Abstract
AIM: Liver regeneration is associated with apoptosis of hepatocytes, which is mediated via tumor necrosis factor receptor 1(TNFR1). The shedding of TNFR1 in liver regeneration and its mechanism to regulate this shedding were investigated.
METHODS: The shedding of TNFR1 in liver regeneration and changes of TNF-α, PMA and plasma membrane purified from hepatocytes on this shedding process were measured with Western blot. Then, the relationship between TNFR1 shedding and apoptosis of hepatocytes induced by TNFα was studied by detecting apoptotic index.
RESULTS: The shedding of TNFR1 began at 4 hours and terminated before 2 months after partial hepatectomy. In culture system, serum from rats at 36 h after partial hepatectomy could also promote this shedding process. With the stimulation of TNF α, PMA or purified plasma membrane from hepatocytes at 36 h after partial hepatectomy or from hepatocytes treated with TNF α for 2 h, membranous TNFR1 was also shed. With the stimulation of both TNF α and plasma membrane from hepatocytes affected with TNF α for 2 h or from hepatocytes at 36 h after partial hepatectomy, apoptotic index of hepatocytes decreased from 21% to 7.52% and 8.45%, respectively. PMA could also reduce apoptotic index to 13.67%. This descent occurred in hepatocytes cultured in serum from rats at 36 h after partial hepatectomy too, but not in serum from rats at 2 months after partial hepatectomy and sham-operated rats.
CONCLUSION: Shedding of TNFR1 may help reduce apoptosis of hepatocytes induced by TNF α. Membrane-anchored metalloprotases could play a role in shedding membranous TNFR1. At the same time, PKC may take part in regulation of this shedding process.
INTRODUCTION
Tumor necrosis factor alpha (TNF-α), secreted predominantly by monocytes and macrophages, is an important mediator of various inflammatory and immune responses[1]. During liver regeneration after a partial hepatectomy, TNFα levels are elevated leading to the activation of a number of transcription factors such as STAT-3, c-jun, c-fos, activating protein-1(AP-1) and nuclear factor-κB (NFκB)[2-4]. And soluble TNF-α exerts its biological functions by binding to special target cell surface receptors[5] which have been identified as TNFR1(p55) and TNFR2 (p75)[6,7]. After ligand binding, TNFR1 or TNFR2 can be bound by TNFR-associated protein-2 and the serine/ threonine kinase receptor-interacting protein, and then mediate survival and proliferating signals via the transcription factor NFκB and via activation of jun N-terminus kinase (JNK), which in turn mediates new gene transcription via AP-1[8]. On the other hand, TNFR1, not TNFR2, could be bound by the death domain of the Fas-associated death domain protein (FADD) via the adapter protein TNF-R1-associated death domain protein (TRADD), which mediates its interaction with caspase 8 and activates the caspase cascade during apoptosis.
Membranous proteins undergo shedding of ectodomain extensively, among these are cytokines such as TNFα[9,10] and kit ligand[11], cytokine receptors like the TNFα receptors[12] and the p75 nerve growth factor receptor[13], adhesion proteins such as L-selectin[14,15], and other proteins, including the β-amyloid precursor protein[16,17],the angiotensin-converting enzyme[18,19], and the protein tyrosine phosphatases LAR and PTPσ[20]. It has been postulated that shedding of membranous protein ectodomain might play a role in controlling a cell’s survival[21]. And this shedding of membranous protein happens extensively in miscellaneous cells, such as monocytes and hepatocytes. For TNFR, both the p55 and p75 form could be shed on the surface of a cell[22-25]. Recently, there had been several reports demonstrating that TNF α could induce the shedding of its own receptor in lymphocyte[26,27]. However, whether membranous TNFR1 of hepatocyte is shed during liver regeneration remains unclear. In present study, we examined this issue in rat regenerative liver.
To clarify which elements affected the shedding of membranous TNFR1, we also determined the shedding of membranous TNFR1 of hepatocyte under stimulations of TNF α, phorbol 12 - myristate 13 -acetate (PMA) or metalloprotease inhibitors. And then the effect of TNFR1 shedding on the apoptosis of hepatocytes was investigated.
MATERIALS AND METHODS
Partial hepatectomy
Partial hepatectomy (PH) was performed on Sprague-Dawley Rattus norvegicus according to Higgins’ method[28].
Separation and purification of parenchymal hepatocytes
After isolated with collagenase according to the method as described previously[29], 4 mL suspension of hepatocytes was laid on 15 mL 60% (v/v) Percoll (Sigma) in 30 mL centrifuge tube, and then centrifugated at 400 g for 5 min at 4 °C. Finally, obtained precipitate was washed three times with PBS at 50 g for 2 min at 4 °C.
Primary culture of hepatocytes and treatment
After isolation, hepatocytes were cultured in 50 mL·L-1 CO2 at 37 °C in RPMI1640 containing 10% fetal bovine serum (BSA) and penicillin/streptomycin for 2 h according to standard protocols. Then the medium was replaced by fresh RPMI 1640 deprived of BSA to remove the non-attached cells.
To determine the effect of serum on shedding of TNFR1, serum from rats after partial hepatectomy for 36 h was added into culture medium. Thirty minutes later, cultured hepatocytes were harvested with policeman from culture dish.
For other incubations, plasma membrane purified from hepatocytes after partial hepatectomy for 48 h or from cultured hepatocytes induced by TNF-α at a concentration of 5 nmol· L-1 for 2 h were added at a concentration of 2 μg membranous protein per milliliter culture medium. Addition of plasma membrane boiled for 5 min and plasma membrane from hepatocytes of rats without partial hepatectomy were used as control. Then 2 mmol·L-1 BB-1101 (Sigma), and staurosporine at 5 ng·L-1 were respectively added into culture medium. Two hours later after various stimulations as above, hepatocytes were collected.
Phorbol 12-myristate 13-acetate (PMA, Sigma) alone concentrated at 10 µmol·L-1 (in DMSO) or PMA accompanied with staurosporine (3 nmol·L-1, Sigma) were added into culture medium of attached hepatocytes for 30 min.
Purification of plasma membrane and membranous protein
Isolated or cultured hepatocytes (1 × 107) were homogenized in buffer A (1 mmol·L-1 NaHCO3, pH7.5; 0.5 mmol·L-1 CaCL2; 2 μmol·L-1 aprotinin, 10 μmol·L-1 E-64, 100 μmol·L-1 PMSF, 100 μmol·L-1 TPCK) on ice. After centrifuged at 1500 g for 3 × 10 min at 4 °C, the precipitate was suspended with 5 mL buffer A, and then mixed with 5 mL 69% (w/v) sucrose solution. And 5 mL 42.3% (w/v) sucrose solution was added on top of the mixture. They were then centrifuged at 100000 g for 2 h at 4 °C. The snip on the top of centrifugal solution was washed with buffer A at 100000 g for 3 × 10 min at 4 °C. Finally, the membranes were purified about 25-fold from homogenate, judging by their 5’-nucleatidase activity. Obtained membranes were resolved in 1 mL TrittonX114 buffer (2% TrittonX114, 50 mmol·L-1 Tris- HCL, pH7.5) on ice for 15 min, then centrifuged at 10000 g for 5 min at 4 °C, and then incubated at 37 °C for 10 min and centrifuged at 2000 g at 37 °C for 5 min. Detergent fraction was collected to perform procedures as described above once again. Finally, pure membranous proteins were in TrittonX114 detergent phase.
TNFR1 and membrane-anchored metalloprotease assays
Total membranous proteins were prepared as described above. Concentration of protein was determined according to described method[30]. Ninety micrograms membranous protein was separated by SDS-PAGE and electroblotted to nitrocellulose. After incubation with fresh blocking solution, blots were exposed to TNFR1 primary antibody (1:1000, Santa cruz, USA). Blots were then incubated with a 1:1000 dilution of rat alkaline phosphatase-colligated secondary antibody (Zhong Shan Biotech Co. China) for 1 h at 37 °C. Blots were again washed for 3 × 5 min in PBS and then developed by NBT/Bcip III (Dingguo Biotech Co. China).
Assessment of apoptosis
The percentage of apoptotic cells was determined by evaluating propidium iodide and Hoechst 33342 stained preparations by fluorescent microscopy and scoring 8-10 randomly selected fields containing more than 1000 cells. In the meantime, the results were confirmed by DNA fragmentation by agarose electrophoresis[31].
Statistical analysis
All data values are expressed as means ± SE for statistical analysis, the significance of differences between experimental conditions was determined using the Student’s test for unpaired observations. A P value (two tailed) of less than 0.05, compared with hepatocytes cultured in RPMI1640 with 10% BSA, was considered significant.
RESULTS
Shedding of TNFR1
Shedding of membranous TNFR1 ectodomain after partial hepatectomy was examined by Western blotting. Shedding of TNFR1 began at 4 h after hepatectomy, but not at 2 h, in which process a 55 kD form of TNFR1 was shed into a 39 kD form. And this process lasted at least for 144 h and ended before 2 months after hepatectomy (Figure 1). In culture system in vitro, serum from rats at 36 h after partial hepatectomy could also promote this shedding process. However, serum from sham-operated rats and from rats at 2 months after partial hepatectomy could not (Figure 2). And with the stimulation of TNFα, PMA and purified plasma membrane from hepatocytes at 36 h after partial hepatectomy and from hepatocytes treated with TNFα for 2 h, TNFR1 also could be shed. But this shedding of membranous TNFR1 was inhibited by staurosporine and BB1101 (Figures 2, 3, 4 and 5).
Figure 1.
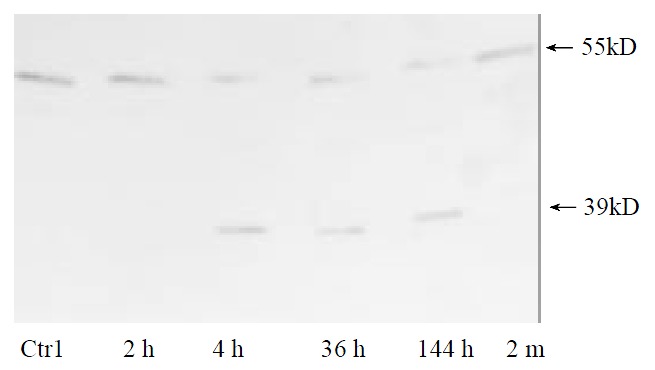
TNFR1 shedding on the surface of parenchymal hepatocytes occurs in regenerative liver.Data shown represent time course of TNFR1 shedding after partial hepatectomy. Plasma membrane from normal liver was used as control.
Figure 2.
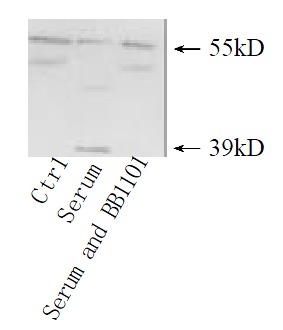
TNFR1 shedding under the stimulation of serum from rats after partial hepatectomy. Cultured hepatocytes were treated with serum from regener-ative liver at 36 h for 30 min, or the serum accompanied with metalloprotase inhibitor BB1101 at 2 mmol·L-1. Cultured hepatocytes under no any treatments were used as control.
Figure 3.
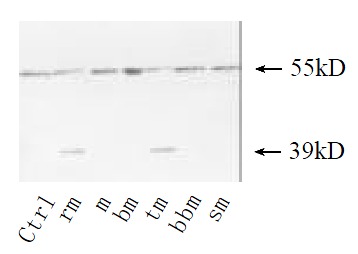
TNFR1 shedding induced with plasma membrane of hepatocytes from regenerative liver. Cultured hepatocytes were treated for 30 min with plasma membrane from hepatocytes at 36 h after hepatectomy (rm) or sham-operated (m) or from hepa-tocytes treated with TNFα at 10 µg·mL-1 for 2 h (tm). Plasma membrane boiled for 5 min (bm) as control. Then metalloprotase inhibitor BB1101 at 2 mmol·L-1 (bbm), or staurosporine at 5 ng·mL-1 e (sm) was added into culture medium. Cultured hepa-tocytes under no any treatments were as control.
Figure 4.
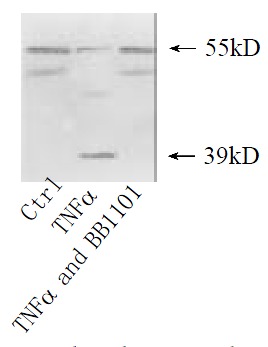
TNFR1 shedding under the stimulation of TNF α. Cultured hepatocytes were treated respectively with TNFα at 10 μg·mL-1 for 2 h, or TNFα accompanied with metalloprotase inhibitor BB1101 at 2 mmol·L-1. Cultured hepatocytes under no any treatments were as control.
Figure 5.
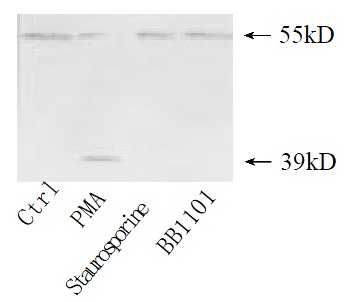
Effect of PMA on TNFR1 shedding. Cultured hepa-tocytes were treated with PMA at 10 µg·mL-1 for 30 min, staurosporine accompanied with PMA at 5 ng·mL-1, or PMA accompanied with BB1101 at 2 mmol·L-1. Confluent cultured hepatocytes without any treatment were used as control.
Effects of TNFR1 shedding on apoptosis of hepatocyte
Purified plasma membrane from hepatocytes at 36 h after partial hepatectomy or from hepatocytes induced with TNFα or PMA reduced the apoptotic index induced by TNFα from 21% to 7.52% , 8.45% and 13.67%, respectively. This descent also occurred in hepatocytes cultured in serum from rats after partial hepatectomy for 36 h. But cultured in serum from rats at 2 months after partial hepatectomy, apoptotic index of hepatocytes was even higher than that in serum from sham-operated rats (Figure 6).
Figure 6.
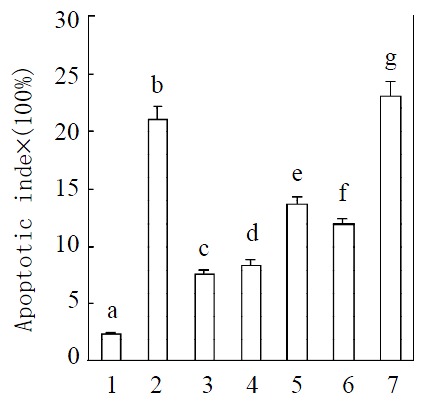
Induction of apoptosis after treatment with TNFα. Means of data shown in figure were: 1. Control; 2. Treated with TNFα at 10 μg·mL-1; 3. Treatment with TNFα at 10 μg·mL-1 after treated with plasma membrane purified from liver at 36 h after partial hepatectomy at 2 μg·mL-1; 4. Treated with plasma membrane at 2 μg·mL-1 purified from hepatocytes induced with TNFa accompanied with TNFa at 10 μg·mL-1; 5. Treated with PMA at a concentration of 10 μmol·L-1 (in DMSO) accompa-nied with TNF at 10 μg·mL-1; 6. Treated with serum from rat after partial hepatectomy for 36 h at 5% accompanied with TNFα at 10 μg·mL-1; 7. Treated with TNFα at 10 μg·mL-1 after treated with plasma membrane purified from rat liver regen-erated for 2 months at 2 μg·mL-1. Apoptotic index = (numbers of apoptotic cells/total cells numbers per well) × 100. Data were means from 6 separate experiments × SE (n = 6 wells). Differ-ent letters over bars indicate significant differences, P < 0.05. The results are confirmed by DNA fragmentation by agarose electrophoresis (data not provided).
DISCUSSION
In adult liver, hepatocytes are highly differentiated and predominantly in G0 state of cell cycle. Partial hepatectomy can induce these hepatocytes to undergo rapid proliferation, leading to organ regeneration[3,32,33]. However, the exact mechanisms that initiate and terminate this highly regulated proliferative event remain unclear. In present studies, we assessed the shedding of TNFR1 during liver regeneration and the association between this shedding and apoptosis of hepatocytes.
Several recent works had provided obvious evidences that tumor necrosis factor-α (TNF-α) functioned as a two-edged sword in the liver. It is an important cytokine of the early signaling pathways leading to regeneration and an antiapoptotic effector. On the other hand, it is also an intensive mediator of apoptosis[2,3]. TNFR should be involved in all these process above because TNFα must bind to TNFR before it can exert its roles. And down-regulation of membranous TNFR1 expression levels of hepatocyte had been previously confirmed as an important pathway to regulate the role of TNF-α[34]. Our results demonstrated that the shedding of TNFR1 occurred during liver regeneration. This shedding of TNFR1 could reduce apoptotic rate of hepatocytes induced by TNF-α.These results suggested that TNFR1 shedding might also be a pathway to down-regulate membranous TNFR1 levels of hepatocyte. Our finding that shedding of TNFR1 induced by serum from rats after partial hepatectomy progressively suggested that some factors were secreted into serum during liver regeneration. These factors might regulate liver regeneration by inducing the shedding of TNFR1.
Several peptide hormones had been shown to down-modulate their own receptors[35-38]. This down-modulation was believed to require the binding of the ligand with its receptor, followed by internalization of the ligand-receptor complex into the cell. After the dissociation of the receptor from its ligand inside the lysosome, the receptor was either degraded or recycled back to the cell surface[39]. Our results showed that the TNFR1 shedding was induced by TNF-α, though only parts of membranous receptor were shed. Higuchi reckoned that TNFR2, not TNFR1, was shed in lysosome[40]. However, our results showed that membranous TNFR1 could be shed on the cell surface.
Our results also showed that metalloprotase inhibitor inhibited the shedding of TNFR1. This suggested that some metalloprotases played a role in this shedding process. Two possible sources of these metalloprotase could be proposed for the shedding of TNFR1. One possibility was that these metalloprotases presented in serum because the shedding of TNFR1 could be induced by serum from rats after partial hepatectomy and inhibited by BB1101. It was also possible that these metalloprotases were membrane-anchored proteins. TNFR1 was shed when cells were treated with plasma membrane purified from hepatocytes of regenerative liver. At the same time, this shedding of TNFR1 could be inhibited by metalloprotase inhibitor either.
We also found that PMA could induce the shedding of TNFR1. This shedding of TNFR1 was inhibited by staurosporine. These results suggested that PKC was involved in regulating the shedding of TNFR1. Perhaps phosphorylating of TNFR1 by PKC made it sensitive to metalloprotase. However, further investigations are needed to identify which protease should be responsible for the shedding of TNFR1.
Footnotes
Supported by Chinese National Natural Science Foundation, No. 39970362 and the Foundation of Bioengineering Key Laboratory in Henan Province, No. PKL99003
Edited by Zhang JZ
References
- 1.Old LJ. Tumor necrosis factor (TNF) Science. 1985;230:630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- 2.Bruccoleri A, Gallucci R, Germolec DR, Blackshear P, Simeonova P, Thurman RG, Luster MI. Induction of early-immediate genes by tumor necrosis factor alpha contribute to liver repair following chemical-induced hepatotoxicity. Hepatology. 1997;25:133–141. doi: 10.1002/hep.510250125. [DOI] [PubMed] [Google Scholar]
- 3.Diehl AM, Rai RM. Liver regeneration 3: Regulation of signal transduction during liver regeneration. FASEB J. 1996;10:215–227. doi: 10.1096/fasebj.10.2.8641555. [DOI] [PubMed] [Google Scholar]
- 4.Diehl AM, Yin M, Fleckenstein J, Yang SQ, Lin HZ, Brenner DA, Westwick J, Bagby G, Nelson S. Tumor necrosis factor-alpha induces c-jun during the regenerative response to liver injury. Am J Physiol. 1994;267:G552–G561. doi: 10.1152/ajpgi.1994.267.4.G552. [DOI] [PubMed] [Google Scholar]
- 5.Decker K. Biologically active products of stimulated liver macrophages (Kupffer cells) Eur J Biochem. 1990;192:245–261. doi: 10.1111/j.1432-1033.1990.tb19222.x. [DOI] [PubMed] [Google Scholar]
- 6.Himmler A, Maurer-Fogy I, Krönke M, Scheurich P, Pfizenmaier K, Lantz M, Olsson I, Hauptmann R, Stratowa C, Adolf GR. Molecular cloning and expression of human and rat tumor necrosis factor receptor chain (p60) and its soluble derivative, tumor necrosis factor-binding protein. DNA Cell Biol. 1990;9:705–715. doi: 10.1089/dna.1990.9.705. [DOI] [PubMed] [Google Scholar]
- 7.Brockhaus M, Schoenfeld HJ, Schlaeger EJ, Hunziker W, Lesslauer W, Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci USA. 1990;87:3127–3131. doi: 10.1073/pnas.87.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West DA, James NH, Cosulich SC, Holden PR, Brindle R, Rolfe M, Roberts RA. Role for tumor necrosis factor alpha receptor 1 and interleukin-1 receptor in the suppression of mouse hepatocyte apoptosis by the peroxisome proliferator nafenopin. Hepatology. 1999;30:1417–1424. doi: 10.1002/hep.510300612. [DOI] [PubMed] [Google Scholar]
- 9.Hooper NM, Karran EH, Turner AJ. Membrane protein secretases. Biochem J. 1997;321(Pt 2):265–279. doi: 10.1042/bj3210265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 11.Huang EJ, Nocka KH, Buck J, Besmer P. Differential expression and processing of two cell associated forms of the kit-ligand: KL-1 and KL-2. Mol Biol Cell. 1992;3:349–362. doi: 10.1091/mbc.3.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porteu F, Nathan C. Shedding of tumor necrosis factor receptors by activated human neutrophils. J Exp Med. 1990;172:599–607. doi: 10.1084/jem.172.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiStefano PS, Johnson EM. Identification of a truncated form of the nerve growth factor receptor. Proc Natl Acad Sci USA. 1988;85:270–274. doi: 10.1073/pnas.85.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 15.Kahn J, Walcheck B, Migaki GI, Jutila MA, Kishimoto TK. Calmodulin regulates L-selectin adhesion molecule expression and function through a protease-dependent mechanism. Cell. 1998;92:809–818. doi: 10.1016/s0092-8674(00)81408-7. [DOI] [PubMed] [Google Scholar]
- 16.Selkoe DJ. Amyloid beta-protein and the genetics of Alzheimer's disease. J Biol Chem. 1996;271:18295–18298. doi: 10.1074/jbc.271.31.18295. [DOI] [PubMed] [Google Scholar]
- 17.Sisodia SS. Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc Natl Acad Sci USA. 1992;89:6075–6079. doi: 10.1073/pnas.89.13.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oppong SY, Hooper NM. Characterization of a secretase activity which releases angiotensin-converting enzyme from the membrane. Biochem J. 1993;292(Pt 2):597–603. doi: 10.1042/bj2920597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramchandran R, Sen I. Cleavage processing of angiotensin-converting enzyme by a membrane-associated metalloprotease. Biochemistry. 1995;34:12645–12652. doi: 10.1021/bi00039a021. [DOI] [PubMed] [Google Scholar]
- 20.Aicher B, Lerch MM, Müller T, Schilling J, Ullrich A. Cellular redistribution of protein tyrosine phosphatases LAR and PTPsigma by inducible proteolytic processing. J Cell Biol. 1997;138:681–696. doi: 10.1083/jcb.138.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone AL, Kroeger M, Sang QX. Structure-function analysis of the ADAM family of disintegrin-like and metalloproteinase-containing proteins (review) J Protein Chem. 1999;18:447–465. doi: 10.1023/a:1020692710029. [DOI] [PubMed] [Google Scholar]
- 22.Kohno T, Brewer MT, Baker SL, Schwartz PE, King MW, Hale KK, Squires CH, Thompson RC, Vannice JL. A second tumor necrosis factor receptor gene product can shed a naturally occurring tumor necrosis factor inhibitor. Proc Natl Acad Sci USA. 1990;87:8331–8335. doi: 10.1073/pnas.87.21.8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porteu F, Nathan C. Shedding of tumor necrosis factor receptors by activated human neutrophils. J Exp Med. 1990;172:599–607. doi: 10.1084/jem.172.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porteu F, Brockhaus M, Wallach D, Engelmann H, Nathan CF. Human neutrophil elastase releases a ligand-binding fragment from the 75-kDa tumor necrosis factor (TNF) receptor. Comparison with the proteolytic activity responsible for shedding of TNF receptors from stimulated neutrophils. J Biol Chem. 1991;266:18846–18853. [PubMed] [Google Scholar]
- 25.Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992;175:323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higuchi M, Aggarwal BB. Inhibition of ligand binding and antiproliferative effects of tumor necrosis factor and lymphotoxin by soluble forms of recombinant P60 and P80 receptors. Biochem Biophys Res Commun. 1992;182:638–643. doi: 10.1016/0006-291x(92)91780-t. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Aggarwal BB. Role of sulfhydryl groups in induction of cell surface down-modulation and shedding of extracellular domain of human TNF receptors in human histiocytic lymphoma U937 cells. J Immunol. 1994;153:3745–3754. [PubMed] [Google Scholar]
- 28.Higgins GM, Anderson RM. Experimental pathology of the liver I: Restoration of the liver of white rat following partial surgical removal. Arch Pathal. 1931;12:186–189. [Google Scholar]
- 29.Zhou JX, Xia M. Rapid isolation and primary culture of the rat hepatocytes. Henan Shifan Daxue Xuebao. 1989;2:46–49. [Google Scholar]
- 30.Marshak DR, Kadonaga JT, Burgess RR, Knuth MW. Strategies for protein purification and characterization: a laboratory course manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press. 1996:82–83. [Google Scholar]
- 31.Martin SJ. Protein or RNA synthesis inhibition induces apoptosis of mature human CD4+ T cell blasts. Immunol Lett. 1993;35:125–134. doi: 10.1016/0165-2478(93)90080-l. [DOI] [PubMed] [Google Scholar]
- 32.Akerman P, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, Diehl AM. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992;263:G579–G585. doi: 10.1152/ajpgi.1992.263.4.G579. [DOI] [PubMed] [Google Scholar]
- 33.Bradham CA, Plümpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387–G392. doi: 10.1152/ajpgi.1998.275.3.G387. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal BB, Eessalu TE. Effect of phorbol esters on down-regulation and redistribution of cell surface receptors for tumor necrosis factor-alpha. J Biol Chem. 1987;262:16450–16455. [PubMed] [Google Scholar]
- 35.Kosmakos FC, Roth J. Insulin-induced loss of the insulin receptor in IM-9 lymphocytes. A biological process mediated through the insulin receptor. J Biol Chem. 1980;255:9860–9869. [PubMed] [Google Scholar]
- 36.Guilbert LJ, Stanley ER. Modulation of receptors for the colony-stimulating factor, CSF-1, by bacterial lipopolysaccharide and CSF-1. J Immunol Methods. 1984;73:17–28. doi: 10.1016/0022-1759(84)90027-9. [DOI] [PubMed] [Google Scholar]
- 37.Lloyd CE, Ascoli M. On the mechanisms involved in the regulation of the cell-surface receptors for human choriogonadotropin and mouse epidermal growth factor in cultured Leydig tumor cells. J Cell Biol. 1983;96:521–526. doi: 10.1083/jcb.96.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heldin CH, Wasteson A, Westermark B. Interaction of platelet-derived growth factor with its fibroblast receptor. Demonstration of ligand degradation and receptor modulation. J Biol Chem. 1982;257:4216–4221. [PubMed] [Google Scholar]
- 39.Wiley HS. Receptors as models for the mechanisms of membrane protein turnover and dynamics. Curr Top Membr Transp. 1985;24:36–41. [Google Scholar]
- 40.Higuchi M, Aggarwal BB. TNF induces internalization of the p60 receptor and shedding of the p80 receptor. J Immunol. 1994;152:3550–3558. [PubMed] [Google Scholar]


