Abstract
AIM: To investigate the expression and function of classical protein kinase C (PKC) isoenzymes in inducing MDR phenotype in gastric cancer cells.
METHODS: Two cell lines were used in the study: gastric cancer cell SGC7901 and its drug-resistant cell SGC7901/VCR stepwise-selected by vincristine 0.3, 0.7 and 1.0 mg·L-1, respectively. The expression of classical PKC (cPKC) isoenzymes in SGC7901 cells and SGC7901/VCR cells were detected using immunofluorescent cytochemistry, laser confocal scanning microscope and Western blot. The effects of anti-PKC isoenzymes antibody on adriamycin accumulation in SGC7901/VCR cells were determined using flow cytometric analysis.
RESULTS: (1) SGC7901 cells exhibited positive staining of PKC-α. SGC7901/VCR cells exhibited stronger staining of PKC-α than SGC7901 cells. The higher dosage vincristine selected, the much stronger staining of PKC-α was observed on SGC7901/VCR cells. (2) Both SGC7901 and SGC7901/VCR cells exhibited positive staining of PKC-β I and PKC-β II with no significant difference. (3) Compared with SGC7901, SGC7901/VCR cells had decreased adriamycin accumulation and retention. Accumulation of adriamycin in SGC7901 was 5.21 ± 2.56 mg·L-1, in SGC7901/VCR 0.3 was 0.85 ± 0.29 mg·L-1, in SGC7901/VCR 0.7 was 0.81 ± 0.32 mg·L-1, and in SGC7901/VCR 1.0 was 0.80 ± 0.33 mg·L-1; Retention of adriamycin in SGC7901 was 2.51 ± 1.23 mg·L-1, in SGC7901/VCR 0.3 was 0.47 ± 0.14 mg·L-1, in SGC7901/VCR 0.7 was 0.44 ± 0.15 mg·L-1, and in SGC7901/VCR 1.0 was 0.41 ± 0.11 mg·L-1. (4) Fluorescence intensity presented adriamycin accumulation in SGC7901/VCR cells was increased from 1.14 ± 0.36 to 2.71 ± 0.94 when cells were co-incubated with anti-PKC-α but not with anti-PKC-β I, PKC-α II and PKCγ antibodies.
CONCLUSION: PKC-α, but not PKC-β I, PKC-β II or PKCγ, may play a role in multidrug resistance of gastric cancer cells SGC7901/VCR.
INTRODUCTION
Multi-drug resistance (MDR), the principal mechanism by which many cancers develop resistance to a variety of chemotherapeutic drugs, is a major factor in the failure of many forms of chemotherapy[1-4]. It affects patients with numerous blood cancers and solid tumors. Cellular drug resistance is mediated by different mechanisms operating at different steps of the cytotoxic action of the drug from a decrease of drug accumulation in the cell to the abrogation of apoptosis induced by the chemical substance. Several different mechanisms will switch on in the MDR cells, but usually one major mechanism is operating. The most investigated mechanisms with known clinical significance are: (1) activation of transmembrane proteins effluxing different chemical substances from the cells, in which P-glycoprotein (P-gp) is the most known efflux pump; (2) activation of the enzymes of the glutathione detoxification system; (3) alterations of the genes and the proteins involved into the control of apoptosis (especially p53 and Bcl-2)[5-12]. PKC comprises a family of at least 13 distinct serine/threonine kinase isoenzymes involved in signal transduction pathways that govern a wide range of physiological processes including differentiation, proliferation, gene expression, brain function, membrane transport and the organization of cytoskeletal and extracellular matrix proteins[13-26]. Recently accumulated evidence indicates that PKC activity, especially cPKC, plays a significant role in the formation of tumor MDR. The isoenzymes possess distinct differences in localization in different cells. Within a single cell, PKC isoforms also exhibit differences in expression and function, so research on distinct function in tumor MDR of isoenzymes has important significance in screening drugs with high specificity that could reverse MDR and in disclosing the mechanism of MDR formation and its regularity of the reversion.
MATESIALS AND METHODS
Materials
Human gastric cancer cell line SGC7901 was reserved by our institute and its drug-resistant sublines SGC7901/VCR were stepwise-selected by vincristine 0.3, 0.7 and 1.0 mg·L-1, respectively. RPMI 1640 medium was the product of Gibco (U.S.A.). Newborn bovine serum was purchased from Hyclone (U.S.A.). Chemical drugs vincristine and adriamycin were purchased from Farmitalia Carlo Erba (U.S.A.) and Minsheng (Hangzhou, China). Rabbit-anti-human polyclonal antibody PKC-α, PKC-β I, PKC-β II and PKCγ were the products of Santa Cruz Biotechnology. SABC immunohischemistry kit and the HRP labeled goat-anti-rabbit IgG was purchased from Boste (Wuhan, China). FITC labeled goat-anti-rabbit IgG was purchased from Zhongshan (China).
Methods
Immunofluorescent cytochemistry The expression of PKC isoenzymes were detected by routine immunocytochemical fluorescence method[27,28].The procedures were as follows. Cells were maintained at 37 °C in a 50 mL·L-1 CO2-humidified incubator in RPMI 1640 medium supplemented with 25 mmol·L-1 HEPES buffer and 100 mL·L-1 new born bovine serum. SGC7901/VCR cells were cultured in the medium with extra adding vincristine at the concentration of 0.3, 0.7 and 1.0 mg·L-1, respectively. Cells at exponential phage were harvested, digested by 2.5 g·L-1 trypsin and then cultured on the slides in the medium described above at 37 °C for further 24 h; RPMI 1640 medium was then washed by PBS and cells were fixed in cold acetone for 5 min; 50 mL·L-1 H2O2 was added and incubated at room temperature for 10-15 min and added 3 g·L-1 TritonX-100 for another 15 min; normal goat serum (1:10) was added and incubated at room temperature for 30 min; rabbit-anti-human polyclonal antibody PKC-α, PKC-β I, PKC-β II and PKCγ (1:100) were added respectively and incubated at 4 °C over night; FITC labeled goat-anti-rabbit IgG was added and incubated at 37 °C for 1 h; the slides were sealed by 500 mL·L-1 glycerin buffer and observed with a fluorescence microscope. Unrelated monoclonal antibody and PBS were used as negative controls.
Laser confocal scanning microscope The laser confocal scanning microscope protocols used were as described[29,30]. Methods of cell culture and stain with Ab were carried out as described in immunocytochemical fluorescence method. FITC labeled goat-anti-rabbit IgG was added in the darkness and incubated at room temperature for 3 h; the slides were sealed by 500 mL·L-1 glycerin buffer and observed with a laser confocal scanning fluorescence microscope. Unrelated monoclonal antibody and PBS were used as negative controls.
SDS-PAGE According to Chen et al[31,33] and Xiao et al[32], cells at exponential phase were harvested and washed by cold PBS and suspended in extraction buffer (50 mmol·L-1 Tris-Cl (pH7.5), 150 mmol·L-1 NaCl, 0.2 mmol·L-1 EDTA, 1 mmol·L-1 PMSF and 10 g·L-1 NP-40). The homogenate was heated for 5 min in a boiling water bath and then centrifuged. The supernatants were harvested and the protein concentrations were assayed by Bradford method. 150 μg total protein were electrophoresed on SDS-polyacrylamide gels with the stacking and the separating gels containing 50 and 100 g·L-1 acrylamide, respectively, and the gels were stained with Coomassie brilliant blue dye.
Western blot analysis According to She et al[34], after SDS-PAGE, proteins were transferred onto nitrocellulose membrane under a constant current of mA for 1 h. Non-specific binding sites were blocked by PBS with 50 mL·L-1 milk plus 1 g·L-1 Tween-20 at room temperature. Primary and secondary antibodies were rabbit-anti-human polyclonal antibody PKC and HRP labeled goat-anti-rabbit IgG, respectively. Films were exposed in DAB detection reagent to develop color of bands.
Flow cytometric analysis According to Jiang et al[35] and Feng et al[36], cells were cultured in 6-well culture plates at 37 °C for 48 h, adriamycin was added to the final concentration of 5 mg·L-1. After further culture for 1 h, rabbit-anti-human polyclonal antibody against different cPKC isoenzymes was added and incubated for 40 min, PBS and normal rabbit serum were used as negative controls. And then, cells were harvested or cultured in drug-free medium for another 30 min and harvested. The harvested cells of the phases were suspended in cold PBS, intracellular adriamycin fluorescence intensity was determined by flow cytometric analysis with the stimulative and acceptant wave length at 488 nm and 575 nm, respectively.
Statistical analysis Data were presented as -x ± s. Significant differences were determined by using ANOVA in statistical software SPSS10.0.
RESULTS
Immunofluorescent cytochemistry
To investigate the expression of PKC isoenzymes of SGC7901 cells and its drug-resistant cell subline SGC7901/VCR. immunofluorescent cytochemistry was performed. The positive signals were of fluorescent signals. Both SGC7901 cells and SGC7901/VCR cells expressed PKC-α, PKC-β I, PKC-β II and PKCγ. The expression of PKC-α was stronger in SGC7901/VCR cells than that in SGC7901 cells. There was no significant difference in the expression of PKC-β I and PKC-β II between SGC7901/VCR cells and SGC7901 cells. And the expression of PKCγ in SGC7901/VCR cells was positive as strongly as that in SGC7901 cells, and also no significant difference was found.
Laser confocal microscope analysis
PKC-α expressed in both SGC7901 cells and SGC7901/VCR cells. The positive signals were localized in cytoplasm and membrane. Compared with SGC7901 cells, the intensity of fluorescence in SGC7901/VCR cells was increased significantly when analysed by the intensity of pixel by using computer, which was 100 in SGC7901/VCR cells and 80 in SGC7901 cells.
Western blot
The expression of PKC-α was significantly higher in SGC7901/VCR cells than that in SGC7901 cells, in which expression increased with the increase of drug-dose-resistance of SGC7901/VCR cells. No significant difference was found in the expression of PKC-β I, PKC-β II and PKCγ between SGC7901/VCR cells and SGC7901 cells (Figure 1).
Figure 1.
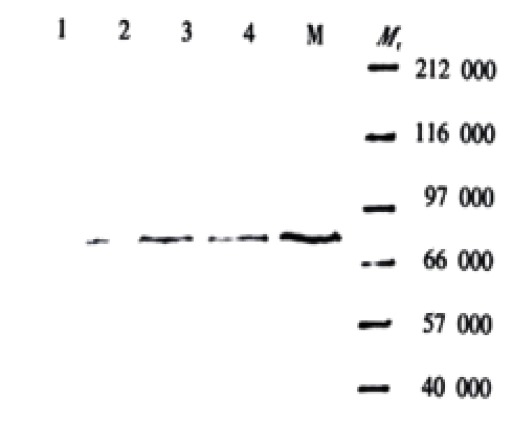
Western blot (detected with anti-PKC-α antibody)
Flow cytometric analysis
The effects of anti-PKC-α or β I antibody on adriamycin accumulation and retention in SGC7901/VCR cells were determined by flow cytometric analysis. When cells were cultured in drug-RPM1640, intracellular drug concentration would increase and finally stabilised at the highest plateau value, which was called adriamycin accumulation. When cells were cultured in drug-free medium, drug was effluxed from cells and subsequently, drug concentration stabilised at a lower plateau value, which was still higher than the initial value and so called adriamycin retention. The results presented by the values of fluorescence intensity showed that adriamycin accumulation and retention decreased in SGC7901/VCR cells than that in SGC7901 cells. When co-incubated with anti-PKC-α antibody, the accumulation of adriamycin in MDR cells increased and showed partly dose-dependent effect, while PKC-β I, PKC-β II and PKCγ could not influence the ADR accumulation in SGC7901/VCR cells (Table 1, Table 2).
Table 1.
Adriamycin accumulation and retention in cells by flow cytometric analysis (-x ± s fluorescence intensity)
| Adriamycin | SGC7901 |
SGC7901/VCR in differentresistant drug dose (VCR, mg·L-1) |
||
| 0.3 | 0.7 | 1.0 | ||
| Accumulation | 5.21 ± 2.56 | 0.85 ± 0.29b | 0.81 ± 0.32b | 0.80 ± 0.33b |
| Retention | 2.51 ± 1.23 | 0.47 ± 0.14b | 0.44 ± 0.15b | 0.41 ± 0.11b |
P < 0.01, vs SGC7901.
Table 2.
Effects of adriamycin accumulation in SGC7901/VCR cells by anti-PKC isoenzymes Ab (-x ± s fluorescence intensity)
| Group |
ρ (anti-PKC isoenzymes Ab)/(μg·L-1) |
|||
| 0 | 25 | 250 | 500 | |
| Anti-PKC-α Ab | 1.14 ± 0.36 | 1.09 ± 0.32 | 2.49 ± 0.84b | 2.71 ± 0.94b |
| Anti-PKC-β IAb | 1.14 ± 0.36 | 1.13 ± 0.38 | 1.14 ± 0.39 | 1.14 ± 0.39 |
| Anti-PKC-α IIAb | 1.14 ± 0.36 | 1.14 ± 0.38 | 1.14 ± 0.40 | 1.14 ± 0.39 |
| Anti-PKC-γ Ab | 1.14 ± 0.36 | 1.14 ± 0.39 | 1.14 ± 0.40 | 1.14 ± 0.39 |
P < 0.01, vs 0 μg·L-1 anti-PKC isoenzymes Ab
DISCUSSION
The development of resistance to chemotherapeutic agents remains one of the major obstacles for successful cure of cancer patients. Tumor cells may acquire MDR in the course of exposure to various compounds that are used in modern anticancer therapy, including cytotoxic drugs and differentiating agents. Therefore, the recurrence of the disease after the initial treatment may be associated with establishment of secondary MDR in the residual tumor. Research on resistance to cancer treatment was mainly focused for 20 years on MDR. No useful method of reversing MDR, suitable for clinical use, has yet emerged from this large quantity of work. The reason could be an complicated mechanism involved in it. There are several ways for cancer cells to develop resistance or defense mechanisms against cytotoxic drugs[6,37-42].
Resistance to therapy has been correlated to the presence of at least two molecular "pumps" that actively expel chemotherapeutic drugs from the tumor cells. This action thus spares tumor cells from the effects of the drug, which has to act inside the cells at the nucleus or the cytoplasm. The two pumps commonly found to confer MDR in cancer are P-gp and multidrug resistance-associated protein (MRP). But they can not explicate the phenomenon of MDR fully. It also reported that some cancer cells are resistant to signal of apoptosis and so making cell life longer might confer to the MDR phenotype.
Recent studies have indicated that the signal of phosphorylation might be an important part of MDR mechanisms. PKC isoforms are often overexpressed in disease states such as cancer and play a critical role in regulation of long term cellular events such as proliferation, differentiation and tumorigenesis. An increase in PKC activity might result in an oncogenic role and in MDR. Several studies indicate a role for PKC in the regulation of the MDR phenotype, since several PKC inhibitors are able to partially reverse MDR and inhibit P-gp phosphorylation.
The PKC family consists of several isoforms comprising three groups: classical, novel and atypical. PKC isoforms are widely distributed in mammalian tissues and have many important physiological functions[43-46]. cPKC subfamily shows significant specificity in tissue distribution. The isoenzymes possess distinct differences in localization in different cells. Within a single cell, PKC isoforms also exhibit differences in their distribution before and after their translocation following activation. For example, thymus cells express PKC-α and PKC-β I but not PKC-β II and PKCγ; Cortical and medullary cells of suprarenal gland express PKC-α, while the cortical cells also express PKC-β I and PKCγ.
To date in recent years, the MDR phenotype is also associated with variation in content of PKC isoenzymes. Different isoforms possess distinct differences in expression and function in different MDR cells. It has been confirmed that sensitive cells show the phenotype of MDR when transfected with cDNA encoding PKC-α, which indicats the effect of PKC on MDR. Resistance to ADR of mouse leukemia MDR cell-line could be reversed by anti-PKC-β mAb when it was incubated with anti-PKC-α or anti-PKC-β mAb[47-59].
This study confirmed that PKC-α, PKC-β I, PKC-β II and PKCγ were expressed in both SGC7901 cells and SGC7901/VCR cells. Our results showed that the expression of PKC-α was significantly higher in SGC7901/VCR cells than that in SGC7901 cells and the expression increased with the increase of drug-dose-resistance of SGC7901/VCR cells. There was no significant difference in the expression of PKC-β I, PKC-β II and PKCγ between SGC7901/VCR cells and SGC7901 cells. The result of flow cytometric analysis showed that ADR accumulation decreased in SGC7901/VCR cells much than that in SGC7901 cells, together with increase of the expression of PKC-α. Further study confirmed that anti-PKC-α antibody could reverse ADR accumulation in MDR cells to some degree and showed partly does-dependent effect, while PKC-β I, PKC-β II and PKCγ could not influence the ADR accumulation in SGC7901/VCR cells. The results suggested that the formation of MDR in SGC7901/VCR cells was associated with over expression of PKC-α but not with PKC-β I, PKC-α II and PKCγ. Since isoenzymes of PKC possess only 1-10 amino acid in there pseudo substrate action site in C1 domain, research on distinct function in tumor MDR of isoenzymes has important significance in screening effective drugs with high specificity that could reverse MDR and in disclosing the mechanism of MDR formation and its regularity of the reversion. Because gastric cancer is common in China and some areas in the world[60-79], this results may be important for further study.
Footnotes
Supported by the National Nature Science Fundation of China, No. 30030140 and No. 30000066.
Edited by Zhang JZ
References
- 1.Yao XQ, Qing SH. Detection of multidrug resistance gene in progvessive colon cancer and its significance. Shijie Huaren Xiaohua Zazhi. 1999;7:535–536. [Google Scholar]
- 2.Tu SP, Jiang SH, Qiao MM, Cheng SD, Wang LF, Wu YL, Yuan YZ, Wu YX. Effect of trichosanthin on cytotoxicity and induction of apoptosis of multiple drugs resi stence cells in gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:150–152. [Google Scholar]
- 3.Cheng SD, Wu YL, Zhang YP, Qiao MM, Guo QS. Abnormal drug accumulation in multidrug resistant gastric carcinoma cells. Shijie Huaren Xiaohua Zazhi. 2001;9:131–134. [Google Scholar]
- 4.Zhang LJ, Chen KN, Xu GW, Xing HP, Shi XT. Congenital expression of mdr-1 gene in tissues of carcinoma and its relation with pathomorphology and prognosis. World J Gastroenterol. 1999;5:53–56. doi: 10.3748/wjg.v5.i1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhan M, Yu D, Lang A, Li L, Pollock RE. Wild type p53 sensitizes soft tissue sarcoma cells to doxorubicin by down-regulating multidrug resistance-1 expression. Cancer. 2001;92:1556–1566. doi: 10.1002/1097-0142(20010915)92:6<1556::aid-cncr1482>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Liu B, Staren E, Iwamura T, Appert H, Howard J. Effects of Taxotere on invasive potential and multidrug resistance phenotype in pancreatic carcinoma cell line SUIT-2. World J Gastroenterol. 2001;7:143–148. doi: 10.3748/wjg.v7.i1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen B, Zhang XY, Zhang YJ, Zhou P, Gu Y, Fan DM. Antisense to cyclin D1 reverses the transformed phenotype of human gastric cancer cells. World J Gastroenterol. 1999;5:18–21. doi: 10.3748/wjg.v5.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu XL, Xiao B, Yu ZC, Guo JC, Zhao QC, Xu L, Shi YQ, Fan DM. Down-regulation of Hsp90 could change cell cycle distribution and increase drug sensitivity of tumor cells. World J Gastroenterol. 1999;5:199–208. doi: 10.3748/wjg.v5.i3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You H, Xiao B, Cui DX, Shi YQ, Fan DM. Two novel gastric cancer-associated genes identified by differential display. World J Gastroenterol. 1998;4:334–336. doi: 10.3748/wjg.v4.i4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao B, Shi YQ, Zhao YQ, You H, Wang ZY, Liu XL, Yin F, Qiao TD, Fan DM. Transduction of Fas gene or Bcl-2 antisense RNA sensitizes cultured drug resistant gastric cancer cells to chemotherapeutic drugs. World J Gastroenterol. 1998;4:421–425. doi: 10.3748/wjg.v4.i5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warr JR, Bamford A, Quinn DM. The preferential induction of apoptosis in multidrug-resistant KB cells by 5-fluorouracil. Cancer Lett. 2002;175:39–44. doi: 10.1016/s0304-3835(01)00721-2. [DOI] [PubMed] [Google Scholar]
- 12.Roepe PD. pH and multidrug resistance. Novartis Found Symp. 2001;240:232–247; discussion 247-250, 265-268. doi: 10.1002/0470868716.ch16. [DOI] [PubMed] [Google Scholar]
- 13.Caruso-Neves C, Silva IV, Morales MM, Lopes AG. Cytoskeleton elements mediate the inhibition of the (Na++K+)atpase activity by PKC in Rhodnius prolixus malpighian tubules during hyperosmotic shock. Arch Insect Biochem Physiol. 2001;48:81–88. doi: 10.1002/arch.1060. [DOI] [PubMed] [Google Scholar]
- 14.Suga S, Wu J, Ogawa Y, Takeo T, Kanno T, Wakui M. Phorbol ester impairs electrical excitation of rat pancreatic beta-cells through PKC-independent activation of KATP channels. BMC Pharmacol. 2001;1:3. doi: 10.1186/1471-2210-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald JF, Kotecha SA, Lu WY, Jackson MF. Convergence of PKC-dependent kinase signal cascades on NMDA receptors. Curr Drug Targets. 2001;2:299–312. doi: 10.2174/1389450013348452. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, et al. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci USA. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar A, Hawkins KS, Hannan MA, Ganz MB. Activation of PKC-beta(I) in glomerular mesangial cells is associated with specific NF-kappaB subunit translocation. Am J Physiol Renal Physiol. 2001;281:F613–F619. doi: 10.1152/ajprenal.2001.281.4.F613. [DOI] [PubMed] [Google Scholar]
- 18.Rivedal E, Opsahl H. Role of PKC and MAP kinase in EGF- and TPA-induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis. 2001;22:1543–1550. doi: 10.1093/carcin/22.9.1543. [DOI] [PubMed] [Google Scholar]
- 19.Smith J, Yu R, Hinkle PM. Activation of MAPK by TRH requires clathrin-dependent endocytosis and PKC but not receptor interaction with beta-arrestin or receptor endocytosis. Mol Endocrinol. 2001;15:1539–1548. doi: 10.1210/mend.15.9.0695. [DOI] [PubMed] [Google Scholar]
- 20.Banan A, Fields JZ, Talmage DA, Zhang Y, Keshavarzian A. PKC-beta1 mediates EGF protection of microtubules and barrier of intestinal monolayers against oxidants. Am J Physiol Gastrointest Liver Physiol. 2001;281:G833–G847. doi: 10.1152/ajpgi.2001.281.3.G833. [DOI] [PubMed] [Google Scholar]
- 21.Manier DH, Shelton RC, Sulser F. Cross-talk between PKA and PKC in human fibroblasts: what are the pharmacotherapeutic implications? J Affect Disord. 2001;65:275–279. doi: 10.1016/s0165-0327(00)00278-0. [DOI] [PubMed] [Google Scholar]
- 22.Boesch DM, Garvin JL. Age-dependent activation of PKC isoforms by angiotensin II in the proximal nephron. Am J Physiol Regul Integr Comp Physiol. 2001;281:R861–R867. doi: 10.1152/ajpregu.2001.281.3.R861. [DOI] [PubMed] [Google Scholar]
- 23.Lum H, Podolski JL, Gurnack ME, Schulz IT, Huang F, Holian O. Protein phosphatase 2B inhibitor potentiates endothelial PKC activity and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001;281:L546–L555. doi: 10.1152/ajplung.2001.281.3.L546. [DOI] [PubMed] [Google Scholar]
- 24.Shindo M, Irie K, Nakahara A, Ohigashi H, Konishi H, Kikkawa U, Fukuda H, Wender PA. Toward the identification of selective modulators of protein kinase C (PKC) isozymes: establishment of a binding assay for PKC isozymes using synthetic C1 peptide receptors and identification of the critical residues involved in the phorbol ester binding. Bioorg Med Chem. 2001;9:2073–2081. doi: 10.1016/s0968-0896(01)00100-6. [DOI] [PubMed] [Google Scholar]
- 25.Kim MS, Lim WK, Cha JG, An NH, Yoo SJ, Park JH, Kim HM, Lee YM. The activation of PI 3-K and PKC zeta in PMA-induced differentiation of HL-60 cells. Cancer Lett. 2001;171:79–85. doi: 10.1016/s0304-3835(01)00505-5. [DOI] [PubMed] [Google Scholar]
- 26.Radeff JM, Nagy Z, Stern PH. Involvement of PKC-beta in PTH, TNF-alpha, and IL-1 beta effects on IL-6 promoter in osteoblastic cells and on PTH-stimulated bone resorption. Exp Cell Res. 2001;268:179–188. doi: 10.1006/excr.2001.5283. [DOI] [PubMed] [Google Scholar]
- 27.Ma X, Qiu DK, Xu J, Zeng MD. Effects of Cordyceps polysaccharides in patients with chronic hepatitis C. Huaren Xiaohua Zazhi. 1998;6:582–584. [Google Scholar]
- 28.Zhao LF, Han DW. Clinical significance of endotoxemia in liver diseases. Shijie Huaren Xiaohua Zazhi. 1999;7:391–393. [Google Scholar]
- 29.Wang CM, Huang XF, Pan BR, Dai XW, Ma FC, Zhao YM. Expression of chromogranin C/secretogranin II and pancreastatin in the pancreatic ductal carcinoma. Huaren Xiaohua Zazhi. 1998;6:470–473. [Google Scholar]
- 30.Huang XF, Wang CM, Dai XW, Pan BR, Yu LB, Fang L, Qian B, Zhao YL. Significance of cathepsin D and chromogranin A expression in human primary hepatocellular carcinomas. Huaren Xiaohua Zazhi. 1998;6:474–478. [Google Scholar]
- 31.Chen YK, Wu XB, Zhang ZY. Study on causes of serum anti-HBs positive patients with chronic liver diseases. Huaren Xiaohua Zazhi. 1998;6:49–50. [Google Scholar]
- 32.Xiao B, Shi YQ, Zhao YQ, You H, Liu XL, Fan DM. Expression of Fas gene in gastric cancer cells transducted with Fas gene. Huaren Xiaohua Zazhi. 1998;6:400–403. [Google Scholar]
- 33.Yang LJ, Sui YF, Chen ZN. Preparation and activity of conjugate of monoclonal antibody HAb18 against hepatoma F(ab')(2) fragment and staphylococcal enterotoxin A. World J Gastroenterol. 2001;7:216–221. doi: 10.3748/wjg.v7.i2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.She FF, Su DH, Lin JY, Zhou LY. Virulence and potential pathogenicity of coccoid Helicobacter pylori induced by antibiotics. World J Gastroenterol. 2001;7:254–258. doi: 10.3748/wjg.v7.i2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang XL, Quan QZ, Sun ZQ, Wang YJ, Qi F. Expression of adhesion molecules in tissues and peripheral lymphocyte of patients with ulcerative colitis. Huaren Xiaohua Zazhi. 1998;6:54–55. [Google Scholar]
- 36.Feng S, Song JD, Tian XR. Significance of proliferating cell nuclear antigen expression in colorectal carcinomas. Huaren Xiaohua Zazhi. 1998;6:146–147. [Google Scholar]
- 37.Gu SQ, Liang YY, Fan LR, Li BY, Wang DS. Co-regulative effects of the cAMP/PKA and DAG/PKC signal pathways on human gastric cancer cells during differentiation induced by traditional Chinese medicines. China Natl J New Gastroenterol. 1997;3:50–53. doi: 10.3748/wjg.v3.i1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu BH, Zhang RJ, Lu DD, Chen XD, Wang NJ. Expression of mdrl gene coded Pglycoprotein in hepatocellular carcinoma and its clinical significance. Huaren Xiaohua Zazhi. 1998;6:783–785. [Google Scholar]
- 39.Liu ZM, Shou NH. Expression significance of mdr1 gene in gastric carcinoma tissue. Shijie Huaren Xiaohua Zazhi. 1999;7:145–146. [Google Scholar]
- 40.Shi YQ, Xiao B, Miao JY, Zhao YQ, You H, Fan DM. Construction of eukaryotic expression vector pBK fas and MDR reversal test of drug-resistant gastric cancer cells. Shijie Huaren Xiaohua Zazhi. 1999;7:309–312. [Google Scholar]
- 41.Liu Y, Lu MZ, Li QM, Wang YL. The expression of p53 C-myc and P-gp proteins in gastric cancer. Xin Xiaohuabingxue Zazhi. 1997;5:585–586. [Google Scholar]
- 42.Yin F, Shi YQ, Zhao WP, Xiao B, Miao JY, Fan DM. Suppression of P-gp induced multiple drug resistance in a drug resistant gastric cancer cell line by overexpression of Fas. World J Gastroenterol. 2000;6:664–670. doi: 10.3748/wjg.v6.i5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato A, Miyazaki M, Ambiru S, Yoshitomi H, Ito H, Nakagawa K, Shimizu H, Yokosuka O, Nakajima N. Multidrug resistance gene (MDR-1) expression as a useful prognostic factor in patients with human hepatocellular carcinoma after surgical resection. J Surg Oncol. 2001;78:110–115. doi: 10.1002/jso.1129. [DOI] [PubMed] [Google Scholar]
- 44.Kaminski MS, Zelenetz AD, Press OW, Saleh M, Leonard J, Fehrenbacher L, Lister TA, Stagg RJ, Tidmarsh GF, Kroll S, et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin's lymphomas. J Clin Oncol. 2001;19:3918–3928. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 45.Ahlman H, Khorram-Manesh A, Jansson S, Wängberg B, Nilsson O, Jacobsson CE, Lindstedt S. Cytotoxic treatment of adrenocortical carcinoma. World J Surg. 2001;25:927–933. doi: 10.1007/s00268-001-0031-6. [DOI] [PubMed] [Google Scholar]
- 46.Dei S, Teodori E, Garnier-Suillerot A, Gualtieri F, Scapecchi S, Budriesi R, Chiarini A. Structure-activity relationships and optimisation of the selective MDR modulator 2-(3,4-dimethoxyphenyl)-5-(9-fluorenylamino)-2-(methylethyl) pentanenitrile and its N-methyl derivative. Bioorg Med Chem. 2001;9:2673–2682. doi: 10.1016/s0968-0896(01)00191-2. [DOI] [PubMed] [Google Scholar]
- 47.Månsson E, Paul A, Löfgren C, Ullberg K, Paul C, Eriksson S, Albertioni F. Cross-resistance to cytosine arabinoside in a multidrug-resistant human promyelocytic cell line selected for resistance to doxorubicin: implications for combination chemotherapy. Br J Haematol. 2001;114:557–565. doi: 10.1046/j.1365-2141.2001.02979.x. [DOI] [PubMed] [Google Scholar]
- 48.Meng LH, Zhang JS, Ding J. Salvicine, a novel DNA topoisomerase II inhibitor, exerting its effects by trapping enzyme-DNA cleavage complexes. Biochem Pharmacol. 2001;62:733–741. doi: 10.1016/s0006-2952(01)00732-8. [DOI] [PubMed] [Google Scholar]
- 49.Chang G, Roth CB. Structure of MsbA from E. coli: a homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science. 2001;293:1793–1800. doi: 10.1126/science.293.5536.1793. [DOI] [PubMed] [Google Scholar]
- 50.Gill PK, Gescher A, Gant TW. Regulation of MDR1 promoter activity in human breast carcinoma cells by protein kinase C isozymes alpha and theta. Eur J Biochem. 2001;268:4151–4157. doi: 10.1046/j.1432-1327.2001.02326.x. [DOI] [PubMed] [Google Scholar]
- 51.Shtil AA, Ktitorova OV, Kakpakova ES, Holian O. Differential effects of the MDR1 (multidrug resistance) gene-activating agents on protein kinase C: evidence for redundancy of mechanisms of acquired MDR in leukemia cells. Leuk Lymphoma. 2000;40:191–195. doi: 10.3109/10428190009054896. [DOI] [PubMed] [Google Scholar]
- 52.Matsumoto Y, Kunishio K, Nagao S. Increased phosphorylation of DNA topoisomerase II in etoposide resistant mutants of human glioma cell line. J Neurooncol. 1999;45:37–46. doi: 10.1023/a:1006346624083. [DOI] [PubMed] [Google Scholar]
- 53.Pallarés-Trujillo J, López-Soriano FJ, Argilés JM. Lipids: A key role in multidrug resistance? (Review) Int J Oncol. 2000;16:783–798. doi: 10.3892/ijo.16.4.783. [DOI] [PubMed] [Google Scholar]
- 54.van Gijn R, van Tellingen O, Haverkate E, Kettenes-van den Bosch JJ, Bult A, Beijnen JH. Pharmacokinetics and metabolism of the staurosporine analogue CGP 41 251 in mice. Invest New Drugs. 1999;17:29–41. doi: 10.1023/a:1006260217400. [DOI] [PubMed] [Google Scholar]
- 55.Merritt JE, Sullivan JA, Drew L, Khan A, Wilson K, Mulqueen M, Harris W, Bradshaw D, Hill CH, Rumsby M, et al. The bisindolylmaleimide protein kinase C inhibitor, Ro 32-2241, reverses multidrug resistance in KB tumour cells. Cancer Chemother Pharmacol. 1999;43:371–378. doi: 10.1007/s002800050909. [DOI] [PubMed] [Google Scholar]
- 56.Han Y, Cao YX, Shi YQ, Fan DM. Expression of P-gp and PKC isoenzymes in multidrug resistance gastric cancer cell-line SGC7901/VCR. Disi Junyi Daxue Xuebao. 2000;21:1454–1456. [Google Scholar]
- 57.Han Y, Shi Y, Li L. [Expression and function of protein kinase C-alpha and beta I isoenzymes in drug-resistant gastric cancer cells] Zhonghua Zhongliu Zazhi. 2001;23:103–106. [PubMed] [Google Scholar]
- 58.Han Y, Shi YQ, Zheng Y, Nie YZ, Zhang HB, Zhang ML, Pan BR, Fan DM. Protein kinase C is related to multidrug resistance by MGr1-Ag. Shijie Huaren Xiaohua Zazhi. 2001;9:517–521. [Google Scholar]
- 59.Han Y, Shi Y, Zhang H. [Alteration of subcellular distribution of protein kinase C isoforms in swelling-activated multi-drug-resistant gastric cancer cells and its significance] Zhonghua Yixue Zazhi. 2001;81:328–331. [PubMed] [Google Scholar]
- 60.Xu L, Zhang SM, Wang YP, Zhao FK, Wu DY, Yan X. Relationship between DNA ploidy,expression of ki-67 antigen and gastric cancer metastasis. World J Gastroenterol. 1999;5:10–11. doi: 10.3748/wjg.v5.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu YA, Lu B, Liu J, Li J, Chen JR, Hu SX. Consequence alimentary reconstruction in nutritional status after total gastrectomy for gastric cancer. World J Gastroenterol. 1999;5:34–37. doi: 10.3748/wjg.v5.i1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ji F, Peng QB, Zhan JB, Li YM. Study of differential polymerase chain reaction of C-erbB-2 oncogene amplification in gastric cancer. World J Gastroenterol. 1999;5:152–155. doi: 10.3748/wjg.v5.i2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhan WH, Ma JP, Peng JS, Gao JS, Cai SR, Wang JP, Zheng ZQ, Wang L. Telomerase activity in gastric cancer and its clinical implications. World J Gastroenterol. 1999;5:316–319. doi: 10.3748/wjg.v5.i4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ji F, Wang WL, Yang ZL, Li YM, Huang HD, Chen WD. Study on the expression of matrix metallo proteinase-2 mRNA in human gastric cancer. World J Gastroenterol. 1999;5:455–457. doi: 10.3748/wjg.v5.i5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang H, Zheng MH, Zhang HB, Zhu J, He JR, Lu AG, Ji YB, Zhang MJ, Jiang Y, Yu BM, et al. Study on incisional implantation of tumor cells by carbon dioxide pneumo peritoneum in gastric cancer of a murine model. World J Gastroenterol. 1999;5:544–546. doi: 10.3748/wjg.v5.i6.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zou SC, Qiu HS, Zhang CW, Tao HQ. A clinical and long-term follow-up study of peri-operative sequential triple therapy for gastric cancer. World J Gastroenterol. 2000;6:284–286. doi: 10.3748/wjg.v6.i2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cai L, Yu SZ, Zhang ZF. Helicobacter pylori infection and risk of gastric cancer in Changle County,Fujian Province,China. World J Gastroenterol. 2000;6:374–376. doi: 10.3748/wjg.v6.i3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang FX, Zhang XY, Fan DM, Deng ZY, Yan Y, Wu HP, Fan JJ. Antisense telomerase RNA induced human gastric cancer cell apoptosis. World J Gastroenterol. 2000;6:430–432. doi: 10.3748/wjg.v6.i3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu QL, Li NL, Zhu ZG, Yin HR, Lin YZ. A study on arsenic trioxide inducing in vitro apoptosis of gastric cancer cell lines. World J Gastroenterol. 2000;6:435–437. doi: 10.3748/wjg.v6.i3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang ZN, Xu HM. Relationship between collagen IV expression and biological behavior of gastric cancer. World J Gastroenterol. 2000;6:438–439. doi: 10.3748/wjg.v6.i3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tu SP, Zhong J, Tan JH, Jiang XH, Qiao MM, Wu YX, Jiang SH. Induction of apoptosis by arsenic trioxide and hydroxy camptothecin in gastriccancer cells in vitro. World J Gastroenterol. 2000;6:532–539. doi: 10.3748/wjg.v6.i4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang BJ, Sun RX, Lin H, Gao YF. Study on the risk factors of lymphatic metastasis and the indications of less in vasive operations in early gastric cancer. World J Gastroenterol. 2000;6:553–556. doi: 10.3748/wjg.v6.i4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng DJ. progress of gastric cancer etiology: N-nitrosamides 1999s. World J Gastroenterol. 2000;6:613–618. doi: 10.3748/wjg.v6.i4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao HJ, Yu LZ, Bai JF, Peng YS, Sun G, Zhao HL, Miu K, L XZ, Zhang XY, Zhao ZQ. Multiple genetic alterations and behavior of cellular biology in gastric cancer and other gastric mucosal lesions: H.pylori infection, histological types and staging. World J Gastroenterol. 2000;6:848–854. doi: 10.3748/wjg.v6.i6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miehlke S, Kirsch C, Dragosics B, Gschwantler M, Oberhuber G, Antos D, Dite P, Läuter J, Labenz J, Leodolter A, et al. Helicobacter pylori and gastric cancer: current status of the Austrain Czech German gastric cancer prevention trial (PRISMA Study) World J Gastroenterol. 2001;7:243–247. doi: 10.3748/wjg.v7.i2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403–406. doi: 10.3748/wjg.v7.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cai L, Yu SZ, Zhang ZF. Glutathione S-transferases M1, T1 genotypes and the risk of gastric cancer: a case-control study. World J Gastroenterol. 2001;7:506–509. doi: 10.3748/wjg.v7.i4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He XS, Su Q, Chen ZC, He XT, Long ZF, Ling H, Zhang LR. Expression, deletion [was deleton] and mutation of p16 gene in human gastric cancer. World J Gastroenterol. 2001;7:515–521. doi: 10.3748/wjg.v7.i4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang DC, Yang SM, Zhou XD, Wang DX, Luo YH. Telomere erosion is independent of microsatellite instability but related to loss of heterozygosity in gastric cancer. World J Gastroenterol. 2001;7:522–526. doi: 10.3748/wjg.v7.i4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]


