Abstract
AIM: To clone flagellin genes A (flaA) and B (flaB) from a clinical strain of Helicobacter pylori (H pylori) and to construct prokaryotic expression systems of the genes and identify immunity of the fusion proteins.
METHODS: The flaA and flaB genes from a clinical H pylori isolate Y06 were amplified by high fidelity PCR. The nucleotide sequences of target DNA amplification fragments from the two genes were sequenced after T-A cloning. The recombinant expression vector pET32a inserted with flaA and flaB genes was constructed, respectively. The expressions of FlaA and FlaB fusion proteins in E. coli BL21DE3 induced by isopropylthio-β-D-galactoside (IPTG) at different concentrations were examined by SDS-PAGE. Western blot using commercial antibodies against whole cell of H pylori and immunodiffusion assay using self-prepared rabbit antiserum against FlaA (rFlaA) or FlaB (rFlaB) recombinant proteins were applied to the determination of the fusion proteins immunity. ELISA was used to detect the antibodies against rFlaA and rFlaB in sera of 125 H pylori infected patients and to examine rFlaA and rFlaB expression in 98 clinical isolates of H pylori, respectively.
RESULTS: In comparison with the reported corresponding sequences, the nucleotide sequence homologies of the cloned flaA and flaB genes were from 96.28%-97.13% and 96.31%-97.73%, and their putative amino acid sequence homologies were 99.61%-99.80% and 99.41%-100% for the two genes, respectively. The output of rFlaA and rFlaB expressed by pET32a-flaA-BL21DE3 and pET32a-flaB-BL21DE3 systems was as high as 40%-50% of the total bacterial proteins. Both rFlaA and rFlaB were able to combine with the commercial antibodies against whole cell of H pylori and to induce rabbits to produce specific antibodies with the same 1:2 immunodiffusion titers after the animals were immunized with the two recombinant proteins. Ninety-eight and zero point 4 and 92.80% of the serum samples from 125 patients infected with H pylori were positive for rFlaA and rFlaB antibodies, respectively. One hundred percent and 98.98% of the 98 tested isolates of H pylori were detectable for rFlaA and rFlaB epitopes, respectively.
CONCLUSION: Two prokaryotic expression systems with high efficiency of H pylori flaA and flaB genes were successfully established. The expressed rFlaA and rFlaB showed satisfactory immunoreactivity and antigenicity. High frequencies of FlaA and FlaB expression in different H pylori clinical strains and the general existence of specific antibodies against FlaA and FlaB in H pylori infected patients strongly indicate that FlaA and FlaB are excellent antigen candidates for developing H pylori vaccine.
INTRODUCTION
In China, chronic gastritis and peptic ulceration are two most common gastric diseases, and gastric cancer is one of the malignant tumors with high mortalities and morbidities[1-34]. Helicobacter pylori (H pylori), a microaerophilic, spiral and Gram-negative bacterium, is considered as a human-specific gastric pathogen that colonizes the stomach of at least half of the world population[35]. Most infected individuals are asymptomatic. However, in some subjects, the infection causes acute, chronic gastritis and peptic ulceration, and plays important roles in the development of peptic ulcer and gastric adenocarcinoma, mucosa-associated lymphoid tissue (MALT) lymphoma and primary gastric non-Hodgkin’s lymphoma[36-43]. This microorganism has been categorized as class I carcinogen by the World Health Organization[44], and direct evidence of carcinogenesis has been recently demonstrated in animal models[45,46]. Immunization against the bacterium represents a cost-effective strategy to prevent H pylori-associated peptic ulcer diseases and to reduce the incidence of global gastric cancer[47]. Selection of antigenic targets is critical in design of H pylori vaccine. So far, no vaccine preventing H pylori infection has been commercially available. The majority of studies attempting to produce a vaccine have focused on urease enzyme, heat shock protein, and vacuolating cytotoxin[35,48-50], but rarely on H pylori flagellin. H pylori flagellin is composed of two subunits, named as FlaA with 53 KDa and FlaB with 54 KDa respectively. The flagellin plays a main role in motility and is necessary for colonization or persistence of H pylori infection[51]. The motility of H pylori is a virulent factor in the pathogenesis of gastric mucosal injury[52]. The data mentioned above indicate that FlaA as well as FlaB may be used as antigen candidates for H pylori vaccine. Therefore, in this study, two prokaryotic vectors responsible for expressing recombinant FlaA (rFlaA) and FlaB (rFlaB) were constructed . Immunoreactivity and antigenicity of rFlaA and rFlaB were further examined. Furthermore, these two recombinant proteins were used for detecting specific antibodies in sera from H pylori infected patients, and rabbit anti-rFlaA and anti-rFlaB sera were prepared for examining the corresponding epitopes of H pylori clinical isolates. The results of this study may contribute to the development of H pylori vaccines.
MATERIALS AND METHODS
Materials
A clinical strain of H pylori was used in this study, which was provisionally named Y06, and well-characterized by the Department of Medical Microbiology and Parasitology, College of Medical Sciences, Zhejing University. A plasmid pET32a (Novagen) and an E. coli strain BL21DE3 (Novagen) were used as the expression vector and host cell, respectively. Primers for PCR amplification, the Pfu-Taq high fidelity PCR kit and restriction endonucleases were purchased from BioAsia (Shanghai, China). The T-A cloning kit and sequencing service were provided by BBST (Shanghai, China). Rabbit antiserum against the whole cell of H pylori, HRP-labeling sheep antisera against rabbit IgG and against human IgG were purchased from DAKO and Jackson ImmunoResearch, respectively. Agents used in isolation and identification of H pylori were purchased from Sigma and bioMérieux. Gastric biopsy specimens with positive H pylori isolation from 126 patients (86 males and 40 females, age range: from 6-78 years old, mean age: 40.5 years old) referred for gastroduodenoscopic examination in four different hospitals in Hangzhou were collected during the period between December 2001 and June 2002. Each of the patients gave a written informed content for this study. Of the 126 patients, 68 had chronic gastritis (CG, 48 superficial, 10 active and 10 atrophic), and the other 58 had peptic ulcer disease (PUD, 12 gastric ulcer, 40 duodenal ulcer and 6 gastric and duodenal ulcer). None of the patients had taken nonsteroidal anti-inflammatory drugs, antacids and antibiotics during the two weeks before seeking medical advice. At the same time, serum specimens were also collected from these patients.
Methods
Isolation and identification of H pylori Each gastric biopsy specimen was homogenized with a tissue grinder and then inoculated on Columbia agar plates supplemented with 8.0% (V/V) sheep blood, 0.5% (W/V) cyclodextrin, 5 mg/L trimethoprim, 10 mg/L vancomycin, 2500 U/L cefsoludin and 2.5 mg/L amphotericin B. The plates were incubated at 37 °C under microaerobic conditions (5% O2, 10% CO2 and 85% N2) for 3 to 5 d. A bacterial isolate was identified as H pylori according to typical Gram staining morphology, biochemical tests positive for urease and oxidase, and agglutination with the commercial rabbit antibody against whole cell of the microbe. All of H pylori isolates were stored at -70 °C for ELISA.
Preparation of DNA template Genomic DNA of H pylori strain Y06 was extracted by the conventional phenol-chloroform method and DNase-free RNase treatment[52]. The obtained DNA was dissolved in TE buffer, and its concentration and purity were determined by ultraviolet spectrophotometry[52].
Polymerase chain reaction Oligonucleotide primers were designed to amplify the whole sequence of flaA and flaB genes from H pylori strain Y06 based on the published corresponding genomic sequences[53-56]. The sequence of flaA sense primer with an endonuclease site of EcoRV was 5’-CCGGATATCATGGCTTTTCAGGTCAA-3’. The sequence of flaA antisense primer with an endonuclease site of XhoI was 5’-CCGCTCGAGAAACTAAGTTAAAAGCC-3’. The sequence of flaB sense primer with an endonuclease site of EcoRI was 5’-CCGGAATTCATGAGTTTTAGGATAAA-3’. The sequence of flaB antisense primer with an endonuclease site of XhoI was 5’-CCGCTCGAGCTGTTATTGTAAAA GCC-3’. The total volume per PCR was 100 µL containing 2.5 mol·L-1 each dNTP, 500 nmol·L-1 each of the two primers, 15 mol·L-1 MgCl2, 3.0 U Pfu-Taq polymerase, 100 ng DNA template and 1 × PCR buffer (pH8.8). The parameters for PCR were at 94 °C for 5 min, × 1; at 94 °C for 30 s, at 52 °C for 30 s, at 72 °C for 90 s, × 10; at 94 °C for 30 s, at 52 °C for 30 s, at 72 °C for 100 s (10 s addition for the each of the following cycles), × 15; then at 72 °C for 10 min, × 1. The results of PCR were observed under UV light after electrophoresis in 15 g·L-1 agarose pre-stained with ethidium bromide. The expected sizes of target amplification fragments were 1530 bp for flaA gene and 1542 bp for flaB gene.
Cloning and sequencing The target amplification DNA fragments from flaA and flaB genes were respectively cloned into pUCm-T vectors (pUCm-T-flaA and pUCm-T-flaB) by using the T-A cloning kit according to the manufacturer’s instructions. The recombinant plasmids were amplified in an E. coli strain DH5α and then extracted by the Sambrook’s method[57]. A professional company (BBST) was responsible for nucleotide sequence analysis of the inserted fragments. Three strains of E. coli DH5α containing pUCm-T-flaA, pUCm-T-flaB and expression vector pET32a were amplified in BL medium, and the three plasmids were extracted, respectively[57]. These plasmids were digested with EcoRV and XhoI, EcoRI and XhoI, respectively. The flaA target fragment and pET32a, and the flaB fragment and pET32a were recovered and then ligased. The recombinant expression vectors pET32a-flaA and pET32a-flaB were respectively transformed into E.coli BL21DE3, and the expression systems were named as pET32a-flaA-BL21DE3 and pET32a-flaB-BL21DE3. The target fragments of flaA and flaB genes inserted in pET32a plasmid were sequenced again.
Expression and identification of fusion proteins pET32a-flaA-BL21DE3 and pET32a-flaB-BL21DE3 were rotatively cultured in LB medium at 37 °C induced by isopropylthio-β-D-galactoside (IPTG) at different concentrations of 1.0, 0.5 and 0.1 mmol·L-1. The supernatant and precipitate were separated through centrifugation after the bacterial pallet was ultrasonically broken (300 V, 5 s × 3). The molecular weight and output of rFlaA and rFlaB were examined by SDS-PAGE. The two recombinant proteins were collected by Ni-NTA affinity chromatography. The commercial rabbit antiserum against whole cell of H pylori and HRP-labeling sheep antiserum against rabbit IgG were used as the first and second antibodies to determine the immunoreactivity of rFlaA and rFlaB by Western blot. Rabbits were immunized with rFlaA and rFlaB, respectively, for preparation of antisera. Immunodiffusion assay was applied to the determination of the antigenicity of rFlaA and rFlaB.
ELISA The specific antibodies against FlaA and FlaB in sera of the 126 patients infected with H pylori were detected by ELISA, by using rFlaA and rFlaB as antigens at the coated concentration of 20 µg/mL and a patient serum sample (1:400 dilution) as the first antibody and HRP-labeling sheep antibody against human IgG (1:4000 dilution) as the second antibody. The result of ELISA for a patient’s serum sample was considered as positive if the optical density at 490 nm (OD490) was over the mean plus 3 SD of five negative serum samples[58]. FlaA and FlaB expression in clinical isolates of H pylori was detected by ELISA using the ultrasonic supernatant of each H pylori isolate (50 µg/mL) as a coated antigen, the self-prepared rabbit antisera against rFlaA and rFlaB (1:800 dilution in both) as the first antibody and HRP-labeling sheep antibody against rabbit IgG (1:3000 dilution) as the second antibody. The result of ELISA for a H pylori ultrasonic supernatant sample was considered as positive if its OD490 value was over the mean plus 3 SD of five ultrasonic supernatant samples at the same protein concentration of E. coli ATCC 25922[58].
Date analysis The nucleotide sequences of the cloned flaA and flaB genes were compared for homology with the 3 published flaA gene sequences (NC000915, NC000921, X60746)[53-55] and the 4 published flaB gene sequences (NC000915, NC000921, L08907, AF479024)[53,54,56,59] by using a molecular biological analysis software.
RESULTS
PCR
Target fragments of flaA and flaB genes with expected sizes amplified from DNA template of H pylori stain Y06 are shown in Figure 1.
Figure 1.
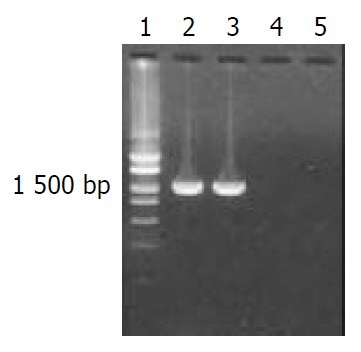
Target fragments of flaA and flaB genes amplified from H pylori strain Y06 DNA.
Nucleotide sequence analysis
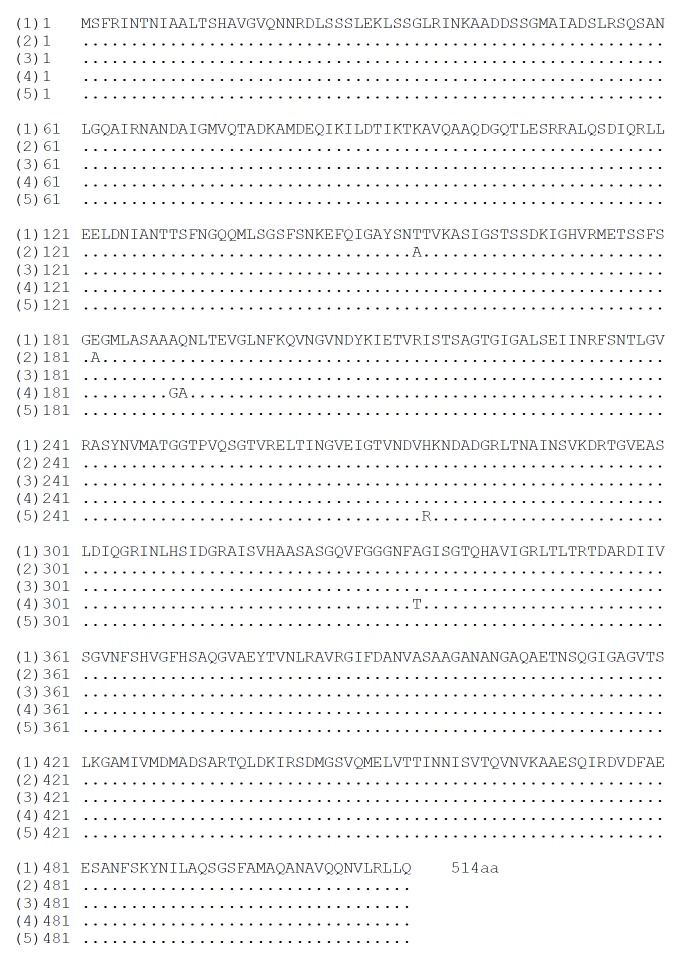
The nucleotide sequences of flaA gene in pUCm-T-flaA and pET32a-flaA were completely the same and so as for flaB gene. The homologies of nucleotide and putative amino acid sequences of the cloned flaA gene compared with the published flaA sequences[53-55] were from 96.28% to 97.13% and from 99.61% to 99.80%, respectively (Figure 2 and Figure 3). The homologies of nucleotide and putative amino acid sequences of the cloned flaB gene were 96.31%-97.73% and 99.41%-100%, compared with the published flaB sequences (Figure 4 and Figure 5)[53,54,56,59].
Figure 2.
Homologies of nucleotide sequence of cloned H pylori flaA gene with reported sequences. (1): the sequencing result of H pylori strain Y06 flaA gene; (2)-(4): the reported sequences from GenBank (No. NC000915, strain 26695; No. NC_000921, strain J99; No. X60746, strain 898-1). Underlined areas indicate the positions of primer sequences.
Figure 3.
Homologies of putative amino acid sequence of H pylori flaA gene with reported sequences. (1): the sequencing result of cloned H pylori strain Y06 flaA gene; (2)-(4): the reported sequences from GenBank (No. NC000915, strain 26695; No. NC_000921, strain J99; No. X60746, strain 898-1).
Figure 4.
Homologies of nucleotide sequence of cloned H pylori flaB gene with reported sequences. (1) the sequencing result of H pylori strain Y06 flaB gene; (2)-(5): the reported sequences from GenBank (No. NC000915, strain 26695; No. NC_000921, strain J99; No. L08907, strain 85P; No. AF479024, strain CH-CTX1). Underlined areas indicate the positions of primer sequences.
Figure 5.
Homologies of putative amino acid sequences of H pylori flaB gene with reported sequences. (1): the sequencing result of H pylori strain Y06 flaB gene; (2)-(5): the reported sequences from GenBank (No. NC000915, strain 26695; No. NC_000921, strain J99; No. L08907, strain 85P; No. AF479024, strain CH-CTX1).
Expression of target fusion proteins
IPTG at concentrations of 1.0, 0.5 and 0.1 mmol·L-1 efficiently induced the expression of rFlaA and rFlaB in pET32a-flaA-BL21DE3 and pET32a-flaB-BL21DE3 systems. The products of rFlaA and rFlaB were mainly presented in ultrasonic precipitates, and the output was 40%-50% of the total bacterial proteins (Figure 6 and Figure 7).
Figure 6.
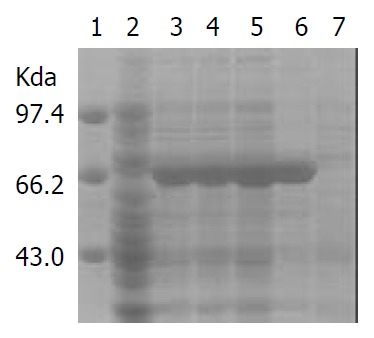
Expression of rFlaA induced by IPTG at different concentrations.
Figure 7.
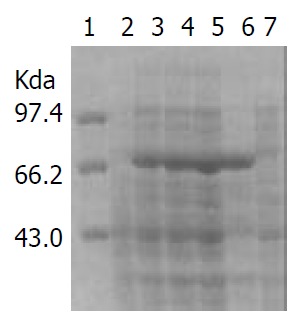
Expression of rFlaB induced by IPTG at different concentrations.
Immunoreactivity and antigenicity of rFlaA and rFlaB
The commercial rabbit antibodies against the whole cell of H pylori combined with rFlaA and rFlaB as confirmed by Western blot (Figure 8 and Figure 9). Both the titer of immunodiffusion assay between rFlaA and its rabbit antiserum, rFlaB and its rabbit antiserum was 1:2.
Figure 8.
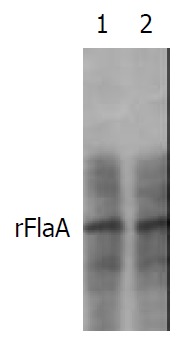
Western blot result of rabbit antibodies against whole cell of H pylori and rFlaA.
Figure 9.

Western blot result of rabbit antibodies against whole cell of H pylori and rFlaB.
ELISA
Since the mean ± SD of OD490 values of the five negative serum samples were 0.338 ± 0.036 for rFlaA and 0.102 ± 0.051 for rFlaB in the detection of specific antibodies in patients’ sera, the positive reference value was 0.446 for FlaA and 0.255 for FlaB. According to the reference values, 98.4% (123/125, one serum sample was contaminated) of the tested patients’ serum samples were positive for antibodies against rFlaA with an OD490 value range of 0.52-1.76, and 92.8% (116/125) were positive for antibodies against rFlaB with an OD490 value range of 0.26-1.50. Since the mean ± SD of OD490 of the five negative bacterial controls was 0.200 ± 0.046 for FlaA and 0.170 ± 0.044 for FlaB in the detection of clinical H pylori isolates, the positive reference value was 0.338 for FlaA and 0.302 for FlaB. According to the reference values, 100% (98/98) of the tested H pylori isolates were detectable for the epitope of rFlaA with an OD490 value range of 0.36-2.01 and 99% (97/98) of the isolates were detectable for the epitope of rFlaB with an OD490 value range of 0.31-1.78.
DISCUSSION
In the present study, H pylori flaA and flaB genes were detected in genomic DNA of almost all H pylori isolates, and their nucleotide and amino acid sequences were considerably conserved[53,54]. The FlaA and FlaB expressed by H pylori rendered the organism strong motility in mucous environment, induced IL-8 secretion and facilitated inflammation in gastric tissue[51,52]. Furthermore, we observed that serum antibodies against FlaA and FlaB were present in approximate 98.4% and 92.8% of H pylori infected patients, respectively, the rates were significantly higher than those of heat shock protein (68%) and vacuolating cytotoxin (68%)[60]. These data indicate that flaA and flaB genes express their products in majority of H pylori strains and efficiently induce specific antibodies, implying a brilliant potential for developing H pylori vaccine.
The flaA gene from H pylori strain Y06, cloned in this study, showed high homologies of nucleotide and putative amino acid sequences compared with the published corresponding sequences (Figure 2 and Figure 3)[53-55]. Similarly, the homologies of nucleotide and putative amino acid sequences of the cloned flaB gene from H pylori strain Y06 were quite high when compared with the published corresponding sequences (Figure 4 and Figure 5)[53,54,56-59]. The high conservation of nucleotide and putative amino acid sequences found in the cloned flaA and flaB genes were probably due to their expression products just as the structural peptides of H pylori.
In the present study, SDS-PAGE demonstrated that the constructed expression systems pET32a-flaA-BL21DE3 and pET32a-flaB-BL21DE3 were able to efficiently produce the target recombinant proteins. However, rFlaA and rFlaB were mainly presented with the form of inclusion body even if they were induced by IPTG at a lower concentration. The high output of rFlaA and rFlaB (40%-50%) was beneficial to the production of a possible H pylori vaccine.
The rabbit antiserum against the whole cell of H pylori recognizes and combined with rFlaA and rFlaB as confirmed by Western blot, indicated that the two recombinant proteins had a relatively high immunoreactivity. The immunodiffusion assay performed in this study demonstrated that rFlaA and rFlaB could efficiently induce rabbit to produce specific antibodies with a higher titer, which indicated that these two recombinant proteins exhibited favorable antigenicity.
All tested H pylori isolates (98/98) expressed FlaA while 99.0% (97/98) of the tested isolates expressed FlaB, as detected by ELISA. Of the H pylori infected patients, 98.4% (123/125) and 92.8% (116/125) were seropositive for the specific antibodies against rFlaA and against rFlaB, respectively. The universal existence of FlaA and FlaB in H pylori strains and the efficient induction of specific antibodies against FlaA and FlaB in patients were the strong favorable evidences for using these two recombinant proteins as the potential antigens in the development of H pylori vaccine.
In conclusion, FlaA and FlaB are excellent and ideal antigens that can be potentially used for the development of H pylori vaccine, and the expression systems of FlaA and FlaB with a high efficiency has been successfully constructed.
ACKNOWLEDGEMENTS
We are grateful to the four hospitals in Hangzhou that provided gastric biopsies in this study, which help us to complete the research subject.
Footnotes
Supported by the Excellent Young Teacher Fund of Chinese Education Ministry and the General Research Plan of the Science and Technology Department of Zhejiang Province, No. 001110438
Edited by Xia HHX and Wang XL
References
- 1.Ye GA, Zhang WD, Liu LM, Shi L, Xu ZM, Chen Y, Zhou DY. Helicobacter pylori vacA gene polymorphism and chronic gastrosis. Shijie Huaren Xiaohua Zazhi. 2001;9:593–595. [Google Scholar]
- 2.Lu SY, Pan XZ, Peng XW, Shi ZL. Effect of Hp infection on gas-tric epithelial cell kinetics in stomach diseases. Shijie Huaren Xiaohua Zazhi. 1999;7:760–762. [Google Scholar]
- 3.Zhang Z, Yuan Y, Gao H, Dong M, Wang L, Gong YH. Apoptosis, proliferation and p53 gene expression of H. pylori associated gastric epithelial lesions. World J Gastroenterol. 2001;7:779–782. doi: 10.3748/wjg.v7.i6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu XL, Qian KD, Tang XQ, Zhu YL, Du Q. Detection of H.pylori DNA in gastric epithelial cells by in situ hybridization. World J Gastroenterol. 2002;8:305–307. doi: 10.3748/wjg.v8.i2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao YL, Xu B, Song YG, Zhang WD. Overexpression of cyclin E in Mongolian gerbil with Helicobacter pylori-induced gastric precancerosis. World J Gastroenterol. 2002;8:60–63. doi: 10.3748/wjg.v8.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo DL, Dong M, Wang L, Sun LP, Yuan Y. Expression of gastric cancer-associated MG7 antigen in gastric cancer, precancerous lesions and H. pylori -associated gastric diseases. World J Gastroenterol. 2002;8:1009–1013. doi: 10.3748/wjg.v8.i6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng ZS, Liang ZC, Liu MC, Ouyang NT. Studies on gastric epi-thelial cell proliferation and apoptosis in Hp associated gastric ulcer. Shijie Huaren Xiaohua Zazhi. 1999;7:218–219. [Google Scholar]
- 8.Hiyama T, Haruma K, Kitadai Y, Miyamoto M, Tanaka S, Yoshihara M, Sumii K, Shimamoto F, Kajiyama G. B-cell monoclonality in Helicobacter pylori-associated chronic atrophic gastritis. Virchows Arch. 2001;438:232–237. doi: 10.1007/s004280000331. [DOI] [PubMed] [Google Scholar]
- 9.Harry XH. Association between Helicobacter pylori and gastric cancer: current knowledge and future research. World J Gastroenterol. 1998;4:93–96. doi: 10.3748/wjg.v4.i2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quan J, Fan XG. Progress in experimental research of Helicobacter pylori infection and gastric carcinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:1068–1069. [Google Scholar]
- 11.Liu HF, Liu WW, Fang DC. Study of the relationship between apoptosis and proliferation in gastric carcinoma and its precan-cerous lesion. Shijie Huaren Xiaohua Zazhi. 1999;7:649–651. [Google Scholar]
- 12.Zhu ZH, Xia ZS, He SG. The effects of ATRA and 5-Fu on telomerase activity and cell growth of gastric cancer cells in vitro. Shijie Huaren Xiaohua Zazhi. 2000;8:669–673. [Google Scholar]
- 13.Tu SP, Zhong J, Tan JH, Jiang XH, Qiao MM, Wu YX, Jiang SH. Induction of apoptosis by arsenic trioxide and hydroxy camptothecin in gastriccancer cells in vitro. World J Gastroenterol. 2000;6:532–539. doi: 10.3748/wjg.v6.i4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai L, Yu SZ, Zhang ZF. Helicobacter pylori infection and risk of gastric cancer in Changle County,Fujian Province,China. World J Gastroenterol. 2000;6:374–376. doi: 10.3748/wjg.v6.i3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao XX, Yin L, Zhang JY, Bai WY, Li YM, Sun ZC. Htert expres-sion and cellular immunity in gastric cancer and precancerosis. Shijie Huaren Xiaohua Zazhi. 2001;9:508–512. [Google Scholar]
- 16.Xu AG, Li SG, Liu JH, Gan AH. Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 2001;7:403–406. doi: 10.3748/wjg.v7.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Lan M, Shi YQ, Lu J, Zhong YX, Wu HP, Zai HH, Ding J, Wu KC, Pan BR, et al. Differential display of vincristine-resistance-related genes in gastric cancer SGC7901 cell. World J Gastroenterol. 2002;8:54–59. doi: 10.3748/wjg.v8.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu JR, Li BX, Chen BQ, Han XH, Xue YB, Yang YM, Zheng YM, Liu RH. Effect of cis-9, trans-11-conjugated linoleic acid on cell cycle of gastric adenocarcinoma cell line (SGC-7901) World J Gastroenterol. 2002;8:224–229. doi: 10.3748/wjg.v8.i2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai L, Yu SZ. A molecular epidemiologic study on gastric cancer in Changle, Fujian Province. Shijie Huaren Xiaohua Zazhi. 1999;7:652–655. [Google Scholar]
- 20.Gao GL, Yang Y, Yang SF, Ren CW. Relationship between pro-liferation of vascular andothelial cells and gastric cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:282–284. [Google Scholar]
- 21.Xue XC, Fang GE, Hua JD. Gastric cancer and apoptosis. Shijie Huaren Xiaohua Zazhi. 1999;7:359–361. [Google Scholar]
- 22.Niu WX, Qin XY, Liu H, Wang CP. Clinicopathological analysis of patients with gastric cancer in 1200 cases. World J Gastroenterol. 2001;7:281–284. doi: 10.3748/wjg.v7.i2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li XY, Wei PK. Diagnosis of stomach cancer by serum tumor markers. Shijie Huaren Xiaohua Zazhi. 2001;9:568–570. [Google Scholar]
- 24.Fang DC, Yang SM, Zhou XD, Wang DX, Luo YH. Telomere erosion is independent of microsatellite instability but related to loss of heterozygosity in gastric cancer. World J Gastroenterol. 2001;7:522–526. doi: 10.3748/wjg.v7.i4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgner A, Miehlke S, Stolte M, Neubauer A, Alpen B, Thiede C, Klann H, Hierlmeier FX, Ell C, Ehninger G, et al. Development of early gastric cancer 4 and 5 years after complete remission of Helicobacter pylori associated gastric low grade marginal zone B cell lymphoma of MALT type. World J Gastroenterol. 2001;7:248–253. doi: 10.3748/wjg.v7.i2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng DJ. progress of gastric cancer etiology: N-nitrosamides 1999s. World J Gastroenterol. 2000;6:613–618. doi: 10.3748/wjg.v6.i4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu ZM, Shou NH, Jiang XH. Expression of lung resistance protein in patients with gastric carcinoma and its clinical significance. World J Gastroenterol. 2000;6:433–434. doi: 10.3748/wjg.v6.i3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo CQ, Wang YP, Liu GY, Ma SW, Ding GY, Li JC. Study on Helicobacter pylori infection and p53, c-erbB-2 gene expression in carcinogenesis of gastric mucosa. Shijie Huaren Xiaohua Zazhi. 1999;7:313–315. [Google Scholar]
- 29.Cai L, Yu SZ, Ye WM, Yi YN. Fish sauce and gastric cancer: an ecological study in Fujian Province,China. World J Gastroenterol. 2000;6:671–675. doi: 10.3748/wjg.v6.i5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801–804. doi: 10.3748/wjg.v7.i6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang RT, Wang T, Chen K, Wang JY, Zhang JP, Lin SR, Zhu YM, Zhang WM, Cao YX, Zhu CW, et al. Helicobacter pylori infection and gastric cancer: evidence from a retrospective cohort study and nested case-control study in China. World J Gastroenterol. 2002;8:1103–1107. doi: 10.3748/wjg.v8.i6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hua JS. Effect of Hp: cell proliferation and apoptosis on stomach cancer. Shijie Huaren Xiaohua Zazhi. 1999;7:647–648. [Google Scholar]
- 33.Liu DH, Zhang XY, Fan DM, Huang YX, Zhang JS, Huang WQ, Zhang YQ, Huang QS, Ma WY, Chai YB, et al. Expression of vascular endothelial growth factor and its role in oncogenesis of human gastric carcinoma. World J Gastroenterol. 2001;7:500–505. doi: 10.3748/wjg.v7.i4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao WX, Ou JM, Fei XF, Zhu ZG, Yin HR, Yan M, Lin YZ. Methionine-dependence and combination chemotherapy on human gastric cancer cells in vitro. World J Gastroenterol. 2002;8:230–232. doi: 10.3748/wjg.v8.i2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michetti P, Kreiss C, Kotloff KL, Porta N, Blanco JL, Bachmann D, Herranz M, Saldinger PF, Corthésy-Theulaz I, Losonsky G, et al. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology. 1999;116:804–812. doi: 10.1016/s0016-5085(99)70063-6. [DOI] [PubMed] [Google Scholar]
- 36.Suganuma M, Kurusu M, Okabe S, Sueoka N, Yoshida M, Wakatsuki Y, Fujiki H. Helicobacter pylori membrane protein 1: a new carcinogenic factor of Helicobacter pylori. Cancer Res. 2001;61:6356–6359. [PubMed] [Google Scholar]
- 37.Nakamura S, Matsumoto T, Suekane H, Takeshita M, Hizawa K, Kawasaki M, Yao T, Tsuneyoshi M, Iida M, Fujishima M. Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori. Gut. 2001;48:454–460. doi: 10.1136/gut.48.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 39.Morgner A, Miehlke S, Fischbach W, Schmitt W, Müller-Hermelink H, Greiner A, Thiede C, Schetelig J, Neubauer A, Stolte M, et al. Complete remission of primary high-grade B-cell gastric lymphoma after cure of Helicobacter pylori infection. J Clin Oncol. 2001;19:2041–2048. doi: 10.1200/JCO.2001.19.7.2041. [DOI] [PubMed] [Google Scholar]
- 40.Kate V, Ananthakrishnan N, Badrinath S. Effect of Helicobacter pylori eradication on the ulcer recurrence rate after simple closure of perforated duodenal ulcer: retrospective and prospective randomized controlled studies. Br J Surg. 2001;88:1054–1058. doi: 10.1046/j.0007-1323.2001.01831.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhuang XQ, Lin SR. Progress in research on the relationship be-tween Hp and stomach cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:206–207. [Google Scholar]
- 42.Gao HJ, Yu LZ, Bai JF, Peng YS, Sun G, Zhao HL, Miu K, Lü XZ, Zhang XY, Zhao ZQ. Multiple genetic alterations and behavior of cellular biology in gastric cancer and other gastric mucosal lesions: H pylori infection, histological types and staging. World J Gastroenterol. 2000;6:848–854. doi: 10.3748/wjg.v6.i6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao YL, Zhang WD. Relation between Helicobacter pylori and gas-tric cancer. Shijie Huaren Xiaohua Zazhi. 2001;9:1045–1049. [Google Scholar]
- 44.Goto T, Nishizono A, Fujioka T, Ikewaki J, Mifune K, Nasu M. Local secretory immunoglobulin A and postimmunization gastritis correlated with protection against Helicobacter pylori infection after oral vaccination of mice. Infect Immun. 1999;67:2531–2539. doi: 10.1128/iai.67.5.2531-2539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 46.Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255–4259. [PubMed] [Google Scholar]
- 47.Hatzifoti C, Wren BW, Morrow WJ. Helicobacter pylori vaccine strategies--triggering a gut reaction. Immunol Today. 2000;21:615–619. doi: 10.1016/s0167-5699(00)01753-9. [DOI] [PubMed] [Google Scholar]
- 48.Kotloff KL, Sztein MB, Wasserman SS, Losonsky GA, DiLorenzo SC, Walker RI. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect Immun. 2001;69:3581–3590. doi: 10.1128/IAI.69.6.3581-3590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubois A, Lee CK, Fiala N, Kleanthous H, Mehlman PT, Monath T. Immunization against natural Helicobacter pylori infection in nonhuman primates. Infect Immun. 1998;66:4340–4346. doi: 10.1128/iai.66.9.4340-4346.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikewaki J, Nishizono A, Goto T, Fujioka T, Mifune K. Therapeutic oral vaccination induces mucosal immune response sufficient to eliminate long-term Helicobacter pylori infection. Microbiol Immunol. 2000;44:29–39. doi: 10.1111/j.1348-0421.2000.tb01243.x. [DOI] [PubMed] [Google Scholar]
- 51.Eaton KA, Suerbaum S, Josenhans C, Krakowka S. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect Immun. 1996;64:2445–2448. doi: 10.1128/iai.64.7.2445-2448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe S, Takagi A, Tada U, Kabir AM, Koga Y, Kamiya S, Osaki T, Miwa T. Cytotoxicity and motility of Helicobacter pylori. J Clin Gastroenterol. 1997;25 Suppl 1:S169–S171. doi: 10.1097/00004836-199700001-00027. [DOI] [PubMed] [Google Scholar]
- 53.Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 54.Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 55.Leying H, Suerbaum S, Geis G, Haas R. Cloning and genetic characterization of a Helicobacter pylori flagellin gene. Mol Microbiol. 1992;6:2863–2874. doi: 10.1111/j.1365-2958.1992.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 56.Suerbaum S, Josenhans C, Labigne A. Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J Bacteriol. 1993;175:3278–3288. doi: 10.1128/jb.175.11.3278-3288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning, A Labo-ratory Manual [M]. 2nd edition. New York: Cold Spring Harbor Laboratory Press; 1989. pp. 1.21–1.52, 2.60-2.80, 7.3-7.35, 9.14-9.22. [Google Scholar]
- 58.Chen Y, Wang J, Shi L. [In vitro study of the biological activities and immunogenicity of recombinant adhesin of Heliobacter pylori rHpaA] Zhonghua Yixue Zazhi. 2001;81:276–279. [PubMed] [Google Scholar]
- 59.Josenhans C, Labigne A, Suerbaum S. Comparative ultrastructural and functional studies of Helicobacter pylori and Helicobacter mustelae flagellin mutants: both flagellin subunits, FlaA and FlaB, are necessary for full motility in Helicobacter species. J Bacteriol. 1995;177:3010–3020. doi: 10.1128/jb.177.11.3010-3020.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Opazo P, Muller I, Rollan A, Valenzuela P, Yudelevich A, Garcia-de la Guarda R, Urra S, Venegas A. Serological response to Helicobacter pylori recombinant antigens in Chilean infected patients with duodenal ulcer, non-ulcer dyspepsia and gastric cancer. APMIS. 1999;107:1069–1078. [PubMed] [Google Scholar]