with antigen every second week, for three times. The mice were bled 10 days after the final boost and sera were collected and stored under -20 °C.
Western blotting
Western blotting was performed as follows. Protein samples, rat liver HO-1 and rat brain HO-2 fractions, were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) in 1.5 mm slab gels according to the procedure of Laemmli and the separated poly peptides were then transferred electrophoretically from the gel to a nitrocellulose sheet using a transfer buffer consisting of 48 mmol/L Tris base, 39 mmol/L glycine, 20% (v/v) methanol and 0.07% (w/v) SDS, pH 8.3. Following electroblotting, the nitrocellulose paper was stained with Ponceau S for protein detection.Destaining was performed with distilled water. For immunostaining the nitrocellulose blot was incubated at 37 °C for 1 h with bovine serum albumin in 20.0 mmol/L Tris-HCl buffer, pH 7.5, containing 0.5 mol/L sodium chloride in order to saturate all protein binding sites. The nitrocellulose blot was then incubated with antiserum against rat liver HO-2 at 37 °C for 1 h, and washed several times with peroxidase-anti-mouse IgG diluted with PBS solution. The blot was stained with diaminobenzidine (DAB) solution, washed with distilled water.
RESULTS
Purification and identification of the two forms of HO from different tissues
Two peaks exhibiting HO activity from rat liver and spleen treated with hematin and phenylhydrazine were eluted using DEAE-Sephacel chromatography. The peaks were designated as HO-1 and HO-2 according to the sequence of their elution from column. The pooled DEAE-Sephacel fractions containing HO-1 and HO-2 activities were loaded onto hydroxylapatite column, and the final purified HO-1 and HO-2 products exhibited only a single band when visualized by staining of SDS-PAGE with Coomassie Blue. The values obtained for HO-1 and HO-2 activities were shown in the Figure 1, with the ratio of HO-1 to HO-2 being approximately 2.0:1 and 3.2:1, respectively.
Figure 1.
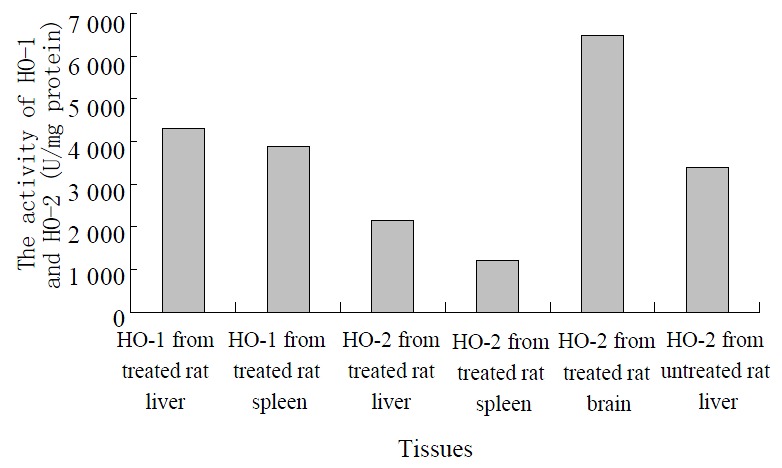
The measurement of HO isomers from different tissues.
The treated rat brain and untreated rat liver microsomal fractions were also solubilized and subjected to ion-exchange chromatography of DEAE-sephacel, respectively. The chromatography elution patterns of HO activity were compared with those of treated rat liver and spleen. Only one peak of HO activity was detected from the treated rat brain and untreated rat liver (Figure 1). An activity peak was not present in the elution at the inducible isomer of HO (HO-1) region.
Characterization of HO-1 and HO-2
Two forms of HO were subjected to electrophoresis, in which two distinct bands exhibiting HO activity were detected, the migration patterns of HO-1 and HO-2 being different. The purified HO-2 on SDS-PAGE displayed a higher monomeric molecular weight. The apparent molecular weight of HO-1 and HO-2 were Mr 30000 and Mr 36000, respectively. The apparent molecular weight in treated brain microsomal preparation was identical to the purified liver and spleen HO-2. The HO-1 activity was increased in response to hematin and phenylhydrazine, while that of HO-2 was fully refractory to these agents. The blot was treated with anti-rat liver HO-2 serum followed by anti-mouse IgG-peroxidase conjugate and then stained for peroxidase activity. As expected, rat brain HO-2 preparation gave a reddish brown band in the region of molecular weight Mr 36000. However, the rat HO-1 preparation failed to show a stained band.
Construction of plasmid pcDNA3HO1 containing rat HO-1 cDNA
The pcDNA3HO1 containing HO-1 cDNA was screened out. The fragments of 916 bp and 5567 bp were observed after digestion of the resulting plasmid with EcoRI indicating that the plasmid had HO-1 cDNA in a correct direction.
Expression of native rat HO-1 cDNA in COS-1 cells
The activity of HO-1 product transiently expressed in COS-1 cells was detected. The results suggested that the specific band was located in the position of Mr 30000, the specific activities of HO-1 in COS-1 cells transfected with plasmid pcDNA3HO1 and plasmid pcDNA3 were 13688 and 2920 units/mg of protein/h, respectively. The activity of expressed enzyme was 4.5-fold, 3.1-fold and 3.5-fold higher in comparison with that of the control transfected with plasmid pcDNA3, purified HO-1 from rat liver and spleen tissue, respectively.
Construction of mutated rat HO-1 cDNA (His25Ala) and assay of its activity
Figure 2 showed the strategy for construction of the expression vector of pcDNA3HO1D25. The histidine-25 residue was replaced with an alanine residue by site-directed mutagenesis. The whole nucleotide sequence of insert of mutant rat HO-1 was confirmed by the dideoxynucleotide chain-termination method. COS-1 cells transfected with pcDNA3HO1D25 were harvested by centrifugation. The activity of mutant rat HO-1 was detected and the value was 1948-2160 units/mg protein per 1 h.
Figure 2.
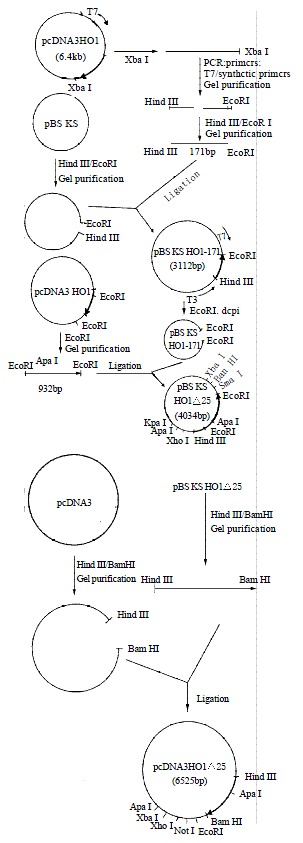
The strategy for construction of the expression vec-tor of pcDNA3HO1△25.
The molecular weight and mobility of native and mutant rat HO-1
The protein concentrations from native and mutant rat HO-1 were 7.38 mg/mL and 7.22 mg/mL, respectively. 10 μL samples were subjected to SDS-PAGE for detecting their molecular weight and mobility. The results revealed that native and mutant rat HO-1 had the same mobility and molecular weight as that of rat liver HO-1 with a molecular mass of about Mr 30000.
The inhibition of mutant to native HO-1
The inhibition of His25Ala mutant was confirmed by detecting the levels of bilirubin. Formation in the reaction system decreased to 42% after addition of the mutant.
DISCUSSION
The oxidation of heme is carried out by three isozymes of microsomal HO. HO-1 is inducible by inflammatory cytokines and oxidants, including nitric oxide (NO), whereas HO-2 and HO-3 are expressed constitutively[31,32]. They are the key enzymes in the catabolic pathway of heme, and the conversion of biliverdin to bilirubin is catalysed by biliverdin reductase[33,34]. So it is highly important to inhibit the activities of HO isomers for reducing the products of bilirubin[35,36], finally to prevent and treat hyperbilirubinemia.
The microsomal HO isomers had been purified in other laboratory. The results showed HO-1, which is inducible and highly expressed in liver and spleen tissues, and HO-2, which is constitutive and distributed throughout the body. HO-3 has only been expressed constitutively in rat neurones[31,32]. The isomers have different molecular masses and are products of distinctly different genes[37]. HO-1 gene from a rat genomic DNA library was isolated using cloned cDNA as hybridization probes and determined its complete nucleotide sequence. The gene is composed of 6 830 nucleotides and consists of four introns and five exons[37]. Preliminary mutagenesis studies on rat HO-1 by Ito-Maki et al[38] have shown that the His25→Ala25 mutation completely abolished the enzyme activity, and histindine has been considered to be an essential residue for the enzyme activity.
In our studies, we have purified and identified two constitutive forms of HO in rat liver, spleen and brain tissues and prov ided evid ences for a decided ly d ifferent characterization for the two forms, HO-1 and HO-2. The results indicate that in the treated rat liver and spleen, there exist two different molecular species of HO. It appears that HO-1 and HO-2 substantially differ in their molecular composition and structure as judged by their chromatographic behavior and electrophoretic migration patterns. Difference in amino acid composition or sequence usually results in such observation. In the induced liver and spleen, the microsomes contain mostly the HO-1, with the ratio of HO-1 being approximately 2.0:1 and 3.2:1, it suggests that the liver and spleen are among the main sites of haemoglobin heme degradation. Hematin and phenylhydrazine are the inducers known for their ability to increase the activity of the microsomal HO-1 in the liver, the activity of which can be induced by up to 100-fold, but the spleen HO-1 activity could not be increased after treatment of hematin and phenylhydrazine, in comparison with the results reported by Maines and Braggins. It suggests that HO activity is constantly maintained in the induced state as a result of sustained exposure to haemoglobin released in the course of disruption of senescent erythrocytes.
With the same procedure, only the form of HO, HO-2, could be clearly detected and the HO-1 was absent in the brain after inducement with hematin and phenylhydrazine. The result, however, does not suggest that this isomer is absent in the tissue, rather, it may reflect the inability of the presently used procedures to detect the exceedingly low level of HO-1, and the expression of the HO-1 isomer in the brain may be suppressed under most conditions. Surprisingly, the brain displayed a higher HO-2 activity than the liver, possibly reflecting a major biological adaptation. HO-1 and HO-2 preparation were analysed by the Western immunoblotting technique. The rat brain HO-2 preparation exhibited immunological reactivity with antibody to rat liver HO-2, but not to the rat liver HO-1, indicating that these two HO preparations are antigenically different.
pRHO1 contains 1557 nucleotides excluding the dG tail and the poly (dA) tract (≈150 residues). The vector pcDNA3 is designed for eukaryotic expression and has an expanded multiple cloning sites to faciliate cloning of inserts besides having the same characterization of other vectors. pcDNA3HO1 harbouring the rat HO-1 cDNA. was constructed and transfected into COS-1 cells for confirming that the isolated cDNA, XhoI(-59)/HindIII(971) fragment, actually encodes HO-1. COS-1 cells have endogenous HO-1 activity and this basal activity did not change after transfection with plasmid pcDNA3HO1. Accordingly, the activity of HO-1 in cells transfected with pcDNA3 was used as control. The results indicated clearly that isolated cDNA encoded HO-1. Furthermore, these results also show that the expressed HO-1 was actually incorporated into the endoplasmic reticulum of COS -1 cells, as we isolated microsomes for assay of HO-1. Shibahara et al constructed the pKCRHO21 carrying the entire protein-coding region in correct orientation as pcDNA3HO1, XhoI(-59)/HindIII(971) fragment. The cells transfected with pKCRHO21 have 2-fold higher activity than that of rat spleen. Ishikawa et al. reported that during cultivation, a few degraded forms of HO-1 that had lost their membrane-associating, C-terminal region appeared. Among these soluble enzymes, a degraded form of HO-1 with molecular mass of Mr 30000 was found to retain ability to decomposed heme to biliverdin[37,38]. These results are in accordance with ours[39]. So it clearly indicates that isolated cDNA encodes HO-1.
There are 10 histindine residues in HO-1 and 5 of them are located between amino acid residues 100-132. Up-to-date, the report showed that the axial heme ligand in the ferrous heme-HO-1 complex is a neutral form of the imidazole of a histindine using optical absorption, EPR, and resonance Raman scattering[38,40,41]. Histindine residues at positions 25, 84, 119 and 132 in the HO-1 sequence are conserved in rat, human, mouse and chicken HO-1. These histindines may be important for heme-binding. Preliminary mutagenesis studies on rat HO-1 by Ito-Maki et al[38] have shown that the His25Ala mutation completely abolished the enzyme activity but that the replacement of either His84 or His119 by Ala did not alter the enzyme activity. On the basis of this work, His25 has been considered to be an essential residue for the enzyme activity. In this study, pcDNA3HO1D25, containing the mutated rat HO-1 cDNA (His25Ala), was constructed using site-directed mutagenesis and transfected to COS-1 cells. The activity of mutant declined but did not completely abolish. At present, no reasoning can be offered for this discrepancy. Maybe it is because the enzyme reaction system is different.
After successfully expressing mutant protein, an equal amount of mutant HO-1 was added in reaction system. The result showed that His25Ala mutant reduced the formation of bilirubin. This suggests that the mutant may competely bind the substrate-heme with native enzyme. Some researches found that the precursor of bilirubin, heme, could be discharged from bile while the metabolic procedure of heme to bilirubin was inhibited by Sn-protoporphyrin.
Further studies are required to identify His25Ala mutant characteristics before using this mutant to prevent and treat hyperbilirubinemia.
Footnotes
Supported by National Natural Scientific Foundation of China, No. 39600159
Edited by Xu JY
References
- 1.Hill-Kapturczak N, Chang SH, Agarwal A. Heme oxygenase and the kidney. DNA Cell Biol. 2002;21:307–321. doi: 10.1089/104454902753759726. [DOI] [PubMed] [Google Scholar]
- 2.Botros FT, Laniado-Schwartzman M, Abraham NG. Regulation of cyclooxygenase- and cytochrome p450-derived eicosanoids by heme oxygenase in the rat kidney. Hypertension. 2002;39:639–644. doi: 10.1161/hy0202.103420. [DOI] [PubMed] [Google Scholar]
- 3.Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 4.Gildemeister OS, Pepe JA, Lambrecht RW, Bonkovsky HL. Induction of heme oxygenase-1 by phenylarsine oxide. Studies in cultured primary liver cells. Mol Cell Biochem. 2001;226:17–26. [PubMed] [Google Scholar]
- 5.Guo X, Shin VY, Cho CH. Modulation of heme oxygenase in tissue injury and its implication in protection against gastrointestinal diseases. Life Sci. 2001;69:3113–3119. doi: 10.1016/s0024-3205(01)01417-5. [DOI] [PubMed] [Google Scholar]
- 6.Haider A, Olszanecki R, Gryglewski R, Schwartzman ML, Lianos E, Kappas A, Nasjletti A, Abraham NG. Regulation of cyclooxygenase by the heme-heme oxygenase system in microvessel endothelial cells. J Pharmacol Exp Ther. 2002;300:188–194. doi: 10.1124/jpet.300.1.188. [DOI] [PubMed] [Google Scholar]
- 7.Lo S, Bell HS, Yamaguchi S, Wharton SB, Whittle IR. Heme oxygenase (HO) isoforms in experimental C6 glioma: an immunocytochemical study. Br J Neurosurg. 2001;15:416–418. doi: 10.1080/02688690120082422. [DOI] [PubMed] [Google Scholar]
- 8.Parfenova H, Neff RA, Alonso JS, Shlopov BV, Jamal CN, Sarkisova SA, Leffler CW. Cerebral vascular endothelial heme oxygenase: expression, localization, and activation by glutamate. Am J Physiol Cell Physiol. 2001;281:C1954–C1963. doi: 10.1152/ajpcell.2001.281.6.C1954. [DOI] [PubMed] [Google Scholar]
- 9.Vaccari A, Ruiu S, Saba P, Fà M, Cagiano R, Coluccia A, Mereu G, Steardo L, Tattoli M, Trabace L, et al. Prenatal low-level exposure to CO alters postnatal development of hippocampal nitric oxide synthase and haem-oxygenase activities in rats. Int J Neuropsychopharmacol. 2001;4:219–222. doi: 10.1017/S1461145701002450. [DOI] [PubMed] [Google Scholar]
- 10.Yoshiki N, Kubota T, Aso T. Identification of heme oxygenase in human endometrium. J Clin Endocrinol Metab. 2001;86:5033–5038. doi: 10.1210/jcem.86.10.7943. [DOI] [PubMed] [Google Scholar]
- 11.Leplingard A, Brung-Lefebvre M, Guédon C, Savoye G, Dechelotte P, Lemoine F, Lebreton JP, Lerebours E. Increase in cyclooxygenase-2 and nitric oxide-synthase-2 mRNAs in pouchitis without modification of inducible isoenzyme heme-oxygenase-1. Am J Gastroenterol. 2001;96:2129–2136. doi: 10.1111/j.1572-0241.2001.03948.x. [DOI] [PubMed] [Google Scholar]
- 12.Vicente AM, Guillén MI, Alcaraz MJ. Modulation of haem oxygenase-1 expression by nitric oxide and leukotrienes in zymosan-activated macrophages. Br J Pharmacol. 2001;133:920–926. doi: 10.1038/sj.bjp.0704145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma HS, Westman J, Gordh T, Alm P. Topical application of brain derived neurotrophic factor influences upregulation of constitutive isoform of heme oxygenase in the spinal cord following trauma an experimental study using immunohistochemistry in the rat. Acta Neurochir Suppl. 2000;76:365–369. doi: 10.1007/978-3-7091-6346-7_76. [DOI] [PubMed] [Google Scholar]
- 14.Ding S, Yao D, Deeni YY, Burchell B, Wolf CR, Friedberg T. Human NADPH-P450 oxidoreductase modulates the level of cytochrome P450 CYP2D6 holoprotein via haem oxygenase-dependent and -independent pathways. Biochem J. 2001;356:613–619. doi: 10.1042/0264-6021:3560613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barber A, Robson SC, Myatt L, Bulmer JN, Lyall F. Heme oxygenase expression in human placenta and placental bed: reduced expression of placenta endothelial HO-2 in preeclampsia and fetal growth restriction. FASEB J. 2001;15:1158–1168. doi: 10.1096/fj.00-0376com. [DOI] [PubMed] [Google Scholar]
- 16.Stevenson DK, Vreman HJ, Wong RJ, Contag CH. Carbon monoxide and bilirubin production in neonates. Semin Perinatol. 2001;25:85–93. doi: 10.1053/sper.2001.23197. [DOI] [PubMed] [Google Scholar]
- 17.Miller SM, Reed D, Sarr MG, Farrugia G, Szurszewski JH. Haem oxygenase in enteric nervous system of human stomach and jejunum and co-localization with nitric oxide synthase. Neurogastroenterol Motil. 2001;13:121–131. doi: 10.1046/j.1365-2982.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- 18.Alcaraz MJ, Habib A, Créminon C, Vicente AM, Lebret M, Lévy-Toledano S, Maclouf J. Heme oxygenase-1 induction by nitric oxide in RAW 264.7 macrophages is upregulated by a cyclo-oxygenase-2 inhibitor. Biochim Biophys Acta. 2001;1526:13–16. doi: 10.1016/s0304-4165(01)00112-x. [DOI] [PubMed] [Google Scholar]
- 19.da Silva JL, Zand BA, Yang LM, Sabaawy HE, Lianos E, Abraham NG. Heme oxygenase isoform-specific expression and distribution in the rat kidney. Kidney Int. 2001;59:1448–1457. doi: 10.1046/j.1523-1755.2001.0590041448.x. [DOI] [PubMed] [Google Scholar]
- 20.Navarra P, Miceli F, Tringali G, Minici F, Pardo MG, Lanzone A, Mancuso S, Apa R. Evidence for a functional link between the heme oxygenase-carbon monoxide pathway and corticotropin-releasing hormone release from primary cultures of human trophoblast cells. J Clin Endocrinol Metab. 2001;86:317–323. doi: 10.1210/jcem.86.1.7091. [DOI] [PubMed] [Google Scholar]
- 21.Giordano A, Nisoli E, Tonello C, Cancello R, Carruba MO, Cinti S. Expression and distribution of heme oxygenase-1 and -2 in rat brown adipose tissue: the modulatory role of the noradrenergic system. FEBS Lett. 2000;487:171–175. doi: 10.1016/s0014-5793(00)02217-1. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi M, Doré S, Ferris CD, Tomita T, Sawa A, Wolosker H, Borchelt DR, Iwatsubo T, Kim SH, Thinakaran G, et al. Amyloid precursor proteins inhibit heme oxygenase activity and augment neurotoxicity in Alzheimer's disease. Neuron. 2000;28:461–473. doi: 10.1016/s0896-6273(00)00125-2. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Song D, Lee SS. Role of heme oxygenase-carbon monoxide pathway in pathogenesis of cirrhotic cardiomyopathy in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G68–G74. doi: 10.1152/ajpgi.2001.280.1.G68. [DOI] [PubMed] [Google Scholar]
- 24.Lim S, Groneberg D, Fischer A, Oates T, Caramori G, Mattos W, Adcock I, Barnes PJ, Chung KF. Expression of heme oxygenase isoenzymes 1 and 2 in normal and asthmatic airways: effect of inhaled corticosteroids. Am J Respir Crit Care Med. 2000;162:1912–1918. doi: 10.1164/ajrccm.162.5.9909081. [DOI] [PubMed] [Google Scholar]
- 25.Yoshiki N, Kubota T, Aso T. Expression and localization of heme oxygenase in human placental villi. Biochem Biophys Res Commun. 2000;276:1136–1142. doi: 10.1006/bbrc.2000.3551. [DOI] [PubMed] [Google Scholar]
- 26.Alm P, Sharma HS, Sjöquist PO, Westman J. A new antioxidant compound H-290/51 attenuates nitric oxide synthase and heme oxygenase expression following hyperthermic brain injury. An experimental study using immunohistochemistry in the rat. Amino Acids. 2000;19:383–394. doi: 10.1007/s007260070069. [DOI] [PubMed] [Google Scholar]
- 27.Gordh T, Sharma HS, Azizi M, Alm P, Westman J. Spinal nerve lesion induces upregulation of constitutive isoform of heme oxygenase in the spinal cord. An immunohistochemical investigation in the rat. Amino Acids. 2000;19:373–381. doi: 10.1007/s007260070068. [DOI] [PubMed] [Google Scholar]
- 28.Beschorner R, Adjodah D, Schwab JM, Mittelbronn M, Pedal I, Mattern R, Schluesener HJ, Meyermann R. Long-term expression of heme oxygenase-1 (HO-1, HSP-32) following focal cerebral infarctions and traumatic brain injury in humans. Acta Neuropathol. 2000;100:377–384. doi: 10.1007/s004010000202. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi Y, Ohshiro N, Okui E, Sasaki T, Tokuyama S, Yoshida T, Yamamoto T. Concurrent induction of rat hepatic microsomal cytochrome P450 and haem oxygenase by 2,2'-dipyridyl ketone: comparison with the effect of 2,2'-dipyridyl amine. Xenobiotica. 2000;30:683–692. doi: 10.1080/00498250050077995. [DOI] [PubMed] [Google Scholar]
- 30.Maines MD. The heme oxygenase system and its functions in the brain. Cell Mol Biol (Noisy-le-grand) 2000;46:573–585. [PubMed] [Google Scholar]
- 31.Donnelly LE, Barnes PJ. Expression of heme oxygenase in human airway epithelial cells. Am J Respir Cell Mol Biol. 2001;24:295–303. doi: 10.1165/ajrcmb.24.3.4001. [DOI] [PubMed] [Google Scholar]
- 32.McCoubrey WK, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 33.Sugishima M, Omata Y, Kakuta Y, Sakamoto H, Noguchi M, Fukuyama K. Crystal structure of rat heme oxygenase-1 in complex with heme. FEBS Lett. 2000;471:61–66. doi: 10.1016/s0014-5793(00)01353-3. [DOI] [PubMed] [Google Scholar]
- 34.Ahmad Z, Salim M, Maines MD. Human biliverdin reductase is a leucine zipper-like DNA-binding protein and functions in transcriptional activation of heme oxygenase-1 by oxidative stress. J Biol Chem. 2002;277:9226–9232. doi: 10.1074/jbc.M108239200. [DOI] [PubMed] [Google Scholar]
- 35.Montellano PR. The mechanism of heme oxygenase. Curr Opin Chem Biol. 2000;4:221–227. doi: 10.1016/s1367-5931(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 36.Sugishima M, Sakamoto H, Kakuta Y, Omata Y, Hayashi S, Noguchi M, Fukuyama K. Crystal structure of rat apo-heme oxygenase-1 (HO-1): mechanism of heme binding in HO-1 inferred from structural comparison of the apo and heme complex forms. Biochemistry. 2002;41:7293–7300. doi: 10.1021/bi025662a. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa K, Sato M, Yoshida T. Expression of rat heme oxygenase in Escherichia coli as a catalytically active, full-length form that binds to bacterial membranes. Eur J Biochem. 1991;202:161–165. doi: 10.1111/j.1432-1033.1991.tb16357.x. [DOI] [PubMed] [Google Scholar]
- 38.Ito-Maki M, Ishikawa K, Matera KM, Sato M, Ikeda-Saito M, Yoshida T. Demonstration that histidine 25, but not 132, is the axial heme ligand in rat heme oxygenase-1. Arch Biochem Biophys. 1995;317:253–258. doi: 10.1006/abbi.1995.1160. [DOI] [PubMed] [Google Scholar]
- 39.Xia Z, Li Y, Chen S, Shen Q, Li C, Shen H, Yu S. The purification and identification of heme oxygenase isoforms from spleen tissue of rat and the expression of heme oxygenase-1 cDNA in COS-1 cells. Chin Med J (Engl) 1998;111:842–846. [PubMed] [Google Scholar]
- 40.Wilks A, Moënne-Loccoz P. Identification of the proximal ligand His-20 in heme oxygenase (Hmu O) from Corynebacterium diphtheriae. Oxidative cleavage of the heme macrocycle does not require the proximal histidine. J Biol Chem. 2000;275:11686–11692. doi: 10.1074/jbc.275.16.11686. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Moënne-Loccoz P, Hildebrand DP, Wilks A, Loehr TM, Mauk AG, Ortiz de Montellano PR. Replacement of the proximal histidine iron ligand by a cysteine or tyrosine converts heme oxygenase to an oxidase. Biochemistry. 1999;38:3733–3743. doi: 10.1021/bi982707s. [DOI] [PubMed] [Google Scholar]


