Abstract
AIM: To determine the effect of apoptosis on gastric cancer cells (SGC-7901) induced by cis-9, trans-11-conjugated linoleic acid (c9, t11-CLA) and its possible mechanism in the inhibition of cancer cells growth.
METHODS: Using cell culture, flow cytometery and immunocytochemical techniques, we examined the cell growth, frequency of apoptosis and distribution of cell cycle, expression of ki67, bcl-2, Fas, and c-myc of SGC-7901 cells which were treated with various c9, t11-CLA concentrations (25, 50, 100 and 200 μmol·L-1) of c9, t11-CLA for 24 h and 48 h, with a negative control (0.1% ethanol).
RESULTS: The growth of SGC-7901 cells was inhibited by c9,t11-CLA. Eight days after treatment with various concentrations of c9,t11-CLA, as mentioned above, the inhibition rates were 5.9%, 20.2%, 75.6% and 82.4%, respectively. The frequency of apoptosis on SGC-7901 cells induced by different concentrations of c9, t11-CLA (except for 25 μmol·L-1, 24 h) was significantly greater than that in the negative control (P < 0.01). To further investigate the influence of the cell cycle progression, we found that apoptosis induced by c9, t11-CLA may be involved in blocking the cell cycle of SGC-7901 cells. Immunocytochemical staining demonstrated that SGC-7901 cells preincubated in media supplemented with different c9, t11-CLA concentrations for various time periods significantly decreased the expressions of ki67 (the expression rates were 18.70%-3.20%, at 24 h and 8.10%-0.20% at 48 h, respectively), bcl-2 (4.30%-0.15% at 24 h and 8.05%-0% at 48 h),and c-myc (4.85%-2.20% at 24 h and 4.75%-0.30% at 48 h) as compared with those in the controls (the expressions of ki67, bcl-2, and c-myc were 15.1% at 24 h and 13.5% at 48 h, 6.80% at 24 h and 8.00% at 48 h, 5.50% at 24 h and 5.30% at 48 h, respectively) (P < 0.01), whereas the expressions of Fas were increased (0.60%-2.75%, 24 h and 0.45%-5.95%, 48 h).
CONCLUSION: The growth and proliferation of SGC-7901 cells are inhibited by c9, t11-CLA via blocking the cell cycle, pathways of bcl-2-associated mitochondria with reduced expression of bcl-2 and Fas-associated death domain protein (FADD) with enhanced expression of Fas. But expression of c-myc on SGC-7901 cells is lower than that in negative control, which needs to be studied further.
INTRODUCTION
Conjugated linoleic acid(CLA), a derivative of a fatty acid linoleic acid (LA), is a minor fatty acid found especially in red meat and in dairy products. The biosynthesis of CLA in ruminants is the result of a rumen bacterium, which is known to convert linoleic acid to stearic acid via CLA. In recent years, CLA has received considerable attention as a chemopreventive agent. This is because CLA was shown to inhibit in vitro the proliferation of human gastric cancer cells(SGC-7901)[1,2], mammary cancer cells(MCF-7)[3-7], of human malignant melanoma cells, colorectal cancer cells[8], and rat hepatoma cells[9] and in animal studies to prevent the development of mouse epidermal carcinogenesis, mouse forestomach cancer[10,11] and of rat mammary tumorigenesis[12-15].
Although the exact mechanisms are not clear, the inhibitory effect of CLA on the proliferation of rapidly dividing cells has been attributed to the induction of cell cycle arrest[1] and the induction of apoptosis[2,16]. In many instances, growth inhibition following terminal differentiation or anticancer drug treatment results in apoptosis. Apoptosis, namely, programmed cell death, is an active and physiological process characterized by a series of morphological and biological alterations in which the cells become smaller, shrinking, the nuclei round up, the chromatin becomes agglutinated and marginated, the nuclear membrane breaks down, and followed by the degenerative changes of the cells. The exact mechanisms of apoptosis are still unclear, but our earlier studies indicated that CLA can induce apoptosis in human gastric cancer SGC-7901 cells[2].
Gastric cancer is both common in China and the other parts of the world[17-30], and chemoprevention is always used as the main treatment for advanced cancer so far, which has become a focus topic in this area[31-54]. In this study, we investigate the pathways of apoptosis induced by cis-9, trans-11-CLA (c9, t11-CLA) which are thought to be high in proportion and activation as potential antioxidant and anticarcinogenic agents in CLA’s isomers and probe into the possible mechanism of apoptosis on human gastric adenocarcinoma cells SGC-7901.
MATERIALS AND METHODS
Materials
c9, t11-CLA, a monoisomer of c-9, t11-octadecadienoic acid with 98% purity, was obtained from Dr. Ruihai Liu (Food Science and Toxicology, Department of Food Science, Cornell University, Ithaca, NY, USA). The c9, t11-CLA was dissolved in 96 mL·L-1 ethanol, and diluted to the following concentrations: 25, 50, 100 and 200 μmol·L-1.
Methods
Cell culture Human gastric adenocarcinoma cells(SGC-7901), purchased from Cancer Research Institute of Beijing (China), were cultured in RPMI 1640 (Gibco) medium, supplemented with calf serum 100 mL·L-1, penicillin (100 × 103U·L-1) and streptomycin (100 mg·L-1). The pH was maintained at 7.2-7.4, by equilibration with 5% CO2. The temperature was kept at 37 °C.The cells were sub-cultured with a mixture of Ethylenedinitrile tetraacetic acid (EDTA) and trypsin.
Cell growth curve The SGC-7901cells were seeded in six 24-well plates (Nuc, Co.), each well containing 2 × 104 cells. After 24 h, the medium of different plates was replaced with media supplemented with c9,t11- CLA at different concentrations. On the next day, the numbers of cells of 3 wells from each plate was determined by using the trypan blue staining. The means were obtained on each of eight days and were used to draw a cellular growth curve. The inhibitory rates (IR) on the 8th day was calculated as follows:
Math 1
Math 1.
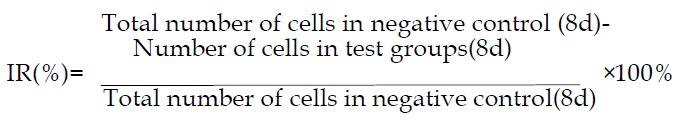
Math(A1).
Apoptosis detection and cell cycle analysis
SGC-7901 cells (5 × 105 cells in 25 mL bottles) were seeded in appropriate medium for 24 h prior to the beginning of the experiment. The medium was then replaced with different concentrations of c9, t11-CLA. After 24 h and 48 h, the cells were harvested using a mixture of trypsin/EDTA, washed twice with cold PBS, fixed in 70% ethanol on ice for 30 minutes, and washed once again. Cells were then stained by adding 1 mL of PI mixture (containing 50 μg propidium iodide, 0.2 mg RNAse, 5 μL Triton X-100, and 1 mg citromalic acid) in the dark (4 °C, 30 min). Cell apoptosis and cell cycle analysis were subsequently performed by flow cytometry using a FACSCalibur Analyzer (BD Biosciences) with a 15-milliwatt air-cooled argon laser (excitation = 488 nm). Sub G1 peak was observed and DNA content in phases of cell cycle was analyzed using software of ModiFix LT.
Cell samples
SGC-7901 cells were treated for 24 h and 48 h with various concentrations of c9, t11-CLA and collected by centrifugation. Specimens were fixed immediately in 40 g·L-1 formaldehydum polymerisatum and embedded in paraffin. Gastric cancer tissue from a patient served as a reference.
Primary antibody
To examine the expression of Ki67 cell proliferation and the expression of bcl-2, c-myc and Fas in SGC-7901 cells, we used four primary antibodies: corresponding rabbit polyclonal antibodies for bcl-2 and Fas and corresponding mouse monoclonal antibodies for ki67 and c-myc. Antibodies of bcl-2 and Fas were purchased from the Calbiochem Co. USA; and others from Zhongshan Co. China.
Immunocytochemistry
Immunocytochemical staining was performed on serial sections at room temperature using the horseradish peroxidase method. The sections were deparaffinized in xylene and rehydrated through graded alcohol. The sections were incubated for 10 min at 95 °C in 10 mmol·L-1 sodium citrate(pH 6.0) buffer for ki67 staining. Endogenous peroxidases were inactivated by immersing the sections in hydrogen peroxide for 10 min, and then were incubated for 10 min with 100 mL·L-1 normal goat serum in PBS to block the non-specific binding. The sections were subsequently incubated overnight at 4 °C with relevant antibodies(1:50 dilution) respectively. The next day, the sections were incubated with biotinylated anti-mouse or anti-rabbit IgG (Zhongshan Co., China) for 30 min, followed by peroxidase-conjugated streptavidin (Zhongshan Co., China) for 30 min. The chromogenic reaction was developed with DAB (diaminobenzidine) for 10 min, and all sections were counterstained with hematoxylin. Controls consisted of omission of the primary antibody. The positive rate (PR) was calculated as follows:
Math 2
Math 2.

Math(A1).
Statistical analysis
Analysis of data was performed using the Student’s t test or χ2 test. A value of P < 0.05 is considered to be statistically significant.
RESULTS
Effect of c9,t11-CLA on SGC-7901 cell growth
As shown in Figure 1, growth of the cells in various concentrations (except for 25 μmol·L-1 and 50 μmol·L-1) of c9, t11-CLA did differ from the negative control within 8d. SGC-7901 cells incubated in 25 μmol·L-1 of c9,t11-CLA grew at a higher rate than that of the negative control, while in 100 and 200 μmol·L-1 concentrations of c9, t11-CLA, proliferation of SGC-7901 cell was significantly inhibited. The inhibitory rate of various c9, t11-CLA concentrations were 5.9%, 20.2%, 75.6% and 82.4%, respectively.
Figure 1.
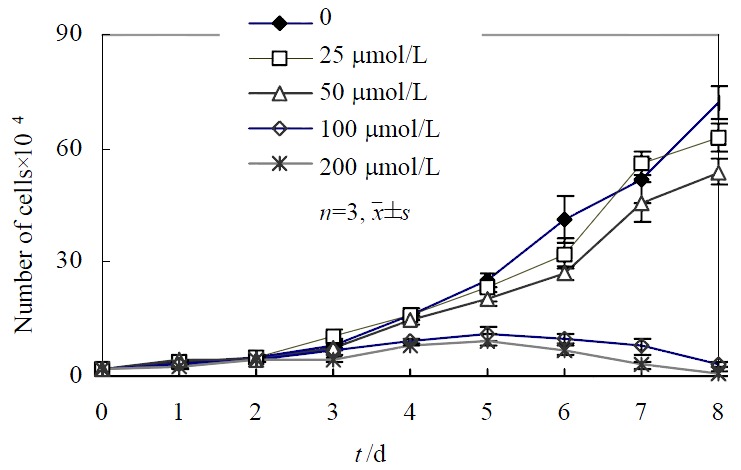
Growth curve of SGC-7901cells cultured in various concentrations of c9,t11-CLA.
Analysis of flow cytometry
To investigate the influence of c9, t11-CLA on apoptosis and cell cycle progression of SGC-7901 cells, we determined apoptosis and cell cycle distribution by flow cytometry. The results are shown in Table 1. We observed that apoptotic peaks and intention of the cells accumulating in the G0/G1 phases and of cells decreased in the S phase of the cell cycle in SGC-7901 cells with different c9, t11-CLA concentrations at various time periods, whereas G2/M did not. The results suggested that c9, t11-CLA may induce apoptosis and arrest the progression of cell cycle of SGC-7901 cells.
Table 1.
Cell cycle analysis of SGC-7901 cells induced by c9, t11-CLA at 24 h and 48 h (mean ± SD, n = 4)
| c9,t11-CLA(μm) |
Apoptosis |
G0/G1 |
G2/M |
S |
||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| 0 | 0.55 ± 0.09 | 0.69 ± 0.21 | 53.13 ± 3.44 | 58.57 ± 0.90 | 11.13 ± 2.75 | 12.64 ± 1.18 | 35.74 ± 2.04 | 28.80 ± 0.89 |
| 25 | 1.27 ± 0.73 | 3.89 ± 2.12 | 53.00 ± 8.35 | 56.81 ± 1.09 | 15.55 ± 1.11 | 15.95 ± 0.36 | 31.40 ± 9.23 | 26.52 ± 1.46 |
| 50 | 4.12 ± 0.55a | 8.18 ± 1.55b | 56.58 ± 0.87 | 56.32 ± 0.78 | 14.55 ± 3.45 | 15.17 ± 0.61 | 28.87 ± 3.32 | 28.51 ± 1.32 |
| 100 | 7.95 ± 0.31b | 12.33 ± 1.53b | 58.35 ± 2.44 | 61.18 ± 4.94 | 13.12 ± 1.50 | 14.49 ± 3.10 | 28.52 ± 0.96 | 25.08 ± 2.85 |
| 200 | 12.79 ± 3.12b | 14.75 ± 5.97b | 60.67 ± 4.28a | 63.82 ± 7.84a | 12.95 ± 4.48 | 13.35 ± 3.91 | 26.38 ± 0.92b | 22.82 ± 4.63b |
P < 0.05,
P < 0.01 vs negative control
Cell proliferation
Ki67 is a marker for the proliferation of cells. To determine the effect of c9, t11-CLA on the proliferation of SGC-7901 cells, we investigated the expression of ki67 using immunocytochemistry. The results are shown in Figure 2. Expression rates of ki67(Figure 3A) on SGC-7901 cells gradually decreased after SGC-7901 cells were incubated with different concentrations of c9,t11-CLA at various time periods. Moreover, SGC-7901 cells expressed significantly less ki67 than did the negative control (P < 0.01). The expression rate of ki67 on SGC-7901 cells displayed a dose-response relationship as the concentrations of CLA increased.
Figure 2.
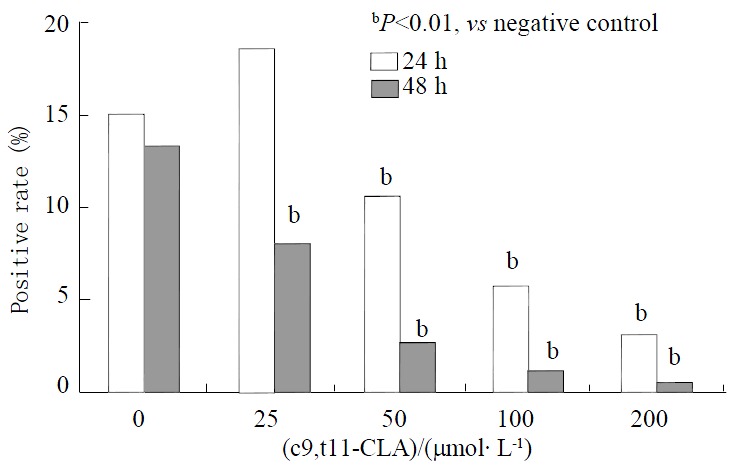
Expression of ki67 on SGC-7901 cells treated with c9, t11-CLA.
Figure 3.
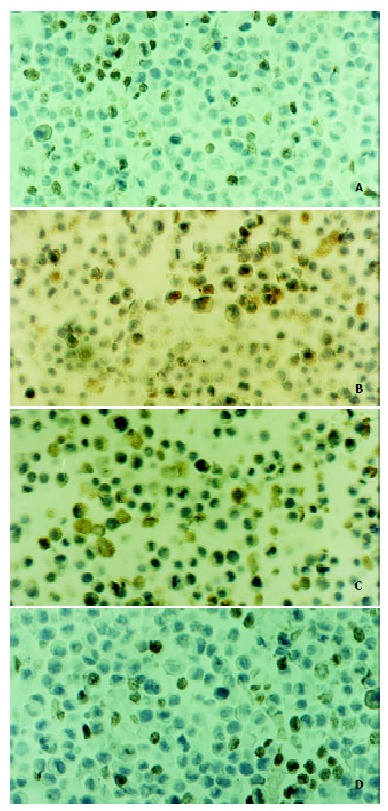
A: The expression of Ki67 on SGC-7901 cells of the negative control. Immunocytochemistry staining SP method, original magnification, × 400; B: The expression of bcl-2 on SGC-7901 cells of the negative control. Immunocytochemistry staining SP method, original magnification, × 400; C: The ex-pression of Fas on SGC-7901 cells of c9, t11-CLA group (200 μmol·L-1 48 h). Immunocytochemistry staining SP method, original magnification, × 400; D:The expression of c-myc on SGC-7901 cells of the negative control. Immunocytochemistry staining SP method, original magnification, × 400.
Expressions of bcl-2, Fas and c-myc
We detected the expression of bcl-2, Fas, and c-myc on SGC-7901 cells treated by various concentrations of c9, t11-CLA with immunocytochemical technique. The expression rates of bcl-2 and c-myc (Figure 3B, D) on SGC-7901 cells was decreased (Table 2) after SGC-7901 cells were incubated with different concentrations of c9, t11-CLA for 24 h and 48 h while expression of Fas increased (Table 2; Figure 3C). In the meantime, there was no expression of Fas at doses of c9,t11-CLA (25 and 50 μmol·L-1 at 24 h).
Table 2.
Positive rates of bcl-2, Fas and c-myc on SGC-7901 cells treated with c9, t11-CLA (%)
| C9,t11-CLA (μmol/L) |
24 h |
48 h |
||||
| bcl-2 | Fas | c-myc | bcl-2 | Fas | c-myc | |
| 0 | 6.80 | 0.60 | 5.50 | 8.00 | 0.45 | 5.30 |
| 25 | 4.30 | 0 | 4.85 | 8.05 | 0.85 | 4.75 |
| 50 | 2.50b | 0 | 4.20a | 3.80b | 2.75b | 3.70a |
| 100 | 1.45b | 1.95b | 3.80a | 0.30b | 4.10b | 1.35b |
| 200 | 0.15b | 2.75b | 2.20b | 0 | 5.95b | 0.30b |
P < 0.05,
P < 0.01 vs negative control.
DISCUSSION
CLA is a natural fatty acid in animal’s food. CLA has a mixture of positional (9/11 or 10/12 double bonds) and geometric (various cis/trans combinations) isomers of linoleic acid (LA) formed by rumen and colonic bacteria. There are eight potential isomers of CLA, but the cis 9, trans 11 and trans 9, cis 11 isomers are thought to be active as potential antioxidant and anticarcinogenic agents. Therefore, it is of interest to investigate extensively the mechanism of anticancer activities of CLA.
Over the past ten years, a number of animal experiments have supported the observation that CLA is an effective chemopreventive agent for cancer, and that it can inhibit carcinogenesis of different tissues at various stages of induction by chemical agents[14,15]. Several investigators in our group have reported that c9, t11-CLA is an effective agent to prevent carcinogenesis[10,11] and cancer[1-3,7]. Zhu’s study[10] demonstrated that c9, t11-CLA could significantly inhibit the mice forestomach neoplasia induced by B(a)P (50 mg·kg-1) in post-initiation in short term (23 weeks). The incidences of tumors in mice of B(a)P group, B(a)P with high dose CLA (5 μL·g-1) group and B(a)P with low dose CLA(2.5 μL·g-1) group were 100%, 60% and 69%, respectively (P < 0.05). Xue’s study[10] also indicated that the incidence of neoplasm in mouse forestomach in the B(a)P group, 75% pure c9, t11-CLA group, 98% pure c9, t11-CLA group and 98% pure t10, c12-CLA group were 100.0%, 75.0%, 69.2% and 53.8%, respectively. This may be due to an inhibiting mitogen of activated protein kinase (MAPK)-an approach to reduce carcinogenesis.
The data in this series suggested that c9,t11-CLA could inhibit the proliferation of cancer cells, i.e. SGC-7901 cells[1,2] and MCF-7 cells[3,7], and induced cancer cell (SGC-7901) apoptosis[2]. Liu’s study[1] indicated that cell growth and proliferation and DNA synthesis of SGC-7901 cells were inhibited and SGC-7901 cells preincubated in media supplemented with different c9,t11-CLA concentrations at various time periods significantly decreased the expressions of PCNA (the expression rates were 7.2%-3.0%, at 24 h and 9.1%-0.9% at 48 h, respectively), Cyclin A (11.0%-2.3%, at 24 h and 8.5%-0.5%, at 48 h), B1 (4.8%-1.8% at 24 h and 5.5%-0.6% at 48 h) and D1 (3.6%-1.4% at 24 h and 3.7%-0% at 48 h) as compared with those in the negative controls (the expressions of PCNA, cyclin A, B1 and D1 were 6.5% at 24 h and 9.0% at 48 h, 4.2% at 24 h and 5.1% at 48 h, 9.5% at 24 h and 6.0% at 48 h, respectively) (P < 0.01), whereas the expressions of p16ink4a and p21cip/waf1, cyclin-dependent kinases inhibitors (CDKI) were increased. Our results showed that the proliferation marker Ki67 was inhibited and the cells were accumulating in the G0/G1 phase and cells decreasing in the S phases of the cell cycle on SGC-7901 cells with different c9, t11-CLA concentrations at various time periods, whereas G2/M did not have. All these results suggested that c9, t11-CLA may arrest the progression of cell cycle of SGC-7901 cells. Our previous works[2] indicated that at the early stage morphological changes of cell apoptosis were observed using fluorescent dye (Hoechst 33342) under electronic microscope and SGC-7901 cells preincubated in media supplemented with different c9, t11-CLA concentrations at various times significantly decreased the expressions of mutant p53 as compared with those in the negative control. The inhibitory rates of mutant p53 on SGC-7901 cells induced by various c9,t11-CLA concentrations (25-200 μmol·L-1) were -19.2%, 13.7%, 53.4%, and 89.0% at 24 h and 1.8%, 29.1%, 87.3%, and 86.8% at 48 h, respectively. p53 is one of the major factors controlling cell proliferation, suppressing both growth and transformation of cells. A common idea[55] is that p53 acts as “guardian of the genome” by preventing damaging of DNA in cell proliferation or DNA damage leads to an increase in the level of p53, resulting in p21CIP1/WAF1-mediated cell cycle arrest in the G1 phase, which persists until DNA repair is completed or by arresting the cell division cycle induces damaged-cell apoptosis. In the absence of functional wild-type p53, the “guardian” function is lost; cells accumulate genetic damage and show marked genetic instability, often to the extent of gross aneuploidy. However, wild-type p53 protein has a very short half-time, many point mutants have a greatly enhanced stability, allowing for the immunohistochemical detection of mutant p53 in clinical material. Mutant p53 protein loses its biochemical functions which may facilitate DNA repair as well as apoptosis, and displayed over-proliferation of cancer cells. In addition, Natalie et al[56] found that a fraction of p53 protein localizes to mitochondria at the onset of p53-dependent apoptosis. The accumulation of p53 to mitochondria is rapid (within 1 h after p53 activation) and precedes changes in mitochondrial membrane potential, cytochrome c release, and procaspase-3 activation. Overexpression of anti-apoptotic bcl-2 inhibits signal-mediated mitochondrial p53 accumulation and apoptosis but not cell cycle arrest. Our results showed that c9, t11-CLA may reduce the expression of mutant p53 protein and may recover the bio-function of wild p53 protein that blocks the cell cycle of SGC-7901 cells by p21CIP1/WAF1 and processes to another apoptotic pathway of mitochondria by bcl-2.
At the same time, we investigated further the expressions of bcl-2 and Fas from pathways of cell apoptosis such as mitochondria and Fas-associated death domain (FADD) as well as expression of c-myc on SGC-7901 cells treated with various concentrations of c9,t11-CLA. Bcl-2 is an inhibitor of apoptosis and shown to exert anti-apoptotic activity by one of the following mechanisms[57]: 1) sequestration of the proforms of two major initiator caspases, pro-caspase-9 (through binding to Apaf-1) and pro-caspase-8 (through unidentified molecules); 2) inhibition of apoptogenic mitochondrial changes, including cytochrome c release and the mitochondrial membrane potential (△ ψ) loss resulting in AIF (apoptosis-inducing factor) release, as demonstrated using isolated mitochondria bearing endogenous bcl-2 and recombinant forms of these proteins, and 3) Inhibition of accumulation of p53 in mitochondrial membrane[56]. Fas is a potent inducer of apoptosis in tumor cells but not in normal cells. Fas/CD95 requires ligand receptor (FasL/CD95L) which binds to FasL and via downstream signaling molecules FADD activates caspase-8. Fas binding to FasL can activate two routes downstream of caspase-8 activation[58]: type I apoptosis signaling: direct activation of effector’s caspases by caspase-8; type II apoptosis (inhibition of bcl-2 overexpression): cleavage of proapoptotic members of bcl-2 family, △ ψ, release of cytochrome c and activation of caspase-9. The present study indicated that expression of bcl-2 protein decreased and expression of Fas increased on SGC-7901 cells induced by c9, t11-CLA as compared with that in negative control. c9, t11-CLA may induce apoptosis via pathways of bcl-2-assiciated mitochondria, p53-associated cell cycle and FADD on SGC-7901 cells. The relationship among p53, bcl-2 and Fas is still unclear in the effect of apoptosis on SGC-7901 cells induced by c9,t11-CLA. The c-myc oncogene product (c-myc) is a transcription factor that dimerizes with Max and recognized E-box sequence, and it plays key functions in cell proliferation, differentiation and apoptosis[59]. C-myc expression can not only promotes proliferation but also induce or sensitize cells to apoptosis. Overpression of c-myc under the circumstances that this gene is usually down regulated such as serum deprivation, results in apoptotic cells in nonhepatic cells and in a hepatoma cell line[60]. But our result showed that expression of c-myc with 10% calf serum was lowered with c9, t11-CLA in SGC-7901 cells. We have not known the reason why expression of c-myc on SGC-7901 cells is lower than that in negative control, and this needs further studies.
In conclusion, c9, t11-CLA may inhibit proliferation and induce apoptosis by decreasing in the expression of ki67, bcl-2 and increasing that of Fas in SGC-7901 cells. This result suggested that the inhibition effect of c9, t11-CLA on SGC-7901 cell proliferation is related to the pathways of bcl-2-associated mitochondria, p53-associated cell cycle and FADD on SGC-7901 cells. In the meantime, we found that expression of c-myc was lowered, but we do not know its action how to regulate apoptotic progression in SGC-7901 cells. The apoptotic mechanism of c9, t11-CLA in SGC-7901 cells awaits further studies.
Footnotes
Supported by the National Natural Science Foundation of China, No. 39870661
Edited by Ma JY
References
- 1.Liu JR, Li BX, Chen BQ, Han XH, Xue YB, Yang YM, Zheng YM, Liu RH. Effect of cis-9, trans-11-conjugated linoleic acid on cell cycle of gastric adenocarcinoma cell line (SGC-7901) World J Gastroenterol. 2002;8:224–229. doi: 10.3748/wjg.v8.i2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu JR, Chen BQ, Deng H, Han XH, Liu RH. Cell Apoptosis In-duced by Conjugated Linoleic Acid in Human Gastric Cancer (SGC-7901) Cells. Gongye Weisheng Yu Zhiyebing. 2001;27:129–133. [Google Scholar]
- 3.Liu JR, Chen BQ, Xue YB, Han XH, Yang YM, Liu RH. Inhibitory effect of conjugated linoleic acid on the in vitro growth of hu-man mammary cancer cells (MCF-7) Zhonghua Yufang Yixue. 2001;35:244–247. [Google Scholar]
- 4.O'Shea M, Devery R, Lawless F, Murphy J, Stanton C. Milk fat conjugated linoleic acid (CLA) inhibits growth of human mammary MCF-7 cancer cells. Anticancer Res. 2000;20:3591–3601. [PubMed] [Google Scholar]
- 5.Park Y, Allen KG, Shultz TD. Modulation of MCF-7 breast cancer cell signal transduction by linoleic acid and conjugated linoleic acid in culture. Anticancer Res. 2000;20:669–676. [PubMed] [Google Scholar]
- 6.Miller A, Stanton C, Devery R. Modulation of arachidonic acid distribution by conjugated linoleic acid isomers and linoleic acid in MCF-7 and SW480 cancer cells. Lipids. 2001;36:1161–1168. doi: 10.1007/s11745-001-0827-0. [DOI] [PubMed] [Google Scholar]
- 7.Liu JR, Chen BQ, Yang YM, Han XH, Xue YB, Wang XL, Zheng YM, Liu RH. Effects ofcis-9,trans-11-conjugated linoleic acid on cancer cell cycle. Environ Health Prev Med. 2002;7:205–210. doi: 10.1007/BF02898006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palombo JD, Ganguly A, Bistrian BR, Menard MP. The antiproliferative effects of biologically active isomers of conjugated linoleic acid on human colorectal and prostatic cancer cells. Cancer Lett. 2002;177:163–172. doi: 10.1016/s0304-3835(01)00796-0. [DOI] [PubMed] [Google Scholar]
- 9.Yamasaki M, Ikeda A, Hirao A, Tanaka Y, Miyazaki Y, Rikimaru T, Shimada M, Sugimachi K, Tachibana H, Yamada K. Effect of dietary conjugated linoleic acid on the in vivo growth of rat hepatoma dRLh-84. Nutr Cancer. 2001;40:140–148. doi: 10.1207/S15327914NC402_10. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y, Qiou J, Chen B. [The inhibitory effect of CLA on mice forestomach neoplasia induced by B(a)P] Zhonghua Yufang Yixue Zazhi. 2001;35:19–22. [PubMed] [Google Scholar]
- 11.Xue YB, Chen BQ, Liu JR, Zheng YM, Liu RH. The inhibition of mouse forestomach neoplasm induced by B(a)p through MAPKs pathway by conjugated linoleic acid. Zhonghua Yufang Yixue. 2001;35:1–4. [Google Scholar]
- 12.Park HS, Ryu JH, Ha YL, Park JH. Dietary conjugated linoleic acid (CLA) induces apoptosis of colonic mucosa in 1,2-dimethylhydrazine-treated rats: a possible mechanism of the anticarcinogenic effect by CLA. Br J Nutr. 2001;86:549–555. doi: 10.1079/bjn2001445. [DOI] [PubMed] [Google Scholar]
- 13.Ip C, Ip MM, Loftus T, Shoemaker S, Shea-Eaton W. Induction of apoptosis by conjugated linoleic acid in cultured mammary tumor cells and premalignant lesions of the rat mammary gland. Cancer Epidemiol Biomarkers Prev. 2000;9:689–696. [PubMed] [Google Scholar]
- 14.Kimoto N, Hirose M, Futakuchi M, Iwata T, Kasai M, Shirai T. Site-dependent modulating effects of conjugated fatty acids from safflower oil in a rat two-stage carcinogenesis model in female Sprague-Dawley rats. Cancer Lett. 2001;168:15–21. doi: 10.1016/s0304-3835(01)00459-1. [DOI] [PubMed] [Google Scholar]
- 15.Futakuchi M, Cheng JL, Hirose M, Kimoto N, Cho YM, Iwata T, Kasai M, Tokudome S, Shirai T. Inhibition of conjugated fatty acids derived from safflower or perilla oil of induction and development of mammary tumors in rats induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) Cancer Lett. 2002;178:131–139. doi: 10.1016/s0304-3835(01)00860-6. [DOI] [PubMed] [Google Scholar]
- 16.Miner JL, Cederberg CA, Nielsen MK, Chen X, Baile CA. Conjugated linoleic acid (CLA), body fat, and apoptosis. Obes Res. 2001;9:129–134. doi: 10.1038/oby.2001.16. [DOI] [PubMed] [Google Scholar]
- 17.Tovey FI, Hobsley M. Post-gastrectomy patients need to be followed up for 20-30 years. World J Gastroenterol. 2000;6:45–48. doi: 10.3748/wjg.v6.i1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan QS, Fang ZP, Zhao YX. Immunocytochemical identification and localization of APUD cells in the gut of seven stomachless teleost fishes. World J Gastroenterol. 2000;6:96–101. doi: 10.3748/wjg.v6.i1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen GY, Wang DR. The expression and clinical significance of CD44v in human gastric cancers. World J Gastroenterol. 2000;6:125–127. doi: 10.3748/wjg.v6.i1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu CT, Huang LT, Pan BR. Current gene therapy for stomach carcinoma. World J Gastroenterol. 2001;7:752–759. doi: 10.3748/wjg.v7.i6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang DC, Yang SM, Zhou XD, Wang DX, Luo YH. Telomere erosion is independent of microsatellite instability but related to loss of heterozygosity in gastric cancer. World J Gastroenterol. 2001;7:522–526. doi: 10.3748/wjg.v7.i4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403–406. doi: 10.3748/wjg.v7.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgner A, Miehlke S, Stolte M, Neubauer A, Alpen B, Thiede C, Klann H, Hierlmeier FX, Ell C, Ehninger G, et al. Development of early gastric cancer 4 and 5 years after complete remission of Helicobacter pylori associated gastric low grade marginal zone B cell lymphoma of MALT type. World J Gastroenterol. 2001;7:248–253. doi: 10.3748/wjg.v7.i2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu WX, Qin XY, Liu H, Wang CP. Clinicopathological analysis of patients with gastric cancer in 1200 cases. World J Gastroenterol. 2001;7:281–284. doi: 10.3748/wjg.v7.i2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xin Y, Li XL, Wang YP, Zhang SM, Zheng HC, Wu DY, Zhang YC. Relationship between phenotypes of cell-function differentiation and pathobiological behavior of gastric carcinomas. World J Gastroenterol. 2001;7:53–59. doi: 10.3748/wjg.v7.i1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao YL, Xu B, Song YG, Zhang WD. Overexpression of cyclin E in Mongolian gerbil with Helicobacter pylori-induced gastric precancerosis. World J Gastroenterol. 2002;8:60–63. doi: 10.3748/wjg.v8.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao HJ, Yu LZ, Bai JF, Peng YS, Sun G, Zhao HL, Miu K, L XZ, Zhang XY, Zhao ZQ. Multiple genetic alterations and behavior of cellular biology in gastric cancer and other gastric mucosal lesions: H.pylori infection, histological types and staging. World J Gastroenterol. 2000;6:848–854. doi: 10.3748/wjg.v6.i6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng DJ. progress of gastric cancer etiology: N-nitrosamides 1999s. World J Gastroenterol. 2000;6:613–618. doi: 10.3748/wjg.v6.i4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu ZM, Shou NH, Jiang XH. Expression of lung resistance protein in patients with gastric carcinoma and its clinical significance. World J Gastroenterol. 2000;6:433–434. doi: 10.3748/wjg.v6.i3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou SC, Qiu HS, Zhang CW, Tao HQ. A clinical and long-term follow-up study of peri-operative sequential triple therapy for gastric cancer. World J Gastroenterol. 2000;6:284–286. doi: 10.3748/wjg.v6.i2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Liu FK, Li X, Li JS, Xu GX. Inhibitory effect of endostatin expressed by human liver carcinoma SMMC7721 on endothelial cell proliferation in vitro. World J Gastroenterol. 2002;8:253–257. doi: 10.3748/wjg.v8.i2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao WX, Ou JM, Fei XF, Zhu ZG, Yin HR, Yan M, Lin YZ. Methionine-dependence and combination chemotherapy on human gastric cancer cells in vitro. World J Gastroenterol. 2002;8:230–232. doi: 10.3748/wjg.v8.i2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Lu YY. Applying a highly specific and reproducible cDNA RDA method to clone garlic up-regulated genes in human gastric cancer cells. World J Gastroenterol. 2002;8:213–216. doi: 10.3748/wjg.v8.i2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun ZJ, Pan CE, Liu HS, Wang GJ. Anti-hepatoma activity of resveratrol in vitro. World J Gastroenterol. 2002;8:79–81. doi: 10.3748/wjg.v8.i1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Lan M, Shi YQ, Lu J, Zhong YX, Wu HP, Zai HH, Ding J, Wu KC, Pan BR, et al. Differential display of vincristine-resistance-related genes in gastric cancer SGC7901 cell. World J Gastroenterol. 2002;8:54–59. doi: 10.3748/wjg.v8.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao F, Yi J, Shi GY, Li H, Shi XG, Tang XM. The sensitivity of digestive tract tumor cells to As2O3 is associated with the inherent cellular level of reactive oxygen species. World J Gastroenterol. 2002;8:36–39. doi: 10.3748/wjg.v8.i1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu DH, Zhang XY, Fan DM, Huang YX, Zhang JS, Huang WQ, Zhang YQ, Huang QS, Ma WY, Chai YB, et al. Expression of vascular endothelial growth factor and its role in oncogenesis of human gastric carcinoma. World J Gastroenterol. 2001;7:500–505. doi: 10.3748/wjg.v7.i4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu S, Wu Q, Chen ZM, Su WJ. The effect pathway of retinoic acid through regulation of retinoic acid receptor alpha in gastric cancer cells. World J Gastroenterol. 2001;7:662–666. doi: 10.3748/wjg.v7.i5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu K, Shan YJ, Zhao Y, Yu JW, Liu BH. Inhibitory effects of RRR-alpha-tocopheryl succinate on benzo(a)pyrene (B(a)P)-induced forestomach carcinogenesis in female mice. World J Gastroenterol. 2001;7:60–65. doi: 10.3748/wjg.v7.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796–800. doi: 10.3748/wjg.v7.i6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui M, Zhang HJ, An LG. Tumor growth Inhibition by polysac-charide from Coprinus comatus. Shijie Huaren Xiaohua Zazhi. 2002;9:287–290. [Google Scholar]
- 42.Wang XB, Wang X, Zhang NZ. Inhibition of somatostatin ana-log Octreotide on human gastric cancer ce ll MKN45 growth in vitro. Xin Xiaohua Bingxue Zazhi. 2002;10:40–42. [Google Scholar]
- 43.Zhan J, Xie DR, Yao HR, Lin XG, Liang XW, Xiang YQ. Apoptosis of larage intestine cancer cells (SW620) induced by As2O3. Shijie Huaren Xiaohua Zazhi. 2001;9:228–229. [Google Scholar]
- 44.Yu LF, Wu YL, Zhang YP. Reversal of drug resistance in the vin-cristine-resistant human gastric cancer cell lines MKN28/VCR by emulsion of seminal oil of Brucea Javanica. Shijie Huaren Xiaohua Zazhi. 2001;9:376–378. [Google Scholar]
- 45.Liu WC, Mu HX, Ren J, Zhang XY, Pan BR. Anti-tumor activity of defensin on gastric cancer cell line in vitro. Shijie Huaren Xiaohua Zazhi. 2001;9:622–626. [Google Scholar]
- 46.Chen JP, Shen DM, Yang ZB. CagA+ Hp broth culture filtrates induced malignant transformation on human gastric epithelial cells. Shijie Huaren Xiaohua Zazhi. 2001;9:617–621. [Google Scholar]
- 47.Qin YC, Yuan YQ, Si JL, Lin J, Zhu J, Liu JY. The effects of Apoptosis and activation of telomerase on human gastric cancer cells induced by paclitaxel. Shijie Huaren Xiaohua Zazhi. 2001;9:1086–1087. [Google Scholar]
- 48.Zhang L, Fu HM, Jin SZ, Hang R, Zhou CG. Overexpression of P53 and relationship between extracellular matrix and differentiation, invasion and metastasis of gastric carcinoma. Shijie Huaren Xiaohua Zazhi. 2001;9:992–996. [Google Scholar]
- 49.Sun B, Wu YL, Zhang XJ, Wang SN, He HY, Qiao MM, Zhang YP, Zhong J. Effects of Sulindac on growth inhibition and apoptosis induction in human gastric cancer cells. Shijie Huaren Xiaohua Zazhi. 2001;9:997–1002. [Google Scholar]
- 50.Liu HF, Liu WW, Fang DC, Gao JH, Wang ZH. Apoptosis and proliferation induced by Helicobacter pylori and its asso c iation with p53 protein expression in gastric epithelial cells. Shijie Huaren Xiaohua Zazhi. 2001;9:1265–1268. [Google Scholar]
- 51.Tu SP, Jiang SH, Qiao MM, Cheng SD, Wang LF, Wu YL, Yuan YZ, Wu YX. Effect of trichosanthin on cytotoxicity and induc-tion of apoptosis of multiple drugs resistence cells in gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:150–152. [Google Scholar]
- 52.Xia ZS, Zhu ZH, He SG. Effects of ATRA and 5-Fu on growth and telomerase activity of xenografts of gastric cancer in nude mice. Shijie Huaren Xiaohua Zazhi. 2000;8:674–677. [Google Scholar]
- 53.Zhao AG, Yang JK, Zhao HL. Chinese Jianpi herbs induce apoptosis of human gastric cancer grafted onto nude mice. Shijie Huaren Xiaohua Zazhi. 2000;8:737–740. [Google Scholar]
- 54.Liu HF, Liu WW, Fang DC, Yang SM, Zhao L. Gastric epithelial apoptosis induced by Helicobacter pylori and its relationship with Bax protein expression. Shijie Huaren Xiaohua Zazhi. 2000;8:860–862. [Google Scholar]
- 55.Koutsodontis G, Tentes I, Papakosta P, Moustakas A, Kardassis D. Sp1 plays a critical role in the transcriptional activation of the human cyclin-dependent kinase inhibitor p21(WAF1/Cip1) gene by the p53 tumor suppressor protein. J Biol Chem. 2001;276:29116–29125. doi: 10.1074/jbc.M104130200. [DOI] [PubMed] [Google Scholar]
- 56.Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- 57.Wang NS, Unkila MT, Reineks EZ, Distelhorst CW. Transient expression of wild-type or mitochondrially targeted Bcl-2 induces apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death. J Biol Chem. 2001;276:44117–44128. doi: 10.1074/jbc.M101958200. [DOI] [PubMed] [Google Scholar]
- 58.Lüschen S, Ussat S, Scherer G, Kabelitz D, Adam-Klages S. Sensitization to death receptor cytotoxicity by inhibition of fas-associated death domain protein (FADD)/caspase signaling. Requirement of cell cycle progression. J Biol Chem. 2000;275:24670–24678. doi: 10.1074/jbc.M003280200. [DOI] [PubMed] [Google Scholar]
- 59.Fujioka Y, Taira T, Maeda Y, Tanaka S, Nishihara H, Iguchi-Ariga SM, Nagashima K, Ariga H. MM-1, a c-Myc-binding protein, is a candidate for a tumor suppressor in leukemia/lymphoma and tongue cancer. J Biol Chem. 2001;276:45137–45144. doi: 10.1074/jbc.M106127200. [DOI] [PubMed] [Google Scholar]
- 60.Liu H, Lo CR, Jones BE, Pradhan Z, Srinivasan A, Valentino KL, Stockert RJ, Czaja MJ. Inhibition of c-Myc expression sensitizes hepatocytes to tumor necrosis factor-induced apoptosis and necrosis. J Biol Chem. 2000;275:40155–40162. doi: 10.1074/jbc.M001565200. [DOI] [PubMed] [Google Scholar]


