Abstract
AIM: Cyclooxygenase-2 (COX-2) has been suggested to be associated with carcinogenesis. We sought to investigate the effect of the selective COX-2 inhibitor, Nimesulide on proliferation and apoptosis of SMMC-7721 human hepatoma cells.
METHODS: This study was carried out on the culture of hepatic carcinoma SMMC-7721 cell line. Various concentrations of Nimesulide (0, 200 μmol/L, 300 μmol/L, 400 μmol/L) were added and incubated. Cell proliferation was detected with MTT colorimetric assay, cell apoptosis by electron microscopy, flow cytometry and TUNEL.
RESULTS: Nimesulide could significantly inhibit SMMC-7721 cells proliferation dose-dependent and in a dependent manner compared with that of the control group. The duration lowest inhibition rate produced by Nimesulide in SMMC-7721 cells was 19.06%, the highest inhibition rate was 58.49%. After incubation with Nimesulide for 72 h, the most highest apoptosis rate and apoptosis index of SMMC-7721 cells comparing with those of the control were 21.20% ± 1.62% vs 2.24% ± 0.26% and 21.23 ± 1.78 vs 2.01 ± 0.23 (P < 0.05).
CONCLUSION: The selective COX-2 inhibitor, Nimesulide can inhibit the proliferation of SMMC-7721 cells and increase apoptosis rate and apoptosis index of SMMC-7721 cells. The apoptosis rate and the apoptosis index are dose-dependent. Under electron microscope SMMC-7721 cells incubated with 300 μmol and 400 μmol Nimesulide show apoptotic characteristics. With the clarification of the mechanism of selective COX-2 inhibitors, These COX-2 selective inhibitors can become the choice of prevention and treatment of cancers.
INTRODUCTION
Hepatic carcinoma was one of most common malignant tumors in China. Its death rate was the third among all cancers, second to gastric carcinoma and lung carcinoma. Although there is a progress in diagnosis and treatment of hepatic carcinoma, its prognosis is still poor. Investigating its pathogenesis and finding new diagnostic and treatment methods is important. Recent epidemiological studies indicate an inverse relationship between the risk of colorectal cancer and intake of NSAIDs. NSAIDs could reduce the incidence of gastric carcinoma and pancreatic carcinoma. It could inhibit tumor cells proliferation and induce apoptosis[1-41]. Cyclooxygenases (COXS) are key enzymes in the conversion of arachidonic acid to prostaglandins and other eicosanoids. Recently two isoforms of the enzyme have been identified. COX-1 is constitutively expressed in a number of cell types, whereas the isoform designated COX-2 is inducible by a variety of factors, as cytokines, growth factors, and tumor promoters. Some studies have suggested that COX-2, but not COX-1, was involved in colon carcinogensis and might thus be the target of chemopreventive effect by the COX inhibor, nonsteroidal anti-inflammatory drugs. The effects of COX-2 on inflammation, procancarous conditions and cancers have been delineated[42-47]. To date the effects of Nimesulide on the growth and apoptosis of human hepatoma cell line SMMC-7721 in vitro have not been analyzed, and that is the aim of this study.
MATERIALS AND METHODS
RPMI 1640 medium is a product of CIBCO; Nimesulide and MTT were from Sigma; In situ cell death detection kit was from Boehringer Mannheim, Germany; 96-well plates were from Costar.
Cell lines and culture
Human hepatoma SMMC-7721 cells were obtained from the Wuhan University Center for type culture collection. The cells were grown as monolayers in RPMI1640 medium supplemented with 10% fetal calf serum (FCS, Gibco) and incubated at 37 °C in the humidified incubator with 5% CO2 in air.
Assay of cell proliferation
The SMMC-7721 cells were seeded at 5 × 104/mL density in 96-well plates 200 μl cell suspension per well. Each group had four wells with a non-treated group as control. When the cells anchored to the plates, various concentrations (0, 200 μmol/L, 300 μmol/L, 400 μmol/L) of Nimesulide were added and the slides were incubated at 37 °C, 5% CO2 for 5 days. In order to maintain Nimesulide concentrations, we changed the culture medium (included various concentrations of Nimesulide) every day. When the cells described above were cultured for 48 h, 72 h, 96 h, 120 h, 0.5% MTT 20 μl was added to each well and cultured for another 4 h. The supernatant was discarded and dimethyl sulfoxide (DMSO) 200 μl added. When the crystals were dissolved, the optical density (OD) value of the slides was read on an enzyme-labeled Minireader II at 492 nm. Cellular proliferation inhibition rate (CPIR) was calculated using the following equation: CPIR = (1 - average OD value of experimental group/average OD value of control group) × 100%
Electron microscopic observation
The SMMC-7721 cells were seeded in culture flasks. Four culture bottles were divided into normal group and control group. When the cells were anchored to the plates, various concentrations (0, 200 μmol/L, 300 μmol/L, 400 μmol/L) of Nimesulide were added and the cells incubated at 37 °C, 5% CO2 for 3 days. Then hepatoma cells were digested by 0.25% trypsinase and collected. After rinsing with PBS, the cells were fixed with 2.5% glutaraldehyde for 30 min and washed with PBS. After routine embedding and sectioning, the cells were observed by Hitachi H-600 electronic microscope.
Flow cytometric analysis
The SMMC-7721 cells were seeded in culture flasks. The culture bottles were divided into normal and three control groups. Each group had three culture bottles. When the cells were anchored to the plates, various concentrations (0, 200 μmol/L, 300 μmol/L, 400 μmol/L) of Nimesulide were added and the cells incubated at 37 °C, 5% CO2 for 3 days. Then each group of cells were washed with PBS, trypsinized and fixed with 70% ethanol at -20 °C for 30 minutes. Fixed cells were incubated with IP/Rnase solution for 15 minutes and 106 cells of each culture bottle were harvested and analyzed with FACScan Becton Dickeuson Flow Cytometer.
In situ apoptotic cell death detection by TUNEL
A TUNEL kit (Boehringer Mannhein, IN) was used to detect DNA fragmentation, the characteristic of apoptotic cell death. The SMMC-7721 cells were seeded in culture flasks. Culture bottles were divided into normal and three control groups. Each group had three culture bottles. When the cells were anchored to the plates, various concentrations (0, 200 μmol/L, 300 μmol/L, 400 μmol/L) of Nimesulide were added and the cells incubated at 37 °C, 5% CO2 for 3 days. In order to maintain Nimesulide concentrations, we changed the culture medium (including various concentrations of Nimesulide) every day. After having been cultured for 3 days, each culture bottle cells were scraped and centrifuged 800 r/min for 5 minutes. Then the deposited cells were smeared and air-dried. Following the manufacturer’s directions, smears were incubated with the TUNEL reaction mixture for 60 min at 37 °C and then with converter-POD for 30 min. The DAB-substrate solution was added to the smears and kept at room temperature until positive signal appeared. Then they were dried and analyzed under light microscope.
Under light microscope, the TUNEL positive nuclei were stained brown. Selecting 5 fields randomly (the number of cells in each field > 1000).
Apoptosis index (AI) = (number of apoptotic cells/the number of cells in each field) × 100%.
Statistical analysis
Statistical analysis was performed using the student’s t test and analysis of variance. P < 0.05 was considered significant.
RESULTS
Effect of Nimesulide in various concentrations on the growth of SMMC-7721
We analyzed the effects of Nimesulide on cell proliferation in cultured human hepatoma cell line SMMC-7721 after 5 days of treatment. Nimesulide, a selective COX inhibitor, produced a dose-dependent inhibition of cells growth (Table 1 and Figure 1). The lowest inhibition rate produced by Nimesulide in SMMC-7721 cells was 19.06%, the highest being 58.49%.
Table 1.
Inhibition effect of Nimesulide on proliferation and growth in hepatic carcinoma cell line SMMC-7721
| Nimesulide Concentrations (μmol/L) |
OD Value |
|||
| the 2nd day | the 3rd day | the 4th day | the 5th day | |
| 0 | 1.039 ± 0.066 | 1.516 ± 0.117 | 2.142 ± 0.072 | 2.467 ± 0.080 |
| 200 | 0.841 ± 0.027a | 1.109 ± 0.231a | 1.416 ± 0.080a | 1.341 ± 0.021a |
| 300 | 0.796 ± 0.019a | 1.002 ± 0.274a | 1.101 ± 0.028a | 1.243 ± 0.168a |
| 400 | 0.581 ± 0.164a | 0.825 ± 0.016a | 0.943 ± 0.032a | 1.024 ± 0.026a |
P < 0.05 vs control group
Figure 1.
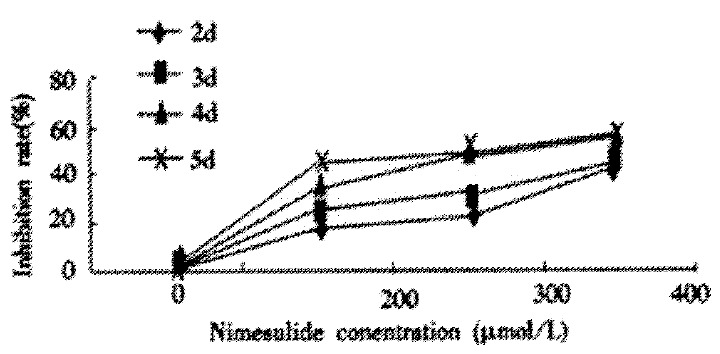
Inhibition rate of Nimesulide on proliferation of SMMC-7721 cells. Cells were incubated with 200 μmol/L, 300 μmol/L, 400 μmol/L Nimesulide for 2 d, 3 d, 4 d, 5 d respectively.
Morphology observation
Under the electron microscope, SMMC-7721 cells exhibited characteristics of apoptosis including plasma membrane blebbing, cytoplasmic condensation, pyknotic nuclei, condensed chromatin and apoptotic bodies. Compared with control groups, 300 μmol/L and 400 μmol/L groups cells had many more cells with apoptotic characteristics (Figure 2).
Figure 2.

Transmission electron micrograph of SMMC-7721 cells treated with Nimesulide at the concentration of 300 μmol/L for 72 h. The picture showed early change of apoptosis, the nuclear chromatin condensation.
Flow-cytometry analysis of cell apoptosis
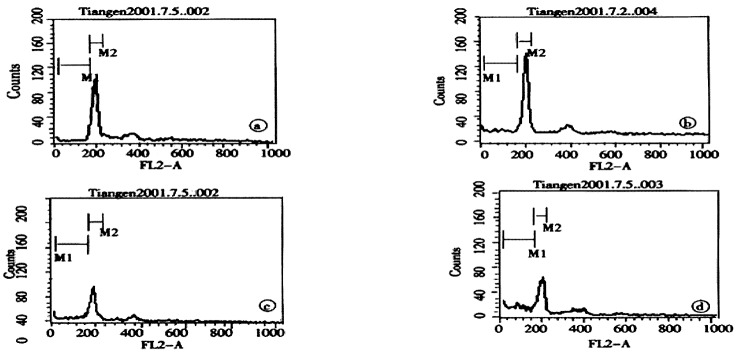
The peak value appearing before the G1 peak is called apoptotic peak. As shown in Figure 3 and Table 2, the apoptotic peak and rate increased with increasing concentrations of Nimesulide. Furthermore, Nimesulide induced cells apoptosis in a dose and time-dependent manner (P < 0.01).
Figure 3.
Cell apoptosis was determined by flow-cytometry, SMMC-7721 cells were treated with Nimesulide at various concentrations (0, 200, 300, 400 μmol/L respectively A to D).
Table 2.
Apoptosis rate of SMMC-7721 cells induced by Nimesulide
| Nimesulide concentration (μmol/L) | Apoptosis rate (%) |
| 0 | 2.24 ± 0.26 |
| 200 | 7.42 ± 0.43b |
| 300 | 9.84 ± 1.54b |
| 400 | 21.20 ± 1.62b |
P < 0.01 vs control group
Analysis of apoptosis by TUNEL
As shown in Figure 4 and Table 3, the apoptotic index increased with increase of Nimesulide concentrations which what appeared to be dose-dependent relationship in the alone groups (P < 0.05).
Figure 4.
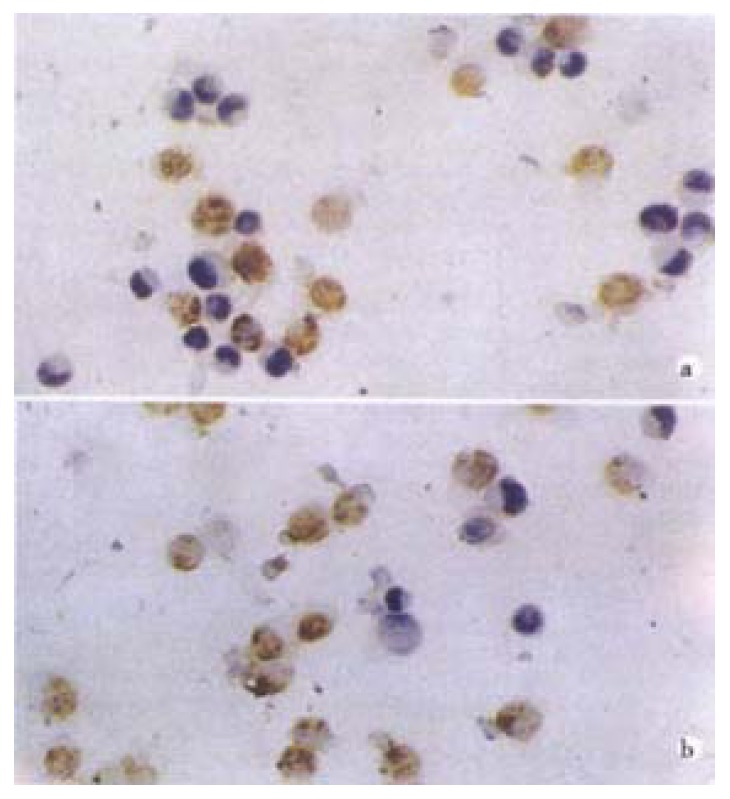
TUNEL stain showed SMMC-7721 cells apoptosis. SMMC-7721 cells were treated with Nimesulide at various concentrations (300, 400 μmol/L A and B).
Table 3.
Aapoptosis index of SMMC-7721 cells induced by Nimesulide
| Nimesulide concentration (μmol/L) | Apoptosis index (%) |
| 0 | 2.016 ± 0.23 |
| 200 | 7.64 ± 0.34a |
| 300 | 10.14 ± 1.42a |
| 400 | 21.23 ± 1.78a |
P < 0.05 vs control group
DISCUSSION
It had been shown that selective COX-2 inhibitors inhibited tumor cells proliferation and induced tumor cells apoptosis, in colon and prostate carcinoma cell lines[48,49]. To date, their effects on human hepatoma SMMC-7721 cell lines have not yet been studied. The aim of this study was to investigate the effect of Nimesulide, a selective COX inhibitor, on the proliferation and apoptosis of SMMC-7721 cell lines. The results indicated that various concentrations of Nimesulide could change the morphology of SMMC-7721 cells and inhibit SMMC-7721 cells proliferation obviously in a dose and time-dependent manner. Nimesulide could induce SMMC-7721 cells apoptosis and cause death in a dose-dependent manner. The precise mechanism by which selective COX-2 inhibitors inhibit tumor cells growth and induce tumor cells apoptosis was not been clearfield. The available data supported the two hypotheses.
Some studies indicate that COX-2 is a key enzyme in the conversion of arachidonic acid to prostaglandins. Selective COX-2 inhibitors can decrease prostaglandins biosynthsis, and prostaglandins can inhibit cell-mediated immunity, which enables the tumor cells escaping the host-immunity[50,51]; PGS also can conjugate with PPARá and activate cell proliferation passage of signal conduction, promote cells proliferation[52]; PGS can also inhibit cells apoptosis and cause cells division uncontrollable, thus accelerating tumor genesis[53,54]; the effects of COX-2 inhibitors might involve prostaglandin biosynthesis. Some studies indicated that the effects of COX-2 were not related to COX-2 expression and PGS. Hanif et al[55] verified that NASIDS (nonselective COX inhibitors) could induce apoptosis of colon carcinoma cell line HCT-15. HCT-15 cells have no COX gene transcription, and does not produce PGS. When adding exogenous PGS to the HCT-15 cells, it could not reverse the induction of HCT-15 cells apoptosis by NASIDS.
On the whole, Nimesulide, a selective COX-2 inhibitor, can inhibit the growth of hepatoma cells and induce tumor cells apoptosis. With the clarification of the mechanism of selective COX-2 inhibitors, These COX-2 selective inhibitors can become the choice of prevention and treatment of cancers.
ACKNOWLEDGMENTS
I would like to thank my wife and all those who provided assistance for this study.
Footnotes
Edited by Wu XN
References
- 1.Gao HJ, Yu LZ, Sun G, Miu K, Bai JF, Zhang XY, Lü XZ, Zhao ZQ. The expression of COX-2 in gastric carcinoma and paracanerous tissues. Shijie Huaren Xiaohua Zazhi. 2000;8:578–579. [Google Scholar]
- 2.Wu HP, Wu KCH, Li L, Yao LP, Lan M, Wang X, Fan DM. Cloning of human cyclooxygenaes-2 (Hcox-2) encoded gene and the study of gastric cancer cell transfected with its antisense vector. Shijie Huaren Xiaohua Zazhi. 2000;8:1211–1217. [Google Scholar]
- 3.Gao HJ, Yu LZ, Bai JF, Peng YS, Sun G, Zhao HL, Miu K, L XZ, Zhang XY, Zhao ZQ. Multiple genetic alterations and behavior of cellular biology in gastric cancer and other gastric mucosal lesions: H.pylori infection, histological types and staging. World J Gastroenterol. 2000;6:848–854. doi: 10.3748/wjg.v6.i6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu QM, Li SB, Wang Q, Wang DH, Li XB, Liu CZ. The expression of COX-2 in esophageal carcinoma and its relationship to clinicopathologic characteristic. Shijie Huaren Xiaohua Zazhi. 2001;9:11–14. [Google Scholar]
- 5.Sun B, Wu YL, Zhang XJ, Wang SN, He HY, Qiao MM, Zhang YP, Zhong J. Effects of Sulindac on growth inhibition and apoptosis induction in human gastric cancer cells. Shijie Huaren Xiaohua Zazhi. 2001;9:997–1002. [Google Scholar]
- 6.Zhuang ZH, Wang LD. Non-steroidal anti-inflammatory drug and digestive tract tumors. Shijie Huaren Xiaohua Zazhi. 2001;9:1050–1053. [Google Scholar]
- 7.Shen ZX, Cao G, Sun J. The effect of COX-2 mRNA expression in colorectal cancer tissues. Shijie Huaren Xiaohua Zazhi. 2001;9:1082–1084. [Google Scholar]
- 8.Mann M, Sheng H, Shao J, Williams CS, Pisacane PI, Sliwkowski MX, DuBois RN. Targeting cyclooxygenase 2 and HER-2/neu pathways inhibits colorectal carcinoma growth. Gastroenterology. 2001;120:1713–1719. doi: 10.1053/gast.2001.24844. [DOI] [PubMed] [Google Scholar]
- 9.Glinghammar B, Rafter J. Colonic luminal contents induce cyclooxygenase 2 transcription in human colon carcinoma cells. Gastroenterology. 2001;120:401–410. doi: 10.1053/gast.2001.21188. [DOI] [PubMed] [Google Scholar]
- 10.Wallace JL, McKnight W, Reuter BK, Vergnolle N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119:706–714. doi: 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- 11.Callejas NA, Boscá L, Williams CS, DuBOIS RN, Martín-Sanz P. Regulation of cyclooxygenase 2 expression in hepatocytes by CCAAT/enhancer-binding proteins. Gastroenterology. 2000;119:493–501. doi: 10.1053/gast.2000.9374. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, DuBois RN. Par-4, a proapoptotic gene, is regulated by NSAIDs in human colon carcinoma cells. Gastroenterology. 2000;118:1012–1017. doi: 10.1016/s0016-5085(00)70352-0. [DOI] [PubMed] [Google Scholar]
- 13.Shirvani VN, Ouatu-Lascar R, Kaur BS, Omary MB, Triadafilopoulos G. Cyclooxygenase 2 expression in Barrett's esophagus and adenocarcinoma: Ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000;118:487–496. doi: 10.1016/s0016-5085(00)70254-x. [DOI] [PubMed] [Google Scholar]
- 14.Shattuck-Brandt RL, Varilek GW, Radhika A, Yang F, Washington MK, DuBois RN. Cyclooxygenase 2 expression is increased in the stroma of colon carcinomas from IL-10(-/-) mice. Gastroenterology. 2000;118:337–345. doi: 10.1016/s0016-5085(00)70216-2. [DOI] [PubMed] [Google Scholar]
- 15.Sinicrope FA, Lemoine M, Xi L, Lynch PM, Cleary KR, Shen Y, Frazier ML. Reduced expression of cyclooxygenase 2 proteins in hereditary nonpolyposis colorectal cancers relative to sporadic cancers. Gastroenterology. 1999;117:350–358. doi: 10.1053/gast.1999.0029900350. [DOI] [PubMed] [Google Scholar]
- 16.Fu S, Ramanujam KS, Wong A, Fantry GT, Drachenberg CB, James SP, Meltzer SJ, Wilson KT. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology. 1999;116:1319–1329. doi: 10.1016/s0016-5085(99)70496-8. [DOI] [PubMed] [Google Scholar]
- 17.Bosch-Marcè M, Clària J, Titos E, Masferrer JL, Altuna R, Poo JL, Jiménez W, Arroyo V, Rivera F, Rodés J. Selective inhibition of cyclooxygenase 2 spares renal function and prostaglandin synthesis in cirrhotic rats with ascites. Gastroenterology. 1999;116:1167–1175. doi: 10.1016/s0016-5085(99)70020-x. [DOI] [PubMed] [Google Scholar]
- 18.Klimp AH, Hollema H, Kempinga C, van der Zee AG, de Vries EG, Daemen T. Expression of cyclooxygenase-2 and inducible nitric oxide synthase in human ovarian tumors and tumor-associated macrophages. Cancer Res. 2001;61:7305–7309. [PubMed] [Google Scholar]
- 19.Lal G, Ash C, Hay K, Redston M, Kwong E, Hancock B, Mak T, Kargman S, Evans JF, Gallinger S. Suppression of intestinal polyps in Msh2-deficient and non-Msh2-deficient multiple intestinal neoplasia mice by a specific cyclooxygenase-2 inhibitor and by a dual cyclooxygenase-1/2 inhibitor. Cancer Res. 2001;61:6131–6136. [PubMed] [Google Scholar]
- 20.Subbarayan V, Sabichi AL, Llansa N, Lippman SM, Menter DG. Differential expression of cyclooxygenase-2 and its regulation by tumor necrosis factor-alpha in normal and malignant prostate cells. Cancer Res. 2001;61:2720–2726. [PubMed] [Google Scholar]
- 21.Oshima M, Murai N, Kargman S, Arguello M, Luk P, Kwong E, Taketo MM, Evans JF. Chemoprevention of intestinal polyposis in the Apcdelta716 mouse by rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer Res. 2001;61:1733–1740. [PubMed] [Google Scholar]
- 22.Shiotani H, Denda A, Yamamoto K, Kitayama W, Endoh T, Sasaki Y, Tsutsumi N, Sugimura M, Konishi Y. Increased expression of cyclooxygenase-2 protein in 4-nitroquinoline-1-oxide-induced rat tongue carcinomas and chemopreventive efficacy of a specific inhibitor, nimesulide. Cancer Res. 2001;61:1451–1456. [PubMed] [Google Scholar]
- 23.Boudreau MD, Sohn KH, Rhee SH, Lee SW, Hunt JD, Hwang DH. Suppression of tumor cell growth both in nude mice and in culture by n-3 polyunsaturated fatty acids: mediation through cyclooxygenase-independent pathways. Cancer Res. 2001;61:1386–1391. [PubMed] [Google Scholar]
- 24.Denkert C, Köbel M, Berger S, Siegert A, Leclere A, Trefzer U, Hauptmann S. Expression of cyclooxygenase 2 in human malignant melanoma. Cancer Res. 2001;61:303–308. [PubMed] [Google Scholar]
- 25.Taylor MT, Lawson KR, Ignatenko NA, Marek SE, Stringer DE, Skovan BA, Gerner EW. Sulindac sulfone inhibits K-ras-dependent cyclooxygenase-2 expression in human colon cancer cells. Cancer Res. 2000;60:6607–6610. [PubMed] [Google Scholar]
- 26.Williams CS, Watson AJ, Sheng H, Helou R, Shao J, DuBois RN. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res. 2000;60:6045–6051. [PubMed] [Google Scholar]
- 27.Souza RF, Shewmake K, Beer DG, Cryer B, Spechler SJ. Selective inhibition of cyclooxygenase-2 suppresses growth and induces apoptosis in human esophageal adenocarcinoma cells. Cancer Res. 2000;60:5767–5772. [PubMed] [Google Scholar]
- 28.Grubbs CJ, Lubet RA, Koki AT, Leahy KM, Masferrer JL, Steele VE, Kelloff GJ, Hill DL, Seibert K. Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res. 2000;60:5599–5602. [PubMed] [Google Scholar]
- 29.Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the min mouse model of adenomatous polyposis. Cancer Res. 2000;60:5040–5044. [PubMed] [Google Scholar]
- 30.Joki T, Heese O, Nikas DC, Bello L, Zhang J, Kraeft SK, Seyfried NT, Abe T, Chen LB, Carroll RS, et al. Expression of cyclooxygenase 2 (COX-2) in human glioma and in vitro inhibition by a specific COX-2 inhibitor, NS-398. Cancer Res. 2000;60:4926–4931. [PubMed] [Google Scholar]
- 31.Attiga FA, Fernandez PM, Weeraratna AT, Manyak MJ, Patierno SR. Inhibitors of prostaglandin synthesis inhibit human prostate tumor cell invasiveness and reduce the release of matrix metalloproteinases. Cancer Res. 2000;60:4629–4637. [PubMed] [Google Scholar]
- 32.Marrogi A, Pass HI, Khan M, Metheny-Barlow LJ, Harris CC, Gerwin BI. Human mesothelioma samples overexpress both cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (NOS2): in vitro antiproliferative effects of a COX-2 inhibitor. Cancer Res. 2000;60:3696–3700. [PubMed] [Google Scholar]
- 33.Cahlin C, Gelin J, Delbro D, Lönnroth C, Doi C, Lundholm K. Effect of cyclooxygenase and nitric oxide synthase inhibitors on tumor growth in mouse tumor models with and without cancer cachexia related to prostanoids. Cancer Res. 2000;60:1742–1749. [PubMed] [Google Scholar]
- 34.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 35.Reddy BS, Hirose Y, Lubet R, Steele V, Kelloff G, Paulson S, Seibert K, Rao CV. Chemoprevention of colon cancer by specific cyclooxygenase-2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer Res. 2000;60:293–297. [PubMed] [Google Scholar]
- 36.Mohammed SI, Knapp DW, Bostwick DG, Foster RS, Khan KN, Masferrer JL, Woerner BM, Snyder PW, Koki AT. Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res. 1999;59:5647–5650. [PubMed] [Google Scholar]
- 37.Molina MA, Sitja-Arnau M, Lemoine MG, Frazier ML, Sinicrope FA. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Res. 1999;59:4356–4362. [PubMed] [Google Scholar]
- 38.Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- 39.Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 40.Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
- 41.Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R, et al. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688–696. doi: 10.1002/hep.510290355. [DOI] [PubMed] [Google Scholar]
- 42.Nanji AA, Jokelainen K, Fotouhinia M, Rahemtulla A, Thomas P, Tipoe GL, Su GL, Dannenberg AJ. Increased severity of alcoholic liver injury in female rats: role of oxidative stress, endotoxin, and chemokines. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1348–G1356. doi: 10.1152/ajpgi.2001.281.6.G1348. [DOI] [PubMed] [Google Scholar]
- 43.Ganey PE, Barton YW, Kinser S, Sneed RA, Barton CC, Roth RA. Involvement of cyclooxygenase-2 in the potentiation of allyl alcohol-induced liver injury by bacterial lipopolysaccharide. Toxicol Appl Pharmacol. 2001;174:113–121. doi: 10.1006/taap.2001.9183. [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto T, Ogino N, Yamamoto S, Hayaishi O. Purification of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1976;251:2629–2636. [PubMed] [Google Scholar]
- 45.Simmons DL, Levy DB, Yannoni Y, Erikson RL. Identification of a phorbol ester-repressible v-src-inducible gene. Proc Natl Acad Sci USA. 1989;86:1178–1182. doi: 10.1073/pnas.86.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian G, Yu JP, Luo HS, Yu BP, Li JY. The expression and effect of cyclo oxygenases-2 in acute hepatic injury. Shijie Huaren Xiaohua Zazhi. 2002;10:24–27. [Google Scholar]
- 47.Tian G, Yu JP, Luo HS, Yu BP, Li JY. The effect of COX-2 and oxidant stress in acute hepatic injury. Yixue Yanjiusheng Xuebao. 2002:6; 165–169. [Google Scholar]
- 48.Elder DJ, Halton DE, Hague A, Paraskeva C. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug: independence from COX-2 protein expression. Clin Cancer Res. 1997;3:1679–1683. [PubMed] [Google Scholar]
- 49.Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998;58:4245–4249. [PubMed] [Google Scholar]
- 50.Yang VW, Shields JM, Hamilton SR, Spannhake EW, Hubbard WC, Hylind LM, Robinson CR, Giardiello FM. Size-dependent increase in prostanoid levels in adenomas of patients with familial adenomatous polyposis. Cancer Res. 1998;58:1750–1753. [PubMed] [Google Scholar]
- 51.Uotila P. The role of cyclic AMP and oxygen intermediates in the inhibition of cellular immunity in cancer. Cancer Immunol Immunother. 1996;43:1–9. doi: 10.1007/BF03354243. [DOI] [PubMed] [Google Scholar]
- 52.Parker J, Kaplon MK, Alvarez CJ, Krishnaswamy G. Prostaglandin H synthase expression is variable in human colorectal adenocarcinoma cell lines. Exp Cell Res. 1997;236:321–329. doi: 10.1006/excr.1997.3741. [DOI] [PubMed] [Google Scholar]
- 53.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 54.Orlov SN, Thorin-Trescases N, Dulin NO, Dam TV, Fortuno MA, Tremblay J, Hamet P. Activation of cAMP signaling transiently inhibits apoptosis in vascular smooth muscle cells in a site upstream of caspase-3. Cell Death Differ. 1999;6:661–672. doi: 10.1038/sj.cdd.4400539. [DOI] [PubMed] [Google Scholar]
- 55.Hanif R, Pittas A, Feng Y, Koutsos MI, Qiao L, Staiano-Coico L, Shiff SI, Rigas B. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]