Abstract
AIM: To investigate the expression of c-myc target from laryngeal cancer cells (MTLC) gene in gastric carcinoma (GC) tissues and the effect of MTLC over-expression on gastric carcinoma cell line BGC823.
METHODS: RT-PCR was performed to determine the expression of MTLC mRNA in GC and matched control tissues. BGC823 cells were transfected with an expression vector pcDNA3.1-MTLC by liposome and screened by G418. Growth of cells expressing MTLC was observed daily by manual counting. Apoptotic cells were determined by TdT-mediated dUTP nick-end labeling (TUNEL) assay.
RESULTS: The expression of MTLC mRNAs was down-regulated in 9 (60%) of 15 cases of GC tissues. The growth rates of the BGC823 cells expressing MTLC were indistinguishable from that of control cells. A marked acceleration of apoptosis was observed in MTLC-expressing cells.
CONCLUSION: MTLC was down-regulated in the majority of GC tissues and could promote apoptosis of GC cell lines, which suggests that MTLC may play an important role in the carcinogenesis of gastric carcinoma.
INTRODUCTION
Gastric carcinoma (GC) is one of the most common malignant tumors in the world[1,2]. Numerous data have shown that some genes such as p53, c-myc, bcl-2, COX-2 and PTEN[3-6] might be associated with the gastric carcinogenesis. However, the exact molecular mechanism underlying GC remains to be fully elucidated. Therefore, it is necessary to look for novel genes to obtain a thorough understanding about gastric carcinogenesis.
c-myc target from laryngeal cancer cells (MTLC) gene, a putative target of c-myc, was recently cloned in our laboratory (GenBank access number AF527367). MTLC was located in 6q25, a chromosome region involved in various kinds of cancers[7-11]. Previous studies have shown that its protein product expressed in nuclei and might take part in the regulation of cell cycle[12], suggesting that MTLC was potentially related to the carcinogenesis. In this study, we therefore performed RT-PCR and eukaryotic transfection to reveal the relationship between MTLC and GC.
MATERIALS AND METHODS
Tissues and cell line
All the gastric cancer and matched control tissues confirmed pathologically were obtained from the First Affiliated Hospital of China Medical University. Tumor tissues were dissected from the resected specimens. The normal tissue block was taken from the distal resection margin and was apart from cancer at least 1 cm. Gastric carcinoma cell line BGC823 was kept in our laboratory.
RT-PCR
Total RNAs were extracted from cancer tissues by TRIZOL reagents (GibcoBRL, Grand Island, NY, USA), and were reverse-transcripted to the first strand of cDNA using reverse transcriptase system (Promega, Madison, WI, USA). MTLC cDNA was amplified by PCR under the following condition: first at 95 °C for 1 min, 30 cycles at 95 °C for 30 s, at 60 °C for 1 min, at 72 °C for 1.5 min, and finally at 72 °C for 10 min. PCR primers consisted of the sequences of forward: 5-ATGGATCCCTGCACTGGCTGATGAGTGTGTA-3 and reverse: 5-GTAAGCTTGAACAGTGCCTTCACCCTCGAGGT-3. β-actin gene was used as internal control.
Construction of MTLC expression vector
MTLC segment amplified by PCR was ligated to pMD-18T vector (Takara, Dalian, China) by TA cloning. The recombinant was digested by BamH I and EcoR I, and then the target fragment was recollected and cloned into pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA). Both PCR product and the expression vector pcDNA3.1-MTLC were confirmed by sequencing to avoid mutation.
Transfection and screening of BGC823 cells
BGC823 cells in logarithmic phase were seeded in 35 mm plates and cultured with DMEM containing 10% serum overnight. Cells were transfected with 1 μg expression vector or empty parental vector by Lipofectamin 2000 (Invitrogen, Carlsbad, CA, USA) and subsequently screened by G418 at a final concentration of 5 g/L after cultured for 24 h.
Observation of cell growth
Cells transfected by pcDNA3.1-MTLC or empty parental vector were plated in 35 mm plates at a concentration of 1 × 105 cells/plate with DMEM culture containing 10% serum. Individual plates were trypsinized daily and the total number of viable cells per plate was determined by manual counting.
Detection of apoptosis
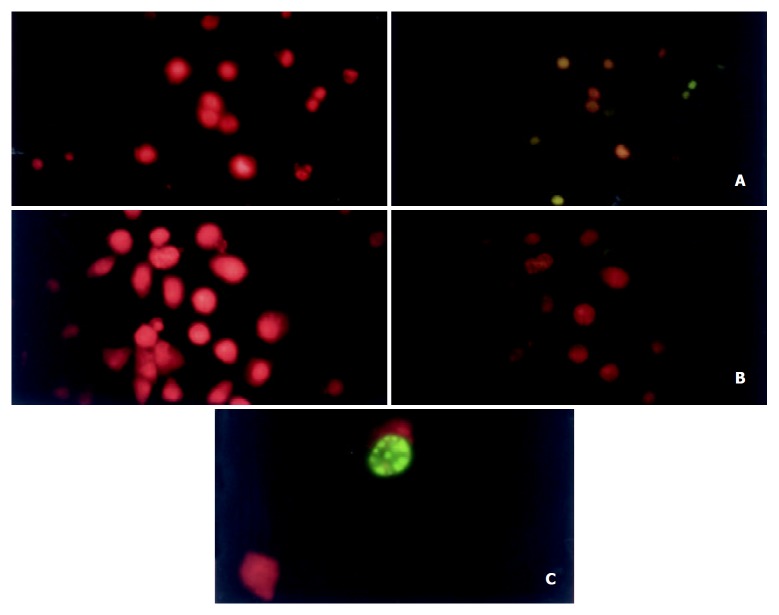
DeadEndTM Fluorometric TUNEL System (Promega, Madison, WI, USA) was used to determine the apoptosis of cells. 1 × 105 cells transfected by pcDNA3.1-MTLC or empty parental vector were seeded into a plate with a poly-L-lysine-coated slide on its center and grown for 24 h in DMEM culture containing 10% serum. The cells were then maintained for additional 18 h in serum-free culture and then detected according to the protocol provided by the manufacturer. The samples were stained with propidium iodide (PI) to make a red background and then observed under fluorescence microscope.
RESULTS
Expression of MTLC mRNA in GC tissues
RT-PCR was performed in 15 paired tissues to reveal the expression levels of MTLC mRNA. The result of electrophoresis showed that the PCR product was a single band on agar gel (Figure 1). MTLC was down-regulated in cancer tissues in 9 (60%) of 15 cases after normalization by comparing the band intensities with software UVP Gelworks ID advanced version 2.5 (Figure 1).
Figure 1.
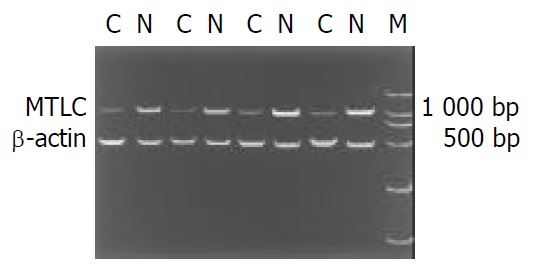
RT-PCR products were electrophoresed on 1% aga-rose gel containing ethidium bromide. The level of β-actin was used as internal control. M: DL2000 DNA marker; C: gastric cancer tissue; N: adjacent normal gastric tissue.
Effects of MTLC expression on cell growth
One of the effects of c-myc on cells is to affect their growth properties. Therefore, we determined whether over-expression of MTLC could recapitulate this character. As seen in Figure 2, the growth rates of MTLC-expressing cells were indistinguishable from those of control cells transfected with the empty parental vector.
Figure 2.
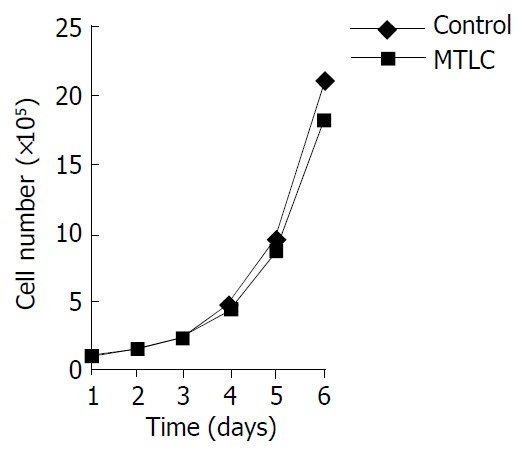
Cells were determined by manual counting daily. The data was analyzed by Microsoft Excel.
Promotion of apoptosis by MTLC
We studied the response of MTLC-expression BGC823 cell line to avoid its growth factors. Compared with the control cells, MTLC-expression cells showed a marked acceleration of apoptosis (Figure 3,Table 1).
Figure 3.
Apoptosis of cells were detected by TUNEL (TdT-mediated dUTP Nick-End Labeling) assay. Green fluorescence of fluores-cein-12-dUTP was detected in apoptotic cells, whereas red fluorescence of PI was observed in all cells. Both signals in a same field were photographed respectively. A: MTLC-expressing cells (× 400); B: control cells (× 400). C: a single apoptotic cell (× 1000).
Table 1.
Relative expressions of MTLC/β-actin in GC tissues and control tissues
| Case No. | GC | Control | Ratioa |
| 1 | 0.27 | 2.56 | 0.10 |
| 2 | 0.16 | 3.78 | 0.04 |
| 3 | 0.56 | 2.64 | 0.21 |
| 4 | 0.93 | 1.56 | 0.59 |
| 5 | 1.25 | 1.17 | 1.06 |
| 6 | 0.52 | 2.31 | 0.22 |
| 7 | 0.35 | 1.75 | 0.20 |
| 8 | 0.35 | 1.21 | 0.29 |
| 9 | 0.64 | 0.73 | 0.87 |
| 10 | 1.21 | 0.98 | 1.29 |
| 11 | 0.39 | 2.35 | 0.16 |
| 12 | 0.57 | 1.54 | 0.37 |
| 13 | 0.47 | 1.56 | 0.30 |
| 14 | 0.69 | 0.76 | 0.90 |
| 15 | 0.83 | 0.93 | 0.89 |
The ratios less than 0.5 were defined as down-regulation.
DISCUSSION
MTLC is a novel gene cloned in our laboratory recently and has no known function. Previous studies showed that it was located in 6q25, a chromosome region involved in a variety of human malignancies, including gastric cancer. Analysis of the 5’flanking sequence also demonstrated two E-boxes on the promoter region of MTLC, suggesting that MTLC may be a target of oncogene c-myc. C-myc, a helix-loop-helix leucine zipper transcription factor, can exert considerable control over transformation, differentiation, apoptosis, and cell cycle progression through a number of target genes, including CAD[13], ODC[14,15], LDH-A[16,17], cyclin E[18], MrDb[19], telomerase/hTERT[20-25], rcl[26], IRP2[27], cdc25A[28], and JPO1[29]. It was also shown that c-myc could contribute to gastric carcinogenesis[30-36], but the exact mechanism is still not clear.
In this study, therefore, we detected the expression of MTLC mRNA in gastric cancer tissues and the effects of MTLC over-expression in gastric carcinoma cell line BGC823 to reveal the relationship between MTLC and gastric cancer. Results of RT-PCR showed that MTLC was down-regulated in 60% (9/15) cases of gastric cancer tissues, a considerable frequency approximating to other genes suppressed in gastric cancer[37-40], suggesting that MTLC may play an important role in carcinogenesis. Furthermore, we performed gene transfection to reveal the possible function of MTLC in gastric carcinogenesis. MTLC did not affect cell growth but remarkably promoted apoptosis in response to growth factor deprivation, although we could not yet explain how this occurred. Over-expression of some other c-myc targets such as p21[41-45] and GADD45 [46-51] has been reported to exhibit similar effects through p53 pathway. However, the mechanism of MTLC promoting GC cells apoptosis needs to be further studied. It is also necessary to verify the down-regulation of MTLC in GC tissues by detecting more samples with various types or clinical stages.
Footnotes
Supported by the National Natural Science Foundation of China, No.30171008; Funds of Educational Department, Liaoning Province, No. 20121034
Edited by Ma JY
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Deng DJ. progress of gastric cancer etiology: N-nitrosamides 1999s. World J Gastroenterol. 2000;6:613–618. doi: 10.3748/wjg.v6.i4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403–406. doi: 10.3748/wjg.v7.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue YW, Zhang QF, Zhu ZB, Wang Q, Fu SB. Expression of cyclooxygenase-2 and clinicopathologic features in human gastric adenocarcinoma. World J Gastroenterol. 2003;9:250–253. doi: 10.3748/wjg.v9.i2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo XL, Wang LE, Du SY, Fan CL, Li L, Wang P, Yuan Y. Association of cyclooxygenase-2 expression with Hp-cagA infection in gastric cancer. World J Gastroenterol. 2003;9:246–249. doi: 10.3748/wjg.v9.i2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Kuang LG, Zheng HC, Li JY, Wu DY, Zhang SM, Xin Y. PTEN encoding product: a marker for tumorigenesis and progression of gastric carcinoma. World J Gastroenterol. 2003;9:35–39. doi: 10.3748/wjg.v9.i1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang VW, Bell DA, Berkowitz RS, Mok SC. Whole genome amplification and high-throughput allelotyping identified five distinct deletion regions on chromosomes 5 and 6 in microdissected early-stage ovarian tumors. Cancer Res. 2001;61:4169–4174. [PubMed] [Google Scholar]
- 8.Rodriguez C, Causse A, Ursule E, Theillet C. At least five regions of imbalance on 6q in breast tumors, combining losses and gains. Genes Chromosomes Cancer. 2000;27:76–84. [PubMed] [Google Scholar]
- 9.Stilgenbauer S, Bullinger L, Benner A, Wildenberger K, Bentz M, Döhner K, Ho AD, Lichter P, Döhner H. Incidence and clinical significance of 6q deletions in B cell chronic lymphocytic leukemia. Leukemia. 1999;13:1331–1334. doi: 10.1038/sj.leu.2401499. [DOI] [PubMed] [Google Scholar]
- 10.Gao H, Wang Q, Wang B, Yan C, Wang S, Wang B, Zhu J, Huang C, Fu S. [Genescan analysis of non-small cell lung cancer in the long arm of chromosome 6] Zhonghua Yixue Yichuanxue Zazhi. 2002;19:14–16. [PubMed] [Google Scholar]
- 11.Acevedo CM, Henríquez M, Emmert-Buck MR, Chuaqui RF. Loss of heterozygosity on chromosome arms 3p and 6q in microdissected adenocarcinomas of the uterine cervix and adenocarcinoma in situ. Cancer. 2002;94:793–802. doi: 10.1002/cncr.10275. [DOI] [PubMed] [Google Scholar]
- 12.Qiu G, Qiu G, Xu Z, Huang D, Gong L, Li C, Sun X, Sun K. [Cloning and characterization of MTLC, a novel gene in 6q25] Zhonghua Yixue Yichuanxue Zazhi. 2003;20:94–97. [PubMed] [Google Scholar]
- 13.Boyd KE, Farnham PJ. Myc versus USF: discrimination at the cad gene is determined by core promoter elements. Mol Cell Biol. 1997;17:2529–2537. doi: 10.1128/mcb.17.5.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bello-Fernandez C, Packham G, Cleveland JL. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc Natl Acad Sci USA. 1993;90:7804–7808. doi: 10.1073/pnas.90.16.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu S, Peña A, Korcz A, Soprano DR, Soprano KJ. Overexpression of Mxi1 inhibits the induction of the human ornithine decarboxylase gene by the Myc/Max protein complex. Oncogene. 1996;12:621–629. [PubMed] [Google Scholar]
- 16.Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci USA. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubank M, Schatz DG. Identifying differences in mRNA expression by representational difference analysis of cDNA. Nucleic Acids Res. 1994;22:5640–5648. doi: 10.1093/nar/22.25.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pérez-Roger I, Solomon DL, Sewing A, Land H. Myc activation of cyclin E/Cdk2 kinase involves induction of cyclin E gene transcription and inhibition of p27(Kip1) binding to newly formed complexes. Oncogene. 1997;14:2373–2381. doi: 10.1038/sj.onc.1201197. [DOI] [PubMed] [Google Scholar]
- 19.Grandori C, Mac J, Siëbelt F, Ayer DE, Eisenman RN. Myc-Max heterodimers activate a DEAD box gene and interact with multiple E box-related sites in vivo. EMBO J. 1996;15:4344–4357. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Xie LY, Allan S, Beach D, Hannon GJ. Myc activates telomerase. Genes Dev. 1998;12:1769–1774. doi: 10.1101/gad.12.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg RA, O'Hagan RC, Deng H, Xiao Q, Hann SR, Adams RR, Lichtsteiner S, Chin L, Morin GB, DePinho RA. Telomerase reverse transcriptase gene is a direct target of c-Myc but is not functionally equivalent in cellular transformation. Oncogene. 1999;18:1219–1226. doi: 10.1038/sj.onc.1202669. [DOI] [PubMed] [Google Scholar]
- 22.Horikawa I, Cable PL, Afshari C, Barrett JC. Cloning and characterization of the promoter region of human telomerase reverse transcriptase gene. Cancer Res. 1999;59:826–830. [PubMed] [Google Scholar]
- 23.Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, Inoue M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551–557. [PubMed] [Google Scholar]
- 24.Wick M, Zubov D, Hagen G. Genomic organization and promoter characterization of the gene encoding the human telomerase reverse transcriptase (hTERT) Gene. 1999;232:97–106. doi: 10.1016/s0378-1119(99)00108-0. [DOI] [PubMed] [Google Scholar]
- 25.Qiu GB, He G, Gong LG, Zhao Z, Pan ZM, Tang YC, Sun KL. Cloning of hTERT cDNA fragment and application of anti-hTERT monoclonal antibody in mechanism of laryngeal carcinogenesis. Yichuan Xuebao. 2003;30:109–113. [PubMed] [Google Scholar]
- 26.Lewis BC, Shim H, Li Q, Wu CS, Lee LA, Maity A, Dang CV. Identification of putative c-Myc-responsive genes: characterization of rcl, a novel growth-related gene. Mol Cell Biol. 1997;17:4967–4978. doi: 10.1128/mcb.17.9.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu KJ, Polack A, Dalla-Favera R. Coordinated regulation of iron-controlling genes, H-ferritin and IRP2, by c-MYC. Science. 1999;283:676–679. doi: 10.1126/science.283.5402.676. [DOI] [PubMed] [Google Scholar]
- 28.Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature. 1996;382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- 29.Prescott JE, Osthus RC, Lee LA, Lewis BC, Shim H, Barrett JF, Guo Q, Hawkins AL, Griffin CA, Dang CV. A novel c-Myc-responsive gene, JPO1, participates in neoplastic transformation. J Biol Chem. 2001;276:48276–48284. doi: 10.1074/jbc.M107357200. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Deng CS, Peng JZ, Wong BC, Lam SK, Xia HH. Effect of Helicobacter pylori on apoptosis and apoptosis related genes in gastric cancer cells. Mol Pathol. 2003;56:19–24. doi: 10.1136/mp.56.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu JR, Chen BQ, Yang YM, Wang XL, Xue YB, Zheng YM, Liu RH. Effect of apoptosis on gastric adenocarcinoma cell line SGC-7901 induced by cis-9, trans-11-conjugated linoleic acid. World J Gastroenterol. 2002;8:999–1004. doi: 10.3748/wjg.v8.i6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen RC, Su JH, Yang SM, Li J, Wang TJ, Zhou H. Effect of isoverbascoside, a phenylpropanoid glycoside antioxidant, on proliferation and differentiation of human gastric cancer cell. Acta Pharmacol Sin. 2002;23:997–1001. [PubMed] [Google Scholar]
- 33.Ishii HH, Gobé GC, Pan W, Yoneyama J, Ebihara Y. Apoptosis and cell proliferation in the development of gastric carcinomas: associations with c-myc and p53 protein expression. J Gastroenterol Hepatol. 2002;17:966–972. doi: 10.1046/j.1440-1746.2002.02805.x. [DOI] [PubMed] [Google Scholar]
- 34.Kawanaka H, Tomikawa M, Baatar D, Jones MK, Pai R, Szabo IL, Sugimachi K, Sarfeh IJ, Tarnawski AS. Despite activation of EGF-receptor-ERK signaling pathway, epithelial proliferation is impaired in portal hypertensive gastric mucosa: relevance of MKP-1, c-fos, c-myc, and cyclin D1 expression. Life Sci. 2001;69:3019–3033. doi: 10.1016/s0024-3205(01)01409-6. [DOI] [PubMed] [Google Scholar]
- 35.Hensel F, Hermann R, Brändlein S, Krenn V, Schmausser B, Geis S, Müller-Hermelink HK, Vollmers HP. Regulation of the new coexpressed CD55 (decay-accelerating factor) receptor on stomach carcinoma cells involved in antibody SC-1-induced apoptosis. Lab Invest. 2001;81:1553–1563. doi: 10.1038/labinvest.3780369. [DOI] [PubMed] [Google Scholar]
- 36.Ye YN, Liu ES, Shin VY, Koo MW, Li Y, Wei EQ, Matsui H, Cho CH. A mechanistic study of proliferation induced by Angelica sinensis in a normal gastric epithelial cell line. Biochem Pharmacol. 2001;61:1439–1448. doi: 10.1016/s0006-2952(01)00625-6. [DOI] [PubMed] [Google Scholar]
- 37.Yoshikawa Y, Mukai H, Hino F, Asada K, Kato I. Isolation of two novel genes, down-regulated in gastric cancer. Jpn J Cancer Res. 2000;91:459–463. doi: 10.1111/j.1349-7006.2000.tb00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu DH, Zhang XY, Fan DM, Huang YX, Zhang JS, Huang WQ, Zhang YQ, Huang QS, Ma WY, Chai YB, et al. Expression of vascular endothelial growth factor and its role in oncogenesis of human gastric carcinoma. World J Gastroenterol. 2001;7:500–505. doi: 10.3748/wjg.v7.i4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Höfler H, Becker KF. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol. 2002;161:1881–1891. doi: 10.1016/S0002-9440(10)64464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Wang Y, Shou C, Xu G, Chen X, Wu J, Xie Y, Li J, So S, Jiafu J. [Detection of Mycoplasma hyorhinis in gastric cancer using bio-chip technology] Zhonghua Yixue Zazhi. 2002;82:961–965. [PubMed] [Google Scholar]
- 41.Bearss DJ, Lee RJ, Troyer DA, Pestell RG, Windle JJ. Differential effects of p21(WAF1/CIP1) deficiency on MMTV-ras and MMTV-myc mammary tumor properties. Cancer Res. 2002;62:2077–2084. [PubMed] [Google Scholar]
- 42.Horiguchi-Yamada J, Fukumi S, Saito S, Nakayama R, Iwase S, Yamada H. DNA topoisomerase II inhibitor, etoposide, induces p21WAF1/CIP1 through down-regulation of c-Myc in K562 cells. Anticancer Res. 2002;22:3827–3832. [PubMed] [Google Scholar]
- 43.Seoane J, Le HV, Massagué J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature. 2002;419:729–734. doi: 10.1038/nature01119. [DOI] [PubMed] [Google Scholar]
- 44.Bergsmedh A, Szeles A, Spetz AL, Holmgren L. Loss of the p21(Cip1/Waf1) cyclin kinase inhibitor results in propagation of horizontally transferred DNA. Cancer Res. 2002;62:575–579. [PubMed] [Google Scholar]
- 45.Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796–800. doi: 10.3748/wjg.v7.i6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conzen SD, Gottlob K, Kandel ES, Khanduri P, Wagner AJ, O'Leary M, Hay N. Induction of cell cycle progression and acceleration of apoptosis are two separable functions of c-Myc: transrepression correlates with acceleration of apoptosis. Mol Cell Biol. 2000;20:6008–6018. doi: 10.1128/mcb.20.16.6008-6018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang A, Gu J, Judson-Kremer K, Powell KL, Mistry H, Simhambhatla P, Aldaz CM, Gaddis S, MacLeod MC. Response of human mammary epithelial cells to DNA damage induced by BPDE: involvement of novel regulatory pathways. Carcinogenesis. 2003;24:225–234. doi: 10.1093/carcin/24.2.225. [DOI] [PubMed] [Google Scholar]
- 48.Chen Z, Clark S, Birkeland M, Sung CM, Lago A, Liu R, Kirkpatrick R, Johanson K, Winkler JD, Hu E. Induction and superinduction of growth arrest and DNA damage gene 45 (GADD45) alpha and beta messenger RNAs by histone deacetylase inhibitors trichostatin A (TSA) and butyrate in SW620 human colon carcinoma cells. Cancer Lett. 2002;188:127–140. doi: 10.1016/s0304-3835(02)00322-1. [DOI] [PubMed] [Google Scholar]
- 49.Vairapandi M, Balliet AG, Hoffman B, Liebermann DA. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J Cell Physiol. 2002;192:327–338. doi: 10.1002/jcp.10140. [DOI] [PubMed] [Google Scholar]
- 50.Uberti D, Carsana T, Bernardi E, Rodella L, Grigolato P, Lanni C, Racchi M, Govoni S, Memo M. Selective impairment of p53-mediated cell death in fibroblasts from sporadic Alzheimer's disease patients. J Cell Sci. 2002;115:3131–3138. doi: 10.1242/jcs.115.15.3131. [DOI] [PubMed] [Google Scholar]
- 51.Okura T, Nakamura M, Takata Y, Watanabe S, Kitami Y, Hiwada K. Troglitazone induces apoptosis via the p53 and Gadd45 pathway in vascular smooth muscle cells. Eur J Pharmacol. 2000;407:227–235. doi: 10.1016/s0014-2999(00)00758-5. [DOI] [PubMed] [Google Scholar]