Abstract
AIM: To investigate the relationship between lymphocyte apoptosis in peripheral blood, spleen and mesenteric lymph nodes (MLN) and endotoxin translocation after thermal injury in rats.
METHODS: In a Wistar rat model inflicted with 30% TBSA III degree scalding, serum LPS levels in portal vein and vena cava were quantified by tachypleus amebocyte lysate (TAL) technique. The analysis of peripheral blood lymphocyte was employed in in situ Cell Death Detection Kit and evaluated by flow cytometry. Apoptotic lymphocytes in paraffin-embedded spleen and MLN sections were examined by histologic analysis, in situ deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) and peroxidase (POD) staining. The imagines were taken by Cooldccd camera system, and the count and optical density value (transmission light) of apoptotic lymphocytes were analyzed with software Spot and Imagine proplus 4.10a (IPP4.10a).
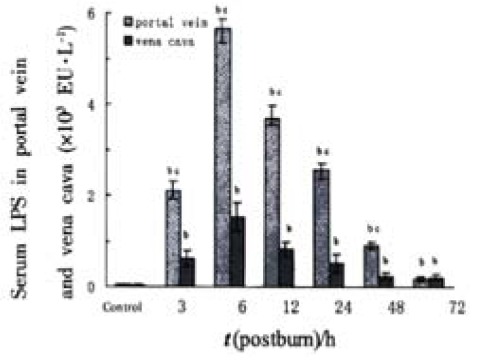
RESULTS: In the period of 3 to 48 postburn hours (PBHs) serum LPS level (× 103 EU·L-1) in portal vein (2.11 ± 0.02, 5.66 ± 0.20, 3.70 ± 0.22, 2.56 ± 0.28, 0.90 ± 0.11) was higher than that in vena cava (0.63 ± 0.01, 1.53 ± 0.18, 0.83 ± 0.32, 0.52 ± 0.12, 0.23 ± 0.02, P < 0.01), but both increased sharply in postburn rats (P < 0.01) and reached a peak at 6 PBH. Analysis of apoptotic lymphocytes showed that the proportion (%) of postburn apoptotic cells was much higher than that in healthy rats (8.34 ± 1.53, 8.13 ± 1.81, 20.77 ± 3.94, 23.90 ± 3.92, 11.23 ± 1.35 and 13.26 ± 2.09 at 3, 6, 12, 24, 48 and 72 PBH, respectively, vs 3.99 ± 1.72, P < 0.01), especially after 6 PBH. The concentrations of lymphocytic apoptosis at 12 and 24 PBH were markedly higher than that at other time points. Meantime, few apoptotic lymphocytes were found in normal MLN, but increased postburn obviously (3 ± 1 vs 546 ± 83, 285 ± 39, 149 ± 30, 58 ± 10, 36 ± 11 and 33 ± 9 in turn, P < 0.01), especially at 3 PBH, whereas apoptotic lymphocytes were concentrated in splenic cortex before the burn and decreased obviously during 72 PBHs (499 ± 186 vs 12 ± 8, 19 ± 15, 12 ± 7, 100 ± 15, 123 ± 25 and 226 ± 26 in turn, P < 0.01) though a slight rise was found in the medulla after 24 PBH. Optical density of apoptotic lymphocytes was significantly reduced in spleen in the 24 PBHs and raised in MLN during 48 PBHs than that prior to the burn, respectively.
CONCLUSION: Gut-origin LPS is a major cause of endotoxemia taken place early in rats following severe thermal injury and could induce extensive lymphocyte apoptosis in blood and MLN, which suggests an immunosuppression state could follow the initial injury and favores a septic state based on apoptotic mechanism.
INTRODUCTION
Endotoxin or lipopolysaccharide (LPS) is a major cause of the local inflammation and septic shock and has been shown to impair host immune defense[1-10]. With the experimental endotoxemia or sepsis in C3H/HeN (endotoxin-sensitive) mice, it was demonstrated in recent studies that LPS could cause thymic atrophy and bring about the increased apoptotic lymphocytes in thymus, spleen and gut-associated lymphatic tissue[11-13]. Since the lymphocytes appear essential to both competent immune function and to the control of inflammatory response[14,15], the lymphocyte apoptosis induced by LPS may play important roles in the development and regulation of the immune system, especially in the gut-dysfunction situations induced by sepsis or severe injuries. Endotoxemia in the patients with severe injury has been suggested to be an important factor in the systemic inflammatory response syndrome (SIRS)[16,17]. It was found in the previous in vivo study that gut-origin endotoxemia following the intestinal mucosal barrier dysfunction could occur in early postburn period and lead to the injury of systemic organs such as lungs, liver etc[18,19]. Although the exact routes by which translocating LPS reach the blood and systemic organs are not known with certainty, most researchers believed that LPS was capable of translocating from the gut via both the mesenteric lymphatics and the portal blood[20]. Because the mesenteric lymph nodes (MLN) receives its lymphatic drainage from the small intestine, cecum and proximal colon, we believed that MLN should be a major site and pathway of translocated gut-origin LPS. However, little has been known about lymphocyte apoptosis in circulating blood and MLN induced by endotoxin translocation after thermal injury and the relationship between them.
The present experiments were performed in severe scalded rats to determine the effects of gut-origin LPS translocation on the apoptosis of lymphocytes in circulating blood, spleen and MLN, and to investigate the relationship between them.
MATERIALS AND METHODS
Animals and reagents
Forty-two adult Wistar rats weighing 235-345 g were randomly distributed in the normal control and 6 thermal injury groups, i.e. 3, 6, 12, 24, 48 and 72 h after scalding. Each group contained 6 animals (half males and half females). Rats in the thermal injury groups were inflicted with 30% total burn surface area (TBSA) III degree scalding on their back after anesthetized with 30 mg/kg of intraperitoneal pentobarbital. Then under general anesthesia at the different time points after the thermal injury, the blood of rats’ portal vein and vena cava were collected for LPS test and the isolation of peripheral blood lymphocyte, respectively. MLN and spleen were harvested by aseptic manipulation and fixed in 10 mL·L-1 paraformaldehyde solution (pH7.4) for 24 h. All chemicals used in this study were purchased from Sigma Chemical Co. (USA) unless specified otherwise. Endotoxin-free glassware and plasticware were prepared by baking at 250 °C for 1.5 h and radiating with 60Co, respectively.
Determination of serum LPS levels in portal vein and vena cava
Sera were obtained from the blood samples of portal vein and vena cava by clotting for 60 min on ice and then centrifuged at 2500 g at 4 °C for 5 min respectively, filtered, aliquoted, and frozen at -70 °C. After all serum samples were collected, the serum LPS levels were determined with a commercially available kit for tachypleus amebocyte lysate (TAL) technique (Zhanjiang A & C Biological Ltd, Zhanjiang, China) according to the manufacture’s guidelines.
Isolation and preparation of lymphocyte
Lymphocyte in the heparinized blood from vena cava were isolated by density gradient centrifugation using ficoll-hypaque (d = 1.077) followed by two washing steps in phosphate buffered saline (PBS) and lysis of residual erythrocytes using nine volumes of an ice-cold isotonic ammonium chloride solution (NH4Cl 155 mmol·L-1, KHCO3 10 mmol·L-1, EDTA 0.1 mmol·L-1) to one volume of cell pellet at 4 °C for 7 min[21]. Lymphocytes were greater than 98% as analyzed by microscopy using Hemacolor staining (Merk, Germany). Cell viability was greater than 97% as assessed by the trypan blue exclusion test. Isolated lymphocytes were maintained in a glass bottle in RPMI 1640-medium without fetal calf serum (endotoxin-free; Gibco BRL, Life Technologies, USA) at a concentration of 1 × 109 cells·L-1 in 5 mL·L-1 CO2 at 37 °C for 2 h to exclude macrophage before the analysis of apoptosis. Then the cells were collected by centrifugation and washed twice in PBS.
Flow cytometric analysis of apoptotic lymphocyte
The analysis of apoptotic lymphocytes was performed by an in situ Cell Death Detection Kit, Fluorescein (Roche, Germany). The procedures in brief was according to the manufacture’s guidelines as follows: lymphocyte were suspended and fixed with 40 mL·L-1 paraformaldehyde solution (pH7.4), rinsed in PBS twice, and incubated in permeabilization solution (1 g·L-1 Triton X-100 in 1 g·L-1 sodium citrate) for 2 min on ice. The cells were again rinsed in PBS, centrifugated and incubated in terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) reaction mixture for 1 h at 37 °C in a humidified atmosphere at dark. Then cells were rinsed in PBS again. TUNEL fluorescence of individual nuclei in a final volume of 500 μL cells solution was analyzed by an FACS Calibur (Becton Dickinson, USA), while gating on physical parameters was enacted to exclude cell debris. A minimum of 10000 events were counted per sample. The results were reported as the percentage of hypodiploid (fragmented) nuclei reflecting the relative proportion of apoptotic cells.
Analysis of apoptotic lymphocytes in spleen and MLN
Paraffin-embedded spleen and MLN tissue were cut into sections of 5 μm and mounted on Vectabond Reagent slides, deparaffinized and rehydrated through xylene, graded ethanol to distilled water. Then the tissue sections were pre-treated with 20 mg·L-1 proteinase K for 30 min, and analyzed with an in situ Cell Death Detection Kit, POD (Roche, USA) according to the manufacture’s guidelines. Each experiment set up by TUNEL reaction mixture without terminal transferase served as negative control. The images were taken by Cooldccd camera system, and the count and optical density (OD) value of apoptotic lymphocytes were analyzed with software Spot and Imagine proplus 4.10a (IPP 4.10). The counts and optical density values (transmission light) of TUNEL-POD positive lymphocytes in spleen and MLN were determined in three high-resolution fields selected randomly in the area concentrated positive apoptotic lymphocytes and two thousand cells per field were counted per slides.
Statistical analysis
All the data was analyzed by Student’s t test and expressed as -x ± s. The statistical difference P < 0.05 was considered as significantly and P < 0.01 as very significant.
RESULTS
Serum LPS in portal vein and vena cava increased after thermal injury
Serum LPS levels in portal vein and vena cava increased sharply postburn (P < 0.01) and reached to a peak level at 6 PBH and decreased thereafter. LPS level in portal vein was higher than that in the vena cava (P < 0.01) in the period of 3 to 48 PBHs, but both decreased to near control level at 72 PBH (Figure 1).
Figure 1.
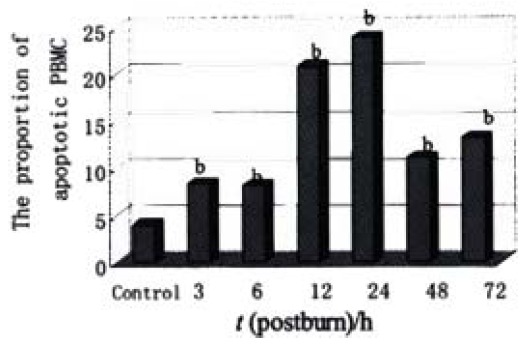
Comparison of the LPS levels postburn in portal vein and in vena cava. bP < 0.01, vs control; cP < 0.01, portal vein vs vena cava
Apoptosis of lymphocytes
Lymphocytes isolated from the circulation of healthy rats exhibited a low proportion of apoptotic cells at (3.99 ± 1.72)%, but increased obviously during the whole postburn period of the experiment (P < 0.01), especially after 6 PBH. The concentrations of lymphocytic apoptosis at 12 and 24 PBH were markedly higher than that at other time points (Figure 2, Figure 3).
Figure 2.
The relative proportions of apoptotic lymphocytes postburn. bP < 0.01, vs control
Figure 3.
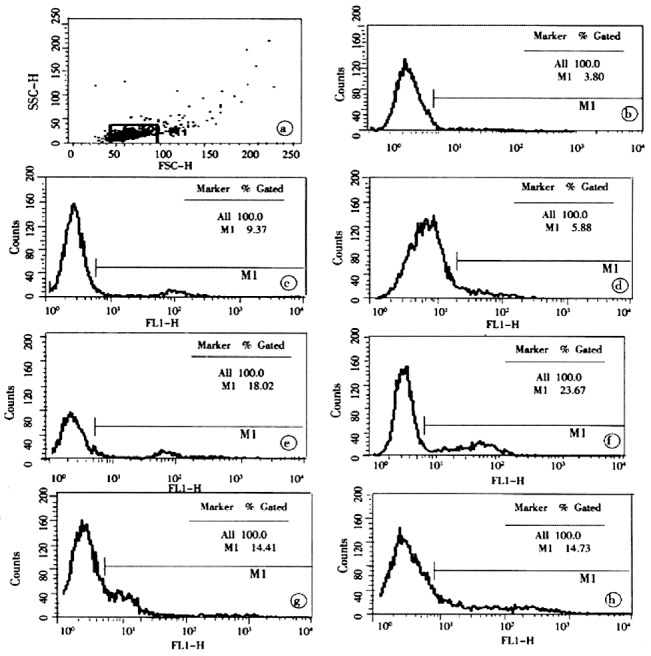
The results of typical flow cytometric analysis of postburn apoptotic lymphocytes. a, b, c, d, e, f, g and h were the relative proportion of apoptotic lymphocytes isolated from one rat in the control and group 3, 6, 12, 24, 48 and 72 PBH, respectively.
Apoptotic lymphocytes in spleen and MLN
It was shown by the results of TUNEL-POD staining and the counts of apoptotic lymphocytes that the apoptotic cells were few in normal MLN (Figure 4A), but increased postburn obviously (P < 0.01), especially at 3 PBH (Figure 4B, Table 1). Opposite to MLN, apoptotic lymphocytes were concentrated in spleen cortex before burn (Figure 4C), but decreased obviously during 3 to 72 PBHs (P < 0.01) though a slight rise was found in the medulla after 24 PBH (Figure 4D, Table 1). Since the principle of TUNEL-POD is to label the fragmented genomic DNA, the biochemical hallmark of apoptotic cell, the color density of nuclei staining could indirectly reflect the extent of DNA fragmentation. Optical density of TUNEL-POD staining in apoptotic lymphocytes was significantly reduced in spleen in the 24 PBHs, but raised in MLN during 48 PBHs than that before burn, respectively (Table 2).
Figure 4.
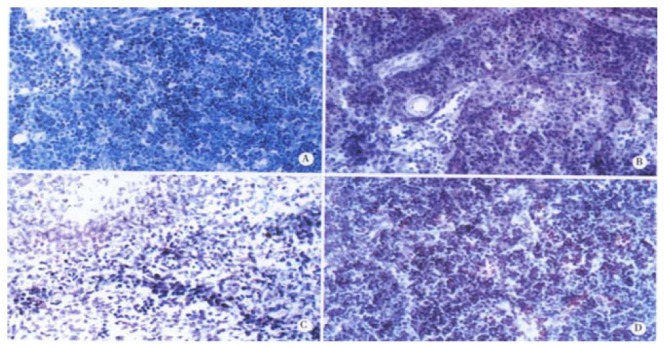
Apoptotic lymphocytes in spleen and MLN. (TUNEL-POD staining × 400, the nuclei stained brown are positive apoptotic cells). A: MLN from the healthy rat; B: MLN from the rat at 3 PBH; C: Spleen from the healthy rat; D: Spleen from the rat 24 PBH.
Table 1.
Apoptotic lymphocyte counts in 2000 total cells of spleen and MLN
| Tissue | Healthy control |
T (postburn)/h |
|||||
| 3 | 6 | 12 | 24 | 48 | 72 | ||
| Spleen | 499 ± 186 | 12 ± 8b | 19 ± 15b | 12 ± 7b | 100 ± 15b | 123 ± 25b | 226 ± 26b |
| MLN | 3 ± 1 | 546 ± 83b | 285 ± 39b | 149 ± 30b | 58 ± 10b | 36 ± 11b | 33 ± 9b |
P < 0.01, vs control
Table 2.
The optical density values (transmission light) of TUNEL-POD positive lymphocytes in spleen and MLN
| Tissue | Healthy control |
T (postburn)/h |
|||||
| 3 | 6 | 12 | 24 | 48 | 72 | ||
| Spleen | 0.54 ± 0.03 | 0.30 ± 0.02b | 0.35 ± 0.12b | 0.36 ± 0.03b | 0.36 ± 0.02b | 0.44 ± 0.19 | 0.52 ± 0.12 |
| MLN | 0.24 ± 0.06 | 0.83 ± 0.16b | 0.96 ± 0.25b | 0.62 ± 0.16b | 0.45 ± 0.04b | 0.35 ± 0.07b | 0.29 ± 0.04 |
P < 0.01, vs control
DISCUSSION
Translocation of gut bacteria and endotoxin is a common situation after severe trauma, probably as a consequence of loss of physical integrity of the mucosal barrier, increments in permeability, or impaired local immune function resulted from ischemia-reperfusion injury of intestine. The gut-origin LPS translocated to the portal circulation or gut lymphatics has been thought to subsequently initiate a septic process leading to the development of SIRS[22]. In this study, the LPS levels in portal vein and vena cava increased sharply after the severe thermal injury (Figure 1) indicating that endotoxin could enter the blood circulation in the early period of trauma and might be a main cause of endotoxemia.
The gastrointestinal tract contains numerous immune effector and regulatory cells of lymphoid and myeloid origin that are thought to play a critical role in host defense against enteric infections[23]. And at a systemic level, the lymphocytes, through the production of regulatory cytokines, are important in mediating and controlling the host immune response. This was well illustrated by the spontaneous appearance of gastrointestinal inflammatory disease in a number of animals deficient in different cytokines, including IL-2 and IL-10, etc[24-26]. Thus, the increased lymphocyte apoptosis in circulatory blood and gut-associated lymphoid tissues must impair the balance between the functions of host defense and immune tolerance in the gut immune system[27,28]. The observation presented here provided some evidence that local and systemic pathologic lymphocyte apoptosis following an initial event of injury could impair the immune system. In the scalded rats, the counts of apoptotic lymphocytes in peripheral blood and MLN increased dramatically during the whole 72 PBHs, and reached a peak level at 12-24 PBHs and 3 PBH, respectively (Figure 2, Figure 3, Figure 4, Table 1, Table 2). The results of lymphocyte apoptosis in MLN were consistent with that reported by Mongini et al[22]. Moreover, It was surprised to observe the apoptotic lymphocytes in the spleen decreased obviously after thermal injury in this study (Figure 4, Table 1, Table 2) though splenic lymphocyte seemed to be less sensitive to apoptosis triggered by LPS and oxidative stress[11,12,29]. Specifically, we also found that splenic apoptotic lymphocytes were located mainly in the cortex in healthy rats, but being present in the medulla after thermal injury. The implications of these results are unclear. During normal lymphocyte development, immature lymphocytes are located in the cortex and migrate to the medulla in the course of maturation. The depletion of cortical lymphocytes by apoptosis may be one mechanism of eliminating potentially autoreactive immune cells[22]. As for the decreased splenic apoptotic lymphocytes in the scalded rats, whether it is a reaction induced by the lymphocyte apoptosis in peripheral blood remains to be clarified.
Taking that both change tendency of lymphocyte apoptosis and serum LPS level after thermal injury into consideration, it was found that the peak level of lymphocyte apoptosis appeared earlier in MLN and much later in circulatory blood than that of LPS in the blood. The results indicated that MLN was an advanced station in the routing of the LPS translocation and suggested that the translocation of gut-origin LPS was a major factor of lymphocyte apoptosis in rats with severe burn injury. Furthermore, LPS translocated into the blood could lead to a further breakdown of gut immune barrier and thus accelerate the entrance of enteric bacteria and their toxins into the circulation[30] and result in an immunosuppressive state due to the induction of lymphocyte apoptosis[14,22,31]. This might explain why the peak level of apoptotic lymphocytes came forth much later in circulatory blood than that in MLN.
In addition to our observations in the present study, thymocyte and mucosal lymphocyte apoptosis after thermal injury had been recently reported[32,33]. Although in these two studies the increased lymphocyte apoptosis in thymus and gut-associated lymphoid tissue seemed mainly due to the increased corticosterone concentration in plasma and in the lymphoid tissue, the presence of lymphocyte apoptosis in the peripheral blood, thymus and gut-associated lymphoid tissue following injury could explain the immunosuppressive state following such injury. In fact, decreased lymphocyte apoptosis in sepsis can improve survival by overexpression of Bcl-2 in transgenic mice[34]. In summary, we can reach the conclusion that an immunosuppression state could follow the initial injury and favor a septic state based on apoptotic mechanism, and that the immunosuppressive state after the thermal injury can be the event induced by translocation of gut-origin LPS and bacteria spreading to other tissues, and which in turn causes a recurrence of sepsis.
Footnotes
Supported by the National Basic Research Priorities Programme of China, No. G199905403
Edited by Wu XN
References
- 1.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 2.Yi JH, Ni RY, Luo DD, Li SL. Intestinal flora translocation and overgrowth in upper gastrointestinal tract induced by hepatic failure. World J Gastroenterol. 1999;5:327–329. doi: 10.3748/wjg.v5.i4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wray GM, Foster SJ, Hinds CJ, Thiemermann C. A cell wall component from pathogenic and non-pathogenic gram-positive bacteria (peptidoglycan) synergises with endotoxin to cause the release of tumour necrosis factor-alpha, nitric oxide production, shock, and multiple organ injury/dysfunction in the rat. Shock. 2001;15:135–142. doi: 10.1097/00024382-200115020-00010. [DOI] [PubMed] [Google Scholar]
- 4.Zhu L, Yang ZC, Li A, Cheng DC. Protective effect of early enteral feeding on postburn impairment of liver function and its mechanism in rats. World J Gastroenterol. 2000;6:79–83. doi: 10.3748/wjg.v6.i1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz MJ, Olszyna DP, de Jonge E, Verbon A, van Deventer SJ, van der Poll T. Reduced ex vivo chemokine production by polymorphonuclear cells after in vivo exposure of normal humans to endotoxin. J Infect Dis. 2000;182:1264–1267. doi: 10.1086/315840. [DOI] [PubMed] [Google Scholar]
- 6.Fu WL, Xiao GX, Yue XL, Hua C, Lei MP. Tracing method study of bacterial translocation in vivo. World J Gastroenterol. 2000;6:153–155. doi: 10.3748/wjg.v6.i1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu BH, Chen HS, Zhou JH, Xiao N. Effects of endotoxin on endothelin receptor in hepatic and intestinal tissues after endotoxemia in rats. World J Gastroenterol. 2000;6:298–300. doi: 10.3748/wjg.v6.i2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuo GQ, Gong JP, Liu CA, Li SW, Wu XC, Yang K, Li Y. Expression of lipopolysaccharide binding protein and its receptor CD14 in experimental alcoholic liver disease. World J Gastroenterol. 2001;7:836–840. doi: 10.3748/wjg.v7.i6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heagy W, Hansen C, Nieman K, Cohen M, Richardson C, Rodriguez JL, West MA. Impaired ex vivo lipopolysaccharide-stimulated whole blood tumor necrosis factor production may identify "septic" intensive care unit patients. Shock. 2000;14:271–276; discussion 271-276;. doi: 10.1097/00024382-200014030-00005. [DOI] [PubMed] [Google Scholar]
- 10.Zhang GL, Wang YH, Teng HL, Lin ZB. Effects of aminoguanidine on nitric oxide production induced by inflammatory cytokines and endotoxin in cultured rat hepatocytes. World J Gastroenterol. 2001;7:331–334. doi: 10.3748/wjg.v7.i3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang YH, Takahashi K, Jiang GZ, Kawai M, Fukada M, Yokochi T. In vivo induction of apoptosis (programmed cell death) in mouse thymus by administration of lipopolysaccharide. Infect Immun. 1993;61:5044–5048. doi: 10.1128/iai.61.12.5044-5048.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manhart N, Vierlinger K, Habel O, Bergmeister LH, Götzinger P, Sautner T, Spittler A, Boltz-Nitulescu G, Marian B, Roth E. Lipopolysaccharide causes atrophy of Peyer's patches and an increased expression of CD28 and B7 costimulatory ligands. Shock. 2000;14:478–483. doi: 10.1097/00024382-200014040-00010. [DOI] [PubMed] [Google Scholar]
- 13.Wang SD, Huang KJ, Lin YS, Lei HY. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994;152:5014–5021. [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, et al. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 16.Pape HC, Remmers D, Grotz M, Schedel I, von Glinski S, Oberbeck R, Dahlweit M, Tscherne H. Levels of antibodies to endotoxin and cytokine release in patients with severe trauma: does posttraumatic dysergy contribute to organ failure? J Trauma. 1999;46:907–913. doi: 10.1097/00005373-199905000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Crespo E, Macías M, Pozo D, Escames G, Martín M, Vives F, Guerrero JM, Acuña-Castroviejo D. Melatonin inhibits expression of the inducible NO synthase II in liver and lung and prevents endotoxemia in lipopolysaccharide-induced multiple organ dysfunction syndrome in rats. FASEB J. 1999;13:1537–1546. [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Schmieg RE, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Karl IE, Buchman TG. Rapid onset of intestinal epithelial and lymphocyte apoptotic cell death in patients with trauma and shock. Crit Care Med. 2000;28:3207–3217. doi: 10.1097/00003246-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Goris RJ, van Bebber IP, Mollen RM, Koopman JP. Does selective decontamination of the gastrointestinal tract prevent multiple organ failure? An experimental study. Arch Surg. 1991;126:561–565. doi: 10.1001/archsurg.1991.01410290033006. [DOI] [PubMed] [Google Scholar]
- 20.Alexander JW, Gianotti L, Pyles T, Carey MA, Babcock GF. Distribution and survival of Escherichia coli translocating from the intestine after thermal injury. Ann Surg. 1991;213:558–566; discussion 566-567. doi: 10.1097/00000658-199106000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ertel W, Keel M, Infanger M, Ungethüm U, Steckholzer U, Trentz O. Circulating mediators in serum of injured patients with septic complications inhibit neutrophil apoptosis through up-regulation of protein-tyrosine phosphorylation. J Trauma. 1998;44:767–775; discussion 775-776. doi: 10.1097/00005373-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Mongini C, Ruybal P, García Rivello H, Mocetti E, Escalada A, Christiansen S, Argibay P. Apoptosis in gut-associated lymphoid tissue: a response to injury or a physiologic mechanism? Transplant Proc. 1998;30:2673–2676. doi: 10.1016/s0041-1345(98)00785-4. [DOI] [PubMed] [Google Scholar]
- 23.Malstrom C, James S. Inhibition of murine splenic and mucosal lymphocyte function by enteric bacterial products. Infect Immun. 1998;66:3120–3127. doi: 10.1128/iai.66.7.3120-3127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 27.Ayala A, Xin Xu Y, Ayala CA, Sonefeld DE, Karr SM, Evans TA, Chaudry IH. Increased mucosal B-lymphocyte apoptosis during polymicrobial sepsis is a Fas ligand but not an endotoxin-mediated process. Blood. 1998;91:1362–1372. [PubMed] [Google Scholar]
- 28.Andjelíc S, Khanna A, Suthanthiran M, Nikolić-Zugić J. Intracellular Ca2+ elevation and cyclosporin A synergistically induce TGF-beta 1-mediated apoptosis in lymphocytes. J Immunol. 1997;158:2527–2534. [PubMed] [Google Scholar]
- 29.Freeman BD, Reaume AG, Swanson PE, Epstein CJ, Carlson EJ, Buchman TG, Karl IE, Hotchkiss RS. Role of CuZn superoxide dismutase in regulating lymphocyte apoptosis during sepsis. Crit Care Med. 2000;28:1701–1708. doi: 10.1097/00003246-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Navaratnam RL, Morris SE, Traber DL, Flynn J, Woodson L, Linares H, Herndon DN. Endotoxin (LPS) increases mesenteric vascular resistance (MVR) and bacterial translocation (BT) J Trauma. 1990;30:1104–1113; discussion 1104-1113;. doi: 10.1097/00005373-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Kurita-Ochiai T, Fukushima K, Ochiai K. Butyric acid-induced apoptosis of murine thymocytes, splenic T cells, and human Jurkat T cells. Infect Immun. 1997;65:35–41. doi: 10.1128/iai.65.1.35-41.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuzuka K, Edwards CK, Clare-Salzer M, Copeland EM, Moldawer LL, Mozingo DW. Glucocorticoid and Fas ligand induced mucosal lymphocyte apoptosis after burn injury. J Trauma. 2000;49:710–716. doi: 10.1097/00005373-200010000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi T, Nishi Y, Sato EF, Ishii M, Hamada T, Inoue M. Thermal injury induces thymocyte apoptosis in the rat. J Trauma. 1998;44:143–148. doi: 10.1097/00005373-199801000-00019. [DOI] [PubMed] [Google Scholar]
- 34.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]


