Abstract
AIM: Esophageal carcinoma is one of the most common malignant tumors in China. But the molecular mechanisms of esophageal carcinoma remains unclear. Gut-enriched Krüppel-like factor (GKLF) is a newly identified transcription factor which is expressed abandantly in the epithelial cells of the gastrointestinal tract and deregulation of GKLF was linked to several types of cancer. It is of interest to study the expression and role of GKLF in esophageal carcinoma.
METHODS: Semi-quantitative RT-PCR was used to compare GKLF expression in esophageal squamous cell carcinoma to normal mucosa of the same patients. The serum deprivation inducibility of GKLF was observed in an esophageal squamous cancer cell line by comparison to the primary culture of human fibroblast. The effect of antisense GKLF transfection on the proliferation and adhesion of esophageal squamous cancer cell line was also observed.
RESULTS: The level of GKLF transcript is lower in esophageal squamous cell carcinoma compared to paired normal-appearing mucosa in 14 of 17 of the tumors analyzed. The serum deprivation inducibility of GKLF was greatly decreased in an esophageal squamous cancer cell line compared to the primary culture of human fibroblast. Decreased expression of GKLF in the esophageal cancer cell by antisense GKLF transfection increased its proliferation rate compared with that of vector transfected cell control (P < 0.05). Transfection of antisense GKLF decreased its adhesion ability (P < 0.05).
CONCLUSION: The findings of this study demonstrate the down-regulation of GKLF in esophageal squamous cancer, and suggest that deregulation of GKLF may play a role in initiation and/or progression as well as the metastasis of esophageal squamous cancer.
INTRODUCTION
The gut-enriched Krüppel-like factor (GKLF), also named as epithelial zinc finger (EZF) or KLF4, is a recently identified transcription factor with three C2H2 zinc fingers in the carboxyl terminus[1-3]. The expression of GKLF is enriched in the epithelial cells of the gastrointestinal tract and skin[1,2]. Although the physiological role of GKLF is not clear, experimental evidences[1] suggest that GKLF is potentially a negative regulator of cell proliferation and involves in the differentiation process of epithelial cells. In the gene targeting experiment[4], GKLF knockout mice showed the impairment of skin barrier function, indicating that GKLF is both vital to and selective for the barrier acquisition.
GKLF expression is decreased in the intestinal adenomas of mice and colon adenomas of patients[5,6]. Experimental data[7] also suggest that down-regulation of GKLF may play a role in the initiation stage of tumorigenesis of colon. In oral dysplastic epithelium and squamous cell carcinoma, GKLF expression was increased and diffusely distributed[8,9]. In breast cancer, GKLF expression was increased and associated with the stages of tumor progression in the breast[9]. These observations indicated that GKLF expression is regulated during neoplastic progression in a tissue and tumor type-specific fashion.
GKLF expression was enriched in esophageal epithelia[2]. In esophageal cancer cell lines, abundant GKLF expression was also observed[10,11]. The regulating effect of GKLF on the squamous epithelium-specific keratin 4 and Epstein-Barr virus ED-L2 promoters[10] suggests GKLF plays an important role in the differentiation of esophageal squamous epithelium. The human GKLF gene was mapped onto chromosome 9q31[3], which is a hot site of chromosome abnormality in esophageal cancer[12,13]. GKLF gene aberration was observed in colon cancer in which the function of GKLF was demolished[14]. These evidences led us to the research on the expression of GKLF in esophageal cancer, and consequent effect on the cancer cells. To our knowledge, this is the first report on the down-regulation of GKLF in esophageal cancer, and the first evidence of association of GKLF function with cell adhesion ability.
MATERIALS AND METHODS
Tissue samples and cell culture
Fresh surgical specimens of 17 pairs of esophageal squamous cell carcinoma and corresponding normal tissues were immediately frozen and stored in liquid nitrogen after resection. Squamous esophageal cancer cell line EC9706 was kindly provided by Dr. Mingrong Wang in the lab. The cell was grown in M199 (Gibco BRL, Gaithersburg, MD) supplemented with 15% FCS. Primary culture of fibroblast was derived from resected normal esophageal tissue of an esophageal cancer patient, and maintained in McCoy 5A (Gibco BRL, Gaithersburg, MD) supplemented with 10% FBS. Cells were incubated at 37 °C with 5% CO2. For the serum deprivation experiments, cells were seeded into 25 mL flasks with 4 ml full medium and cultured until 50% confluency. The content of FCS in the cell media was reduced to 0.5%. Cells were then incubated continuously at 37 °C, in 5% CO2 for various periods.
RNA preparation and RT-PCR
Total RNA was extracted by TRIzol method (Gibco BRL, Gaithersburg, MD). Up to 5 μg of total RNA was reverse transcripted in 20 μL volume of reaction with both oligo (dT) and random primers, using SuperScript Preamplification System (Gibco BRL, Gaithersburg, MD) according to the instruction.
PCR was carried out in 30 μL total reaction mixture which contains 10 mmol/L Tris-HCL, pH 8.3, 50 mmol/L KCL, 1.75 mmol/L MgCl2, 0.1% Triton X-100, 200 μmol/L each of dGTP, dATP, dTTP, and dCTP, and 200 nmol/L each of the forward and reverse primers; 0.5-2 μL of cDNA template after RNase-free DNase I digestion was added. The primers used in the PCR reaction were synthesized according to the published cDNA sequences encoding human GKLF and GAPDH (GeneBank Accession numbers AF105036 and NM_002046, respectively). For the measurement of GKLF mRNA expression in tissue and cell samples, forward primer 5'-GTCGGACCACCTCGCCTTACACAT-3' and reverse primer 5'-GGTCTTCCCTCCCCCAACTCACG-3' of GKLF were used which amplify the region containing 3' noncoding sequence of GKLF cDNA (+1374 to +1749) and the expected PCR product was 376 bp; For the measurement of GKLF mRNA expression in cells after transfection of GKLF cDNA, forward primer 5'-CCACCGGCCGGCTGCACACGACT-3' and reverse primer 5'-TCATCTGAGCGGGCGAATTTCCATCCACA-3' of GKLF were used which amplify the coding sequence of GKLF cDNA (+864 to +1 298) and the expected PCR product was 435 bp. GAPDH primers (forward primer 5'-GGCAAATTCCATGGCACCGTCAAG-3' and reverse primer 5'-GCAATGCCAGCCCCAGCGTCAAA-3') were added in the same PCR reaction, and the expected PCR product of 746 bp was used as internal control. PCR was set up for 27 cycles: 30 s at 94 °C, 30 s at 56 °C 45 s at 72 °C.
GKLF cDNA cloning and sequencing
The full length GKLF cDNA coding sequence was amplified from reverse transcripted cDNA of EC9706. The forward primer 5'-CTGCTTCGGGCTGCCGAGGACCTTCTGGG-3' and reverse primer 5'-GGCAGTGTGGGTCATATCCACTGTCTGGGA-3' of GKLF were used and the expected PCR product was 1511 bp (-67 to + 1444 nucleotides). The cycling condition was 30 s at 94 °C, 30 s at 63 °C 1 min at 72 °C for 30 cycles. The PCR product was electrophoresed onto 0.9% agarose and 1.5 kb fragment was purified with the purification system (Qiagen, Hilden, Germany). The fragment was then cloned into PCR cloning vector pMD18T (TaKaRa, Dalian, China) and sequenced commercially (TaKaRa, Dalian, China).
Transfection
DNA fragment containing GKLF cDNA (-67 to + 1444 nucleotides) was cut out from pMD18T vector by BamHI and HindIII digestion and subcloned into pCDNA3.1 expression vector (Invitrogen, San Diego, CA) in antisense orientation. EC9706 cells were transfected with antisense GKLF cDNA as well as pCDNA3.1 vector using the Lipofectamine method according to the manufacture's protocol (Gibco BRL, Gaithersburg, MD). Briefly, cells were incubated with 1 mL of the transfection mixture containing 4.0 μg plasmid DNA and 25 μL Lipofectamine reagents in serum-free medium for 6 h, followed by adding 1 mL fresh media supplemented with 30% FCS. The medium was exchanged with fresh complete media after overnight incubation.
RNA content determination
For semi-quantification of GKLF mRNA expression, the PCR products were electrophoresed onto 1.2% agarose gel containing ethidium bromide. The images were analyzed with Multi-Analyst software (Bio-Rad, Hercules, CA) after scanned and the densities of GKLF and GAPDH bands were determined. The ratios of GKLF to GAPDH were compared in each paired sample.
Cell proliferation assay
Cells were inoculated into 96 well plates (2000/well) 36 h after transfection and incubated for further 24 h at 37 °C in CO2 incubator. CellTiter 96 AQuous One Reagent (Promega, Madison, WI) was added into each well and OD490 was measured according to manufacture’s method. The cells were washed three times with PBS and incubated in complete media for another 24 h. The assay was repeated and the ratio of OD490 to that of the first assay was calculated as the relative cell proliferate rate.
Cell adhesion assay
Cells were detached from culture flasks by trypsinisation 48 h after transfection and inoculated into 96 well plates (5000/well). After 2 h incubation, unattached cells were then removed by washing 3 times with PBS. CellTiter 96 AQuous One Reagent was added into each well and OD490 was measured. The percentage adhesion was determined by comparing the OD490 of attached cells to that of corresponding cells without washing.
Statistics
Results are expressed as mean ± S.E.M. The statistical significance was determined with paired or grouped t-test.
RESULTS
GKLF expression in esophageal cancer
The GKLF mRNA expression was determined semi-quantitively using RT-PCR method. In 17 pairs of tissue specimens, GKLF expression was detected in all samples as an expected PCR product of 376 bp in length, and 14 of them showed decreased expression in cancer sample compared to corresponding normal tissue. Paired t-test has a significant difference (P < 0.05) between cancer sample and normal tissue. Representative cases of GKLF expression detected by RT-PCR are shown in Figure 1.
Figure 1.
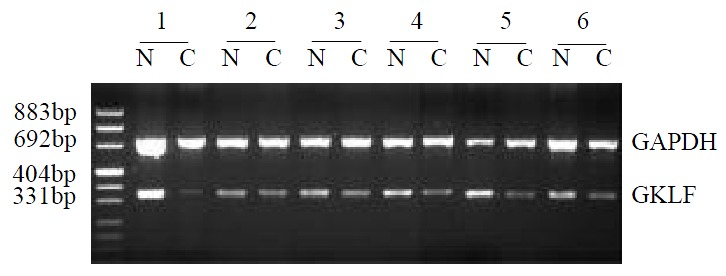
Semi-quantitative RT-PCR of GKLF in esophageal squamous cancer patients. RNA from specimens of normal appearing mucosa (N) and cancer (C) were extracted and GKLF as well as GAPDH were amplified. This figure showed the rep-resentative results from several individual patients.
GKLF cDNA coding sequence
GKLF gene resides at 9q31 of human genome[3], which is a frequent site of LOH in esophageal cancer[15,16]. Gene mutation of GKLF coding sequence was also reported in a colon cancer cell line[14]. In this Laboratory, the genomic alterations were observed in squamous esophageal cancer cell line EC9706 which includes 9q region by comparative genomic hybridization (unpublished data). To find out if GKLF cDNA was mutated in esophageal cancer cell line EC9706, the full length cDNA of coding region of GKLF was amplified by RT-PCR from cDNA of EC9706. An 1.5 kb length of PCR product was detected as expected. The single band was cloned into pMD18T vector and sequenced. The sequence conformed to cDNA of GKLF (GeneBank Accession number AF105036) completely without any mutation.
Serum deprivation induction of GKLF
GKLF expression was induced in murine fibroblast by serum deprivation[1]. As GKLF expression was down-regulated in esophageal squamous cell carcinoma, we were interested in observing the inducibility of GKLF expression in esophageal cancer by serum deprivation. The cell line EC9706 was serum deprived and GKLF mRNA expression was detected by RT-PCR at 0 h, 6 h, 1 d, 2 d and 3 d. A primary culture of human fibroblast was also serum deprived as a positive control. Figure 2 showed the inducibility of GKLF by serum deprivation in both EC9706 and fibroblast. Although GKLF was induced in EC9706, the level was greatly decreased compared to that in human fibroblast.
Figure 2.
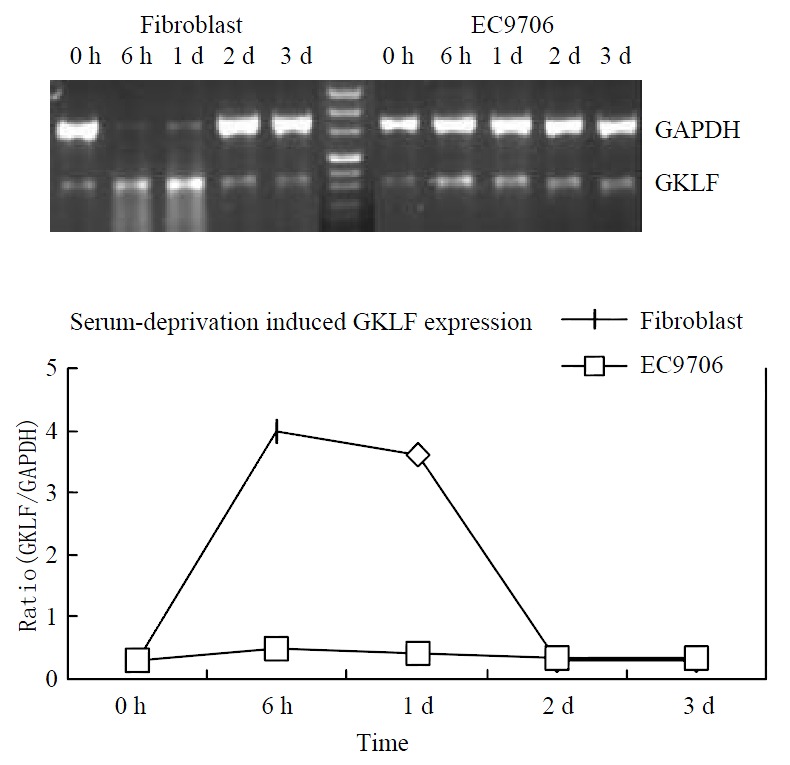
Serum deprivation induced GKLF expression. Both human primary cultured fibroblast and an esophageal squa-mous cancer cell line EC9706 was underwent serum deprivation. GKLF expression was measured semi-quantitively by RT-PCR. The magnitude of GKLF expression was calcu-lated as ratio of GKLF to GAPDH.
Cell growth and adhesion properties in esophageal cancer cells with low GKLF expression
It was reported that forced overexpression of GKLF inhibited DNA synthesis[1] and decreased cell proliferation[17]. We transiently transfected the esophageal cancer cell line EC9706 with antisense GKLF expression vector, which caused low expression of GKLF in the transfected cells (shown in Figure 3). The cell proliferative rate was assayed as described above. Compared to the controlled cells, the low GKLF expression cells grew at a greater rate with a significant difference (P < 0.05). As we noticed that cell adhesion ability changed in individual stable transfectants of EC9706 with GKLF expression vectors (data not shown), cell adhesion assay was also carried out in these transiently transfected cells with low GKLF expression. In these cells, the adhesion ability was decreased significantly compared to the controlled cells (P < 0.05) (Figure 3).
Figure 3.
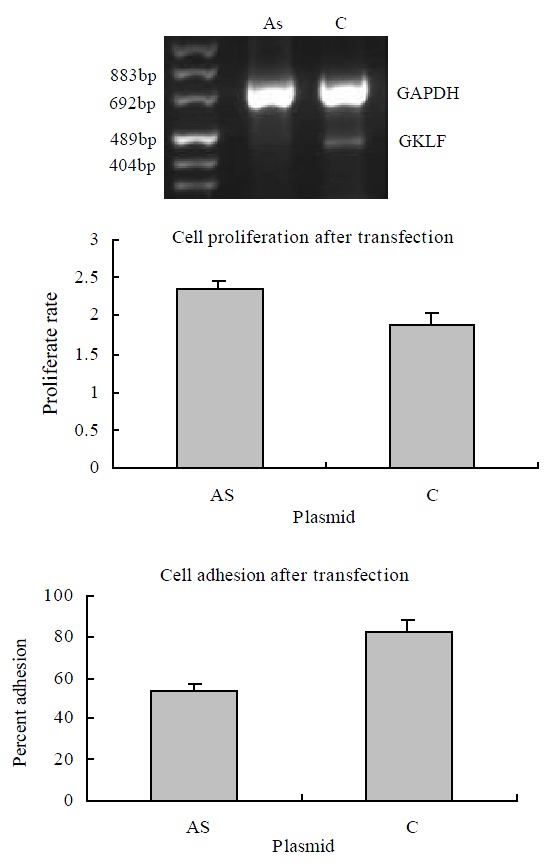
Cell growth and adhesion in GKLF transfected EC9706 cells. The cells were transiently transfected with antisense GKLF expression plasmid (AS) and pCDNA3.1 as control (C). The GKLF expression levels detected by RT-PCR were shown in the upper panel. The cell growth rates were shown in the middle panel; each data point represents mean ± S.E.M. of 7 repeats; there was a significant difference between AS and C (P < 0.05). The cell adhesions were shown in the lower panel; each data point represents mean ± S.E.M. of 4 repeats; AS had significant difference compared to C (P < 0.05).
DISCUSSION
Alterations of GKLF expression in cancer cells are tissue and tumor type-specific. Our current findings showed that GKLF was down-regulated in esophageal squamous cell carcinoma. It is known that down-regulation of GKLF occurred in intestinal adenoma and colonic adenoma[6] while up-regulation of GKLF occurred in breast carcinomas and oral squamous cell carcinoma[9]. The mechanism of altered GKLF expression and its difference among different tumor type is not clear yet. The results from colon cancer cells[6] showed that no involvement of DNA methylation in the down-regulation of GKLF, while mutated CDX2 expression in some colon cancer cell lines may help to explain the low levels of GKLF expression[18].
Serum deprivation induces growth arrest and apoptosis in normal cells while the inducibility of serum deprivation is usually decreased in cancer cells. Experimental evidence showed that p21[19] and cyclin D1[20] involved in the serum deprivation induces growth arrest and apoptosis. In some kind of cancer cells, serum deprivation did not induce the expression of p21[21]. As a transcription factor, GKLF involves in the p53-p21 regulating pathway[22]. It also regulates the cell cycle factors such as cyclin D1[23]. Decreased inducibility of GKLF by serum deprivation in EC9706 cells may suggest a role of GKLF in decreased response of cancer cells to serum deprivation.
GKLF overexpression inhibits proliferation of NIH3T3 and of HT-29 colon cancer cells[1,7]. Similar to the report[7], we found that antisense expression of GKLF promotes cellular proliferation of EC9706 cells. Combined with the finding that GKLF was down-regulated in esophageal cancer tissues, the result suggested that GKLF might play a role in the initiation and/or progression of esophageal cancer. It was shown that GKLF exerted its effect on cell proliferation at G1 phase of cell cycle[7,24]. That GKLF mediates the p53-p21 regulating pathway[22] could be a mechanism of its cell cycle effect as this pathway is important for the regulation of G1/S transition[25]. Cyclin D1-RB pathway is another possible effecting pathway of GKLF for its cell cycle regulation, since GKLF directly down-regulates cyclin D1 expression[23] while cyclin D1 facilitates progression through the G1 phase which plays a role in the initiation of esophageal cancer[26]. Overexpression of cyclin D1 was observed in the early phase of esophageal tumors in zinc-deficient rat model[27], suggesting an important role of zinc proteins in regulating cyclin D1 expression, and conforming to our proposition that down-regulation of GKLF plays a role in the initiation and/or progression of esophageal cancer.
The basic mechanism of tumour cell metastasis is reduced expression of adhesion molecules which results in an increased migratory ability of the cancer cells[28]. We showed here that alteration of GKLF expression in EC9706 cell decreased its adhesion, implicating that GKLF involves in the metastasis of esophageal cancer. Abnormal expression of such cell adhesion molecules as cadherin, integrin and mucin has definite relationship with cancer metastasis[29-31]. It is known that GKLF could regulate MUC5B gene transcription directly[32]. GKLF also regulates laminin expression[33,34] while laminin is a component of extracellular matrix which plays a key role in cell adhesion and migration. More frequent allelic loss at 9q region where the GKLF gene located was reported in esophageal squamous cell carcinoma patients with metastasis[15], suggesting a clinically significant role of GKLF gene in esophageal cancer.
GKLF belongs to a family of Krüppel-like factors that is the most abundant transcription factors in human cells[35]. It targets to the specific DNA sequence[36] which represents one of the most abundant classes of conserved motifs of intergenic regions over the human genome[37], besides to the common DNA sequences through which this family of factors binds and acts[36,38]. More and more target genes are being discovered[39,40], that will expedite our understandings of GKLF mediated action in both normal and malignant cells.
In summary, the results of this study showed that GKLF is down-regulated in esophageal squamous cell cacinoma, and GKLF deregulation in esophageal cancer cell line EC9706 sho wes a manner of decrease inducibility to serum deprivation. Furthermore, down-regulation of GKLF in EC9706 cells results in the proliferation and decreased adhesion of cancer cells, suggesting that down-regulation of GKLF may contribute to malignant phenotype of esophageal cancer.
Footnotes
Supported by China Key Program on Basic Research, No. G1998051021 and National Natural Science Foundation of China, No. 39993420
Edited by Zhang JZ
References
- 1.Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrett-Sinha LA, Eberspaecher H, Seldin MF, de Crombrugghe B. A gene for a novel zinc-finger protein expressed in differentiated epithelial cells and transiently in certain mesenchymal cells. J Biol Chem. 1996;271:31384–31390. doi: 10.1074/jbc.271.49.31384. [DOI] [PubMed] [Google Scholar]
- 3.Yet SF, McA'Nulty MM, Folta SC, Yen HW, Yoshizumi M, Hsieh CM, Layne MD, Chin MT, Wang H, Perrella MA, et al. Human EZF, a Krüppel-like zinc finger protein, is expressed in vascular endothelial cells and contains transcriptional activation and repression domains. J Biol Chem. 1998;273:1026–1031. doi: 10.1074/jbc.273.2.1026. [DOI] [PubMed] [Google Scholar]
- 4.Segre JA, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- 5.Ton-That H, Kaestner KH, Shields JM, Mahatanankoon CS, Yang VW. Expression of the gut-enriched Krüppel-like factor gene during development and intestinal tumorigenesis. FEBS Lett. 1997;419:239–243. doi: 10.1016/s0014-5793(97)01465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dang DT, Bachman KE, Mahatan CS, Dang LH, Giardiello FM, Yang VW. Decreased expression of the gut-enriched Krüppel-like factor gene in intestinal adenomas of multiple intestinal neoplasia mice and in colonic adenomas of familial adenomatous polyposis patients. FEBS Lett. 2000;476:203–207. doi: 10.1016/s0014-5793(00)01727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shie JL, Chen ZY, O'Brien MJ, Pestell RG, Lee ME, Tseng CC. Role of gut-enriched Krüppel-like factor in colonic cell growth and differentiation. Am J Physiol Gastrointest Liver Physiol. 2000;279:G806–G814. doi: 10.1152/ajpgi.2000.279.4.G806. [DOI] [PubMed] [Google Scholar]
- 8.Foster KW, Ren S, Louro ID, Lobo-Ruppert SM, McKie-Bell P, Grizzle W, Hayes MR, Broker TR, Chow LT, Ruppert JM. Oncogene expression cloning by retroviral transduction of adenovirus E1A-immortalized rat kidney RK3E cells: transformation of a host with epithelial features by c-MYC and the zinc finger protein GKLF. Cell Growth Differ. 1999;10:423–434. [PubMed] [Google Scholar]
- 9.Foster KW, Frost AR, McKie-Bell P, Lin CY, Engler JA, Grizzle WE, Ruppert JM. Increase of GKLF messenger RNA and protein expression during progression of breast cancer. Cancer Res. 2000;60:6488–6495. [PubMed] [Google Scholar]
- 10.Jenkins TD, Opitz OG, Okano J, Rustgi AK. Transactivation of the human keratin 4 and Epstein-Barr virus ED-L2 promoters by gut-enriched Krüppel-like factor. J Biol Chem. 1998;273:10747–10754. doi: 10.1074/jbc.273.17.10747. [DOI] [PubMed] [Google Scholar]
- 11.Brembeck FH, Rustgi AK. The tissue-dependent keratin 19 gene transcription is regulated by GKLF/KLF4 and Sp1. J Biol Chem. 2000;275:28230–28239. doi: 10.1074/jbc.M004013200. [DOI] [PubMed] [Google Scholar]
- 12.Miura K, Suzuki K, Tokino T, Isomura M, Inazawa J, Matsuno S, Nakamura Y. Detailed deletion mapping in squamous cell carcinomas of the esophagus narrows a region containing a putative tumor suppressor gene to about 200 kilobases on distal chromosome 9q. Cancer Res. 1996;56:1629–1634. [PubMed] [Google Scholar]
- 13.Hu N, Roth MJ, Polymeropolous M, Tang ZZ, Emmert-Buck MR, Wang QH, Goldstein AM, Feng SS, Dawsey SM, Ding T, et al. Identification of novel regions of allelic loss from a genomewide scan of esophageal squamous-cell carcinoma in a high-risk Chinese population. Genes Chromosomes Cancer. 2000;27:217–228. doi: 10.1002/(sici)1098-2264(200003)27:3<217::aid-gcc1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 14.Shie JL, Tseng CC. A nucleus-localization-deficient mutant serves as a dominant-negative inhibitor of gut-enriched Krüppel-like factor function. Biochem Biophys Res Commun. 2001;283:205–208. doi: 10.1006/bbrc.2001.4762. [DOI] [PubMed] [Google Scholar]
- 15.Hu N, Roth MJ, Emmert-Buck MR, Tang ZZ, Polymeropolous M, Wang QH, Goldstein AM, Han XY, Dawsey SM, Ding T, et al. Allelic loss in esophageal squamous cell carcinoma patients with and without family history of upper gastrointestinal tract cancer. Clin Cancer Res. 1999;5:3476–3482. [PubMed] [Google Scholar]
- 16.Roth MJ, Hu N, Emmert-Buck MR, Wang QH, Dawsey SM, Li G, Guo WJ, Zhang YZ, Taylor PR. Genetic progression and heterogeneity associated with the development of esophageal squamous cell carcinoma. Cancer Res. 2001;61:4098–4104. [PubMed] [Google Scholar]
- 17.Chen ZY, Shie J, Tseng C. Up-regulation of gut-enriched krüppel-like factor by interferon-gamma in human colon carcinoma cells. FEBS Lett. 2000;477:67–72. doi: 10.1016/s0014-5793(00)01764-6. [DOI] [PubMed] [Google Scholar]
- 18.Dang DT, Mahatan CS, Dang LH, Agboola IA, Yang VW. Expression of the gut-enriched Krüppel-like factor (Krüppel-like factor 4) gene in the human colon cancer cell line RKO is dependent on CDX2. Oncogene. 2001;20:4884–4890. doi: 10.1038/sj.onc.1204645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duttaroy A, Qian JF, Smith JS, Wang E. Up-regulated P21CIP1 expression is part of the regulation quantitatively controlling serum deprivation-induced apoptosis. J Cell Biochem. 1997;64:434–446. [PubMed] [Google Scholar]
- 20.Driscoll B, Buckley S, Barsky L, Weinberg K, Anderson KD, Warburton D. Abrogation of cyclin D1 expression predisposes lung cancer cells to serum deprivation-induced apoptosis. Am J Physiol. 1999;276:L679–L687. doi: 10.1152/ajplung.1999.276.4.L679. [DOI] [PubMed] [Google Scholar]
- 21.Modiano JF, Ritt MG, Wojcieszyn J, Smith R. Growth arrest of melanoma cells is differentially regulated by contact inhibition and serum deprivation. DNA Cell Biol. 1999;18:357–367. doi: 10.1089/104454999315259. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Geiman DE, Shields JM, Dang DT, Mahatan CS, Kaestner KH, Biggs JR, Kraft AS, Yang VW. The gut-enriched Kruppel-like factor (Kruppel-like factor 4) mediates the transactivating effect of p53 on the p21WAF1/Cip1 promoter. J Biol Chem. 2000;275:18391–18398. doi: 10.1074/jbc.C000062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shie JL, Chen ZY, Fu M, Pestell RG, Tseng CC. Gut-enriched Krüppel-like factor represses cyclin D1 promoter activity through Sp1 motif. Nucleic Acids Res. 2000;28:2969–2976. doi: 10.1093/nar/28.15.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Johns DC, Geiman DE, Marban E, Dang DT, Hamlin G, Sun R, Yang VW. Krüppel-like factor 4 (gut-enriched Krüppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J Biol Chem. 2001;276:30423–30428. doi: 10.1074/jbc.M101194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartek J, Lukas J. Pathways governing G1/S transition and their response to DNA damage. FEBS Lett. 2001;490:117–122. doi: 10.1016/s0014-5793(01)02114-7. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa H, Wang TC, Zukerberg L, Odze R, Togawa K, May GH, Wilson J, Rustgi AK. The targeting of the cyclin D1 oncogene by an Epstein-Barr virus promoter in transgenic mice causes dysplasia in the tongue, esophagus and forestomach. Oncogene. 1997;14:1185–1190. doi: 10.1038/sj.onc.1200937. [DOI] [PubMed] [Google Scholar]
- 27.Fong LY, Nguyen VT, Farber JL, Huebner K, Magee PN. Early deregulation of the the p16ink4a-cyclin D1/cyclin-dependent kinase 4-retinoblastoma pathway in cell proliferation-driven esophageal tumorigenesis in zinc-deficient rats. Cancer Res. 2000;60:4589–4595. [PubMed] [Google Scholar]
- 28.Cavallaro U, Christofori G. Cell adhesion in tumor invasion and metastasis: loss of the glue is not enough. Biochim Biophys Acta. 2001;1552:39–45. doi: 10.1016/s0304-419x(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 29.Madhavan M, Srinivas P, Abraham E, Ahmed I, Mathew A, Vijayalekshmi NR, Balaram P. Cadherins as predictive markers of nodal metastasis in breast cancer. Mod Pathol. 2001;14:423–427. doi: 10.1038/modpathol.3880329. [DOI] [PubMed] [Google Scholar]
- 30.Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001;61:3819–3825. [PubMed] [Google Scholar]
- 31.Kim YS, Gum JR, Crawley SC, Deng G, Ho JJ. Mucin gene and antigen expression in biliopancreatic carcinogenesis. Ann Oncol. 1999;10 Suppl 4:51–55. [PubMed] [Google Scholar]
- 32.Van Seuningen I, Perrais M, Pigny P, Porchet N, Aubert JP. Sequence of the 5'-flanking region and promoter activity of the human mucin gene MUC5B in different phenotypes of colon cancer cells. Biochem J. 2000;348 Pt 3:675–686. [PMC free article] [PubMed] [Google Scholar]
- 33.Miller KA, Eklund EA, Peddinghaus ML, Cao Z, Fernandes N, Turk PW, Thimmapaya B, Weitzman SA. Kruppel-like factor 4 regulates laminin alpha 3A expression in mammary epithelial cells. J Biol Chem. 2001;276:42863–42868. doi: 10.1074/jbc.M108130200. [DOI] [PubMed] [Google Scholar]
- 34.Higaki Y, Schullery D, Kawata Y, Shnyreva M, Abrass C, Bomsztyk K. Synergistic activation of the rat laminin gamma1 chain promoter by the gut-enriched Kruppel-like factor (GKLF/KLF4) and Sp1. Nucleic Acids Res. 2002;30:2270–2279. doi: 10.1093/nar/30.11.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bieker JJ. Krüppel-like factors: three fingers in many pies. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 36.Shields JM, Yang VW. Identification of the DNA sequence that interacts with the gut-enriched Krüppel-like factor. Nucleic Acids Res. 1998;26:796–802. doi: 10.1093/nar/26.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondrashov AS, Shabalina SA. Classification of common conserved sequences in mammalian intergenic regions. Hum Mol Genet. 2002;11:669–674. doi: 10.1093/hmg/11.6.669. [DOI] [PubMed] [Google Scholar]
- 38.Philipsen S, Suske G. A tale of three fingers: the family of mammalian Sp/XKLF transcription factors. Nucleic Acids Res. 1999;27:2991–3000. doi: 10.1093/nar/27.15.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen ZY, Shie JL, Tseng CC. Gut-enriched Kruppel-like factor represses ornithine decarboxylase gene expression and functions as checkpoint regulator in colonic cancer cells. J Biol Chem. 2002;277:46831–46839. doi: 10.1074/jbc.M204816200. [DOI] [PubMed] [Google Scholar]
- 40.Zelko IN, Folz RJ. Myeloid zinc finger (MZF)-like, Kruppel-like and Ets families of transcription factors determine the cell-specific expression of mouse extracellular superoxide dismutase. Biochem J. 2003;369:375–386. doi: 10.1042/BJ20021431. [DOI] [PMC free article] [PubMed] [Google Scholar]


