Abstract
AIM: The E-cadherin-catenin complex is important for cell-cell adhesion of epithelial cells. Impairment of one or more components of this complex is associated with poor differentiation and increased invasiveness of carcinomas. We evaluated the expression pattern of E-cadherin and β-catenin in gastric carcinoma and dysplasia and analyzed their relationship with tumor clinicopathological features and patient survival.
METHODS: Immunohistochemical staining of E-cadherin and β-catenin was performed from paraffin specimens of 163 gastric carcinomas, 44 gastric mucosal dysplasia, and 25 intestinal metaplasia, 28 atrophic gastritis and 12 healthy controls.
RESULTS: Normal membrane staining was observed in intestinal metaplasia, atrophic gastritis and control biopsy specimens for E-cadherin and β-catenin. 36% and 16% of gastric dysplasia were stained abnormally for E-cadherin and β-catenin respectively. Abnormal expression of E-cadherin and β-catenin was demonstrated in 46% and 44% of gastric carcinoma respectively. Abnormal expression of E-cadherin and β- catenin occurred more significantly in Borrmann III/IV than in Borrmann I/II type (P < 0.005, respectively). A significantly higher proportion of signet-ring, mucinous and tubular adenocarcinomas were abnormally expressed for E-cadherin and β-catenin as compared with papillary adenocarcinomas (χ2 = 8.47, P < 0.005, and χ2 = 7.05, P < 0.01, respectively). Morever, abnormal E-cadherin and β-catenin staining occurred more frequently in diffuse than in intestinal type of tumor (χ2 = 18.18 and 17.79, P < 0.005, respectively). There was a significant correlation between abnormal β-catenin expression and positive lymph node metastasis. A survival advantage was noted in tumors retaining normal membranous expression of β-catenin, independent of type, grade, or stage of the disease (P < 0.0005).
CONCLUSION: Abnormal expression of the E-cadherin-catenin complex occurs frequently in gatric carcinoma, closely related to its histogenesis. Abnormal expression of the E-cadherin- catenin complex in gastric dysplasia may be an early event in the tumorigenesis. The close correlation with poor survival suggests that abnormal β-catenin may be a useful prognostic marker.
INTRODUCTION
It is well-known that anchorage of cells to substrate is critical for the integrity of many cell types including epithelial cells. The cadherins are a major class of adhesion molecules which play an important role in the homotypic cell-cell adhesion and hence cancer cell metastasis and invasion. E-cadherin is a member of the cadherin family which is expressed in all epithelial cells[1,2]. It is a calcium-dependent cell adhesion molecule that binds to other E-cadherin molecules on adjacent cells and is located at the adhesion junction of epithelial cells[1]. Functional cadherin-dependent cell adhesion requires the formation of complexes between E-cadherin and the cytoplasmic proteins known as the catenins. The catenin, which interacts with the cytoplasmic domain of E-cadherin, consists of at least three proteins: α-,β-, and γ- catenin. β-catenin forms a complex with E-cadherin, while α- catenin links this complex to the actin cytoskeleton[3,4]. It is postulated that changes in cell-cell and cell-matrix interactions account for the ability of cancer cells to cross normal tissue boundaries and metastasize[5]. In addition, loss of cell adhesion may contribute to loss of contact inhibition and thus play a role at the earlier stage of the neoplastic process.
Previous studies have provided evidence that perturbation of E-cadherin-mediated cell adhesion is involved in tumor progression and metastasis[6,7]. Loss of E-cadherin expression or function in vitro has been associated with loss of differentiation and increased invasive capacity of cancer cell lines[8,9]. A variety of human cancers exhibit altered expression of E-cadherin that correlates with high grade and advanced tumor stage[10-12]. These changes may result from deletions, point mutations of the E-cadherin genes or CpG methylation of the promoter region.
β-catenin appears to bind directly and most tightly to E-cadherin and so affects the strength of cell-cell adhesion[13]. It is also involved in the Wingless/Wnt signaling pathway and interacts with epidermal growth factor receptor[14,15], with the APC tumor suppressor gene, and with two novel nuclear transcription factors,T-cell factor (TCF)-4 and lymphoid enhancing factor (LEF)-1[16,17].
Gastric cancer is the second most common malignancy worldwide, and is among the leading causes of mortality in countries such as Japan, China, and Chile. Even in the developed Western countries, the 5-year survival rate for gastric cancer is only 10%-19%[18]. In China, gastric cancer is the most common malignancy diagnosed annually, with a high cancer-related mortality (25%)[19]. Among the highly prevalent geographic areas of gastric cancer is Lanzhou, a city of northwest China. It is currently unknown what factors contribute to the development, progression, and metastasis of gastric cancers in this geographic area[20,21].
Reduced or completely lost E-cadherin-catenin complex expression has been found in gastric, although results regarding the degree of aberrant E-cadherin-catenin complex expression and its relationship with clinicopathological features and patient survival are contradictory. In this study, by using the immunohistochemical staining, we investigated the expression pattern of E-cadherin or β-catenin in gastric cancers, dysplasia, intestinal metaplasia, atrophic gastritis and normal gastric mucosa and the possible role of E-cadherin- catenin complex in the pathogenesis of gastric cancers in Lanzhou area. The possible relationship between the expression of the E-cadherin and β-catenin and the tumor clinicopathology, as well as its potential value in the evaluation of patient survival were also discussed.
MATERIALS AND METHODS
Patients and tissue samples
The specimens of gastric cancers were obtained from 185 consecutive patients who underwent gastrectomy at the Department of Surgery (First Teaching Hospital, Lanzhou Medical College, Lanzhou, P. R. China) between January 1995 and December 1996. None of the patients received chemotherapy or radiotherapy before surgery. Clinico-pathological information and survival data were obtained from hospital records and patients’ doctor in charge. Six patients who died within 4 weeks following surgery were excluded from the survival analysis for the purpose of eliminating bias caused by surgical operation-related death. In sixteen cases, suitable well-preserved blocks could not be obtained, thus a total of 163 patients were included in the final study. There were 123 males and 40 females (3.08:1), with median age of 54.5 years (range, 28-77 years). Samples were taken from the representative cancerous lesions as well as adjacent non-cancerous mucosa. In addition, mucosal biopsy specimens from 32 patients with dysplasia (plus 12 cases from gastrectomy, total 44 cases), 25 with intestinal metaplasia, 28 with atrophic gastritis and 12 healthy controls were examined.
For microscopic examination, tissues were routinely fixed with formalin before being embedded in paraffin. A 4-μm section from each specimen block was stained with H & E for histological evaluation, and representative blocks were chosen for immunohistochemical study. In addition, normal colonic epithelium was used as positive control and adjacent normal gastric mucosa as internal control. Negative controls included adjacent section of the same block in which the primary antibody was replaced by phosphate-buffered saline (PBS).
Tumor staging and classification
Tumors were staged at the time of surgery by the standard criteria for TNM staging using the unified international gastric cancer staging classification[22] and the following morphological details were recorded: depth of invasion (pT category), lymph node involvement (pN category). By Lauren system[23], tumors were classified into intestinal and diffuse types. According to the criteria of WHO classification, tumors were classified into adenocarcinoma, which was defined as papillary adenocarcinoma, tubular adenocarcinoma, mucinous adenocarcinoma and signet-ring cell carcinoma, and undifferenciated carcinoma. The gross appearance of tumors was diagnosed using the Borrmann’s classification. Dysplasia was diagnosised according to the Padova International Classification[24] for gastric dysplasia.
Antibodies and other chemicals
The following items were purchased from Maxim Biotech (Maxim Biotech Inc., South San Francisco, CA, USA): mouse monoclonal antibodies against human E-cadherin and β-catenin; UltraSensitive S-P Kit; and peroxidase-conjugated streptavidin. Diaminobenzidine tetrahydrochloride (DAB) and other routine chemicals were obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA).
Immunohistochemical staining
To detect the presence and patterns of E-cadherin and β-catenin, we utilized the peroxidase-conjugated streptavidin immunostaining technique, as previously described by others[25]. Briefly, 4 μm tissue sections were dewaxed and rehydrated through changes of xylene and graded alcohol, then to water. Endogenous peroxidase activity was blocked by incubating the sections with 0.6% hydrogen peroxide for 10 min. Heat-mediated antigen retrieval was performed by heating the sections (immersed in 0.01 M citrate buffer, pH 6.0) in a microwave oven (750 W) for 20 min. The slides were then washed with PBS before being exposed to 10% normal goat serum for 10 min to block the non-specific background reaction. The slides were then incubated with respective primary antibody overnight at 4 °C. Following washes with PBS, the slides were incubated for 15 min with the secondary antibody, biotinylated goat anti-mouse IgG.The slides were further washed for 3 × 10 min in PBS, followed by incubation with peroxidase-conjugated streptavidin for 10 min. The peroxidase reaction was developed in PBS using hydrogen peroxide as substrate and DAB as a chromogen. Sections were counterstained with haematoxylin, dehydrated, and evaluated under a light microscope.
Evaluation of immunostaining
Slides were independently examined by two experienced pathologists who were blinded to the stage of the tumor and to the initial score of the other observer, and a high level of concordance (90%) was achieved. In case of disagreement, the slides were reviewed and a consensus view achieved. Staining intensity was graded semiquantitatively from 0 to 3, as previously described[25,26]. 0:negative staining; 1: cytoplasmic staining; 2: heterogeneous staining (tumors composing of both normal and abnormal staining areas); and 3: a normal membranous staining. Because the staining pattern sometimes varied within the same tumor particularly when the differentiation status varied, the final score was based on the dominant pattern. For the ease of data analysis, all tumors with loss of membranous expression were classified as abnormal which included those with absent,heterogeneous or cytoplasmic staining patterns.
Statistical analysis
The correlation between the expression of the E-cadherin or β-catenin and clinicopathological features was analysed for statistical significance by the χ2 (chi-square) test. Survival curves were constructed according to the method of Kaplan and Meier. For differences between curves, the P value was calculated using the log rank test. A multivariate Cox regression analysis was performed to assess the prognostic significance of different staining patterns and their relationship with other pathological variables. A P value of less than 0.05 was considered as statistically significant. All statistical analyses were performed using the SPSS 8.0 statistical package (USA).
RESULTS
Immunohistochemical staining of E-cadherin and β-catenin
Normal gastric mucosa Gastric mucosa specimen from 12 normal health controls were examined. E-cadherin and β-catenin stained intensely in a membranous distribution throughout the epithelium in all the normal control cases (Figure 1).
Figure 1.
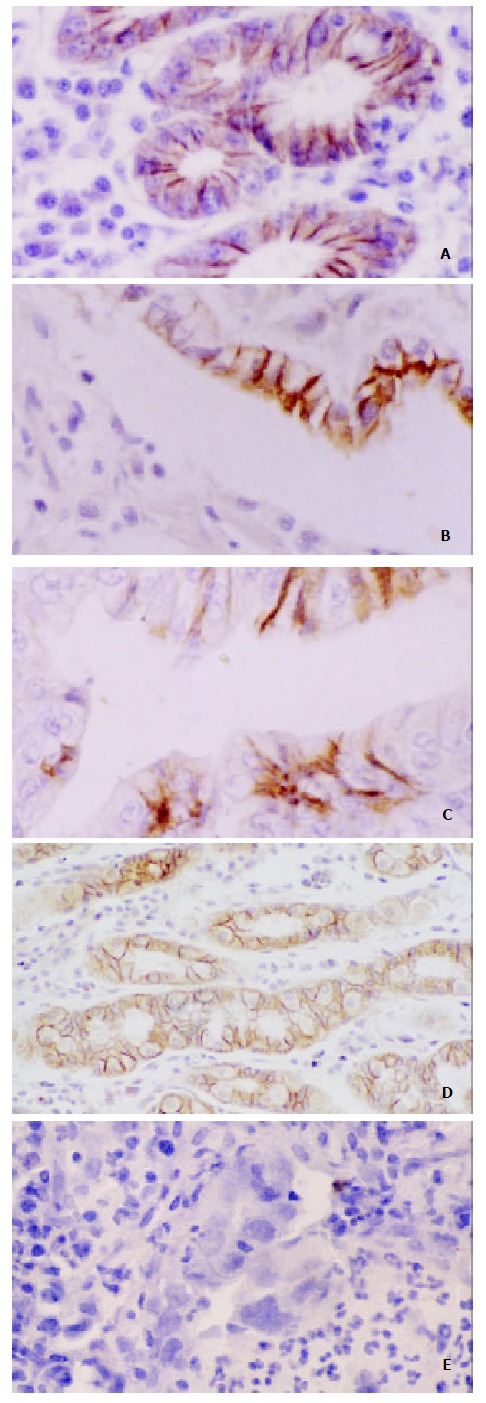
Immunoreactivity of E-cadherin and β-catenin in Gastric Carcinoma. A. Normal membranous staining pattern of E-cadherin (SP × 200); B. Poorly differentiated tumor with complete loss of membraous staining with E-cadherin in the majority of cells. Membranous staining can be seen in a few cells retaining gastric gland (SP×400); C. Morderately differ-entiated tumor showing cytoplasmic staining with β-catenin, partly nuclear β-catenin expression (SP × 400); D. Staining of E-cadherin showing preserved strong membranous expression in gastric mucosa with intestinal metaplasia (SP × 200); E. Mu-cosal biopsy specimen with dysplasia (high grade) showing loss of membranous expression of E-cadherin.(SP × 400)
Atrophic gastritis, intestinal metaplasia, dysplasia and gastric cancer Areas of atrophic gastritis, intestinal metaplasia showed normal membranous distribution of staining for E-cadherin and β-catenin in all cases. Abnormal expression was shown in 36.4% (16/44) of dysplasia for E-cadherin, and in 15.9% (7/44) for β-catenin. Of the total of 163 primary tumors examined, the abnormal expression rate of E-cadherin and β-catenin were 46.0% and 44.8%, respectively (Table 1). Representative examples were shown in Figure 1.
Table 1.
Abnormal expression of E-cadherinin and β-catenin in gastric carcinoma and precancerous condition
| Total n | E-cadherin n(%) | β-catenin n(%) | |
| Gastric carcinoma | 163 | 75 (46.0) | 73 (44.8) |
| Dysplasia | 44 | 16 (36.4) | 7 (15.9) |
| Intestinal metaplasia | 25 | 0 | 0 |
| Atrophic gastritis | 28 | 0 | 0 |
| Health controls | 12 | 0 | 0 |
Expression of E-cadherin or β-catenin and gross appearance in gastric cancer
The frequencies of abnormal expression in tumors less than 5 cm, 5.1-10 cm and more than 10 cm in length were 33.3%, 35.5%, and 74.4% for E-cadherin, respectively,and 28.2%, 36.2%, and 78.9% for β-catenin, respectively (Table 2). The rate of abnormal expression in tumors more than 10 cm was significantly higher than that in tumors less than 10 cm in length (P < 0.005, respectively). As shown in Table 2, analysis of 139 cases of gastric cancer (24 cases were excluded from 163 cases because of incomplete surgical record) revealed that the expression of E-cadherin and β-catenin were significantly correlated with the Borrmann classification: Abnormal expression occurred more significantly often in Borrmann III and Borrmann IVthan in Borrmann Iand Borrmann IItype (P < 0.005, respectively).
Table 2.
Expression of E-cadherin or β-catenin and Gross Ap-pearance
| No |
E-cadherin expression (%) |
β-catenin expression (%) |
|||
| Abnormal | P value | Abnormal | P value | ||
| Tumor size (cm) | 163 | ||||
| 0-4.9 | 48 | 16 (33.3) | 14 (28.2) | ||
| 5.0-9.9 | 76 | 30 (35.5) | 16.58 | 29 (36.2) | 21.41 |
| ≥ 10.0 | 39 | 29 (74.4) | < 0.005a | 30 (78.9) | < 0.005a |
| Gross appearance | 139 | ||||
| BorrmannI | 12 | 1 (8.3) | 2 (16.7) | ||
| BorrmannII | 16 | 3 (18.8) | 3 (18.8) | ||
| BorrmannIII | 80 | 35 (43.8) | 11.92 | 32 (40.0) | 9.65 |
| BorrmannIV | 31 | 21 (67.7) | < 0.005b | 24 (77.4) | < 0.005b |
≥ 10.0 group vs 0-4.9 or 5-9.9 group;
Borrmann IIIand Borrmann IVgroups vs BorrmannI and Borrmann IIgroups
Expression of E-cadherin or β-catenin and histopathological findings
Table 3 shows no correlation could be verified between expression of E-cadherin or β-catenin and the invasion depth of the tumor (T1/T2 vs T3/T4). Among the 163 cases of gastric cancers in our series, lymphatic metastasis was observed in 92 cases (56.4%). We found that there was no correlation between the abnormal expression of E-cadherin and the positivity of lymphatic metastasis. However, the expression of β-catenin was closely correlated with lymphatic metastasis (P < 0.005).
Table 3.
Relationship Between Expression of E-cadherin or β-catenin and Histopathological Features in Gastric Carcinoma
| No |
E-cadherin expression (%) |
β-catenin expression (%) |
|||
| Abnormal | P value | Abnormal | P value | ||
| pT category | |||||
| T1/T2 | 65 | 28 (43.1) | 29 (44.6) | ||
| T3/T4 | 98 | 47 (48.0) | NS | 44 (44.9) | NS |
| pN category | |||||
| N-negative | 71 | 29 (40.8) | 20 (28.2) | 14.56 | |
| N-positive | 92 | 46 (50.0) | NS | 53 (57.6) | < 0.005 |
| WHO classification | |||||
| Adenocarcinoma | 160 | ||||
| Papillary | 16 | 3 (18.8)a | 2 (7.1)a | ||
| Tubular | 109 | 48 (44.0) | 46 (42.2) | ||
| Mucinous | 24 | 13 (54.2) | 8.47 | 15 (62.5) | 7.05 |
| Signet ring | 11 | 8 (72.7) | < 0.005 | 7 (63.3) | < 0.01 |
| Undiff. carcinoma | 3 | 3 (100) | 3 (100) | ||
| Lauren classification | |||||
| Intestinal type | 108 | 36 (33.3)b | 18.18 | 34 (31.5)b | 17.79 |
| Diffuse type | 40 | 29 (72.5) | < 0.005 | 28 (70.0) | < 0.005 |
| Mixed type | 15 | 10 (66.7) | 11 (73.3) | ||
Papillary vs Tubular, Mucinous and Signet ring;
Intestinal type vs Diffuse type. NS: No Significance
According to the WHO classification, papillary adenocarci-noma showed abnormal expression of E-cadherin and β-catenin more frequently compared with signet-ring carcinoma and mucinous and tubular adenocarcinoma (P < 0.005 and P < 0.01, respectively). Furthermore, according to the histological classification of Lauren, abnormal expression of E-cadherin and β-catenin was significantly higher in diffuse than in intestinal types (P < 0.005, respectively).
Survival analysis
Survival data were obtained for 113 of the patients. Kaplan-Meier curves were computed to compare survival of patients with normal versus abnormal expression of E-cadherin and β-catenin. The overall median survival of patients in this study was 1067 days (range 78-2365). Average survival time for patients showing abnormal expression of E-cadherin was 1065 ± 118 days, normal expression of E-cadherin was 1508 ± 121 days. Patients with normal expression of E-cadherin appeared to have a longer survival time compared with those who have abnormal E-cadherin expression, although the difference was not statistically significant (P = 0.058). The average survival time for patients with abnormal expression of β-catenin was 764 ± 110 days, as opposed to the survival time of 1798 ± 105 days for patients with normal expression (P < 0.0005) (Figure 2). A multivariate Cox regression analysis confirmed statistically significant association of abnormal β-catenin expression with poor survival (P < 0.0005), independent of tumor type, grade, or stage.
Figure 2.
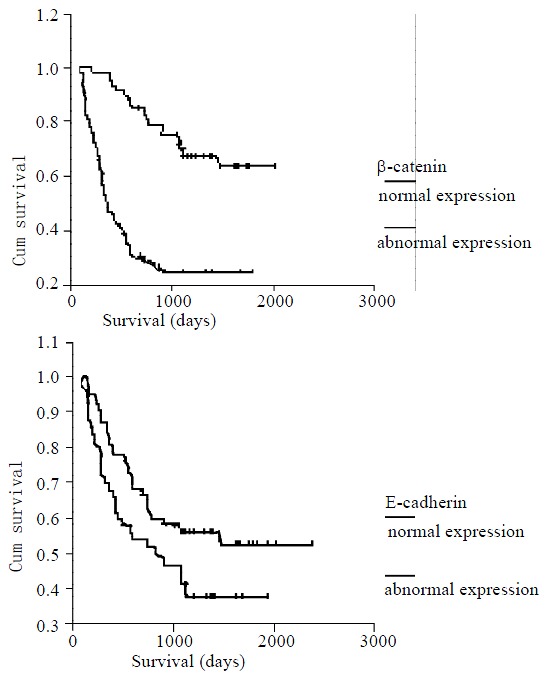
Kaplan-Meier survival curves showing a statistically significant survival advantage found in all tumors with nor-mal membranous expression of β-catenin (B) compared with those with loss of membranous expression (P < 0.0005). Abnormol E-cadherin (A) staining pattern showed a trend to-ward worse survival, but not statistically significant (P = 0.058).
DISCUSSION
Aberrant expression of E-cadherin and β-catenin with loss of membranous localization has been detected in a number of human cancers. Previous studies[27-30] have shown variable abnormal expression of E-cadherin-catenin complex ranging from 20% up to 90% of tumors. In gastric carcinoma or other human carcinomas, the overall expression of E-cadherin-catenin complex as reported in different studies is difficult to compare due to different score systems and different antibodies used. In this study, 46% of gastric carcinomas showed abnormal E-cadherin expression and 44% showed abnormal β-catenin expression, so our data are in line with previous reports which revealed alteration of E-cadherin and β-catenin expression in gastric carcinomas. In addition, by using the Kaplan-Meier curve and A multivariate Cox regression analysis, we found that β-catenin but not E-cadherin expression abnormalities were associated with poor survival, confirming the findings of Jawhari et al[25] and Ramesh et al[48]. However, the use of E-cadherin as a patient survival indicator needs to be further verified, as previous findings by Gabbertz et al[30], Shun et al[29], and Grabsch et al[31] indicated that there was correlation between abnormal E-cadherin expression and patient survival in gastric carcinoma.
We demonstrated a significant correlation between expression of E-cadherin-catenin complex and tumor differentiation. Aberrant E-cadherin or β-catenin expression was associated with diffuse-type (poorly differentiated) carcinomas. Similarly, abnormal expression rate of E-cadherin and β-catenin was significantly higher in low- and un-differentiated adenocarcinomas than in well-differentiated adenocarcinomas. Previous in vitro studies[8] have shown that loss of E-cadherin in human carcinoma cell lines is associated with poor differentiation and fibroblastoid morphology. Furthermore, down-regulation of E-cadherin protein product after E-cadherin-specific antisense RNA transfection results in an invasive and de-differentiated phenotype[9]. Transfection of E-cadherin cDNA into undifferentiated carcinoma cells produces an epithelioid morphology and increases intercellular adhesion. These effects are reversible using anti-E-cadherin antibodies and provide direct in vitro evidence of the crucial role of E-cadherin in the regulation of cell polarity, cellular differentiation, and epithelial morphology[9,32]. Our results support the above findings, suggesting that E-cadherin-catenin complex may play an important role in the genesis of histological differentiation and may be used as a differentiation marker in gastric carcinoma[33,34].
The E-cadherin-mediated cell adhesion system is known to act as an “invasive suppressor system”[24,35,36] and tumors with reduced E-cadherin expression were also reported to have a higher frequency of lymph node involvement, distant metastasis and morphologic degree of invasiveness than those with preserved E-cadherin expression[37,38]. In this study, decreased E-cadherin expression occurred more frequently in tumors of more infiltrative growth on gross appearance or in those of larger size than in their counterparts, but showed no association with the invasion depth of the tumor, and lymph node metastasis. This findings strongly suggests that the presence of E-cadherin as revealed by immunohistochemistry might not indicate that E-cadherin is necessarily functional. For instance, certain mutations in the E-cadherin gene or changes in E-cadherin associated cytoplasmic proteins, the catenin, may alter their adhesive functions. Mutations affecting intercellular adhesion mechanisms have emerged as important steps in the development and progression of many human epithelial tumors. In gastric carcinomas, mutations of the E- cadherin gene have been reported[39-42]. A germline mutation in E-cadherin associated with familial gastric carcinoma was recently reported in a New Zealand kindred[43]. Moreover, in vitro studies with E-cadherin-negative carcinoma cell lines suggested that methylation around the promoter region CpG island may be one mechanism of E-cadherin inactivation in human carcinomas[44,45]. Catenins have been classified into α, β,and γ. The cytoplasmic tail of E-cadherin interacts with either β- or γ-catenins which bridge E-cadherin to the cytoskeleton through α-catenin[46,47]. Many studies indicate that the association of E-cadherin with catenins is essential for their cell-cell adhesion[25,48]. Mutations in α-and β-catenins have not been convincingly demonstrated[49], but protein expression abnormalities are relatively frequent, and occur in both diffuse and intestinal carcinomas[50,51]. As the function of E-cadherin is modulated by α-catenin, loss of α-catenin expression or deletion of α-catenin could suppress E-cadherin-mediated cell-cell adhesion activity, despite normal E-cadherin and β-catenin expression[52]. β-catenin is a multifunctional protein, and plays an important role in Wingless/Wnt signal transduction in addition to its function as a cell-adhesion components[53]. Posttranslation modification of β-catenin molecule by phosphorylation has been shown to disrupt the interaction between E-cadherin and α-catenin, causing loss of E-cadherin-dependent intercellular adhesiveness and in turn potentiating the neoplastic process[53,54]. Furthermore, the association of abnormal β-catenin expression with a worse survival, independent of tumor type, grade, or stage, seems to suggest that it acts as an independent prognostic variable.
The pathogenesis of gastric carcinoma remains largely unknown. Although a number of genetic and molecular alterations have been described in gastric carcinoma[55-57], the exact sequence and number of genetic or molecular alterations are not yet known. There were several patterns postulated on how histomorphological changes of the gastric mucosa will progress to the development of gastric carcinoma. The best known was proposed by Correa et al[58] who described the development of gastric tumors from gastritis through intestinal metaplasia, dysplasia, and eventually to gastric carcinoma. Recent in vitro and in vivo studies have shown that alteration or loss of E-cadherin expression seems to be critical for the development of gastric carcinomas. Furthermore, Ohene et al[59], Xiangming et al[60], Blok et al[61] and Shun et al[62] reported that abnormal E-cadherin-catenin complex expression occured in early gastric carcinomas and changes in E-cadherin expression might be early events in gastric carcinoma. Our analysis aimed at investigating E-cadherin-catenin complex expression in the possible precancerous conditions, such as atrophic gastritis, intestinal metaplasia and dysplasia and evaluating the role of E-cadherin-catenin complex expression in the development of gastric carcinoma. In this study, we found that abnormal expression was shown in 36.4% of dysplasia for E-cadherin and 16% for β-catenin, respectively, while atrophic gastritis, intestinal metaplasia showed normal expression. Our observations of decreased E-cadherin and β-catenin expression in dysplasia raises the possibility that changes in the E-cadherin-catenin complex occur at an early stage in the neoplastic process.
In conclusion, abnormal expression of E-cadherin and β-catenin occurs in a considerable proportion of gastric carcinomas and correlates with loss of differentiation. Loss of normal β-catenin expression showed a close correlation with poor survival, independent of tumor type, grade, and stage, suggesting that it may be an independent prognostic marker. Our results also indicate that changes in abnormal expression of E-cadherin-catenin complex may be early events in gastric carcinomas.
ACKNOWLEDGEMENTS
We are grateful to Drs. M. Jiang, MT. Gao and JS. Wang, Department of Pathology, Lanzhou Medical College, Lanzhou, China, for providing the tissue specimens.
Footnotes
Edited by Xu JY
References
- 1.Uemura T. The cadherin superfamily at the synapse: more members, more missions. Cell. 1998;93:1095–1098. doi: 10.1016/s0092-8674(00)81452-x. [DOI] [PubMed] [Google Scholar]
- 2.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 3.Zhou YN, Wu ZD, Xu CP, Fang DC. E-cadherin-catenin com-plex in gastric carcinoma. Shijie Huaren Xiaohua Zazhi. 2002;10:436–440. [Google Scholar]
- 4.Jawhari A, Farthing M, Pignatelli M. The importance of the E-cadherin-catenin complex in the maintenance of intestinal epithelial homoeostasis: more than intercellular glue. Gut. 1997;41:581–584. doi: 10.1136/gut.41.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pignatelli M, Vessey CJ. Adhesion molecules: novel molecular tools in tumor pathology. Hum Pathol. 1994;25:849–856. doi: 10.1016/0046-8177(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 6.Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, Kobayashi T, Takatsuka Y, Matsuyoshi N, Hirano S, Takeichi M. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993;53:1696–1701. [PubMed] [Google Scholar]
- 7.Pignatelli M, Ansari TW, Gunter P, Liu D, Hirano S, Takeichi M, Klöppel G, Lemoine NR. Loss of membranous E-cadherin expression in pancreatic cancer: correlation with lymph node metastasis, high grade, and advanced stage. J Pathol. 1994;174:243–248. doi: 10.1002/path.1711740403. [DOI] [PubMed] [Google Scholar]
- 8.Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Löchner D, Birchmeier W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vleminckx K, Vakaet L, Mareel M, Fiers W, van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 10.Richmond PJ, Karayiannakis AJ, Nagafuchi A, Kaisary AV, Pignatelli M. Aberrant E-cadherin and alpha-catenin expression in prostate cancer: correlation with patient survival. Cancer Res. 1997;57:3189–3193. [PubMed] [Google Scholar]
- 11.Syrigos KN, Krausz T, Waxman J, Pandha H, Rowlinson-Busza G, Verne J, Epenetos AA, Pignatelli M. E-cadherin expression in bladder cancer using formalin-fixed, paraffin-embedded tissues: correlation with histopathological grade, tumour stage and survival. Int J Cancer. 1995;64:367–370. doi: 10.1002/ijc.2910640603. [DOI] [PubMed] [Google Scholar]
- 12.Siitonen SM, Kononen JT, Helin HJ, Rantala IS, Holli KA, Isola JJ. Reduced E-cadherin expression is associated with invasiveness and unfavorable prognosis in breast cancer. Am J Clin Pathol. 1996;105:394–402. doi: 10.1093/ajcp/105.4.394. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, Kitajima Y, Edakuni G, Sato S, Miyazaki K. Abnormal expression of E-cadherin and beta-catenin may be a molecular marker of submucosal invasion and lymph node metastasis in early gastric cancer. Br J Surg. 2002;89:236–244. doi: 10.1046/j.0007-1323.2001.01985.x. [DOI] [PubMed] [Google Scholar]
- 14.Katoh M. Frequent up-regulation of WNT2 in primary gastric cancer and colorectal cancer. Int J Oncol. 2001;19:1003–1007. doi: 10.3892/ijo.19.5.1003. [DOI] [PubMed] [Google Scholar]
- 15.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 16.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 17.Porfiri E, Rubinfeld B, Albert I, Hovanes K, Waterman M, Polakis P. Induction of a beta-catenin-LEF-1 complex by wnt-1 and transforming mutants of beta-catenin. Oncogene. 1997;15:2833–2839. doi: 10.1038/sj.onc.1201462. [DOI] [PubMed] [Google Scholar]
- 18.Hansson LE, Sparén P, Nyrén O. Survival in stomach cancer is improving: results of a nationwide population-based Swedish study. Ann Surg. 1999;230:162–169. doi: 10.1097/00000658-199908000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, Mu R, Zhou Y, Dai X, Qiao Y, Zhang S, Huangfu X, Sun J, Li L, Lu F. [1990-1992 mortality of stomach cancer in China] Zhonghua Zhongliu Zazhi. 2002;24:4–8. [PubMed] [Google Scholar]
- 20.Werner M, Becker KF, Keller G, Höfler H. Gastric adenocarcinoma: pathomorphology and molecular pathology. J Cancer Res Clin Oncol. 2001;127:207–216. doi: 10.1007/s004320000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker KF, Keller G, Hoefler H. The use of molecular biology in diagnosis and prognosis of gastric cancer. Surg Oncol. 2000;9:5–11. doi: 10.1016/s0960-7404(00)00016-5. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy BJ. The unified international gastric cancer staging classification. Scand J Gastroenterol. 1987;22:11–13. [Google Scholar]
- 23.LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 24.Rugge M, Correa P, Dixon MF, Hattori T, Leandro G, Lewin K, Riddell RH, Sipponen P, Watanabe H. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000;24:167–176. doi: 10.1097/00000478-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Jawhari A, Jordan S, Poole S, Browne P, Pignatelli M, Farthing MJ. Abnormal immunoreactivity of the E-cadherin-catenin complex in gastric carcinoma: relationship with patient survival. Gastroenterology. 1997;112:46–54. doi: 10.1016/s0016-5085(97)70218-x. [DOI] [PubMed] [Google Scholar]
- 26.Shiozaki H, Tahara H, Oka H, Miyata M, Kobayashi K, Tamura S, Iihara K, Doki Y, Hirano S, Takeichi M. Expression of immunoreactive E-cadherin adhesion molecules in human cancers. Am J Pathol. 1991;139:17–23. [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer B, Johnson JP, Leitl F, Jauch KW, Heiss MM, Schildberg FW, Birchmeier W, Funke I. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53:1690–1695. [PubMed] [Google Scholar]
- 28.Karayiannakis AJ, Syrigos KN, Chatzigianni E, Papanikolaou S, Karatzas G. E-cadherin expression as a differentiation marker in gastric cancer. Hepatogastroenterology. 1998;45:2437–2442. [PubMed] [Google Scholar]
- 29.Shun CT, Wu MS, Lin JT, Wang HP, Houng RL, Lee WJ, Wang TH, Chuang SM. An immunohistochemical study of E-cadherin expression with correlations to clinicopathological features in gastric cancer. Hepatogastroenterology. 1998;45:944–949. [PubMed] [Google Scholar]
- 30.Gabbert HE, Mueller W, Schneiders A, Meier S, Moll R, Birchmeier W, Hommel G. Prognostic value of E-cadherin expression in 413 gastric carcinomas. Int J Cancer. 1996;69:184–189. doi: 10.1002/(SICI)1097-0215(19960621)69:3<184::AID-IJC6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 31.Grabsch H, Takeno S, Noguchi T, Hommel G, Gabbert HE, Mueller W. Different patterns of beta-catenin expression in gastric carcinomas: relationship with clinicopathological parameters and prognostic outcome. Histopathology. 2001;39:141–149. doi: 10.1046/j.1365-2559.2001.01177.x. [DOI] [PubMed] [Google Scholar]
- 32.Fleming S. C. L. Oakley Lecture (1991). Cell adhesion and epithelial differentiation. J Pathol. 1991;164:95–100. doi: 10.1002/path.1711640202. [DOI] [PubMed] [Google Scholar]
- 33.Chan AO, Lam SK, Chu KM, Lam CM, Kwok E, Leung SY, Yuen ST, Law SY, Hui WM, Lai KC, et al. Soluble E-cadherin is a valid prognostic marker in gastric carcinoma. Gut. 2001;48:808–811. doi: 10.1136/gut.48.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ougolkov A, Mai M, Takahashi Y, Omote K, Bilim V, Shimizu A, Minamoto T. Altered expression of beta-catenin and c-erbB-2 in early gastric cancer. J Exp Clin Cancer Res. 2000;19:349–355. [PubMed] [Google Scholar]
- 35.Shiozaki H, Oka H, Inoue M, Tamura S, Monden M. E-cadherin mediated adhesion system in cancer cells. Cancer. 1996;77:1605–1613. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1605::AID-CNCR28>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 36.Lee JH, Koh JT, Shin BA, Ahn KY, Roh JH, Kim YJ, Kim KK. Comparative study of angiostatic and anti-invasive gene expressions as prognostic factors in gastric cancer. Int J Oncol. 2001;18:355–361. [PubMed] [Google Scholar]
- 37.Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- 38.Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 39.Machado JC, Soares P, Carneiro F, Rocha A, Beck S, Blin N, Berx G, Sobrinho-Simões M. E-cadherin gene mutations provide a genetic basis for the phenotypic divergence of mixed gastric carcinomas. Lab Invest. 1999;79:459–465. [PubMed] [Google Scholar]
- 40.Fukudome Y, Yanagihara K, Takeichi M, Ito F, Shibamoto S. Characterization of a mutant E-cadherin protein encoded by a mutant gene frequently seen in diffuse-type human gastric carcinoma. Int J Cancer. 2000;88:579–583. doi: 10.1002/1097-0215(20001115)88:4<579::aid-ijc10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 41.Chun YS, Lindor NM, Smyrk TC, Petersen BT, Burgart LJ, Guilford PJ, Donohue JH. Germline E-cadherin gene mutations: is prophylactic total gastrectomy indicated. Cancer. 2001;92:181–187. doi: 10.1002/1097-0142(20010701)92:1<181::aid-cncr1307>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 42.Zheng ZH, Sun XJ, Qiu GR, Liu YH, Wang MX, Sun KL. E-cadherin gene mutation in precancerous condition,early and advanced stages of gastric cancer. Shijie Huaren Xiaohua Zazhi. 2002;10:153–156. [Google Scholar]
- 43.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 44.Tamura G, Yin J, Wang S, Fleisher AS, Zou T, Abraham JM, Kong D, Smolinski KN, Wilson KT, James SP, et al. E-Cadherin gene promoter hypermethylation in primary human gastric carcinomas. J Natl Cancer Inst. 2000;92:569–573. doi: 10.1093/jnci/92.7.569. [DOI] [PubMed] [Google Scholar]
- 45.Leung WK, Yu J, Ng EK, To KF, Ma PK, Lee TL, Go MY, Chung SC, Sung JJ. Concurrent hypermethylation of multiple tumor-related genes in gastric carcinoma and adjacent normal tissues. Cancer. 2001;91:2294–2301. [PubMed] [Google Scholar]
- 46.Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinck L, Näthke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramesh S, Nash J, McCulloch PG. Reduction in membranous expression of beta-catenin and increased cytoplasmic E-cadherin expression predict poor survival in gastric cancer. Br J Cancer. 1999;81:1392–1397. doi: 10.1038/sj.bjc.6693437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tong JH, To KF, Ng EK, Lau JY, Lee TL, Lo KW, Leung WK, Tang NL, Chan FK, Sung JJ, et al. Somatic beta-catenin mutation in gastric carcinoma--an infrequent event that is not specific for microsatellite instability. Cancer Lett. 2001;163:125–130. doi: 10.1016/s0304-3835(00)00681-9. [DOI] [PubMed] [Google Scholar]
- 50.Karatzas G, Karayiannakis AJ, Syrigos KN, Chatzigianni E, Papanikolaou S, Simatos G, Papanikolaou D, Bogris S. Expression patterns of the E-cadherin-catenin cell-cell adhesion complex in gastric cancer. Hepatogastroenterology. 2000;47:1465–1469. [PubMed] [Google Scholar]
- 51.Joo YE, Rew JS, Kim HS, Choi SH, Park CS, Kim SJ. Changes in the E-cadherin-catenin complex expression in early and advanced gastric cancers. Digestion. 2001;64:111–119. doi: 10.1159/000048849. [DOI] [PubMed] [Google Scholar]
- 52.Yu J, Ebert MP, Miehlke S, Rost H, Lendeckel U, Leodolter A, Stolte M, Bayerdörffer E, Malfertheiner P. alpha-catenin expression is decreased in human gastric cancers and in the gastric mucosa of first degree relatives. Gut. 2000;46:639–644. doi: 10.1136/gut.46.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 54.Takayama T, Shiozaki H, Doki Y, Oka H, Inoue M, Yamamoto M, Tamura S, Shibamoto S, Ito F, Monden M. Aberrant expression and phosphorylation of beta-catenin in human colorectal cancer. Br J Cancer. 1998;77:605–613. doi: 10.1038/bjc.1998.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yokozaki H, Yasui W, Tahara E. Genetic and epigenetic changes in stomach cancer. Int Rev Cytol. 2001;204:49–95. doi: 10.1016/s0074-7696(01)04003-7. [DOI] [PubMed] [Google Scholar]
- 56.Tamura G, Sato K, Akiyama S, Tsuchiya T, Endoh Y, Usuba O, Kimura W, Nishizuka S, Motoyama T. Molecular characterization of undifferentiated-type gastric carcinoma. Lab Invest. 2001;81:593–598. doi: 10.1038/labinvest.3780268. [DOI] [PubMed] [Google Scholar]
- 57.Gao HJ, Yu LZ, Bai JF, Peng YS, Sun G, Zhao HL, Miu K, L XZ, Zhang XY, Zhao ZQ. Multiple genetic alterations and behavior of cellular biology in gastric cancer and other gastric mucosal lesions: H.pylori infection, histological types and staging. World J Gastroenterol. 2000;6:848–854. doi: 10.3748/wjg.v6.i6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 59.Ohene-Abuakwa Y, Noda M, Perenyi M, Kobayashi N, Kashima K, Hattori T, Pignatelli M. Expression of the E-cadherin/catenin (alpha-, beta-, and gamma-) complex correlates with the macroscopic appearance of early gastric cancer. J Pathol. 2000;192:433–439. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH723>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 60.Xiangming C, Hokita S, Natsugoe S, Tanabe G, Baba M, Takao S, Kuroshima K, Aikou T. Cooccurrence of reduced expression of alpha-catenin and overexpression of p53 is a predictor of lymph node metastasis in early gastric cancer. Oncology. 1999;57:131–137. doi: 10.1159/000012020. [DOI] [PubMed] [Google Scholar]
- 61.Blok P, Craanen ME, Dekker W, Tytgat GN. Loss of E-cadherin expression in early gastric cancer. Histopathology. 1999;34:410–415. doi: 10.1046/j.1365-2559.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 62.Shun CT, Wu MS, Lin MT, Chang MC, Lin JT, Chuang SM. Immunohistochemical evaluation of cadherin and catenin expression in early gastric carcinomas: correlation with clinicopathologic characteristics and Helicobacter pylori infection. Oncology. 2001;60:339–345. doi: 10.1159/000058530. [DOI] [PubMed] [Google Scholar]


