Abstract
AIM: Psychological factors, altered motility and sensation disorders of the intestine can be variably associated with irritable bowel syndrome (IBS). Such aspects have not been investigated simultaneously. The aim of this paper was to evaluate gastrointestinal motility and symptoms, psychological spectrum and quality of life in a large group of IBS patients in southern Italy.
METHODS: One hundred IBS patients (F:M = 73:27, age 48 ± 2 years, mean ± SE) fulfilling ROME II criteria matched with 100 healthy subjects (F:M = 70:30, 45 ± 2 years). Dyspepsia, bowel habit, alexithymia, psycho-affective profile and quality of life were assessed using specific questionnaires. Basally and postprandially, changes in gallbladder volumes and antral areas after liquid meal and orocaecal transit time (OCTT) were measured respectively by ultrasonography and H2-breath test. Appetite, satiety, fullness, nausea, and epigastric pain/discomfort were monitored using visual-analogue scales.
RESULTS: Compared with controls, IBS patients had increased dyspepsia (score 12.6 ± 0.7 vs 5.1 ± 0.2, P < 0.0001), weekly bowel movements (12.3 ± 0.4 vs 5.5 ± 0.2, P < 0.00001, comparable stool shape), alexithymia (score 59.1 ± 1.1 vs 40.5 ± 1.0, P = 0.001), poor quality of life and psycho-affective profile. IBS patients had normal gallbladder emptying, but delayed gastric emptying (T50: 35.5 ± 1.0 vs 26.1 ± 0.6 min, P = 0.00001) and OCTT (163.0 ± 5.4 vs 96.6 ± 1.8 min, P = 0.00001). Fullness, nausea, and epigastric pain/discomfort were greater in IBS than in controls.
CONCLUSION: ROME II IBS patients have a pan-enteric dysmotility with frequent dyspepsia, associated with psychological morbidity and greatly impaired quality of life. The presence of alexithymia, a stable trait, is a novel finding of potential interest to detect subgroups of IBS patients with different patterns recoveed after therapy.
INTRODUCTION
Irritable bowel syndrome (IBS) is a common, chronic biopsychological functional disorder of unknown aetiology, affecting 10%-20% of all individuals any one time. Psychological factors, altered motility and sensation disorders of the intestine can be variably associated[1]. Dysfunctions of the gastrointestinal tract in IBS appear as altered bowel function[2], associated with pain or discomfort, without organic disease[3-5]. Besides lowered visceral perception thresholds involving the rectum[6] and more proximal districts[7], motility defects seem to develop. Thus, IBS patients may complain of a constellation of both gastrointestinal and extra-intestinal symptoms[7], and a significant proportion of patients may have disordered perception[8,9] resulting in more readily feeling of normal intestinal contractions[10]. Psychological factors, such as abnormal illness attitute[11], may also play a role in the pathogenesis of IBS[5], with a significant impact on health-related quality of life (HRQOL)[12]. More recently, revised diagnostic criteria for IBS have been proposed (ROME II criteria)[4]. Moreover, sophisticated questionnaires are now available for studying both quality of life and specific psychological aspects like the alexithymia construct. The latter includes difficulties in identifying and describing feelings, impoverishment of fantasy life and excessive preoccupation with physical symptoms and external events[13].
Such aspects have not been investigated simultaneously in IBS patients. Therefore, the aim of the present study was to evaluate gastrointestinal motility and symptoms, psychological status, including alexithymia and HRQOL in a large group of IBS patients from a referral center in southern Italy.
MATERIALS AND METHODS
Subjects
A total number of 200 adult subjects were studied prospectively and divided into 2 cohorts: 100 consecutive patients with IBS (27 males and 73 females) and 100 healthy subjects (30 males and 70 females) with their age and body size matched. The two groups were also comparable for years of education (9.3 ± 1.5 years and 9.6 ± 1.3 years in healthy and IBS, respectively). The characteristics of patients are depicted in Table 1. There was no difference in sex ratio, age and body size. The percentage of non-coffee drinkers and smokers was greater in IBS than in healthy subjects (0.02 < P < 0.03). The IBS group was composed of out-patients, with a firm diagnosis through positive identification of the ROME II diagnostic criteria[4]. With the inclusion criteria used in this study, the specificity of IBS diagnosis was about 98%[14,15]. Mean years since first diagnosis have been 5.9 years with bowel habit alternating between diarrhea and constipation, according to standardized criteria[4]. During the above mentioned period, patients had tried several -albeit poorly effective- therapeutic trials with the help of their family practices. All patients were symptomatic at the time of the study, and lower abdominal discomfort or pain was the main reason for seeking medical advice, and none was on specific therapy for IBS at the time of evaluation. There was no coexistent disease and all patients had normal haematology, biochemistry, urinalysis, together with a normal colonoscopy or barium enema if aged over 50 years. Excluded were subjects if they had a history of gastrointestinal surgery. Female subjects were excluded if they were pregnant, breast feeding, or hysterectomised and were studied during the first phase of the menstrual cycle or while taking oestrogen/progesterone contraceptive medication. Drugs and cigarette smoking were not allowed for 48 hours prior to the study while alcohol and caffeine containing drinks were stopped 24 hours prior to the study. All subjects smoked less than 5 cigarettes per day and drank below the recommended safe alcohol limit (that is less than 21 units/week).
Table 1.
Patient characteristics in study group (mean ± SE)
| IBS | Healthy | P* | |
| No of subjects | 100 | 100 | |
| Female: Male ratio | 73:27 | 70:30 | NS |
| Age (yr) | 48 ± 2 | 45 ± 2 | NS |
| BMI (Kg/m2) | 23.5 ± 0.4 | 22.9 ± 0.3 | NS |
| Coffee-drinkers No (%) | |||
| None | 43 (43%) | 24 (24%) | 0.03° |
| < 1 per day | 9 (9%) | 22 (22%)° | |
| 1-5 per day | 48 (48%) | 54 (54%)° | |
| Smokers No (%) | 30 (30%) | 15 (15%) | 0.02° |
| Lower abdominal | 5.8 ± 0.1 | 0.04 ± 0.01 | 0.0001 |
| pain (VAS, cm) | |||
| Abdominal bloating | 6.2 ± 0.1 | 0.1 ± 0.02 | 0.0001 |
| (VAS, cm) |
*Student’s t test or χ2 for comparison of proportions, VAS = visual analogue scale.
Because lactose maldigestion-intolerance and IBS may have almost identical symptoms[16], all patients were screened by lactose H2-breath test, which was invariably negative. Healthy volunteers were recruited from staff members and with the help of local family practices. Laboratory investigations were normal (as above) in all healthy subjects and there was a negative toxicology for substances of abuse. All subjects gave informed consent to join the study which was approved by the Ethics Committee of Bari University Hospital.
Measures
Symptomatology was assessed by focusing on an estimate of the maximum degree of lower abdominal pain or discomfort, and abdominal bloating in the previous 12 months was assessed by visual analogue scales (VAS)[17]. The weekly frequency of bowel movements over a time span of one month was estimated by a self-assessed diary. A specific stool form scale was also used based on a semi-quantitative score[18]. To carefully characterise the association between IBS and functional dyspepsia[19], the presence and severity of dyspeptic symptoms were quantified in two ways: validated semiquantitative score[20]; and self-assessed VAS of upper gastrointestinal perception monitoring appetite, satiety, nausea, abdominal fullness and upper abdominal (epigastric) pain or discomfort[21]. In the latter case, scores were obtained at baseline (i.e. time 0) and at 15, 30, 45, 60, 90 and 120 min postprandially. The Middlesex Hospital Questionnaire (MHQ) symptom check list was used to investigate six symptoms: anxiety, phobic behavior, obsessive-compulsive behavior, somatization, depression, and hysteria[22]. Traits of alexithymia were assessed by the Toronto Alexithymia scale based on a 20-item scale (TAS-20)[23] with the validated Italian translation[24,25]. A final score ≥ 61 was considered positive for alexithymia, while a score ranging from 50 to 60 was “border line”[25]. The short form check list (SF-36) was used to assess HRQOL in the following 8 domains: general health, physical functioning, role-physical, role-emotion, social functioning, mental health, body pain, and vitality[26,27].
Motility studies
After an overnight fast, subjects attended the Functional Investigations Unit. Standard criteria recently reviewed by our group[28] were used to study gallbladder and gastric emptying by functional ultrasonography in response to a liquid test meal, consisting of 200 mL solution totalling 13 g (39%) fat, 10 g (13%) protein and 35 g (48%) carbohydrates, calorie content 1270 kJ, 365 mmol/L (Nutridrink®, Nutricia, Milano, Italy), which was consumed within 2 min. Gallbladder emptying was assessed by monitoring fasting and postmeal course of gallbladder volumes. Sagittal and transverse scans of the gallbladder at its largest dimension were obtained at 5-15 minute intervals over 2 hours. Gastric emptying was assessed by monitoring fasting and postmeal course of antral areas[17,29]. Oro-caecal transit time was measured simultaneously with ultrasonographic studies by hydrogen breath test using 10 g lactulose and collecting the breath with a portable equipment (EC60-Gastrolyzer, Bedfont, USA)[30].
Statistical analysis
All calculations were performed with the NCSS 2001 statistical software (Kaysville, UT, USA, see http://www.ncss.com/). Results were given as mean ± standard error (SE). Differences in emptying curves were evaluated by two way ANOVA repeated-measures followed by Fisher’s LSD multiple comparison test. Differences of means between healthy and IBS were evaluated using the Student’s t test for unpaired data. Linear regression analysis was performed by the method of least square. The chi-square test was used to assess associations between categorical data. A two-tailed probability (P) value of less than 0.05 was considered statistically significant[31,32].
RESULTS
All subjects tolerated the tests well. Table 1 also shows that VAS scores for lower abdominal pain and bloating were significantly higher in IBS patients than in healthy subjects. Whereas the frequency of bowel movements was greater in IBS patients than in healthy subjects (12.3 ± 0.4 vs 5.5 ± 0.2 evacuations/wk, P = 0.00001) (Figure 1A), stool form was similar (Buckley score: 3.9 ± 0.1 vs 3.5 ± 0.1 in patients and controls, respectively), (Figure 1B).
Figure 1.
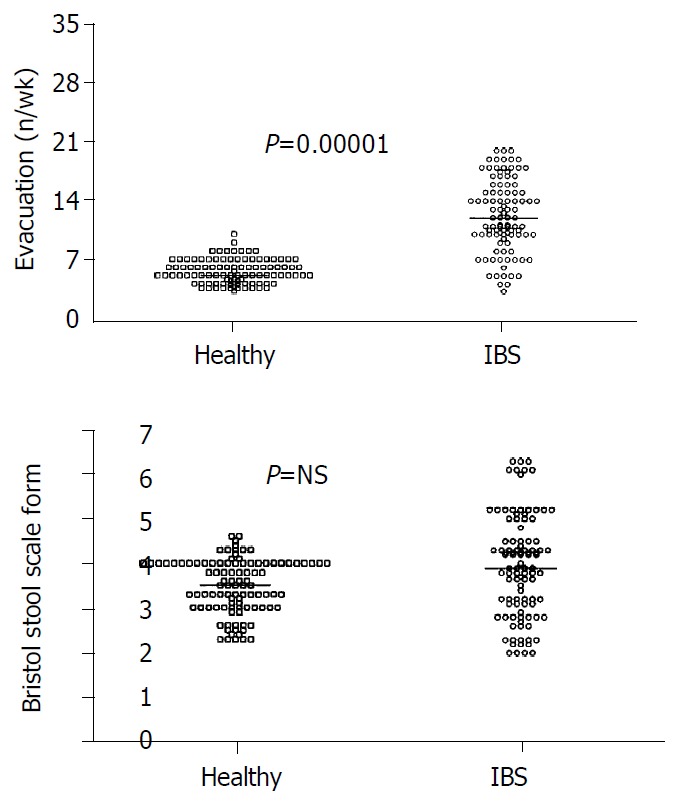
A: Significantly more bowel movements in IBS pa-tients than in healthy subjects. B: Comparable score for stool form between IBS patients and healthy subjects. Results are expressed as individual data and median (horizontal line).
Mean score of dyspepsia was greater in patients than in healthy subjects (12.6 ± 0.7 vs 5.1 ± 0.2, P < 0.0001), an abnormal score was found in 75% and 5% of patients and healthy subjects, respectively (P < 0.0001, Figure 2). As expected, the perception of satiety and appetite (both at fasting and as postprandial AUC) showed a strong negative correlation in IBS patients and healthy subjects (0.91 < r < 0.92, P < 0.0001). There was no difference in appetite and satiety between IBS patients and healthy subjects (either at baseline and postprandially), By contrast, the AUC during 120 min was invariably greater in IBS than in control subjects for nausea (58.0 ± 14.1 vs 3.9 ± 1.0, P = 0.002), fullness (133.5 ± 26.1 vs 17.1 ± 1.9, P = 0.00002) and pain/discomfort (75.1 ± 24.2 vs 2.5 ± 0.6, P = 0.002). Time-dependent profiles for these scores are depicted in Figure 3.
Figure 2.
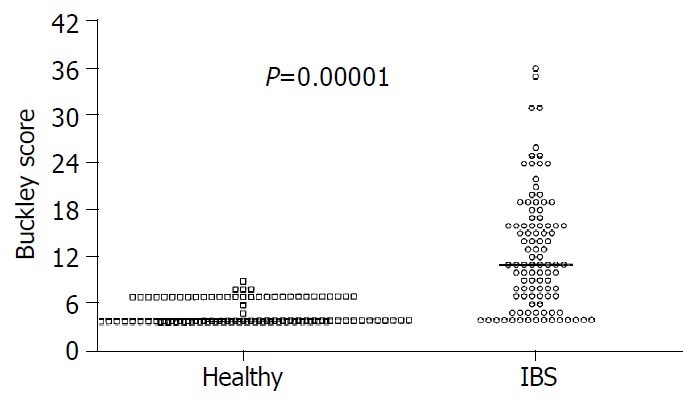
Significant dyspepsia in IBS patients compared with healthy subjects. Results are expressed as individual data and median.
Figure 3.
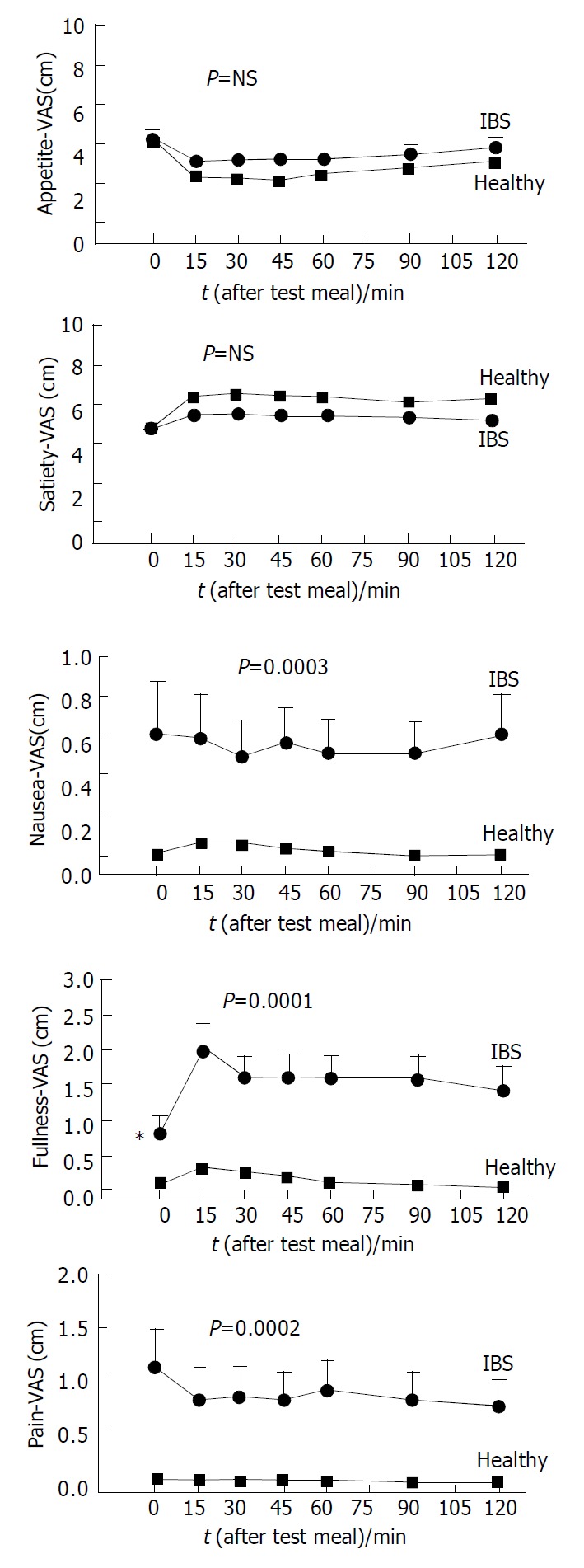
Time-course of visual analogue scale (VAS) for appetite, satiety, nausea, abdominal fullness and epigastric pain (or discomfort) in healthy subjects. On the X-axis time “0” is before ingestion of test meal. Asterisks indicate significant differences (0.0001 < P < 0.001) of IBS patients vs healthy subjects at baseline. Significant differences for nausea, fullness and epi-gastric pain/discomfort of IBS patients vs healthy subjects (area under curve).
Results from the MHQ questionnaire are given in Figure 4A which shows that IBS patients had significantly higher scores for anxiety, somatization, phobia and depression than healthy subjects. Results from the TAS 20 questionnaire on alexithymia are given in Figure 4B which shows a significantly increased score in IBS patients (59.1 ± 1.1) compared to healthy subjects (40.5 ± 1.0). An abnormal (i.e. > 61) score for alexithymia was found in 43% of patients and in 2% of healthy subjects. Mean scores of SF-36 are reported in Figure 5 which shows that HRQOL was significantly poorer for all domains in IBS patients compared with healthy subjects. There was no difference between IBS patients and healthy subjects with respect to gallbladder motility, i.e. fasting and postprandial volumes and half-emptying times (Table 2). Concerning gastric motility, the mean cross-sectional antral areas at fasting and immediately after the test meal were comparable between IBS patients and healthy subjects (Table 2). However, postprandial minimal antral areas were significantly larger and half-emptying time longer in IBS than in healthy subjects (Table 2, Figure 6). With respect to OCTT, basal levels of H2 were invariably < 10 p.p.m. in all subjects (i.e. absence of bacterial overgrowth) and did not differ between IBS patients and healthy subjects. OCTT, however, was increased by 62% in IBS patients compared to healthy subjects (Table 2) with scattered distribution depicted in Figure 7. A value of OCTT above 130 min, representing the upper limit of normal (mean + 2SDs), was found in 70% of patients and in 2% of healthy subjects (P = 0.000001).
Figure 4.
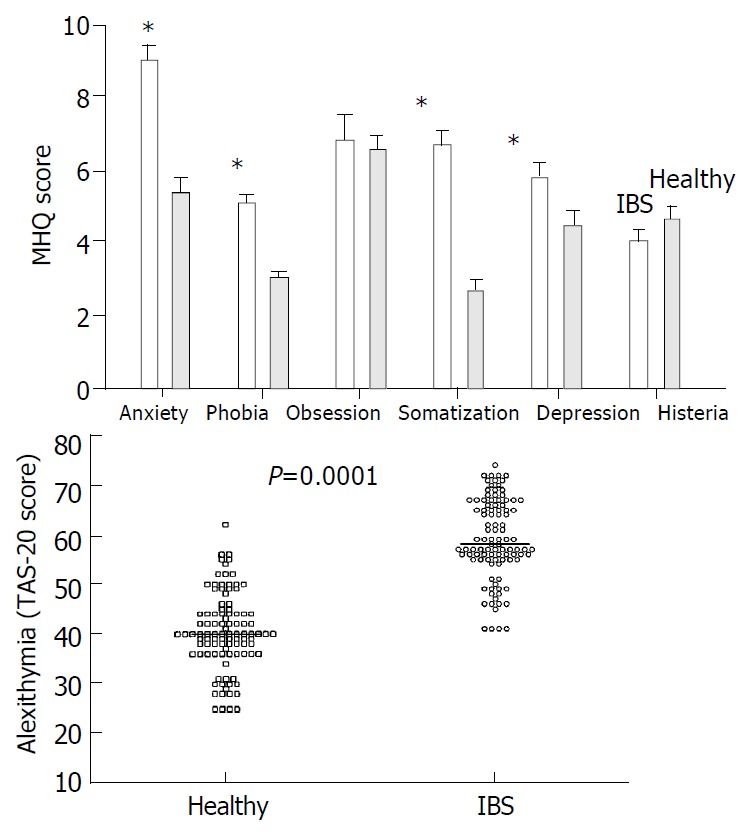
A: Scores of psychological disorders (mean ± SE, *0.009 < p < 0.04). B: Scores of alexithymia (individual data and median) in IBS patients and healthy subjects.
Figure 5.
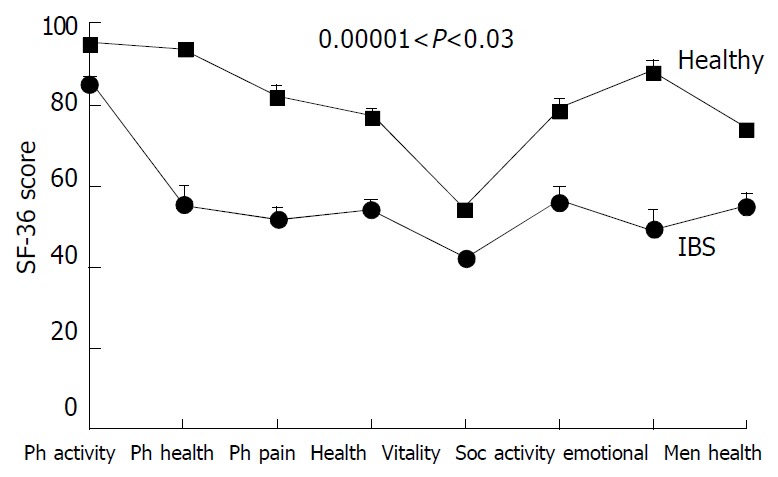
Profiles of health-related quality of life-HRQOL (SF-36 questionnaire) for IBS patients and healthy subjects. Data are mean ± SE. Significantly poorer HRQOL for all 8 scales in IBS patients compared with healthy subjects. Legend: Ph, physical; soc, social.
Table 2.
Motility indices in study group (mean ± SE)
| IBS | Healthy | P* | |
| No of subjects | 100 | 100 | |
| Gallbladder (volume) | |||
| Fasting (mL) | 21.4 ± 1.0 | 22.0 ± 0.9 | NS |
| Postprandial residual,mL (%) | 5.3 ± 0.6 | 5.7 ± 0.3 | NS |
| (23.4 ± 1.2) | (25.7 ± 0.9) | ||
| T50 (min.) | 20.1 ± 0.9 | 21.4 ± 0.6 | NS |
| Stomach (antral area) | |||
| Fasting (cm2) | 3.1 ± 0.1 | 3.4 ± 0.1 | NS |
| Postprandial maximal (cm2) | 10.4 ± 0.2 | 11.8 ± 0.2 | 0.001 |
| Postprandial minimal (%) | 6.1 ± 1.0 | 2.8 ± 0.5 | 0.02 |
| T50 (min.) | 35.5 ± 1.0 | 26.1 ± 0.6 | 0.00001 |
| Small bowel | |||
| Orocaecal transit time (min.) | 161.9 ± 5.5 | 96.6 ± 1.8 | 0.00001 |
*Student’s t test, OCTT = orocaecal transit time, NS = not significant. Indices of gallbladder motility: fasting volume (mean of 3 measurements at -15, -5 and 0 min before test meal, ex-pressed in mL), residual volume (minimum volume measured postprandially, in mL) and half-emptying time (T50, time to achieve 50% decrease of fasting volume). Indices of stomach emptying: fasting antral area (mean of 3 measurements at -15, -5 and 0 min before test meal, expressed in cm2), maximal antral area at 2 min postmeal in cm2, minimal postprandial antral area during the 2-hour observation period (expressed in%, normalized to maximal area after subtracting basal areas: i.e. 100 × (At-a)/(A2-a), where At = postprandial area at any given time; a = basal area; A2 = area at 2 min postprandially[37]) and half-emptying time (T50).
Figure 6.
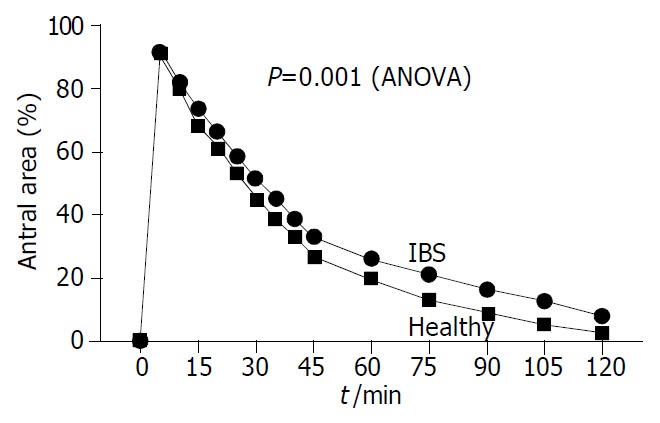
Time-course of gastric emptying in IBS patients and healthy subjects after ingestion of 200 mL of standard liquid meal. Symbols indicate mean (SE is very small and not visible at each time-point). IBS patients had impaired emptying with larger postprandial antral areas (expressed as% of basal area) than healthy subjects. See also Table 2 for half-emptying time differences.
Figure 7.
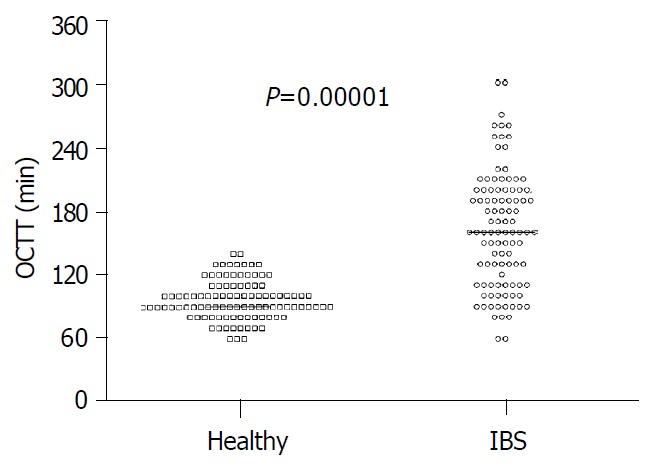
Significant delay of orocaecal transit time (OCTT) in IBS patients compared with healthy subjects. Results are ex-pressed as individual data and median.
Overall, the score of dyspepsia was positively correlated with OCTT (r = 0.38, P = 0.0001) but not with gastric emptying speed. Also, the dyspepsia score was negatively correlated with all domains of HRQOL (-0.34 < r < -0.43, P < 0.001), while there was a positive correlation with somatization (r = 0.32, P = 0.001), anxiety (r = 0.29, P = 0.002) and alexithymia (r = 0.47, P = 0.00001). Frequency of bowel movements increased with anxiety (r = 0.39, P = 0.00001), somatization (r = 0.34, P = 0.0004) and alexithymia (r = 0.52, P = 0.000001).
DISCUSSION
We used an integrated approach in a large group of IBS patients to investigate gastrointestinal motility patterns in relation to symptoms, quality of life and psychological comorbidity. We believe that the present study may offer a fairly representative picture of IBS characteristics in southern Italy. The ROME II criteria[4] were chosen because they perform well in the clinic and they have greater simplicity than other less recent criteria[1]. Due to the setting where the study was performed (i.e. a tertiary referral center), caution must be expressed in interpreting our results, for at least two reasons. Firstly, only a minority of IBS patients were thought to consult physicians[33] and secondly, characteristics of patients seen in a third referral center miqht differ from those of patients referred to primary or secondary care[25,34]. Nevertheless, IBS patients had multiple and simultaneous gastrointestinal motility defects involving stomach and intestine at a different extent, and were associated with d iffuse gastrointestinal symptoms, ab normal psychological status, including alexythimia, and poor quality of life.
Ultrasonography was chosen as a non-invasive and validated technique to assess both gallbladder[35,36] and gastric emptying[17,29,37-40] simultaneously[28]. The protocol we employed, moreover, was further informative due to the simultaneous assessment of small bowel transit by H2-breath test. Such a novel combined procedure allows a one-day (and time-saving) test for studying upper gastrointestinal motility in a clinical setting.
Despite “normal” feeling of appetite and satiety, IBS patients had strikingly abnormal upper gastrointestinal perception for nausea, fullness, epigastric pain/discomfort, both at fasting and postprandially. This was also the case in gastrectomized patients[21], Thus, ingestion of 200 mL of a moderately caloric, isosmotic test meal might prove useful in identifying groups of patients with dyspepsia, as also proposed in different clinical settings[41]. About 30% of IBS patients included in this study had delayed gastric emptying, as was found in other gastrointestinal functional disorders such as dyspepsia and slow transit constipation[30,42]. Although this study was primarily focusing on patients with lower abdominal symptoms for IBS, we found that the score for dyspepsia was abnormal in about two-thirds of patients, as was also reported by Agreus et al[43] in the Swedish population. Taken together, these findings suggest that dyspepsia and IBS are closely related and develop as a continuum[19]. No close correlation existed between delayed gastric emptying and dyspepsia. Indeed, gastric emptying was defective only in a subgroup of dyspeptic patients and this also seemed to be the case in IBS patients. Moreover, both symptoms or gastric half emptying times were poor predictors of gastrointestinal dysmotility in functional dyspepsia[44]. Also, dyspeptic symptoms might originate from an altered fundic receptive relaxation (not measured in the present study) and/or from disorders of other organs, including duodenum under acidic stress[45].
At variance with an early study in a scant number of IBS patients[46], we found no gross evidence for impaired gallbladder motility. Differences in selection criteria might partly explain the variability. CCK played a key role in postprandial gallbladder contraction, and abnormal sensitivity of the gallbladder smooth muscle to exogenous CCK has been reported in IBS[47]. Apparently, the defect was absent postprandially, since either a high-fat[47] or a low-fat (this study) liquid meal yielded similar gallbladder contractions. Whether or not the trend we showed of faster refilling in IBS points to an abnormality of the smooth muscle in the digestive tract[47], remains to be established. Impaired gallbladder motility has been found in a subgroup of patients with functional dyspepsia[42] and delayed transit constipation[30]. Disturbed motilin and CCK release might be a potential cause of intestinal dysmotility in IBS[48].
This study also showed that OCTT was delayed in IBS. Changes in phase II and phase III components of the migrating motor complex suggested that both local (i.e. enteric) and central mechanisms might operate to produce intestinal dysmotility[49]. Delayed OCTT could be independent of colonic transit (as seen in patients with functional dyspepsia[42]) or might be associated with delayed colonic transit (as seen in patients with functional constipation[30]). Although accelerated small bowel and colonic transit have been reported in diarrhea-predominant IBS[50], a similar conclusion could not be drawn from this study, since patients alternated between diarrhea and constipation in their history. IBS patients had rather increased bowel habits, despite delayed OCTT. This finding pointed to a diffuse impairment of visceral sensitivity and perception, involving not only the rectum[6], but also the colon or even more proximal districts such as small bowel and stomach[7,51]. Abnormal motor patterns in the small bowel might be associated with symptoms in patients with IBS (e.g. clustered jejunal contractions and propagated giant ileal contractions during abdominal colic[52]). It must be stressed, however, that no motor abnormality in either small or large intestine was pathognomonic for IBS[53]. Whether or not delayed OCTT in IBS could contribute to accumulation of gas in the intestine[54,55] and/or abnormal colonic fermentation in the colon[56], remains to be determined. Interestingly, we found delayed OCTT with increased bowel movements in chronic alcoholic patients during abstinence, associated with dysfunction of autonomic nervous system[57]. This possibility deserves further attention, since a form of subclinical autonomic neuropathy might predispose to a diffuse disorder of smooth muscle,which was suggestive for the multi-organic involvement of the gastrointestinal tract[7].
It is known that visceral hyperalgesia in IBS could exacerbate symptoms due to lactose maldigestion[58]. A recent study found that 24% of previously diagnosed IBS patients had lactose intolerance[16], this was not the case in the present study where lactose maldigestion or intolerance was an exclusion criterion. It is still controversial whether or not a subset of patients with IBS might be positive for coeliac disease[59,60]. Since the recent British Society of Gastroenterology guidelines estimated that routine antiendomysial antibodies would reveal only 1%-2% of abnormalities[3], systematic screening for coeliac disease in this study was not believed to be cost-effective and was not performed.
It is believed that up to 60% of IBS patients seen at referral centers might have psychological morbidity[5,61-64]. An abnormal psychological profile in IBS patients emerged also from the present study. Psychological disturbances could influence aspects of bowel habit[1,65]. We found increased bowel movements to be associated with anxiety, somatisation and also with alexithymia. Alexithymia construct is one of the four syndromes in the Diagnostic Criteria for Psychosomatic Research[34]. A stronger positive association was reported between alexithymia and somatoform rather than chronic somatic disorders[25,66,67]. In the present study we employed TAS-20, the most validated questionnaire available so far[23] and found significant alexithymia in over 40% of IBS patients (increasing to about 80% if patients with border-line scores were included). These results are in line with those from another although smaller study conducted in a closed geographical area, investigating alexithymia in patients with IBS and other functional gastrointestinal disorders[25]. The finding may have at least two practical implications. Firstly, whether IBS patients are first seen in a gastroenterological or psychiatric setting might determine if patients are classified as suffering mainly from IBS or somatoform disturbances, respectively. Secondly, as alexithymia is seen as a stable feature, it might act as an important prognostic factor related to treatment outcomes in subgroups of IBS patients. If the IBS patients were seen within the broader spectrum of functional gastrointestinal diseases, our findings pointed to an association between alexithymia and tendency to negative affectivity[25,67].
IBS patients showed a significantly poorer HRQOL than healthy subjects. This finding was in accordance with previous studies[12,61] and pointed to related problems, namely absenteeism, the social indirect costs and, ultimately, the need for appropriate treatment of IBS patients. Interestingly, both IBS patients and healthy subjects in this setting had a HRQOL profile remarkably similar to that derived from subjects across different cultures in the USA and UK[12]. This finding underscored the internal consistency of HRQOL questionnaires. Taken together, our findings confirmed that although IBS was not a life-threatening condition, it could lead to significant impairment of quality of life, at least in the subgroup of patients seen in a tertiary referral center.
In conclusion, these data show that IBS patients have a pan-enteric dysmotility with frequent dyspepsia, associated with psychological morbidity and greatly impaired quality of life. The presence of alexithymia, a stable trait, is a novel finding of potential interest to detect subgroups of IBS patients with different patterns of recovery after therapy.
Footnotes
Edited by Wang XL
References
- 1.Camilleri M, Heading RC, Thompson WG. Consensus report: clinical perspectives, mechanisms, diagnosis and management of irritable bowel syndrome. Aliment Pharmacol Ther. 2002;16:1407–1430. doi: 10.1046/j.1365-2036.2002.01305.x. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M. Motor function in irritable bowel syndrome. Can J Gastroenterol. 1999;13 Suppl A:8A–11A. doi: 10.1155/1999/240329. [DOI] [PubMed] [Google Scholar]
- 3.Jones J, Boorman J, Cann P, Forbes A, Gomborone J, Heaton K, Hungin P, Kumar D, Libby G, Spiller R, et al. British Society of Gastroenterology guidelines for the management of the irritable bowel syndrome. Gut. 2000;47 Suppl 2:ii1–i19. doi: 10.1136/gut.47.suppl_2.ii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horwitz BJ, Fisher RS. The irritable bowel syndrome. N Engl J Med. 2001;344:1846–1850. doi: 10.1056/NEJM200106143442407. [DOI] [PubMed] [Google Scholar]
- 6.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 7.Whorwell PJ, McCallum M, Creed FH, Roberts CT. Non-colonic features of irritable bowel syndrome. Gut. 1986;27:37–40. doi: 10.1136/gut.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houghton LA, Whorwell PJ. Opening the doors of perception in the irritable bowel syndrome. Gut. 1997;41:567–568. doi: 10.1136/gut.41.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azpiroz F. Hypersensitivity in functional gastrointestinal disorders. Gut. 2002;51 Suppl 1:i25–i28. doi: 10.1136/gut.51.suppl_1.i25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naliboff BD, Munakata J, Fullerton S, Gracely RH, Kodner A, Harraf F, Mayer EA. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomborone J, Dewsnap P, Libby G, Farthing M. Abnormal illness attitudes in patients with irritable bowel syndrome. J Psychosom Res. 1995;39:227–230. doi: 10.1016/0022-3999(94)00126-p. [DOI] [PubMed] [Google Scholar]
- 12.Hahn BA, Yan S, Strassels S. Impact of irritable bowel syndrome on quality of life and resource use in the United States and United Kingdom. Digestion. 1999;60:77–81. doi: 10.1159/000007593. [DOI] [PubMed] [Google Scholar]
- 13.Kosturek A, Gregory RJ, Sousou AJ, Trief P. Alexithymia and somatic amplification in chronic pain. Psychosomatics. 1998;39:399–404. doi: 10.1016/S0033-3182(98)71298-8. [DOI] [PubMed] [Google Scholar]
- 14.Kruis W, Thieme C, Weinzierl M, Schüssler P, Holl J, Paulus W. A diagnostic score for the irritable bowel syndrome. Its value in the exclusion of organic disease. Gastroenterology. 1984;87:1–7. [PubMed] [Google Scholar]
- 15.Vanner SJ, Depew WT, Paterson WG, DaCosta LR, Groll AG, Simon JB, Djurfeldt M. Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. Am J Gastroenterol. 1999;94:2912–2917. doi: 10.1111/j.1572-0241.1999.01437.x. [DOI] [PubMed] [Google Scholar]
- 16.Böhmer CJ, Tuynman HA. The effect of a lactose-restricted diet in patients with a positive lactose tolerance test, earlier diagnosed as irritable bowel syndrome: a 5-year follow-up study. Eur J Gastroenterol Hepatol. 2001;13:941–944. doi: 10.1097/00042737-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Hveem K, Jones KL, Chatterton BE, Horowitz M. Scintigraphic measurement of gastric emptying and ultrasonographic assessment of antral area: relation to appetite. Gut. 1996;38:816–821. doi: 10.1136/gut.38.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Donnell MR, Virjee J, Heaton KW. Detection of pseudo diar-rhoea by simple assessment of intestinal transit rate. Br Med J. 1990;300:439–440. doi: 10.1136/bmj.300.6722.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talley NJ, Fett SL, Zinsmeister AR, Melton LJ. Gastrointestinal tract symptoms and self-reported abuse: a population-based study. Gastroenterology. 1994;107:1040–1049. doi: 10.1016/0016-5085(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 20.Buckley MJ, Scanlon C, McGurgan P, O'Morain CA. A validated dyspepsia symptom score. Ital J Gastroenterol Hepatol. 1997;29:495–500. [PubMed] [Google Scholar]
- 21.Portincasa P, Altomare DF, Moschetta A, Baldassarre G, Di Ciaula A, Venneman NG, Rinaldi M, Vendemiale G, Memeo V, vanBerge-Henegouwen GP, et al. The effect of acute oral erythromycin on gallbladder motility and on upper gastrointestinal symptoms in gastrectomized patients with and without gallstones: a randomized, placebo-controlled ultrasonographic study. Am J Gastroenterol. 2000;95:3444–3451. doi: 10.1111/j.1572-0241.2000.03282.x. [DOI] [PubMed] [Google Scholar]
- 22.Crown S, Crisp AH. A short clinical diagnostic self-rating scale for psychoneurotic patients. The Middlesex Hospital Question-naire (M.H.Q.) Br J Psychiatry. 1966;112:917–923. doi: 10.1192/bjp.112.490.917. [DOI] [PubMed] [Google Scholar]
- 23.Taylor GJ, Ryan D, Bagby RM. Toward the development of a new self-report alexithymia scale. Psychother Psychosom. 1985;44:191–199. doi: 10.1159/000287912. [DOI] [PubMed] [Google Scholar]
- 24.Bressi C, Taylor G, Parker J, Bressi S, Brambilla V, Aguglia E, Allegranti I, Bongiorno A, Giberti F, Bucca M, et al. Cross validation of the factor structure of the 20-item Toronto Alexithymia Scale: an Italian multicenter study. J Psychosom Res. 1996;41:551–559. doi: 10.1016/s0022-3999(96)00228-0. [DOI] [PubMed] [Google Scholar]
- 25.Porcelli P, Taylor GJ, Bagby RM, De Carne M. Alexithymia and functional gastrointestinal disorders. A comparison with inflammatory bowel disease. Psychother Psychosom. 1999;68:263–269. doi: 10.1159/000012342. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Snow KK, Kosinski M. SF-36 health survey. Manual and interpretation guide. Boston: The Health Institute: New En-gland Medical Center; 1993. [Google Scholar]
- 27.Stewart AL, Greenfield S, Hays RD, Wells K, Rogers WH, Berry SD, McGlynn EA, Ware JE. Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA. 1989;262:907–913. [PubMed] [Google Scholar]
- 28.Portincasa P, Colecchia A, Di Ciaula A, Larocca A, Muraca M, Palasciano G, Roda E, Festi D. Standards for diagnosis of gastrointestinal motility disorders. Section: ultrasonography. A position statement from the Gruppo Italiano di Studio Motilità Apparato Digerente. Dig Liver Dis. 2000;32:160–172. doi: 10.1016/s1590-8658(00)80404-1. [DOI] [PubMed] [Google Scholar]
- 29.Bolondi L, Bortolotti M, Santi V, Calletti T, Gaiani S, Labò G. Measurement of gastric emptying time by real-time ultrasonography. Gastroenterology. 1985;89:752–759. doi: 10.1016/0016-5085(85)90569-4. [DOI] [PubMed] [Google Scholar]
- 30.Altomare DF, Portincasa P, Rinaldi M, Di Ciaula A, Martinelli E, Amoruso A, Palasciano G, Memeo V. Slow-transit constipation: solitary symptom of a systemic gastrointestinal disease. Dis Colon Rectum. 1999;42:231–240. doi: 10.1007/BF02237134. [DOI] [PubMed] [Google Scholar]
- 31.Armitage P, Berry G. Statistical methods in medical research. 3rd ed. Oxford: Blackwell Science Ltd; 1994. [Google Scholar]
- 32.Dawson B, Trapp RG. Basic & Clinical Biostatistics. 3rd ed. New York: McGraw-Hill; 2001. [Google Scholar]
- 33.Jones R, Lydeard S. Irritable bowel syndrome in the general population. BMJ. 1992;304:87–90. doi: 10.1136/bmj.304.6819.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porcelli P, De Carne M, Fava GA. Assessing somatization in functional gastrointestinal disorders: integration of different criteria. Psychother Psychosom. 2000;69:198–204. doi: 10.1159/000012394. [DOI] [PubMed] [Google Scholar]
- 35.Everson GT, Braverman DZ, Johnson ML, Kern F. A critical evaluation of real-time ultrasonography for the study of gallbladder volume and contraction. Gastroenterology. 1980;79:40–46. [PubMed] [Google Scholar]
- 36.Portincasa P, Di Ciaula A, Baldassarre G, Palmieri VO, Gentile A, Cimmino A, Palasciano G. Gallbladder motor function in gall-stone patients: sonographic and in vitro studies on the role of gallstones, smooth muscle function and gallbladder wall inflammation. J Hepatol. 1994;21:430–440. doi: 10.1016/s0168-8278(05)80324-1. [DOI] [PubMed] [Google Scholar]
- 37.Wedmann B, Schmidt G, Wegener M, Coenen C, Ricken D, Althoff J. Effects of age and gender on fat-induced gallbladder contraction and gastric emptying of a caloric liquid meal: a sonographic study. Am J Gastroenterol. 1991;86:1765–1770. [PubMed] [Google Scholar]
- 38.Ricci R, Bontempo I, Corazziari E, La Bella A, Torsoli A. Real time ultrasonography of the gastric antrum. Gut. 1993;34:173–176. doi: 10.1136/gut.34.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergmann JF, Chassany O, Petit A, Triki R, Caulin C, Segrestaa JM. Correlation between echographic gastric emptying and appetite: influence of psyllium. Gut. 1992;33:1042–1043. doi: 10.1136/gut.33.8.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darwiche G, Almér LO, Björgell O, Cederholm C, Nilsson P. Measurement of gastric emptying by standardized real-time ultrasonography in healthy subjects and diabetic patients. J Ultrasound Med. 1999;18:673–682. doi: 10.7863/jum.1999.18.10.673. [DOI] [PubMed] [Google Scholar]
- 41.Strid H, Norström M, Sjöberg J, Simrén M, Svedlund J, Abrahamsson H, Björnsson ES. Impact of sex and psychological factors on the water loading test in functional dyspepsia. Scand J Gastroenterol. 2001;36:725–730. doi: 10.1080/003655201300191987. [DOI] [PubMed] [Google Scholar]
- 42.Portincasa P, Moschetta A, Venneman NG, Palasciano G. Gas-trointestinal motility in patients with chronic functional dyspepsia. Dig Liver Dis (Ital J Gastroenterol) 30 (Suppl. II), A111. 1998 [Google Scholar]
- 43.Agréus L, Svärdsudd K, Nyrén O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995;109:671–680. doi: 10.1016/0016-5085(95)90373-9. [DOI] [PubMed] [Google Scholar]
- 44.Wilmer A, Van Cutsem E, Andrioli A, Tack J, Coremans G, Janssens J. Ambulatory gastrojejunal manometry in severe motility-like dyspepsia: lack of correlation between dysmotility, symptoms, and gastric emptying. Gut. 1998;42:235–242. doi: 10.1136/gut.42.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samsom M, Verhagen MA, vanBerge Henegouwen GP, Smout AJ. Abnormal clearance of exogenous acid and increased acid sensitivity of the proximal duodenum in dyspeptic patients. Gastroenterology. 1999;116:515–520. doi: 10.1016/s0016-5085(99)70171-x. [DOI] [PubMed] [Google Scholar]
- 46.Braverman DZ. Gallbladder contraction in patients with irritable bowel syndrome. Isr J Med Sci. 1987;23:181–184. [PubMed] [Google Scholar]
- 47.Kellow JE, Miller LJ, Phillips SF, Zinsmeister AR, Charboneau JW. Altered sensitivity of the gallbladder to cholecystokinin octapeptide in irritable bowel syndrome. Am J Physiol. 1987;253:G650–G655. doi: 10.1152/ajpgi.1987.253.5.G650. [DOI] [PubMed] [Google Scholar]
- 48.Sjölund K, Ekman R, Lindgren S, Rehfeld JF. Disturbed motilin and cholecystokinin release in the irritable bowel syndrome. Scand J Gastroenterol. 1996;31:1110–1114. doi: 10.3109/00365529609036895. [DOI] [PubMed] [Google Scholar]
- 49.Kellow JE, Eckersley GM, Jones M. Enteric and central contributions to intestinal dysmotility in irritable bowel syndrome. Dig Dis Sci. 1992;37:168–174. doi: 10.1007/BF01308167. [DOI] [PubMed] [Google Scholar]
- 50.Vassallo M, Camilleri M, Phillips SF, Brown ML, Chapman NJ, Thomforde GM. Transit through the proximal colon influences stool weight in the irritable bowel syndrome. Gastroenterology. 1992;102:102–108. doi: 10.1016/0016-5085(92)91789-7. [DOI] [PubMed] [Google Scholar]
- 51.Kellow JE, Phillips SF. Functional disorders of the small intestine. In: Snape WJJ, editor. Pathogenesis of functional bowel disorders. New York: Plenum Publ Corp; 1989. pp. 171–198. [Google Scholar]
- 52.Stivland T, Camilleri M, Vassallo M, Proano M, Rath D, Brown M, Thomforde G, Pemberton J, Phillips S. Scintigraphic measurement of regional gut transit in idiopathic constipation. Gastroenterology. 1991;101:107–115. doi: 10.1016/0016-5085(91)90466-x. [DOI] [PubMed] [Google Scholar]
- 53.Kellow JE, Phillips SF. Altered small bowel motility in irritable bowel syndrome is correlated with symptoms. Gastroenterology. 1987;92:1885–1893. doi: 10.1016/0016-5085(87)90620-2. [DOI] [PubMed] [Google Scholar]
- 54.Whorwell PJ. The problem of gas in irritable bowel syndrome. Am J Gastroenterol. 2000;95:1618–1619. doi: 10.1111/j.1572-0241.2000.02169.x. [DOI] [PubMed] [Google Scholar]
- 55.Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut. 2001;48:14–19. doi: 10.1136/gut.48.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352:1187–1189. doi: 10.1016/s0140-6736(98)02146-1. [DOI] [PubMed] [Google Scholar]
- 57.Portincasa P, Moschetta A, Radicione T, Pugliese S, Castore A, Salerno MT, Palasciano G. Coexistence of diffuse gastrointesti-nal dysmotility, dyspepsia and autonomic dysfunction in chronic alcoholism. Gastroenterology. 2001;120(Suppl1):A1496. [Google Scholar]
- 58.Tolliver BA, Jackson MS, Jackson KL, Barnett ED, Chastang JF, DiPalma JA. Does lactose maldigestion really play a role in the irritable bowel? J Clin Gastroenterol. 1996;23:15–17. doi: 10.1097/00004836-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Sanders DS, Carter MJ, Hurlstone DP, Pearce A, Ward AM, McAlindon ME, Lobo AJ. Association of adult coeliac disease with irritable bowel syndrome: a case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet. 2001;358:1504–1508. doi: 10.1016/S0140-6736(01)06581-3. [DOI] [PubMed] [Google Scholar]
- 60.Hamm LR, Sorrells SC, Harding JP, Northcutt AR, Heath AT, Kapke GF, Hunt CM, Mangel AW. Additional investigations fail to alter the diagnosis of irritable bowel syndrome in subjects fulfilling the Rome criteria. Am J Gastroenterol. 1999;94:1279–1282. doi: 10.1111/j.1572-0241.1999.01077.x. [DOI] [PubMed] [Google Scholar]
- 61.Whitehead WE, Burnett CK, Cook EW, Taub E. Impact of irritable bowel syndrome on quality of life. Dig Dis Sci. 1996;41:2248–2253. doi: 10.1007/BF02071408. [DOI] [PubMed] [Google Scholar]
- 62.Drossman DA, McKee DC, Sandler RS, Mitchell CM, Cramer EM, Lowman BC, Burger AL. Psychosocial factors in the irritable bowel syndrome. A multivariate study of patients and nonpatients with irritable bowel syndrome. Gastroenterology. 1988;95:701–708. doi: 10.1016/s0016-5085(88)80017-9. [DOI] [PubMed] [Google Scholar]
- 63.Fullwood A, Drossman DA. The relationship of psychiatric illness with gastrointestinal disease. Annu Rev Med. 1995;46:483–496. doi: 10.1146/annurev.med.46.1.483. [DOI] [PubMed] [Google Scholar]
- 64.Whitehead WE, Bosmajian L, Zonderman AB, Costa PT, Schuster MM. Symptoms of psychologic distress associated with irritable bowel syndrome. Comparison of community and medical clinic samples. Gastroenterology. 1988;95:709–714. doi: 10.1016/s0016-5085(88)80018-0. [DOI] [PubMed] [Google Scholar]
- 65.Gorard DA, Gomborone JE, Libby GW, Farthing MJ. Intestinal transit in anxiety and depression. Gut. 1996;39:551–555. doi: 10.1136/gut.39.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bagby RM, Taylor GJ, Parker JD. Construct validity of the Toronto Alexithymia Scale. Psychother Psychosom. 1988;50:29–34. doi: 10.1159/000288097. [DOI] [PubMed] [Google Scholar]
- 67.Taylor GJ, Parker JD, Bagby RM, Acklin MW. Alexithymia and somatic complaints in psychiatric out-patients. J Psychosom Res. 1992;36:417–424. doi: 10.1016/0022-3999(92)90002-j. [DOI] [PubMed] [Google Scholar]


