Abstract
AIM: To study different approaches to caudate lobectomy with “curettage and aspiration” technique using Peng’s multifunctional operative dissector (PMOD). The surgical procedure of isolated complete caudate lobectomy was specially discussed.
METHODS: In 76 cases of various types of caudate lobectomy, three approaches were used including left side approach, right side approach, and anterior approach. Among the 76 cases, isolated complete caudate lobectomy was carried out in 6 cases with transhepatic anterior approach. The surgical procedure consisted of mobilization of the total liver, ligation and separation of the short hepatic veins, splitting the liver parenchyma through the Cantlie’s plane, ligation and division of the caudate portal triads from the hilum, dissection of the root of major hepatic veins, detachment of the caudate lobe from liver parenchyma.
RESULTS: The mean operative time was 285 ± 51 min, the mean blood loss was 1600 mL. No severe complications were observed. Among the 6 cases receiving isolated complete caudate lobectomy with transhepatic anterior approach, one case died 17 months after operation due to disease recurrence and liver failure, the other 5 cases have been alive without recurrence, with one longest survival of 49 months.
CONCLUSION: The choice of approach is essential to the success of caudate lobectomy. As PMOD and “curettage and aspiration” technique can delineate intrahepatic or extrahepatic vessels clearly, caudate lobe resection has become safer, easier and faster.
INTRODUCTION
The caudate lobe of the liver is difficult to resect because it lies deep beneath the confluence of main hepatic veins and between porta hepatis and inferior vena cava[1]. Isolated or combined caudate lobectomy is the treatment of choice for a mass originating in caudate lobe or for hepatobiliary cancer invading the hepatic hilum. Among various types of caudate lobectomy, isolated complete resection of caudate lobe is technically the most difficult one. Anterior approach is considered to be a safe, potentially curative option for isolated resection of the entire caudate lobe, especially in the presence of cirrhosis, as we can spare innocent hepatic parenchyma using this approach[2,3]. With the use of a specially designed instrument-Peng’s multifunctional operative dissector (PMOD), we developed a new surgical technique called “curettage and aspiration” technique with which transection of the liver parenchyma can be carried out in a nearly bloodless field[4]. Precise anatomy of caudate lobe can be achieved with this technique and instrument. Therefore isolated complete resection of caudate lobe has become easier and faster as the liver is split into two halves by anterior approach. From 1994 to June 2003, we performed 76 cases of various types of hepatectomy with caudate lobectomy, which included 6 isolated complete caudate lobectomies by anterior transhepatic approach. This review studied different approaches to caudate lobectomy especially the anterior transhepatic approach for isolated complete caudate lobectomy with “curettage and aspiration” technique using PMOD.
MATERIALS AND METHODS
Patients
Seventy-six cases were enrolled in the study. Various types of hepatectomy with caudate lobectomy were performed in 51 cases, isolated caudate lobectomy was performed in 25 cases. Forty-five cases had hepatocellular carcinoma (HCC) originating from or invading caudate lobe, 7 cases had benign tumors and stone of caudate lobe, 17 cases had cholangiocarcinoma, 7 cases had metastatic tumor in the liver (6 cases of colonic carcinoma, 1 case of adrenal carcinoma). These 6 patients received isolated complete caudate lobectomy by anterior approach. Among them, four were male and 2 female, their age ranged from 32 to 65 years (mean 52 years); five cases had HCC accompanied by cirrhosis, and one had hemoangioma.
Surgical procedures
In the majority of cases, a reversed L-shaped skin incision from xiphoid to the tip of the twelfth right rib was used, giving an excellent exposure, which was of vital importance for caudate lobectomy. The whole abdominal cavity was explored to rule out intra-abdominal metastasis.
The choice of approach is essential to the success of caudate lobectomy. Approaches are dependent largely on the size, location of the lesion and the severity of cirrhosis. In this series, four approaches were used for various types of caudate lobectomy. Left side approach was suitable for small tumors situated in Spiegelian lobe or when caudate lobe was to be resected combined with the left liver. Left lateral segmentectomy, left hemi-hepatectomy or left trisegmentectomy was carried out before caudate lobe was exposed and resected. Right side approach was more suitable for tumor located in the caudate process or when the caudate lobe was resected together with the right liver, mostly right hemi-hepatectomy. The combined approach was a combination of the left side and right side approach. The caudate lobe might be approached mainly from the right or left side although dissection from both sides was necessary in many cases. Anterior transhepatic approach was suitable for cases when isolated complete resection of caudate lobe was indicated and innocent liver parenchyma should not be resected due to cirrhosis of the liver (Figure 1). The characteristics of this approach were that the liver was split through the interlobar plane into two halves, so as to fully expose the caudate lobe.
Figure 1.
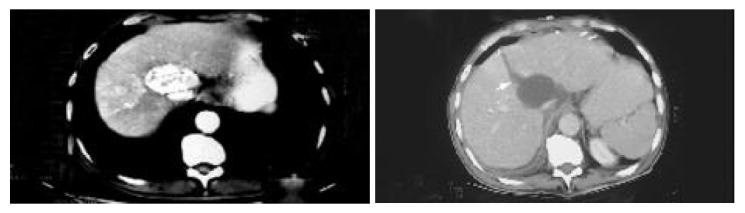
Pre-operative CT scan shows the tumor originating in caudate lobe, post-opera-tive CT scan shows splitting line (arrow).
The anterior transhepatic approach included 7 steps. (1) The falciform ligament was separated up to the front of suprahepatic IVC, then the incision was turned to the right and left. The coronary ligaments, triangular ligaments and hepato-renal ligament were separated, respectively. The adrenal gland was detached from the liver, and hepatogastric ligament was completely separated. (2) The short hepatic veins (SHV) were dissected and ligated caudal cranially, three to five thick short hepatic veins were separated in this process (Figure 2). (3) Tapes were used to encircle the suprahepatic and infrahepatic IVC, respectively (Figure 3). (4) The interlobar plane was split and the anterior surface of the paracaval portion and the hilar plate were explored. (5) The ascending caudate portal triads were ligated and separated (Figure 4). (6) The caudate lobe was separated from the major hepatic veins (MHV) (Figure 5). (7) The caudate lobe was detached from the neighboring liver parenchyma. No large branches here needed to be ligated, the small vessels encountered could be cauterized with PMOD. Thus isolated complete caudate lobe was resected and two halves of the liver were sutured (Figure 6).
Figure 2.
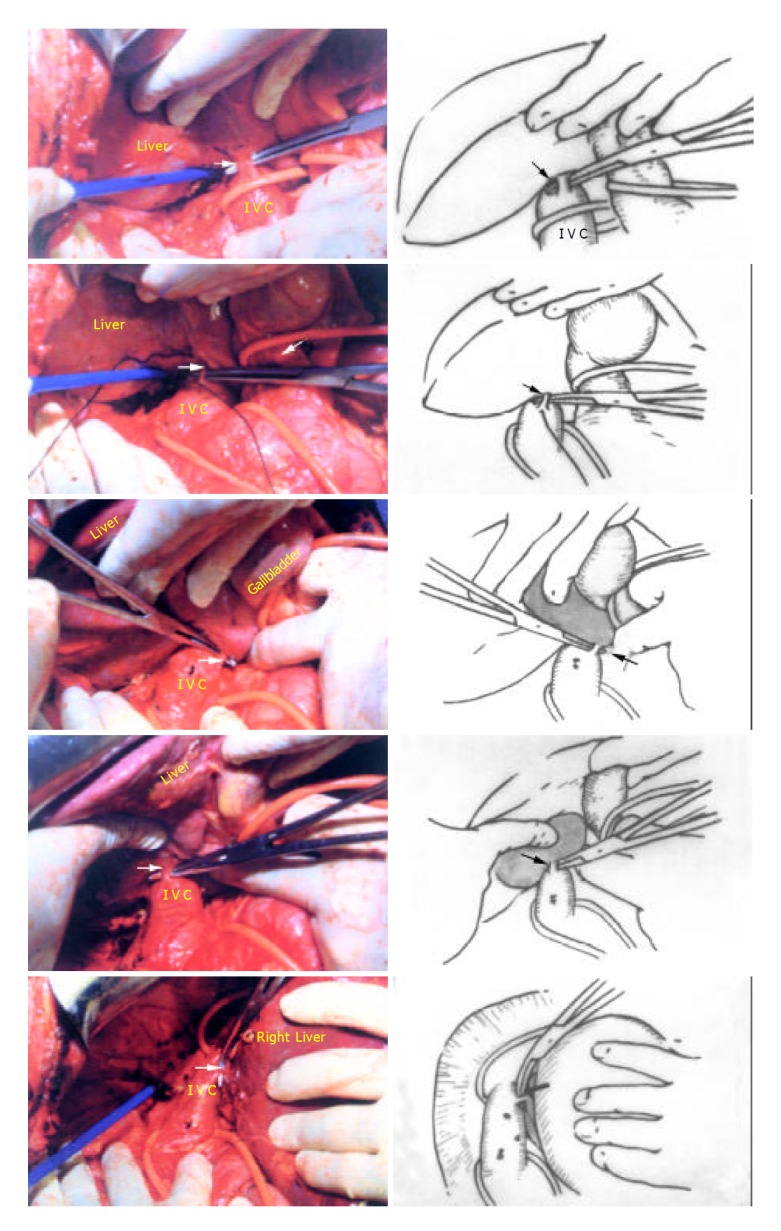
Five short hepatic veins ligated from caudal direction to cranial direction respestively (Arrow).
Figure 3.
The suprahepatic inferior vena cava and right hepatic vein (RHV) dissected and encircled with tapes (thick arrow: RHV, thin arrow: suprahepatic IVC).
Figure 4.
Four groups of portal triads to the caudate lobe(Arrow) were divided, the tumor was detached from the hilum.
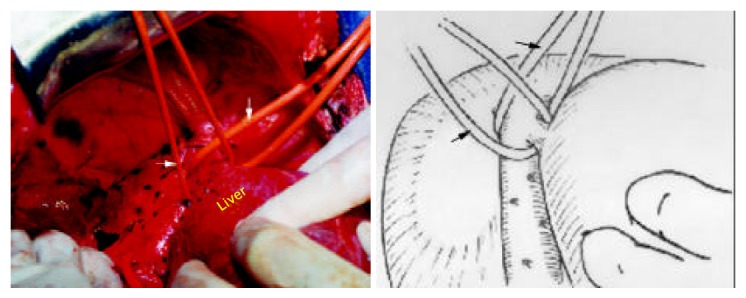
Figure 5.
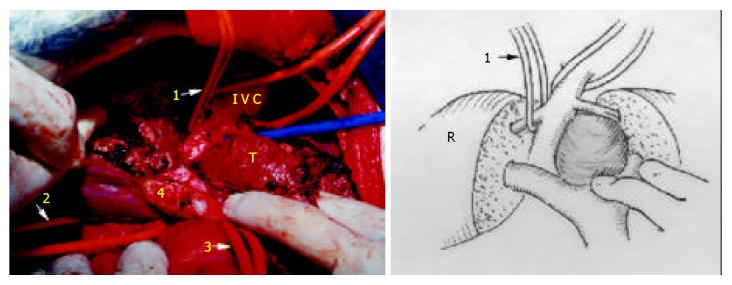
The tumor still attaches to MHV (1: tape across RHV, 2: tape across IVC, 3: tape across pedicle, 4: the portion of bifurcation, T: tumor).
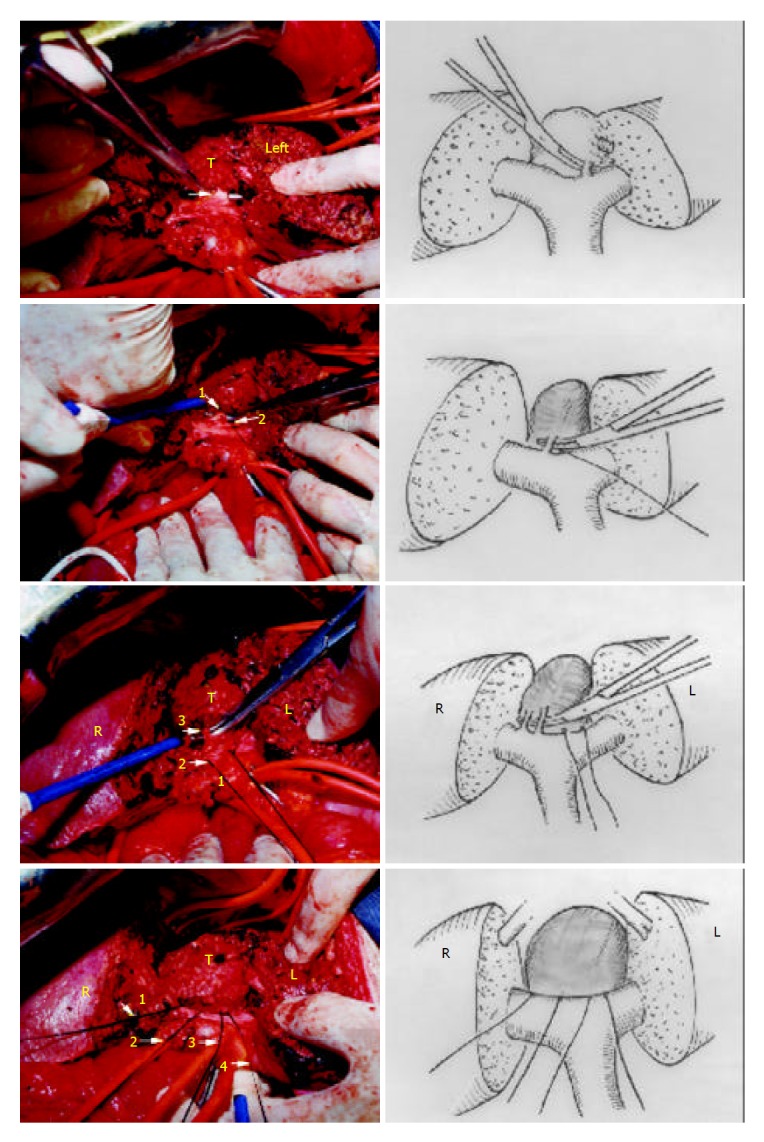
Figure 6.

Completely resected tumor. Two halves of the liver were sutured. (1: RHV, 2: common trunk of MHV and LHV, 3: IVC, R: right liver, L: left liver, Arrow: interlobar plane, T: tumor).
In this series, the mobilization of caudate lobe was from left side first in most of the cases. Bartlett et al[5] reported that the ligamentous attachment should be separated and the tip of caudate lobe was mobilized before separation of the caudate veins.
The liver parenchyma was transected by means of “curettage and aspiration” technique using PMOD with intermittent inflow occlusion at the hepatoduodenal ligament (Pringle’s maneuver), the time limit was 10 min each time with reperfusion for 2 min. Total vascular exclusion was seldom necessary except that when the tumor involved IVC or major hepatic veins. PMOD is a special instrument, it has the functions of dissection, coagulation and aspiration separately or synchronously. As a result, the surgical field was nearly bloodless and the intrahepatic duct structures could be identified, isolated and treated individually.
RESULTS
Isolated or combined caudate lobectomy was successfully performed in 76 patients (Table 1). Isolated caudate lobectomy was performed in 25 cases. Of these 25 cases, isolated complete caudate lobectomy was performed in 19 cases, 6 cases (31.6%, 6/19) underwent complete resection by anterior transhepatic approach. In these 6 cases, the size of tumor was from 3 cm to 8 cm, the mean operating time was 285 ± 51 min, and the total occlusion time ranged from 50 to 110 min, the operative blood loss ranged from 500-3000 mL (mean 1600 mL).
Table 1.
Resection procedures
| Operation | No. of patients |
| Complete caudate lobectomy (49 cases) | |
| Isolated | 19 (total) |
| Anterior transhepatic approach | 6 |
| Other approaches | 13 |
| Combined | 30 (total) |
| Right hepatectomy +S1(C) | 13 |
| Left hepatectomy +S1(C) | 14 |
| Extend left hepatectomy +S1(C) | 2 |
| VI segmentectomy +S1(C) +T colon | 1 |
| Partial caudate lobectomy (27 cases) | |
| Isolated | 6 (total) |
| Combined | 21 (total) |
| Left hepatectomy +S1(P) | 5 |
| Right hepatectomy +S1(P) | 8 |
| Right hepatectomy +S1(P)+adrenal gland | 1 |
| V segmentectomy +S1(P) | 1 |
| VI segmentectomy +S1(P) | 4 |
| VII segmentectomy +S1(P) | 1 |
| V + VI segmentectomy +S1(P) | 1 |
| Total | 76 |
S1: Caudate lobe, T Colon: Transverse colon, P: Partial, C:Complete.
No intraoperative death and signs of liver failure occurred in all the 6 cases, serum aspartate transaminase level recovered to normal range within one week postoperatively. One case had ascites because of liver cirrhosis, one case had right pleural effusion and was cured after aspiration two times. One case had bile leakage and was cured after two weeks’ conservative treatment. Abdominal drains were placed in all the patients and removed within one week except the case with bile leakage.
All the 6 cases received a long-term follow-up. One case died in the 17th month postoperatively due to disease recurrence and liver failure, the other 5 cases have been alive without recurrence, with the longest survival of 49 months in 1 case.
DISCUSSION
The caudate lobe is a single anatomic segment that is defined by the presence of portal venous and hepatic arterial branches, which supply the lobe, drain biliary ducts and hepatic veins[6]. Three parts make up the entire caudate lobe, namely, the usual caudate lobe (Spiegelian lobe), the caudate process, and the paracaval portion[7]. Partial or complete caudate lobectomy with major hepatectomy is often necessary for extirpation of the tumor. But in China, most liver cancers occur in cirrhotic liver. The poor liver function of our cases prevented us from performing a combined or preparatory resection of major segment or other liver segments for tumors situated only in the caudate lobe.
Caudate lobectomy is classified by complete and partial resection, it is also classified by isolated and combined resection. Therefore caudate lobectomy is generally composed of four types: isolated complete resection, combined complete resection, isolated partial resection and combined partial resection. To select an appropriate surgical approach is essential for resection of caudate lobe. In our experience, right side approach is suitable for the tumor confined in caudate process or for cases when hepatectomy is indicated for cancer involvement. Left side approach is suitable for the combined resection of the left liver overlying the caudate lobe or for tumor confined in Spiegelian lobe. Isolated complete caudate lobectomy without resection of innocent parenchyma is sometimes performed by splitting the parenchyma through interlobar plane as anterior approach. In addition, central segmentectomy is also classified as anterior approach, anterior segmentectomy together with caudate lobectomy has been considered as an appropriate treatment for hilar cholangiocarcinoma without infiltration of the posterior hepatic branch[8,9].
Some of the isolated caudate lobectomies could be performed through both left side and right side approaches when the tumor was small, but when the tumor was large or IVC and/or major hepatic veins were compressed by the tumor, the above methods might not be appropriate due to the possibility of laceration of major hepatic veins. Under such circumstances, anterior transhepatic approach is the best choice for isolated complete caudate lobectomy. In 1992,Yamamoto et al[10] described a patient with cirrhosis and a 3 × 3 cm HCC in the paracaval portion of the caudate lobe for whom they performed isolated caudate lobectomy by splitting the cirrhotic liver into two halves. The anterior transhepatic approach could provide a safe strategic alternative for isolated complete caudate lobectomy. The separation of hepatic parenchyma overlying the caudate lobe could expose the major hepatic veins and the hilar plate to direct view, facilitating control of venous bleeding and interruption of the ascending paracaval portal branches along the hilar plate. Usually it would be associated with a significant amount of blood loss. In this series, we used PMOD to transect the liver parenchyma by means of “curettage and aspiration” technique, all of the intrahepatic ducts could be identified and isolated before it was transected. Asahara et al[2] reported that the minor operative time and blood loss were 355 min and 1100 mL. Because PMOD could facilitate the transection of cirrhotic liver, the minor operation time and blood loss were 234 min and 500 mL respectively. In our series with “curettage and aspiration” technique, not only the blood loss was decreased obviously, but also no atrophy of the medial segment was observed on postoperative computed tomography. So the benefit exceeded the risk of splitting the medium hepatic plane. For selective caudate lobectomy, the most dangerous part is the parenchyma division of its anterosuperior portion, where the roots of major hepatic veins are running next to the line of dissection. Without an outflow occlusion, any inadvertently deep cleavage could result in sudden and massive hemorrhage from a hepatic vein. Although total vascular exclusion could limit blood loss from the hepatic venous system and decrease the risk of air embolism. Such a maneuver would result in hemodynamic instability in up to 40%[11]. In our series, Tapes were used to encircle the suprahepatic and infrahepatic IVC for precautions. The author usually isolated the common trunk and the right hepatic vein to pre-place the tapes for controlling the three major hepatic veins. In case massive hemorrhage occurred from a hepatic vein, we could control the hepatic vein to substitute the total vascular exclusion. In addition, during hepatic transection, usually dissection was carried out along the section plane, but when large vessels were shown, curettage was proceeded in parenchyma in parallel with the vessels in deep cleavage plane by altering direction, so sudden and massive hemorrhage occurred rarely, and we could treat vessels in direct view even if there was hemorrhage. In fact, we did not use tapes in all the 6 cases.
The anterior transhepatic approach for isolated complete caudate lobectomy is a curative procedure for primary or metastatic hepatobiliary neoplasm originating from caudate lobe, especially in the presence of cirrhosis when the tumor is large and involves IVC and/or the major hepatic veins. As PMOD and “curettage and aspiration” technique can delineate intrahepatic or extra hepatic vessels clearly, caudate lobe resection has become safer, easier and faster.
ACKNOWLEDGMENT
We are deeply grateful to Professor Fang Zheng (Department of Anatomy, School of Medicine, Zhejiang University) for producing the figures of this paper.
Footnotes
Edited by Wang XL and Zhu LH
References
- 1.Lucandri G, Stipa F, Sapienza P, Ziparo V, Stipa S. Resection of the caudate lobe for hepatocellular carcinoma. Eur J Surg. 1998;164:395–398. doi: 10.1080/110241598750004445. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Dohi K, Hino H, Nakahara H, Katayama K, Itamoto T, Ono E, Moriwaki K, Yuge O, Nakanishi T, et al. Isolated caudate lobectomy by anterior approach for hepatocellular carcinoma originating in the paracaval portion of the caudate lobe. J Hepatobiliary Pancreat Surg. 1998;5:416–421. doi: 10.1007/s005340050066. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto J, Kosuge T, Shimada K, Yamasaki S, Takayama T, Makuuchi M. Anterior transhepatic approach for isolated resection of the caudate lobe of the liver. World J Surg. 1999;23:97–101. doi: 10.1007/s002689900572. [DOI] [PubMed] [Google Scholar]
- 4.Peng S, Mou Y, Peng C, Cai X, Jiang X, Li J. [Resection of caudate lobe of liver: report of 26 cases] Zhonghua Waike Zazhi. 1999;37:12–13. [PubMed] [Google Scholar]
- 5.Bartlett D, Fong Y, Blumgart LH. Complete resection of the caudate lobe of the liver: technique and results. Br J Surg. 1996;83:1076–1081. doi: 10.1002/bjs.1800830812. [DOI] [PubMed] [Google Scholar]
- 6.Blumgart LH, Fong Y. Surgery of the liver and biliary tract. 3rd ed. Health Science Asia Elsevier Science. 2000:1639. [Google Scholar]
- 7.Takayama T, Makuuchi M. Segmental liver resections, present and future-caudate lobe resection for liver tumors. Hepatogastroenterology. 1998;45:20–23. [PubMed] [Google Scholar]
- 8.Shimada H, Izumi T, Note M, Seki H, Nakagawara G. Anterior segmentectomy with caudate lobectomy for hilar cholangiocarcinoma. Hepatogastroenterology. 1993;40:61–64. [PubMed] [Google Scholar]
- 9.Nagino M, Nimura Y, Kamiya J, Kanai M, Uesaka K, Hayakawa N, Yamamoto H, Kondo S, Nishio H. Segmental liver resections for hilar cholangiocarcinoma. Hepatogastroenterology. 1998;45:7–13. [PubMed] [Google Scholar]
- 10.Yamamoto J, Takayama T, Kosuge T, Yoshida J, Shimada K, Yamasaki S, Hasegawa H. An isolated caudate lobectomy by the transhepatic approach for hepatocellular carcinoma in cirrhotic liver. Surgery. 1992;111:699–702. [PubMed] [Google Scholar]
- 11.Yanaga K, Matsumata T, Hayashi H, Shimada M, Urata K, Sugimachi K. Isolated hepatic caudate lobectomy. Surgery. 1994;115:757–761. [PubMed] [Google Scholar]


